Abstract
As newer methods of management are made available, and accessible, survival rates with human immunodeficiency virus (HIV) are increasing. This means that chronic, metabolic complications of HIV are becoming more frequent in clinical practice, as acute morbidity is controlled. Management of HIV/acquired immunodeficiency syndrome (AIDS) is gradually expanding to include these chronic and metabolic complications of the disease, and the adverse effects associated with its treatments, including diabetes. Unfortunately, no guidelines are available to help the medical practitioners choose appropriate therapy for patients with these conditions. The aim of the South Asian Consensus Guidelines is to provide evidence-based recommendations to assist healthcare providers in the rational management of type 2 diabetes mellitus in patients with HIV. The development of these guidelines used systematic reviews of available evidence to form its key recommendations. These guidelines and associated review of literature represent a compilation of available knowledge regarding rational management of diabetes in HIV. Patients of diabetes with concomitant HIV infection are managed optimally with insulin therapy and judicious use of highly active antiretroviral therapy with suitable alternatives is also recommended. These guidelines should prove helpful to physicians, not only in South Asia, but also across the globe, while managing patients with coexistent HIV and diabetes.
Keywords: Diabetes, human immunodeficiency virus, South Asian Guidelines
INTRODUCTION
Patients with human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) are increasing in number, partly due to improved methods of screening, diagnosis and treatment. Figures reveal that the total number of patients with HIV is 33 million, with 2.7 million new infections in 2007.[1] Most of these patients are in sub-Saharan Africa and Asia.
As newer methods of management are made available, and accessible, survival rates with HIV are increasing. This means that chronic metabolic complications of HIV are becoming more frequent in clinical practice, as acute morbidity is controlled.
Management of HIV/AIDS is gradually expanding to include these chronic and metabolic complications of the disease, and the adverse effects associated with its treatments, including diabetes. Unfortunately, no guidelines are available to help medical practitioners to choose appropriate therapy for patients with these conditions.
An international cross-sectional study of 788 HIV-infected adults recruited at 32 centers was carried out to assess the metabolic syndrome prevalence using International Diabetes Federation (IDF) and US National Cholesterol Education Program Adult Treatment Panel III (ATPIII) criteria, relative to body composition (whole-body dual-energy X-ray absorptiometry and abdominal computed tomography), lipids, glycemic parameters, insulin resistance, leptin, adiponectin, and C-reactive protein (CRP).[2]
The prevalence of metabolic syndrome was 14% (n = 114; 83 men) by IDF criteria and 18% (n = 139; 118 men) by ATPIII criteria. Half of the patients (49%) exhibited two or more features of metabolic syndrome but were not classified as having the syndrome because they had normal or low waist circumferences or waist-to-hip ratios. Metabolic syndrome was more common in those currently receiving protease inhibitors (PIs; P = 0.04). Type 2 diabetes prevalence was fivefold to ninefold higher in those with metabolic syndrome.[2]
In another study, the incidence of new-onset diabetes in HIV-infected persons was significantly high. Over 130,151 person-years of follow-up (PYFU) in the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study, diabetes was diagnosed in 744 patients [incidence rate of 5.72 per 1000 PYFU (95% CI 5.31–6.13)]. The incidence of diabetes increased with cumulative exposure to combination ART. The strongest relationship with diabetes was exposure to stavudine, while treatment with zidovudine and didanosine was also associated with an increased risk of diabetes.[3]
A retrospective study examined whether the goals set forth by the American Diabetes Association were being attained in an HIV specialty clinic run by internal medicine physicians. Overall, less than 50% of patients were achieving goals of therapy for hemoglobin A1c (HbA1c), cholesterol, triglycerides, and blood pressure. The findings emphasize that clinicians need to be aware of the concomitant disease states that HIV patients have and to treat those disease states to the standard of care set forward.[4]
Thus, there is a need for definitive guidelines to help physicians improve glycemic and metabolic control in patients infected with HIV.
Guideline objectives
The aim of the consensus guidelines is to provide recommendations to assist healthcare providers in the rational management of diabetes and HIV. These guidelines do not cover the clinical examination, investigations or treatment of other complications of HIV/AIDS.
Clinical questions
The clinical questions answered by these guidelines are:
Why are patients with HIV more prone to diabetes?
What methods should be used to screen, diagnose and monitor diabetes in HIV patients?
What drugs should be used for rational management of diabetes in HIV?
METHODOLOGY
Evidence, identification and search strategy
The questions to be answered were approved by the consensus group members who met during various occasions in 2010 and 2011. A search of the literature was done for systematic reviews, graded by Amstar, randomized controlled trials, graded by graded score, observational studies, graded by SIGN 50, letters to editors and case reports. The evidence presented in these guidelines was collated from a systematic review of relevant published literature (up to 2011) as identified by electronic (e.g. Medline) search, and standard textbooks.
Consensus group felt that therapy of diabetes in HIV was a priority area being neglected by HIV specialists, for want of appropriate guidelines.
A first-level selection of abstracts was done by the task force, followed by a second-level selection of full text articles. Quality assessment of these articles was done as detailed above. Data selection and data description was done for the chosen articles, focusing on the rationale of management. The task force used systematic reviews of available evidence to write its key recommendations.
A first draft of the guidelines was prepared by the task force in August 2010. It was then passed on to the expert committee members for their suggestions and recommendations. The second, corrected draft was circulated to professional leaders from different specialities (endocrinology, internal medicine, HIV, pharmacology), and lay members of institutional ethics committees, to ensure involvement of all stakeholders, across both countries, for correction and suggestion.
Two external reviewers representing basic and clinical sciences from outside South Asia then read the third draft of the consensus statement and made recommendations, which were incorporated in the document.
Various corrections, additions and suggestions were incorporated, and a fourth draft was presented to all members by e-mail. After incorporating suggestions and corrections, a fifth version of the consensus guidelines was prepared, and is being published.
PATHOGENESIS OF DIABETES
Risk factors that contribute to development of metabolic syndrome in HIV-infected patients are age, gender, duration of HIV infection, CD4 count, viral burden, body mass index, and waist circumference, waist-to-hip ratio, socioeconomic class, and culture.[3]
Impaired glucose tolerance and insulin resistance are noted to precede weight loss in patients with HIV.[3,5] Insulin resistance, rather than insulin deficiency, is implicated in the pathogenesis of diabetes in HIV-infected patients. According to early reports, evidence of islet cell autoimmunity or beta cell destruction is not seen in HIV patients.[6] However, autoimmune diabetes has recently been reported in HIV-infected patients.[7]
Concurrent use of opiates, however, may alter beta cell function,[8] while heroin addiction is associated with insulin resistance.
HIV infection is linked with hepatitis C infection, which is associated with insulin resistance and diabetes due to increased intrahepatic tumor necrosis factor (TNF-α) and hepatic steatosis.[8]
HIV is also associated with various endocrine abnormalities, including those of the growth hormone axis. Growth hormone deficiency may contribute to insulin resistance in HIV-infected patients.[9]
The increased accumulation of visceral fat, with wasting of subcutaneous fat, noted in these patients, creates higher levels of inflammatory cytokines such as TNF-α and increases insulin resistance.[10]
Viral factors which contribute to diabetes risk are an increase in viral burden of 0.5 log over a 6-month period, a lower CD4 count, and longer duration of HIV infection.
The major contributor to hyperglycemia in HIV/AIDS, however, is iatrogenic.[11] The past few decades have seen remarkable improvement in the clinical outcome of HIV patients, thanks to highly active antiretroviral therapy (HAART). Benefits include suppression of viral load, improvement in CD4 count, decrease in opportunistic infections and length of hospital stay, and reduction in mortality.[12]
HAART, however, has also led to an increase in metabolic dysfunction, including insulin resistance, diabetes dyslipidemia and lipodystrophy.[12]
A recent analysis has found that diabetes is fourfold more common is HIV-infected men exposed to HAART than in HIV-seronegative men.[13] PIs which have been used extensively as antiretroviral agents are the mainstay of HAART. This class of drugs includes atazanavir, darunavir and saquinair.
PIs have been shown to increase insulin resistance and reduce insulin secretion by interfering with GLUT-4 mediated glucose transport. A positive family history of diabetes, weight gain, lipodystrophy, old age and hepatitis C infection[12] predisposes to diabetes. PIs interfere with cellular retinoic acid-binding protein type 1 (CRABP 1) that interacts with peroxisomal proliferator-activated receptor (PPAR)-γ. Inhibition of PPAR-γ promotes adipocyte inflammation, release of free fatty acids and insulin resistance.[10] Hyperglycemia due to PIs usually resolves when the offending drug is stopped.
All PIs do not have the same metabolic effects [Table 1]. Indinavir induces insulin resistance with no effect on lipid metabolism, while lopinavir and ritonavir increase fasting triglycerides and free fatty acids, without affecting insulin sensitivity. Indinavir and ritonavir both block GLUT-4, but no such effect is noted with amprenavir and atazanazvir. Patients treated with nelfinavir, indinavir, liponavir or saquinavir demonstrate alterations in first-phase insulin release with a 25% reduction in β-cell dysfunction.[14]
Table 1.
Drug treatment for human immunodeficiency virus and the impact of drugs on pathogenesis of diabetes mellitus
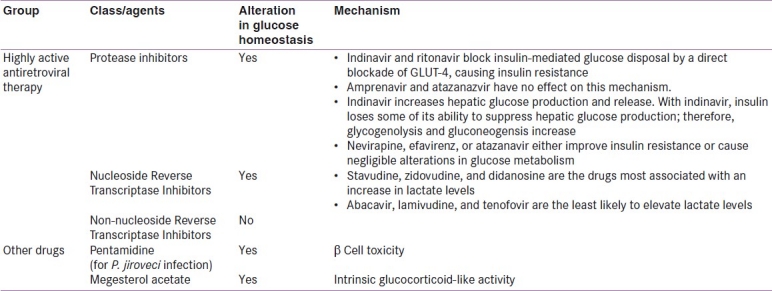
Thus, there is no class effect of PIs on diabetes and various PIs should be studied individually with respect to their metabolic effects.[15]
The other class of drugs which is used is the nucleoside analogs (Nucleoside Reverse Transcriptase Inhibitors or NRTIs). It was earlier felt that NRTIs were less likely to cause metabolic abnormalities. A recent study which analyzed 130,151 p erson years of exposure, however, has shown that these drugs increase the risk of diabetes.[16]
The risk is highest with stavudine, but is also significant with zidovudine and didanosine. Proposed mechanisms include insulin resistance, lipodystrophy, and mitochondrial dysfunction.[17]
PIs seem to confer acute metabolic risks, while NRTIs confer cumulative risks of diabetes in predisposed, exposed persons. Exposure to a combination of NRTI and indinavir (a PI) has been shown to be an additional risk factor for the onset of diabetes.[16]
Drugs used to manage comorbid conditions associated with AIDS may also cause diabetes. Pentamidine, which is used to prevent and treat Pneumocystis jiroveci associated pneumonia, can cause β-cell toxicity, with acute hypoglycemia followed by later diabetes. Factors associated with increased risk of hypoglycemia are longer and higher dosage of pentamidine, as well as renal insufficiency. The group of patients who progressed to diabetes had low C peptide levels, suggestive of β-cell destruction.[17]
Megestrol acetate, an appetite stimulant, predisposes to diabetes because of its intrinsic glucocorticoid like activity, increased caloric intake and weight gain.[18] Hypoglycemia has been noted to resolve once megestrol is stopped, and to recur on rechallenging.[19]
Patients on HAART may also be predisposed to diabetes because of the improved nutritional status and weight gain that accompanies effective treatment of HIV.
EVIDENCE/RECOMMENDATIONS FOR SCREENING
Glycemic values
Studies have shown that screening for diabetes should be done in the HIV care setting. The AIDS Society-USA panel recommends fasting plasma glucose as the test of choice for screening.[20] This should be tested before initiating HAART, and periodically thereafter. Other studies have shown that an oral glucose tolerance test is a better method of detecting diabetes.[21] In the Asian setting, postprandial glucose values should also be checked, as the role of insulin resistance in the pathogenesis of diabetes is important.
HbA1c is not an accurate assessment of glycemic control in HIV patients, as hemolysis, caused by dapsone and ribavarin, and other infections can shorten the lifespan of the red blood cell.[22] HbA1c may be falsely lowered in such cases. Fructosamine, too, may be inaccurate in patients with low serum albumin levels.[22]
Recommendations
The South Asian Consensus Guidelines recommend that both fasting and postprandial glucose values should be checked at screening and during monitoring of therapy. Venous samples should be taken for diagnosis. All aseptic precautions should be followed while handling HIV-contaminated blood samples.
EVIDENCE/RECOMMENDATIONS FOR NON-PHARMACOLOGICAL AND NON-GLYCEMIC THERAPY
Management strategies for diabetes are somewhat different in HIV patients than in the general population.
General measures
Modification of risk factors such as hypertension, dyslipidemia, platelet function should be done through non-pharmacological and pharmacological methods, as in non-HIV infected patients. Precipitating factors such as tuberculosis, which is present in one-third of all HIV patients,[1] should be searched for and treated aggressively.
Dyslipidemia is common in HIV, and treatment guidelines are available to help practitioners handle this complication.[23] Pravastatin and fluvastatin are safe to use with ritonavir, atorvastatin and rosuvastatin should be used with caution, while simvastatin is contraindicated in patients on these PIs.[24]
Hypertension, too, is a frequent comorbid condition. The routinely used antihypertensives, such as angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), may not be optimal choices in patients with HIV.[24] Captopril has been associated with Kaposi's sarcoma, hepatotoxicity and neurotoxicity. Enalapril is linked with muscle pain, weakness and diarrhea. ARBs may compete with other drugs that are metabolized by the cytochrome P450 isoenzyme.
Lipid elevation with triglycerides remains the most commonly observed change in HIV patients. Ritonavir is the principal antiretroviral (ARV) drug most commonly associated with this lipid change; other ARVs have also been implicated. Data suggest that fish oil and fenofibrate alone produce significant decreases in triglyceride (TG) levels.[25] However, the proportion of patients achieving the National Cholesterol Education Programme (NCEP) recommended target of ≤200 mg/dL using either of the two medications alone is quite low. The combination of these two agents has been found to reduce TG levels beyond monotherapy with each of these agents and also increases the proportion of subjects with a TG level ≤200 mg/dL. Bezafibrate also seems to be safe and effective for the reduction of hypertriglyceridemia in HIV-infected patients on HAART.[26] Though little is known about the comparative effectiveness of statins among HIV-infected patients, it appears that atorvastatin and rosuvastatin are preferable to pravastatin for the treatment of HIV-infected patients with dyslipidemia. This may be due to greater declines in total cholesterol, low density lipoprotein-cholesterol (LDL-C), and non–high density lipoprotein-cholesterol (non–HDL-C), with similar lower toxicity rates. These findings are also consistent with the recent British guidelines that recommend the use of rosuvastatin in HIV patients with dyslipidemia.[27]
The South Asian Consensus Guidelines advocate appropriate modification of all associated metabolic risk factors in HIV by using appropriate non-pharmacological and pharmacological methods, as detailed above.
Lifestyle modification
Diet, physical activity/exercise and cessation of smoking are as important in HIV-infected as in non-infected persons. Weekly counseling by a dietician with main emphasis on healthy eating and limited emphasis on weight loss has been shown to reduce blood pressure, waist circumference and HbA1c significantly,[28] along with a reduction in caloric intake and percentage of calories from saturated fat, as well as increased fiber intake. Many patients of HIV are cachexic and need increased caloric intake to improve general health. There should be limited emphasis on weight loss and more emphasis on healthy eating.
Endurance and resistance exercise has been shown to have positive effects on metabolic parameters in HIV-infected patients.[29] Similar results, including decreased blood pressure, increased strength and endurance, lower cholesterol and increased insulin sensitivity,[30] are seen with weight lifting and aerobic/endurance exercise.
Smoking cessation is critical for optimal health. Newer non-nicotine replacement therapy should be used in caution in HIV patients. Varenicline and bupropion may interact with drugs metabolized by the cytochrome P450 isoenzyme, e.g., ritonavir, efavirenz and nelfinavir.[31]
Psychosocial management
While psychosocial support is an integral part of effective diabetes management, it is of utmost importance in patients who have to handle the double stress of diabetes and HIV.
Strategies to support behavior change need to be individualized, culture-, age-, and gender-appropriate, negotiable, flexible and dynamic. The WATER (welcome warmly, ask and assess, tell truthfully, explain with empathy, reassure and return) approach coined at Bharti Hospital, Karnal, is an effective method of motivational interviewing.[32] Coping skills training should be provided to all HIV patients and should focus on enhancing posture coping strategies, confidence and self-esteem. The AEIOU (ask and analyze, eliminate negative strategies, internalize posture skills, observe on ongoing basis, upgrade) method is a simple and time-efficient way of doing so.[33]
Recommendations
The South Asian Consensus Guidelines recommend diet, physical activity and pyschosocial support should be emphasized in patients with diabetes and HIV.
EVIDENCE/RECOMMENDATIONS FOR ANTIDIABETIC THERAPY
Oral antidiabetic drugs
Oral antidiabetic drugs (OADs) are frequently used in patients with type 2 diabetes mellitus. The patient with coexistent HIV infection, however, poses a special challenge to the treating physician. The choice of OADs is influenced by many factors unique to the HIV setting.
This patient is at greater risk of comorbidities such as hepatitis C, tuberculosis or other opportunistic infections and may have severe insulin resistance. The number of concomitant medications is greater, leading to an increased chance of drug interactions. Because of impaired renal and hepatic function, the risk of adverse events and drug toxicity may be higher.[7]
One should therefore choose an OAD regime in patients with diabetes and HIV with great care.[7]
Metformin is the first-line drug of choice in most persons with type 2 diabetes, but should be used with caution in HIV.[7]
Though it improves insulin sensitivity, it may not be well tolerated by cachexic patients. Metformin is more likely to cause diarrhea than other drugs.[34]
It is contraindicated in renal or hepatic dysfunction and may lead to metformin-associated lactic acidosis (MALA). It should be avoided in combination with drugs such as stavudine and tenofovir, which also increase the risk of lactic acidosis.[7] Abacavir, lamivudine and tenofovir are the least likely drugs to cause elevation of lactate levels. However, there is no rationale for ordering lactic acid tests for asymptomatic patients at any time during HIV care. Interruption of NRTI therapy is recommended for symptomatic patients with a venous lactate level of 15 mmol/L.[35]
HIV patients on metformin should be educated about the symptoms of lactic acidosis, including fatigue, weight loss, nausea, abdominal pain, dyspnea, and arrhythmias. Liver-related symptoms such as tender hepatomegaly, edema, ascites and encephalopathy may occur, but jaundice is uncommon.[36]
Metformin should be avoided in patients with comorbid conditions such as tuberculosis, weight loss, cachexia, ketonuria and lipoatrophy. Further reductions in subcutaneous fat can occur with this drug.[37]
The thiazolidinediones have a mechanism of action which should make them drugs of choice in HIV. The possibility of a slight increase in subcutaneous fat makes them the preferred drug class in patients with lipodystrophy.[7] However, they are contraindicated in hepatic dysfunction and heart failure, may cause edema, increase cardiovascular morbidity, worsen osteoporosis, predispose to bladder cancer, and decrease hematocrit. These side effects prevent wide usage of these drugs in type 2 diabetes as well as in HIV-associated diabetes.
Insulin secretagogues, such as repaglinide and sulfonylureas (glimiperide, glicazide, glibenclamide) are safe, but may not be effective in the face of severe insulin resistance. However, amongst the OADs, they have a faster onset of action, and may be used in appropriate doses, provided there is no ketonuria. The meglitinides address the defect in first-phase insulin secretion that is seen with certain PIs and may be an appropriate choice of OADs.[24]
The newer class of OADs, the dipeptidyl peptidase (IV) inhibitors, i.e., saxagliptin, sitagliptin, and vildagliptin, have not been studied in patients with HIV and diabetes. There is a theoretical risk of immunodeficiency and exacerbation of infections[38] with these drugs, however, which prevent them from being used as first-line drugs in HIV.
Recommendations
The South Asian Consensus suggests OADs should be prescribed with care in patients with diabetes and HIV. The prescribing physician should be aware of the contraindications and side effects of various drugs. Patients should be educated about warning symptoms of lactic acidosis.
Insulin therapy
Few drug interaction studies have been done between antidiabetic drugs and antiretroviral molecules, but the potential for these, and the frequent administration of polypharmacy for other comorbid conditions, implies that insulin is a safer alternative in HIV-infected patients. HIV-infected patients should be taught how to dispose of lancets, glucose strips, insulin syringes, pens and needles, to prevent HIV transmission.
Insulin is the drug of choice for management of diabetes with HIV. Insulin has an anabolic effect, is known to reduce inflammatory markers such as TNF-α, does not have any interactions with antiretroviral or other drugs, is not contraindicated with renal or hepatic dysfunction, does not reduce appetite or cause gastrointestinal side effects, can correct both insulin deficiency and resistance when given in appropriate doses, and does not increase the risk of cardiovascular disease.[39]
Insulin therapy should be initiated at the outset, using basal bolus regime or premixed insulin. The American Association of Clinical Endocrinologists (AACE) recommends the use of modern insulins or insulin analogues, as they are more predictable in action and cause less hypoglycemia. The use of traditional human insulin is discouraged by AACE.[40] However, human insulins can be used as suitable alternatives to modern insulins where the latter may not be available.
Insulin requirements are high to begin with and fall after a few weeks, once glucotoxicity is corrected and infection controlled. Insulin requirements may rise as appetite returns to normal and caloric intake increases. Sick patients should be tested for ketonuria.[41] Rapid acting analogues such as aspart insulin may obviate the need for admission in patients with ketonuria[42] and are useful for critically ill patients as well.[43] The Indian Insulin guidelines suggest the use of premixed analogues as a safe, effective and acceptable method of insulin initiation on OPD basis. The initial recommended dose is 10 units daily.[44]
Lipodystrophy may add to the confounding factors of insulin absorption and actions in patients with HIV/AIDS. This has not been studied well in HIV patients and requires attention when dealing with patients having substantial lipodystrophy.[44]
Recommendations
The South Asian Consensus Guidelines suggests that insulin is the drug of choice for patients with diabetes and HIV, and should be prescribed as indicated.
Other injectable drugs
Effects similar to those seen in the general population may be expected with incretin mimetics in HIV-infected patients.[23] Liraglutide has recently been reported to improve various indices of insulin sensitivity, including HOMA-IR, blood pressure and weight, apart from achieving effective glycemic control.[45] These properties may make it worthwhile to study the effect of liraglutide in HIV-associated diabetes.
Off label use of exenatide for the management of insulin-resistant patients has been seen.[46] However, there is a need to have more extensive experience and clinical data before these modalities become standard of care in patients with HIV infection.
EVIDENCE/RECOMMENDATION FOR CHANGES IN HAART
PIs-based regimes should be avoided in patients at high risk of developing diabetes, e.g., those with a history of gestational diabetes, a positive family history of diabetes, or impaired glucose tolerance on screening. Indinavir should be avoided and replaced with less toxic drugs.
Structured treatment interruption should be avoided as it increases the risk of death, opportunistic disease and myocardial infarction.[47,48]
Patients should be counseled about the potential risks, discomforts and benefits of HAART, and encouraged to follow a healthy lifestyle while monitoring glycemia regularly.
Recommendations
The South Asian Consensus Guidelines recommend rational choice of HAART, using safest possible alternatives.
CONCLUSIONS
Multiple well-conducted studies with adequate power are required to assess the efficacy of various antidiabetics and their combinations in patients with HIV.
As the management of HIV improves and access to HAART becomes more undesired, the incidence of HIV-related diabetes will rise.
Thus, there will be a greater need for resources to help physicians manage this condition. However, insulin happens to be the optimal choice for management of diabetes in patients afflicted with diabetes. At the same time, the judicious use of HAART is as important for managing the primary pathophysiology of HIV/AIDS.
These guidelines and associated review of literature represent a compilation of the available knowledge regarding rational management of diabetes in HIV. These guidelines should prove helpful to physicians, not only in South Asia, but also across the globe, while managing patients with coexistent HIV and diabetes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1. [Last Accessed 2010 Apr 25]. Available from: http://data.unaids.org/pub/GlobalReport/2008/jc1510_2008_global_report_pp29_62_en.pdf .
- 2.Samaras K, Wand H, Law M, Emery S, Cooper D, Carr A. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Federation and Adult treatment Panel III criteria: Associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes Care. 2007;30:113–9. doi: 10.2337/dc06-1075. [DOI] [PubMed] [Google Scholar]
- 3.De Wit S, Sabin CA, Weber R, Worm SW, Reiss P, Cazanave C, et al. Incidence and Risk Factors for New-Onset Diabetes in HIV-Infected Patients.The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D). Study. Diabetes Care. 2008;31:1224–9. doi: 10.2337/dc07-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bury JE, Stroup JS, Stephens JR, Baker DL. Achieving American Diabetes Association goals in HIV-seropositive patients with diabetes mellitus. Proc (Bayl Univ Med Cent) 2007;20:118–23. doi: 10.1080/08998280.2007.11928265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mondy K, Oovertan ET, Grubb J, Tong S, Seyfried W, Powderly W, et al. Metabolic syndrome in HIV- infected patiets from an urban, Midwestern US outpatient population. Clin Infec Dis. 2007;44:726–34. doi: 10.1086/511679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagogo-Jack S. HIV therapy and diabetes risk. Diabetes Care. 2008;31:1267–8. doi: 10.2337/dc08-0459. [DOI] [PubMed] [Google Scholar]
- 7.Kalra S, Kalra B, Agrawal N, Unnikrishnan AG. Understanding diabetes in patients with HIV/AIDS. Diabetol Metab Syndr. 2011;3:2. doi: 10.1186/1758-5996-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsson R, Capili B, Eckert- Norton M, Colagreco JP, Anastasi JK. Disorders of glucose metabolism in the context of human immunodeficiency virus infection. J Am Acad Nurse Pract. 2006;18:92–103. doi: 10.1111/j.1745-7599.2006.00109.x. [DOI] [PubMed] [Google Scholar]
- 9.Smith JC, Evans LM, Wilkinson I, Goodfellow J, Cockcroft JR, Scanlon MF, et al. Effects of GH replacement on endothelial function and large artery stiffness in GH- deficient adults: A randomized, double-blind, placebo-controlled study. Clin Endocrinol (Oxf) 2002;56:493–501. doi: 10.1046/j.1365-2265.2002.01514.x. [DOI] [PubMed] [Google Scholar]
- 10.Vigouroux C, Maachi M, Nguyen TH, Coussieu C, Gharakhanian S, Funahashi T, et al. Serum adipocytokines are related to lipodystrophy and metabolic disorder in HIV- infected men under antiretroviral therapy. AIDS. 2003;17:1503–11. doi: 10.1097/00002030-200307040-00011. [DOI] [PubMed] [Google Scholar]
- 11.Dagogo-Jack S. New drugs and diabetes risk: antipsychotic and antiretroviral agents. In: Fonseca VA, editor. Clinical diabetes. Philadelphia: Saunders; 2006. pp. 569–81. [Google Scholar]
- 12.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes in a multicenter AIDS Cohort study. Arch Intern Med. 2005;165:1179–84. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 13.Woerle HJ, Marivz PR, Meyer C, Reichman RC, Popa EM, Dostou JM, et al. Mechanisms for the deterioration in glucose tolerance associated with protease inhibitor regumem. Diabetes. 2003;52:918–25. doi: 10.2337/diabetes.52.4.918. [DOI] [PubMed] [Google Scholar]
- 14.Lee GA, Rao MN, Greenfeld C. The effects of HIV Protease inhibitors on carbohydrate and lipid metabolism. Curr Infect Dis Rep. 2004;6:471–82. doi: 10.1007/s11908-004-0067-5. [DOI] [PubMed] [Google Scholar]
- 15.De Wit S, Sabin CA, Weber R, Worm SW, Reiss P, Cazanave C, et al. Incidence and risk factors for new - onset Diabetes in HIV -infected patients: the data collection on adverse events of anti- HIV drugs (D:A:D) study. Diabetes Care. 2008;31:1224–9. doi: 10.2337/dc07-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleishman A, Johnsen S, Systrom DM, Hrovst M, Farrar CT, Frontea W, et al. Effects of a nucleoside reverse transcriptase inhibitor, stavudine, on glucose disposal and mitochondrial function in muscle of healthy adults. Am J Physiol Endocrinol Metab. 2007;292:E1666–73. doi: 10.1152/ajpendo.00550.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waskin H, Stehr-Green JK, Helmick CG, Sattler FR. Risk factors for hypoglycemia associated with pentamidine therapy for Pneumocystis pneumonia. JAMA. 1988;260:345–7. [PubMed] [Google Scholar]
- 18.Schambelan M, Benson CA, Carr A, Currier JS, Dube MP, Gerber JG, et al. Management of metabolic complications associated with antiretroviral therapy for HIV-1 infection: Recommendations of an International AIDS Society-USA Panel. J Acquir Immune Defic Syndr. 2002;31:257–75. doi: 10.1097/00126334-200211010-00001. [DOI] [PubMed] [Google Scholar]
- 19.Shing-shing Y, Schuster MW. Megestrol acetate in cachexia and anorexia. Int J Nanomedicine. 2006;1:411–6. doi: 10.2147/nano.2006.1.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beatty G, Khalili M, Abbasi F, Chu J, Reaven GM, Rosen A, et al. Quantification of insulin-mediated glucose-disposal in HIV-infected individual: Comparision of patients treated and untreated with protease inhibitors. J Acquir Immune Defic Syndr. 2003;33:34–40. doi: 10.1097/00126334-200305010-00006. [DOI] [PubMed] [Google Scholar]
- 21.Polgreen PM, Putz D, Stapleton JT. Inaccurate glycosylated hemoglobin A1c measurements in human immunodeficiency virus-positive patients with diabetes mellitus. Clin Infec Dis. 2003;37:e53–6. doi: 10.1086/376633. [DOI] [PubMed] [Google Scholar]
- 22.Lundgren JD, Battegay M, Behrens G, De Wit S, Guaraldi G, Katlame C, et al. European AIDS clinical Society (EACS) guidelines on the prevention and management of metabolic diseases in HIV. HIV Med. 2008;9:72–81. doi: 10.1111/j.1468-1293.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 23.Brown TT. Approach to the Human immunodeficiency virus- infected patient with lipodystrophy. J Clin Endocrinol Metab. 2008;93:2937–45. doi: 10.1210/jc.2008-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feigenbaum K, Longstaff L. Management of the metabolic syndrome in patients with human immunodeficency virus. [Last accessed on 2010 Apr 21];The Diabetes Educator. 2010 doi: 10.1177/0145721710363619. Published online as doi:10.1177/0145721710363610. [DOI] [PubMed] [Google Scholar]
- 25.Gerber JG, Kitch DW, Fichtenbaum CJ, Zackin RA, Charles S, Hogg E, et al. Fish Oil and Fenofibrate for the Treatment of Hypertriglyceridemia in HIV-infected Subjects on Antiretroviral Therapy: Results of ACTG A5186. J Acquir Immune Defic Syndr. 2008;47:459–66. doi: 10.1097/QAI.0b013e31815bace2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geraix J, de Souza ME, Delatim FC, Pereira PC. Bezafibrate for the treatment of hypertriglyceridemia in HIV1-infected patients on highly active antiretroviral therapy. Braz J Infect Dis. 2006;10:159–64. doi: 10.1590/s1413-86702006000300001. [DOI] [PubMed] [Google Scholar]
- 27.Singh S, Willig JH, Mugavero MJ, Crane PK, Harrington RD, Knopp RH, et al. Comparative effectiveness and toxicity of statins among HIV-Infected patients. Clin Infect Dis. 2011;52:387–95. doi: 10.1093/cid/ciq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitch KV, Anderson EJ, Hubbard JL, Carpenter SJ, Waddell WR, Caliendo AM, et al. Effects of lifestyle modification programs in HIV-infected patients with metabolic syndrome. AIDS. 2006;20:1843–50. doi: 10.1097/01.aids.0000244203.95758.db. [DOI] [PubMed] [Google Scholar]
- 29.Robinson FP, Quinn LT, Rimmer JH. Effects of high-intensity endurance and resistance exercise on HIV metabolic abnormalities: A pilot study. Bio Res Nurs. 2007;8:177–85. doi: 10.1177/1099800406295520. [DOI] [PubMed] [Google Scholar]
- 30.Yarasheski KE, Roubenoff R. Exercise treatment for HIV associated metabolic and anthropomorphic complications. Exerc Sport Sci Rev. 2001;29:170–4. doi: 10.1097/00003677-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Thomson Reuters. Micromedex. [Last accessed on 2008 Jan 25]. Available from: http://www.thomsonhc/hcs/librarian .
- 32.Kalra S, Kalra B, Sharma A, Sirka M. Motivational interviewing: The WATER approach. Endocr J. 2010;57(suppl 2):S391. [Google Scholar]
- 33.Kalra S, Kalra B, Sharma A, Sirka M. Coping skills training: The AEIOU approach. Endocr J. 2010;57(suppl 2):S39. [Google Scholar]
- 34.Clinician Summary Guide: Comparing Oral Medications for Adults With Type 2 Diabetes. Rockville, Maryland: Agency for Healthcare Research of Quality; 2007. Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- 35.Aberg JA, Kaplan JE, Libman H, Emmanuel P, Anderson JR, Stone VE, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 Update by the HIV medicine association of the infectious diseases society of America. Clin Infect Dis. 2009;49:651–81. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 36.Spollett GR. Hyperglycemia in HIV/AIDS. Diabetes Spectr. 2006;19:163–6. [Google Scholar]
- 37.Kohli R, Shevitz A, Gorbach S, Wanke C. A randomized placebo-controlled trial of metformin for the treatment of HIV lipodystrophy. HIV Med. 2007;8:420–6. doi: 10.1111/j.1468-1293.2007.00488.x. [DOI] [PubMed] [Google Scholar]
- 38.Gadsby R. Efficacy and safety of sitagliptin in the treatment of type 2 diabetes. Clinical Medicine: Therapeutics. 2009;1:53–62. [Google Scholar]
- 39.Rao PV. Persons with type 2 diabetes and co-morbid active tuberculosis should be treated with insulin. Int J Diabetes Dev Ctries. 1999;19:79–86. [Google Scholar]
- 40.Rodbard HW, Jelleinger PS, Davidson JA, Einhorn D, Garber AJ, Grunberger G, et al. Statement by an AACE/ ACE Consensus Panel on type 2 diabetes mellitus.An algorithm for glycemic control. Endocr Pract. 2009;15:540–59. doi: 10.4158/EP.15.6.540. [DOI] [PubMed] [Google Scholar]
- 41.Kalra S, Kalra B, Sharma A. Ketonuria and ketonemia in type 2 diabetes mellitus patients attending an Indian endocrine clinic. Indian J Endocrinol Metab. 2007;11:7–10. [Google Scholar]
- 42.Kalra S, Kalra B, Nanda G. OPD management of ketosis in pregnancy: Aspart vs.regular insulin. Diabet Med. 2006;23(Suppl 4):504. [Google Scholar]
- 43.Kalra S. Walking the tightrope in critical care: Role of rapid-acting analogue insulin. In: Agarwal AK, editor. Medicine Update. Vol. 19. New Delhi: Part I. Jay Pee Bros; 2009. pp. 394–88. [Google Scholar]
- 44.Heinemann L. Insulin absorption from lipodystrophic areas: A (neglected) source of trouble for insulin therapy? J Diabetes Sci Technol. 2010;4:750–3. doi: 10.1177/193229681000400332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalra S, Kalra B, Sharma A. Liraglutide-A Novel GLP-1 Analogue. Recent Pat Endocr, Metab Immune Drug Discov. 2009;3:200–4. [Google Scholar]
- 46.Sheffield CA, Kane MP, Busch RS. Off-label use of exenatide for the management of insulin-resistant type 1 diabetes mellitus in an obese patient with human immunodeficiency virus infection. Pharmacotherapy. 2007;27:1449–55. doi: 10.1592/phco.27.10.1449. [DOI] [PubMed] [Google Scholar]
- 47.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, et al. Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 48.Ananworanich J, Gayet-Ageron A, Le Braz M, Prasithsirikul W, Chetchotisaked P, Kiertiburanakul S, et al. CD4-guided scheduled treatment interruptions compared with continuous therapy for patients infected with HIV-1: Results of the Staccato randomised trial. Lancet. 2006;368:459–65. doi: 10.1016/S0140-6736(06)69153-8. [DOI] [PubMed] [Google Scholar]


