Abstract
Human immunodeficiency virus (HIV) endocrinopathy encompasses a broad spectrum of disorders. Almost all the endocrine organs are virtually affected by HIV infection. HIV can directly alter glandular function. More commonly secondary endocrine dysfunction occurs due to opportunistic infections and neoplasms in immunocompromised state. The complex interaction between HIV infection and endocrine system may be manifested as subtle biochemical and hormonal perturbation to overt glandular failure. Antiretroviral therapy as well as other essential medications often result in adverse endocrinal consequences. Apart from adrenal insufficiency, hypogonadism, diabetes and bone loss, AIDS wasting syndrome and HIV lipodystrophy need special reference. Endocrinal evaluation should proceed as in other patients with suspected endocrine dysfunction. Available treatment options have been shown to improve quality of life and long-term mortality in AIDS patients.
Keywords: Antiretroviral therapy, endocrinopathy, human immunodeficiency virus, lipodystrophy, wasting
INTRODUCTION
Human immunodeficiency virus (HIV) infection is unique among all infectious diseases to cause functional derangement of virtually every endocrine organ system of human body. Additionally, a pandemic of HIV infection and acquired immunodeficiency syndrome (AIDS) worldwide as well as the survival benefit of highly active antiretroviral therapy (HAART) gave rise to the high incidence of endocrinopathies in HIV-infected patients in the last two decades. HIV-endocrinology is thus evolving as a new research corner in the field of modern medicine and endocrinology as well.
The spectrum of HIV endocrinopathy is broad. There may be primary endocrinopathy as a result of direct HIV effect as well as secondary endocrine dysfunction due to indirect effects of cytokines, opportunistic infections and rarely neoplasms. Adrenal, gonadal, thyroid, bone and metabolic abnormalities are common in both early and late stages of HIV syndrome, resulting in poor quality of life and significant mobidity and mortality.[1–3] In many situations, HAART and other medications used in HIV care are critically responsible for the detrimental changes in body composition and metabolic parameters known to cause AIDS wasting syndrome and HIV lipodystrophy syndrome.
The interaction between HIV infection and endocrine sytem is complex, starting from subtle abnormalities in hormone secretion, transport and metabolism to rare instances of hormonal resistance and organ failure. Causes and clinical significance of many of these altered physiology are still unknown. Disseminated infection, varying nutritional status and drug effects often mimic a primary glandular dysfunction. This article reviews the variety of endocrinal abnormalities reported to be associated with HIV infection, along with the possible underlying pathophysiologic mechanisms and optimal treatment strategies for the patients. A timely suspicion, appropriate diagnosis, correct evaluation and early intervention are often required in the long-term management of AIDS patients.
PATHOPHYSIOLOGIC BASIS OF HIV ENDOCRINOPATHY
In HIV-infected patients, endocrine functions of differrent organs may be interfered by differrent means [Table 1]. Excluding acute and chronic illnesses, the relentless progression of immune dysfunction in AIDS alters the internal environment through activation of a couple of cytokines, chemokines and antibody formation and constitutes the direct effect of HIV over endocrine function.
Table 1.
Common endocrine abnormalities in human immunodeficiency virus disease

FUNCTIONAL DERANGEMENT OF ENDOCRINE ORGANS: DIRECT EFFECT OF HIV
The mechanism by which HIV might affect glandular function and hormonal secretion mainly centers on immunomodulatory effects of cytokines at every tier of endocrinal axis (hypothalamic–pituitary–effector organ like adrenal, thyroid, gonads). Adrenal gland has always received most attention as a prime target organ in HIV disease, and HIV adrenalitis is a well-known entity.[4,5] It is postulated that HIV infection triggers macrophages to secrete interleukins (IL-1) and tumor necrosis factor (TNF) and these are likely to act as potent adrenal stimulators.[6,7] In contrary, HIV also has the potential to cause polyclonal B cell activation and production of antibodies against glandular cells, thereby inhibiting glandular endocrine function.[8]
IL-1 produced in median eminence can also affect hypothalamus and pituitary. Increased release of corticotropin releasing hormone (CRH) from the hypothalamus may cause increase in corticotropin or adrenocorticotropic hormone (ACTH) secretion reported in early HIV disease.[9–11] However, IL-1 has been found to directly stimulate cultured pituitary cells to secrete ACTH.[12,13] HIV-infected mononuclear cells stimulate the production of interferons (IFN) whose serum concentration increases with advanced HIV disease.[14] AIDS patients with advanced disease can rarely develop glucocorticoid resistance with hypercortisolism and increased ACTH level. In such patients, glucocorticoid receptor function has been found abnormal in association with increased IFNα level.[15] Whether it is the result of immunomodulation by IFNα is not clear. Pituitary involvement also occurs in AIDS. Idiopathic adenohypophyseal necrosis observed in 10% HIV-infected patients at autopsy is thought to be due to direct effect of HIV.[16] Increased prolactin levels and gynecomastia have been demonstrated among these patients. More than 20% of HIV-infected men with stable disease were reported having hyperprolactinemeia and this was significantly associated with opioid use and increased CD4 count but not with gynecomastia.[17] Current postualtion is that HIV infection reduces dopaminergic tone and thereby increases the bioactivity of prolactin, though the machanism of this effect remains unclear.[18]
Hypogonadism common to HIV-infected men is mostly secondary. But HIV-induced primary hypogonadism is also seen and may be caused by the effects of cytokine on testes. Virus-induced immunomodulators like TNF can inhibit steroidogenesis by altering the side chain cleavage enzyme and IL-1 prevents Leydig cell steroid production and leutinizing hormone (LH) binding to Leydig cells.[19–21]
Reduced bone density is common in HIV-infected patients due to multifactorial causes like hypogonadism, relative growth hormone (GH) deficiency with excess visceral adiposity. Debate persists on whether HIV can directly infect osteocytes, but priliminary research works suggest that HIV is very unlikely to affect osteoblasts as reservoir cells for HIV infection.[22]
STRUCTURAL DESTRUCTION OF ENDOCRINE TISSUE: SECONDARY PHENOMENON
In HIV disease, almost every endocrine organ of the human body can secondarily be affected by means of opportunistic infections, hemorrhage, neoplastic process and other nonspecific inflammation. Regarding structural destruction of adrenal gland, cytomegalovirus (CMV) adrenalitis is the commonest, seen in approximately 40–90% of patients with CMV infection at autopsy. However, glandular destruction by CMV being less than 50% is thus very unlikely to cause adrenal insufficiency.[23] Other causes of adrenal destruction in HIV disease include Mycobacterium tuberculosis, Mycobacterium avium-intracellulare complex (MAC), cryptococcus and hemorrhages. Similarly, pituitary and hypothalamic destruction can also rarely happen by toxoplasmosis, cryptococcosis and CMV infection.
Pneumocystis thyroiditis has been reported to cause a painful thyroiditis resembling the picture of hyperthyroidism followed by hypothyroidism.[24] Thyroid getting affected by CMV, MAC, cryptococcus, Kaposi's sarcoma, abscesses from aspergillosis, rhodococcus infection, etc. has been demonstrated at autopsy, but neither of these manifests clinically as thyroid dysfunction.
ENDOCRINE EFFECTS OF MEDICATIONS
Medications are a common cause of endocrine dysfunction in HIV-infected patients. More than 20 antiretrovirals from differrent groups [protease inhibitors (PIs), nucleoside reverse transcriptase inhibitors (NRTIs), nucleotide reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors (NNRTIs) and entry inhibitor] have been approved in the treatment of AIDS. They are mostly metabolized in liver, and also in kidney and plasma. They primarily contribue to the alteration in lipid metabolism, insulin sensitivity and bone mineral homeostasis. A short account of these drugs is given here.
Protease inhibitors
Lopinavir/ritonavir, nelfinavir, amprenavir, saquinavir, etc. are the drugs that are metabolized in liver mainly via CYP3A pathway. Relevant endocrine effects are seen in the form of fat redistribution, hyperlipidemia with hypertriglyceridemia, diabetes mellitus and increased production of small, dense, atherogenic LDL2 molecules. PIs may have direct effect on adipogenesis [via decreased nuclear localization of sterol regulatory element binding protein 1 (SREBP1) and reduction in peroxisome proliferator-activated receptor gamma (PPARγ) expression] and on glucose metabolism (by decreasing GLUT4 activity and insulin sensitivity, resulting in hyperglycemia).[25,26] Specific PIs can cause direct stimulation of prolactin.[27] These drugs have also been known to decrease bone density and rarely cause avascular necrosis (AVN) of the bone, but the relationship of AVN and HAART is unclear. PIs can decrease the level of 1α-hydroxy vitamin D, causing osteomalcia.[28]
Nucleoside reverse transcriptase inhibitors
These first-line antiretrovirals include lamivudine, zidovudine, emtricitabine, didanosine, abacavir, etc. Just like PIs, they alter lipid and carbohydrate metabolism, decrease insulin resistance, inhibit mitochondrial polymerase γ activity, and thereby cause fat redistribution, subcutaneous fat loss, gynecomastia, hyperglycemia and loss of appetite.[29,30] NRTI drugs (especially didanosine and zalcitabine) can also affect renal tubular function, causing hypophosphatemia, hypomagnesemia, hypocalcemia and hypokalemia.
Nucleotide reverse transcriptase inhibitors
Tenofovir, fumarate, and disoproxil comprise this group. All the drugs are metabolized by esterases in plasma. They have been associated with fat redistribution and hypophosphatemia in the long run.
Non-nucleoside reverse transcriptase inhibitors
NNRTI drugs include delavirdine, nevirapine and efavirenz. All are metabolized in the liver by cytochrome pathways (CYP3A, CYP2B6, CYP2C9, etc.) and are associated with hyperlipidemia and fat redistribution in AIDS patients.
Additional medications
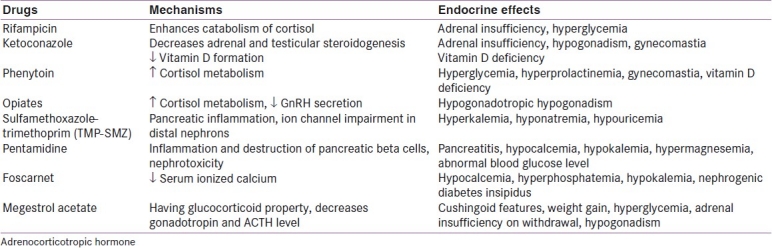
Drugs other than antiretrovirals, like antibiotics, antifungals and chemotherapeutic agents, often used in AIDS patients for controlling several opportunistic infections, can also have adverse consequences on endocrine function. These are listed in Table 2.
Table 2.
Endocrine effects of additional medications
ENDOCRINE DISORDERS
Adrenal dysfunction
Adrenal dysfunction may be suspected in HIV-infected patients with advanced stage of AIDS. In AIDS patients, subtle impairment of adrenal function is manifested as fatigue, hyponatremia or rarely with clinical symptoms of adrenal insufficiency. Biochemical presentation of adrenal insufficiency is relatively common in hospitalized AIDS patients (17%) in comparison to clinically symptomatic patients (4%).[31]
Impaired adrenal reserve is a common finding in HIV-infected patients. It is evident by reduced aldosterone and dehydroepiandrosterone (DHEA) levels with normal cortisol in response to ACTH challenge. In progressive disease state, ACTH levels increase over time, suggesting impaired adrenal reserve.[32] Inadequate adrenal stimulation is also demonstrated in the studies involving hospitalized AIDS patients after cosyntropin challenge test. Increased cortisol levels may be seen in HIV-infected patients; mostly, it is a stress response to the disease burden. Few studies have suggested an intra-adrenal shunting toward cortisol synthesis due to 17,20 lyase dysfunction resulting in a reduced DHEA to cortisol ratio on cosyntropin stimulation.[33]
Adrenal gland shows evidence of both inflammation and necrosis at autopsy. It is likely that the observed pathology may be insufficient to cause clinical problem. Cushing's syndrome has also been demonstrated. Two cases of Cushing's syndrome with secondary adrenal insufficiency have been reported from concomitant use of ritonavir and inhaled fluticasone in children with HIV.[34] Rarely, glucocorticoid resistance at receptor level has been shown in advanced disease state. Such patients demonstrated increased cortisol and ACTH level with Addisonian symptoms. Monocytes from these patients were found to be dexamethasone resistant, with increase in glucocorticoid receptors and decreased affinity for dexamethasone.
In suspected conditions, all the risk factors (opportunistic infections, drugs) for adrenal insufficiency should be evaluated. Cortisol axis is first evaluated by cosyntropin stimulation test in primary adrenal insufficiency. If secondary adrenal dysfunction (pituitary/hypothalamic lesion) is suspected, morning cortisol level or insulin tolerance test may be necessary. Next step is ACTH testing and appropriate imaging for disease localization. Though chronic steroid replacement at supraphysiologic doses is not advocated in AIDS to prevent further immunosuppression, HIV is not a contraindication to pharmacologic glucocorticoid therapy (as in CNS toxoplasmosis).
Thyroid dysfunction
Thyroid function tests are often abnormal in HIV patients. But prevalence of overt thyroid disorder is not found more than in general population.[35] CD4 counts correlate inversely with thyroid binding globulin (TBG), with TBG being high in advanced AIDS patients.[36] Until now, this thyroid dysfunction has been explained by hormonal changes as a stress response to the advanced disease state or comorbidities. However, in HIV-infected patients, the biochemical picture does not fully fit with sick euthyroid syndrome. Progression of HIV disease exhibits decreased T3 level, increased TBG and reduced reverse triiodothyronine (rT3) level over time.[37]
Recent observations have demonstrated an increased prevalence of primary hypothyroidism. Among 350 HIV-infected patients studied in France, 2.6% had clinically evident hypothyroidism, about 6.6% had subclinical hypothyroidism with increased thyroid stimulating hormone (TSH) but normal level of free T4 and 6.8% had a low free T4 level. Male sex, stavudine use and low CD4 count were predictive of hypothyroidism.[38] However, this hypothyroidism is not often seen in association with antithyroid antibodies, and therefore the underlying cause of hypothyroidism remains unclear.[39,40]
Recently, thyroid dysfunction has been described as a component of immune reconstitution syndrome. Autoimmune thyroid disorder has occurred in association with initiation of potent antiretroviral therapy and improved immune function. Graves’ disease is the commonest among immune reconstitution syndromes, showing higher prevalence in women (3% for women and 0.2% for men).[41]
In subclinical hypothyroidism with mildly elevated TSH, the TSH level should be rechecked in 1–3 months. Levothyroxine may be considered in patients with persistently elevated levels of TSH >10 mU/L. However, there is insufficient evidence to recommend routine screening of thyroid function in asymptomatic AIDS patients.
Gonadal dysfunction in male
Male gonadal dysfunction is common among AIDS patients. Clinically, around 67% male patients with advanced HIV disease complained of loss of libido and 33% of them complained of impotence.[42] Increased disease severity increases the chances of developing biochemical hypogonadism (50% of male AIDS patients).[43] With the advent of HAART, this prevalence has become around 20% among treated male patients.[44] Cause of gonadal dysfunction is mostly secondary with low gonadotropin levels due to the effect of undernutrition, infection-inflammation and several drugs on gonadotropin production.
Increased prolactin levels and gynecomastia have been reported among HIV-infected patients. Gynecomastia has been seen in a small percentage of male patients with hypogonadism, hepatitis C and lipodystrophy.[45] It has been found that men with HIV may also be prone to an early “andropause” marked by dysregulation of hypothalamic–pituitary axis and associated signs and symptoms.[46] Crum-Ciamflone has reported the prevalence of 61.4% of erectile dysfunction in HIV-positive men, which had no correlation with hypogonadism.[47]
As sex hormone binding capacity increases in 30–55% patients, use of free testosterone assay is always recommended to diagnose hypogonadism. Hypogonadism is also associated with sarcopenia, weight loss and reduced strength in HIV-infected men.[48] Physiologic testosterone replacement that does not suppress endogenous gonadal function results in increased lean body mass, improved quality of life and reduction in depression. Studies showed sustained gain of lean body mass of around 8% after 1 year treatment with testosterone.[49] Standard therapy for hypogonadism has been intramuscular testosterone (enanthate or ciprionate esters) every 1–3 weeks to provide 100 mg per week. Alternate transdermal or oral routes for androgen administration are also available. Roles of anabolic steroids like nandrolone, oxandrolone (hepatotoxic), pharmacologic dose of testosterone [suppresses high density lipoprotein (HDL) and hypothalamic–pituitary–gonadal axis] with or without progressive resistance training (increases lean mass and HDL) are variable.[50] Regular monitoring of prostate-specific antigen level in blood should be done in elderly patients receiving testosterone. There is no clear benefit of combining testosterone and anabolic steroids in male hypogonadism.
Gonadal dysfunction in female
Information on gonadal function in women with HIV infection is limited. Ovarian dysfunction among female AIDS patients is less common. Amenorrhoea is seen in around 25% women during stress of illness and anovulation is seen among half of the female patients with low CD4 counts.[51] Early menopause has been seen in up to 8% of HIV-infected female patients.[52] Androgen deficiency has been reported more so in women with significant weight loss.[51] It may be due to intra-adrenal shunting toward cortisol production from androgen synthesis in wasted female AIDS patients as human chorionic gonadotropine testing showed intact ovarian androgen production.[53] Studies with the use of transdermal patch to deliver a low physiologic dose of testosterone demonstrated improved functional capacity without hirsuitism or virilization. But this dose appeared too low to increase lean body mass. A doubling of the dose of testosterone in normal weight HIV-infected female patients, though was well tolerated, did not increase lean body mass.[54]
Pancreatic dysfunction
In HIV disease, pancreas is a frequent target organ of opportunistic infections and malignancies (lymphoma, Kaposi's sarcoma), though clinically relevant endocrine dysfunction rarely happens. Major clinical manifestations are pancreatitis and hypoglycemia. Pancreatitis is commonly seen as a drug effect, especially from the use of pentamidine, trimethoprim, didanosine and zalcitabine. Elevated amylase levels in HIV-infected patients may also be due to mcroamylasemia and salivary amylase. Hypoglycemia can result from islet cell inflammation and insulin release by pentamidine with subsequent chronic hyperglycemia. Megestrol acetate is also associated with new-onset diabetes mellitus.
Disorders of glucose homeostasis are relatively common in HIV-infected patients with dyslipidemia and HIV lipodystrophy syndrome. Diabetes mellitus is found to be three times more prevalent among patients receiving HAART.[55] Impaired glucose tolerance and diabetes in these patients occurred due to the primary mechanism of insulin resistance and hyperinsulinemia.[56] Mechanisms of insulin resistance include abnormal fat distribution such as central adiposity, peripheral subcutaneous fat loss, altered cytokines like elevated TNF and low adiponectin or other factors like increased lipolysis, increased accumulation of fat in liver and muscles, mitochondrial toxicity and use of specific antiretrovirals like PIs.[57–61] However, insulin resistance among HIV-infected women has not been seen to be associated with common features like polycystic ovarian syndrome.[56]
Growth failure (GH–IGF1 axis disturbance)
Growth failure, especially failure to thrive, is an important issue in treating HIV-infected children. GH deficiency as well as GH insensitivity has been found to be a potential cause of growth failure in such patients. Reduced level of insulin-like growth factor 1 (IGF1) and reduced IGF binding protein 3 (IGFBP3) due to its enhanced proteolysis have been demonstrated. IGF1 and IGFBP3 responses to GH are also impaired in HIV-infected children.[62–64] Such a degree of GH insensitivity might improve with weight gain and improved immunity by HAART. Normal thymic development cannot happen in GH deficiency state.[65] Among HIV-infected children with lipoatrophy, decreased GH secretion is associated with excess visceral fat.[66]
Adult AIDS patients also exhibit two distinct patterns of abnormalities of GH-IGF1 axis. In patients with AIDS wasting, GH levels are increased with GH resistance state (IGF1 decreased), a typical pattern seen in malnutrition.[67] GH resistance is likely to be a secondary phenomenon in AIDS wasting, but the HIV envelope protein gp120 has been shown to decrease GH in vitro and also in animal studies.[68] In contrast, viscerally obese HIV-infected patients with lipodystrophy show GH deficient state with decreased amplitude of mean overnight GH level and GH pulse. This state of suppression of GH release in lipodystrophy syndrome may be explained by increased somatostatin tone, decreased ghrelin and increased circulatory free fatty acid due to enhanced lipolysis.[69]
Though GH and GH secretagogues (GH releasing hormone 1-29) have already been used in AIDS wasting syndrome and HIV lipodystrophy syndrome, they remain investigational with their large phase III trials to establish long-term clinical benefits in lipodystrophy.[70] However, GH has got approval from US Food and Drug Administration (FDA) in treatment of severe sarcopenia in AIDS wasting syndrome.
Fluid-electrolyte imbalance
Hyponatremia and hyperkalemia are the commonest among all fluid-electrolyte disturbances in AIDS patients. Hyponatremia (Na < 130 mmol/L) is more common among hospitalized (40–60%) AIDS patients than outpatients (20%). It is most often related to the secretion of inappropriate antidiuretic hormone (SIADH) contributing around half of all hyponatremic HIV-infected patients. These patients are euvolemic with low serum sodium but increased urinary sodium excretion and inappropriately elevated urine osmolarity. Various infections and tumors are the commonest underlying cause. Symptomatic treatment with fluid restriction and in severe cases infusion of hypertonic saline should be offered. Among hypovolemic hyponatremic HIV-infected patients, majority (30%) suffer from adrenal insufficiency.[71] Volume-depleted hyponatremia may also be due to diarrhea, vomiting and impaired water clearance (HIV nephropathy). Volume repletion is the required treatment here. Hyporeninemic hypoaldosteronism can rarely be caused by drugs like miconazole and pentamidine.[72] Hypernatremia may be seen in foscarnet-induced nephrogenic diabetes insipidus.
Trimethoprim use has been reported as the commonest cause of hyperkalemia, occurring in nearly 20–50% AIDS patients.[73] Trimethoprim is similar to amiloride in structure and inhibits tubular potassium excretion. Among the other causes, pentamidine tubulopathy, HIV-induced glomerulosclerosis, primary adrenal insufficiency and hyporeninemic hypoaldosteronism are significant.
Bone mineral dysfunction
Both osteoporosis and osteopenia are common among AIDS patients. Reduced bone mineral density is seen in 73% HIV-infected patients versus 30% HIV-negative patients of similar age.[74] Dual energy X-ray absorptiometry (DEXA) is helpful to detect reduced bone density of hip and lumbar spine among HIV-infected men with weight loss and receiving HAART. Though a couple of previous studies suggested an association of PI therapy with reduced bone density, recent research showed that age-adjusted bone density was reduced in association with HIV infection itself and with traditional risk factors like low body weight, smoking and steroid use but was not affected by HAART.[75] Similarly, in HIV-infected women, osteopenia was detected in more than 50% outpatients, about 2.4 times more prevalent in comparison to age-matched control population.[76] No association with specific PI use was found but markers of bone resorption were increased. Reduced vertebral bone density was also associated with increased visceral adiposity among HIV-infected patients.[77]
Reduced bone density has also been reported in HIV-infected children receiving HAART and maximum reduction was found among children with lipodystrophy.[78] Reduced bone formation with increased resorption has been suggested in this group by the presence of increased markers of bone resorption and reduced osteocalcin. A potential effect of low GH and IGF1 on bone density may also be responsible for reduction in total bone density.[79] Other endocrine factors like hypogonadism and excess visceral adiposity have been found to correlate significantly with vertebral bone density. PI-induced relative vitamin D deficiency may also act as an additive factor. Recent studies have shown that alendronate is effective in increasing bone density (5.2% increases in spinal bone density over 48 weeks) among patients with idiopathic bone loss.[80] Male patients with AIDS wasting syndrome are usually benefited by testosterone at high dose (200 mg/week). Relationship between AVN and HAART is unclear.[81] Potential association factors include prior use of systemic cortisol, anticardiolipin antibody and mechanical stress.
Calcium and vitamin D balance
HIV-infected patients often suffer from hypocalcemia, with the prevalence being nearly 6.5% of a large cohort of patients of AIDS. Serum calcium concentration decreases progressively with stage of AIDS. Around half of the patients with hypocalcemia have vitamin D deficiency, but without compensatory increase in parathormone (PTH).[82] Exact cause of reduced PTH is not known though decreased PTH secretion in immunodeficiency and presence of hypomagnesemia may partially explain the situation. Vitamin D deficiency is multifactorial. Causes include malabsorption (AIDS enteropathy), decreased hydroxylation (PI induced) and Fanconi syndrome with hypophosphatemia (tenofovir, cedofovir). A number of drugs, especially foscarnet, pentamidine, and ketoconazole, can affect calcium homeostasis. On rare occasions, hypercalcemia has been reported among AIDS patients. Granuloma (tuberculosis, lymphoma) induced excess production of active vitamin D3, enhanced local osteoclastic resorption of bone (disseminated CMV) or activation of PTH-related protein (HTLV1) explain some of the conditions associated with hypercalcemia in AIDS.
AIDS WASTING SYNDROME
Wasting is an age-old terminal event of AIDS originally termed as “slim disease”. Still in the era of HAART, wasting remains common with high prevalence (34%).[83] Weight loss is a significant predictor of mortality in HIV infection. Body mass index (BMI) of less than 18.4 kg/m2 shows 2.2-fold increased mortality and BMI less than 16 kg/m2 shows associated 4.4-fold increased mortality in HIV-infected patients.[84] Currently, AIDS wasting syndrome is defined as body weight less than 90% of ideal weight or weight loss of more than 10% of body weight over 3 months. A disproportionate loss of lean body mass with a relative sparing of body fat with disease progression in both sexes characterizes the condition. Other important features include muscle wasting, weakness, increased resting energy expenditure and hypertriglyceridemia. Cytokine related increased energy expenditure and decreased appetite, malabsorption and hypogonadism remain central to the potential mechanisms behind such wasting.[85]
Multidisciplinary approach to AIDS wasting syndrome starts with optimal antiretroviral therapy and adequate nutrition. Testosterone (intramuscular/transdermal) has been successfully used to increase lean body mass in men with this syndrome, whereas it has also been shown to be safe and well tolerated in women. A number of agents like anabolic steroids (oxandrolone, nandrolone), megesrtol acetate, thalidomide, human chorionic gonadotropin, pentoxyfiline, amino acid mixtures and omega 3 fatty acids have been tried in this setting with variable efficacy but potential side effects.[86–90] Only testosterone administration has proved useful to increase lean body mass, especially in conjunction with progressive resistance training. Supraphysiologic dose GH administration is currently reserved for severe wasting refractory to other treatments.
HIV LIPODYSTROPHY SYNDROME
HIV lipodystrophy syndrome is associated with metabolic derangements, changes in body composition and abnormal fat distribution. It has been most widely recognized since the era of HAART but can also be seen in antiretroviral-naïve patients. It is characterized by phenotypic changes like truncal obesity, buffalo hump, peripheral fat loss, atrophy of facial fat and breast enlargement in women. Considering some physical similarities to Cushing's syndrome, it has been termed as “a pseudo-Cushing's syndrome” though neither any other specific stigmata of Cushing's syndrome (proximal muscle weakness, bruising and facial plethora) nor biochemical parameters are associated with this syndrome.[91] Normal cortisol level with its normal diurnal variation and adequate suppressibility to dexamethasone are seen in HIV lipodystrophy syndrome. However, it is very often associated with insulin resistance, hyperglycemia, and hypertriglyceridemia. In particular, dyslipidemia and diabetes mellitus are increasingly common among patients receiving HAART.[92] Cardiovascular risk increases many folds, especially the rate of developing myocardial infarction and the prevalence of metabolic syndrome (40% of the HIV-infected patients with fat redistribution having metabolic syndrome as evidenced by Framingham equation) are most increased among HIV-infected patients treated with HAART.[93] But HIV status and viral load have not been shown predictive of cardiovascular disease.[94] Treatment options available consist of exercise and lifestyle modification, switching to less-toxic NRTIs or PIs, fenofibrates to decrease serum triglyceride level, and use of insulin sensitizers like metformin to reduce visceral fat and thiazolidinediones to improve subcutaneous fat loss. Testosterone and anabolic steroids have no effect in reducing visceral fat in lipodystrophy syndrome.[95]
CONCLUSION
HIV endocrinopathy is not so uncommon, though overt glandular failure is a rare entity. Mechanisms by which the virus directly alters the endocrine function are still ill-understood. Adrenal insufficiency is the commonest dysfunction, followed by other hormonal abnormalities complicating the longstanding disease process. Drug-induced endocrine toxicity is another important aspect of patient care. Prospective studies are needed to evaluate the burden of HIV endocrinopathy among Indian population, which will enable us to adopt optimal management strategy for these patients to avert a further compromise in disease and health.
ACKNOWLEDGMENT
We acknowledge all the staff of our concerned departments of NRS Medical College, Kolkata.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Griffin JE. The dilemma of abnormal thyroid function tests: Is thyroid disease present or not? Am J Med Sci. 1985;289:76–88. doi: 10.1097/00000441-198502000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Morley JE, Melmed S. Gonadal dysfunction in systemic disorders. Metabolism. 1979;28:1051–73. doi: 10.1016/0026-0495(79)90010-6. [DOI] [PubMed] [Google Scholar]
- 3.Parker LN, Levin ER, Lifrak ET. Evidence for adrenocortical adaptation to severe illness. J Clin Endocrinol Metab. 1985;60:947–52. doi: 10.1210/jcem-60-5-947. [DOI] [PubMed] [Google Scholar]
- 4.Smith D. Endocrine complications in HIV progression. AIDS Treatment News. 1991:140. [Google Scholar]
- 5.Greene LW, Cole W, Greene JB, Levy B, Louie E, Raphael B, et al. Adrenal insufficiency as a complication of the acquired immunodeficiency syndrome. Ann Intern Med. 1984;101:497–8. doi: 10.7326/0003-4819-101-4-497. [DOI] [PubMed] [Google Scholar]
- 6.Tracey KJ, Cerami A. Metabolic responses to cachectin/TNF: A brief review. Ann N Y Acad Sci. 1990;587:325–31. doi: 10.1111/j.1749-6632.1990.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 7.Merrill JE, Koyanagi Y, Chen ISY. Interleukin-1 and tumor necrosis factor α can be induced from mononuclear phagocytes by human immunodeficiency virus type 1 binding to the CD4 receptor. J Virol. 1989;63:4404–8. doi: 10.1128/jvi.63.10.4404-4408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salim YS, Faber V, Wiik A, Andersen PL, Hoier-Madsen M, Mourtisen S. Anti-corticosteroid antibodies in AIDS patients. APMIS. 1988;96:889–94. doi: 10.1111/j.1699-0463.1988.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 9.Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotrophin-releasing factor. Science. 1987;238:522–4. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- 10.Woloski BM, Smith EM, Meyer WJ, 3rd, Fuller GM, Blalock JE. Corticotropin-releasing activity of monokones. Science. 1985;230:1035–7. doi: 10.1126/science.2997929. [DOI] [PubMed] [Google Scholar]
- 11.Whitcomb RW, Linehan WM, Wahl LM, Knazek RA. Monocytes stimulate cortisol production by cultured human adrenocortical cells. J Clin Endocrinol Metab. 1988;66:33–8. doi: 10.1210/jcem-66-1-33. [DOI] [PubMed] [Google Scholar]
- 12.Szebeni J, Dieffenbach C, Wahl SM, Venkateshan CN, Yeh A, Popovic M, et al. Induction of alpha interferon by human immunodeficiency virus type 1 in human monocyte-macrophage cultures. J Virol. 1991;65:6362–4. doi: 10.1128/jvi.65.11.6362-6364.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer WJ, 3rd, Smith EM, Richards GE, Cavallo A, Morrill AC, Blalock JE. In vivo immunoreactive adrenocorticotropin (ACTH) production by human mononuclear leukocytes from normal and ACTH-deficient individuals. J Clin Endocrinol Metab. 1987;64:98–105. doi: 10.1210/jcem-64-1-98. [DOI] [PubMed] [Google Scholar]
- 14.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–52. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 15.Norbiato G, Bevilacqua M, Vago T, Clerici M. Glucocorticoids and interferon-alpha in the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1996;81:2601–6. doi: 10.1210/jcem.81.7.8675584. [DOI] [PubMed] [Google Scholar]
- 16.Ferrerio J, Vinters HV. Pathology of pituitary gland in patients with acquired immune deficiency syndrome (AIDS) Pathology. 1988;20:211–5. doi: 10.3109/00313028809059495. [DOI] [PubMed] [Google Scholar]
- 17.Collazos J, Ibarra S, Martinez E, Mayo J. Serum prolactin concentrations in patients infected with human immunodeficiency virus. HIV Clin Trials. 2002;3:133–8. doi: 10.1310/QAQQ-XTCJ-8AL4-6F5P. [DOI] [PubMed] [Google Scholar]
- 18.Parra A, Ramirez-Peredo J, Larrea F, Cabrera V, Coutiño B, Torres I, et al. Decreased dopaminergic tone and increased basal bioactive prolactin in men with human immunodeficiency virus infection. Clin Endocrinol (Oxf) 2001;54:731–8. doi: 10.1046/j.1365-2265.2001.01262.x. [DOI] [PubMed] [Google Scholar]
- 19.Xiong Y, Hales DB. The role of the tumor necrosis factor-alpha in the regulation of mouse Leydig cell steroidogenesis. Endocrinology. 1993;132:2438–44. doi: 10.1210/endo.132.6.8504748. [DOI] [PubMed] [Google Scholar]
- 20.Hales DB. Interleukin1 inhibits Leydig cell steroidogenesis primarily by decreasing 17α-hydoxylase/C17-20 lyase cytochrome P450 expression. Endocrinology. 1992;131:2165–72. doi: 10.1210/endo.131.5.1425417. [DOI] [PubMed] [Google Scholar]
- 21.Calkins JH, Siegel MM, Nankin HR, Lin T. Interleukin-1 inhibits leydig cell steroidogenesis in primary cell culture. J Clin Endocrinol Metab. 1988;123:1605–10. doi: 10.1210/endo-123-3-1605. [DOI] [PubMed] [Google Scholar]
- 22.Nacher M, Serrano S, Gonzales A, Hernández A, Mariñoso ML, Vilella R, et al. Osteoblasts in HIV-infected patients: HIV-1 infection and cell function. AIDS. 2001;15:2239–43. doi: 10.1097/00002030-200111230-00004. [DOI] [PubMed] [Google Scholar]
- 23.Glasgow BJ, Steinsapir KD, Anders K, Layfield LJ. Adrenal pathology in the acquired immune deficiency syndrome. Am J Clin Pathol. 1985;84:594–7. doi: 10.1093/ajcp/84.5.594. [DOI] [PubMed] [Google Scholar]
- 24.Drucker DJ, Bailey D, Rotstein L. Thyroiditis as the presenting manifestation of disseminated extrapulmonary Pneumocystis carinii infection. J Clin Endocrinol Metab. 1990;71:1663–5. doi: 10.1210/jcem-71-6-1663. [DOI] [PubMed] [Google Scholar]
- 25.Caron M, Auclair M, Vigouroux C, Glorian M, Forest C, Capeau J. The HIV protease inhibitor indinavir impairs sterol regulatory element-binding protein-1 intranuclear localization, inhibits preadipocyte differentiation, and induces insulin resistance. Diabetes. 2001;50:1378–88. doi: 10.2337/diabetes.50.6.1378. [DOI] [PubMed] [Google Scholar]
- 26.Murata H, Hruz PW, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem. 2000;275:20251–4. doi: 10.1074/jbc.C000228200. [DOI] [PubMed] [Google Scholar]
- 27.Hutchinson J, Murphy M, Harries R, Skinner CJ. Galactorrhoea and hyper-prolactinoma associated with protease inhibitors. Lancet. 2000;356:1003–4. doi: 10.1016/S0140-6736(00)02697-0. [DOI] [PubMed] [Google Scholar]
- 28.Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. HIV-protease inhibitors impair vitamin D bioactivation to 1, 25-dihydroxyvitamin D. AIDS. 2003;17:513–20. doi: 10.1097/00002030-200303070-00006. [DOI] [PubMed] [Google Scholar]
- 29.Hadigan C, Borgonha S, Rabe J, Young V, Grinspoon S. Increased rates of lipolysis among HIV-infected men receiving highly active antiretroviral therapy. Metabolism. 2002;51:1143–7. doi: 10.1053/meta.2002.34704. [DOI] [PubMed] [Google Scholar]
- 30.Shikuma CM, Hu N, Milne C, Yost F, Waslien C, Shimizu S, et al. Mitochondrial DNA decrease in subcutaneous adipose tissue of HIV-infected individuals with peripheral lipoatrophy. AIDS. 2001;15:1801–9. doi: 10.1097/00002030-200109280-00009. [DOI] [PubMed] [Google Scholar]
- 31.Membreno L, Irony I, Dere W, Klein R, Biglieri EG, Cobb E. Adrenocortical function in acquired immune deficiency syndrome. J Clin Endocrinol Metab. 1987;65:482–7. doi: 10.1210/jcem-65-3-482. [DOI] [PubMed] [Google Scholar]
- 32.Findling JW, Buggy BP, Gilson IH, Brummitt CF, Bernstein BM, Raff H. Longitudinal evaluation of adrenocortical function in patients with the human immunodeficiency virus. J Clin Endocrinol Metab. 1994;79:1091–6. doi: 10.1210/jcem.79.4.7962279. [DOI] [PubMed] [Google Scholar]
- 33.Grinspoon S, Corcoran C, Stanley T, Rabe J, Wilkie S. Mechanisms of androgen deficiency in human immunodeficiency virus-infected women with the wasting syndrome. J Clin Endocrinol Metab. 2001;86:4120–6. doi: 10.1210/jcem.86.9.7843. [DOI] [PubMed] [Google Scholar]
- 34.Johnson SR. Cushing syndrome with secondary adrenal insufficiency from concomitant therapy with ritonavir and fluticasone. J Pediatr. 2006;148:386–8. doi: 10.1016/j.jpeds.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman CJ, Brown TT. Thyroid function abnormalities in HIV infected patients. Clin Infect Dis. 2007;45:488–94. doi: 10.1086/519978. [DOI] [PubMed] [Google Scholar]
- 36.Bourdoux PP, De Wit SA, Servais GM, Clumeck N, Bonnyns MA. Biochemical thyroid profile in patients infected with human immunodeficiency virus. Thyroid. 1991;1:147–9. doi: 10.1089/thy.1991.1.147. [DOI] [PubMed] [Google Scholar]
- 37.Grinspoon SK, Bilezikian JB. HIV disease and the endocrine system. N Engl J Med. 1992;327:1360–5. doi: 10.1056/NEJM199211053271906. [DOI] [PubMed] [Google Scholar]
- 38.Beltran S, Lescure FX, Desailloud R, Douadi Y, Smail A, El Esper I, et al. Increased prevalence of hypothyroidism among human immunodeficiency virus-infected patients: A need for screening. Clin Infect Dis. 2003;37:579–83. doi: 10.1086/376626. [DOI] [PubMed] [Google Scholar]
- 39.Calza L, Manfredi R, Chiodo F. Subclinical hypothyroidism in HIV-infected patients receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31:361–3. doi: 10.1097/00126334-200211010-00014. [DOI] [PubMed] [Google Scholar]
- 40.Quirino T, Bongiovanni M, Ricci E, Chebat E, Carradori S, Martinelli C, et al. Hypothyroidism in HIV-infected patients who have or have not received HAART. Clin Infect Dis. 2004;38:596–7. doi: 10.1086/381442. [DOI] [PubMed] [Google Scholar]
- 41.Chen F, Day SL, Metcalfe RA, Sethi G, Kapembwa MS, Brook MG, et al. Characteristics of autoimmune thyroid disease occurring as a late complication of immune reconstitution in patients with advanced human immunodeficiency virus (HIV) disease. Medicine (Baltimore) 2005;84:98–106. doi: 10.1097/01.md.0000159082.45703.90. [DOI] [PubMed] [Google Scholar]
- 42.Mylonakis E, Koutkia P, Grinspoon S. Diagnosis and treatment of androgen deficiency in human immunodeficiency virus-infected men and women. Clin Infect Dis. 2001;33:857–64. doi: 10.1086/322695. [DOI] [PubMed] [Google Scholar]
- 43.Dobs AS, Dempsey MA, Ladenson PW, Polk BF. Endocrine disorders in men infected with human immunodeficiency virus. Am J Med. 1988;84:611–6. doi: 10.1016/0002-9343(88)90144-1. [DOI] [PubMed] [Google Scholar]
- 44.Rietschel P, Corcoran C, Stanley T, Basgoz N, Klibanski A, Grinspoon S. Prevalence of hypogonadism among men with weight loss related to human immunodeficiency virus infection who were receiving highly active antiretroviral therapy. Clin Infect Dis. 2000;31:1240–4. doi: 10.1086/317457. [DOI] [PubMed] [Google Scholar]
- 45.Biglia A, Blanco JL, Martinez E, Domingo P, Casamitjana R, Sambeat M, et al. Gynecomastia among HIV-infected patients is associated with hypogonadism: A case-control study. Clin Infect Dis. 2004;39:1514–9. doi: 10.1086/425363. [DOI] [PubMed] [Google Scholar]
- 46.Cohan GR HIV-associated hypogonadism. (352-4).AIDS Read. 2006;16:341–5. [PubMed] [Google Scholar]
- 47.Crum-Ciaflone NM. Erectile dysfunction and hypogonadism amongmen with HIV. AIDS Patient Care STDS. 2007;21:9–19. doi: 10.1089/apc.2006.0071. [DOI] [PubMed] [Google Scholar]
- 48.Grinspoon S, Corcoran C, Lee K, Burrows B, Hubbard J, Katznelson L, et al. Loss of lean body and muscle mass correlates with androgen levels in hypogonadal men with acquired immunodeficiency syndrome and wasting. J Clin Endocrinol Metab. 1996;81:4051–8. doi: 10.1210/jcem.81.11.8923860. [DOI] [PubMed] [Google Scholar]
- 49.Grinspoon S, Corcoran C, Anderson E, Hubbard J, Stanley T, Basgoz N, et al. Sustained anabolic effects of long-term androgen administration in men with AIDS and wasting. Clin Infect Dis. 1999;28:634–6. doi: 10.1086/515162. [DOI] [PubMed] [Google Scholar]
- 50.Grinspoon S, Corcoran C, Parlman K, Costello M, Rosenthal D, Anderson E, et al. Effects of testosterone and progressive resistance training in Eugonadal men with AIDS and wasting. Ann Int Med. 2000;133:348–55. doi: 10.7326/0003-4819-133-5-200009050-00010. [DOI] [PubMed] [Google Scholar]
- 51.Grinspoon S, Corcoran C, Miller K, Biller BM, Askari H, Wang E, et al. Body composition and endocrine function in women with acquired immunodeficiency syndrome wasting. J Clin Endocrinol Metab. 1997;82:1332–7. doi: 10.1210/jcem.82.5.3907. [DOI] [PubMed] [Google Scholar]
- 52.Clark RA, Mulligan K, Stamenovic E, Chang B, Watts H, Andersen J, et al. Frequency of anovulation and early menopause among women enrolled in selected adult AIDS clinical trials group studies. J Infect Dis. 2001;184:1325–7. doi: 10.1086/323999. [DOI] [PubMed] [Google Scholar]
- 53.Grinspoon S, Corcoran C, Stanley T, Rabe J, Wilkie S. Mechanisms of androgen deficiency in human immunodeficiency virus-infected women with the wasting syndrome. J Clin Endocrinol Metab. 2001;86:4120–6. doi: 10.1210/jcem.86.9.7843. [DOI] [PubMed] [Google Scholar]
- 54.Choi HH, Gray PB, Storer TW, Calof OM, Woodhouse L, Singh AB, et al. Effects of testosterone replacement in human immunodeficiency virus-infected women with weight loss. J Clin Endocrinol Metab. 2005;90:1531–41. doi: 10.1210/jc.2004-1677. [DOI] [PubMed] [Google Scholar]
- 55.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–84. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 56.Johnsen S, Dolan SE, Fitch KV, Killilea KM, Shifren JL, Grinspoon SK. Absence of polycystic ovary syndrome features in human immunodeficiency virus-infected women despite significant hyperinsulinemia and truncal adiposity. J Clin Endocrinol Metab. 2005;90:5596–604. doi: 10.1210/jc.2005-1083. [DOI] [PubMed] [Google Scholar]
- 57.Meininger G, Hadigan C, Rietschel P, Grinspoon S. Body-composition measurements as predictors of glucose and insulin abnormalities in HIV-positive men. Am J Clin Nutr. 2002;76:460–5. doi: 10.1093/ajcn/76.2.460. [DOI] [PubMed] [Google Scholar]
- 58.Myanrcik DC, McNurlan MA, Steigbigel RT, Fuhrer J, Gelato MC. Association of severe insulin resistance with both loss of limb fat and elevated serum tumor necrosis factor receptor levels in HIV lipodystrophy. J Acquir Immune Defic Syndr. 2000;25:312–21. doi: 10.1097/00042560-200012010-00004. [DOI] [PubMed] [Google Scholar]
- 59.Tong Q, Sankale JL, Hadigan CM, Tan G, Rosenberg ES, Kanki PJ, et al. Regulation of adiponectin in human immunodeficiency virus-infected patients: Relationship to body composition and metabolic indices. J Clin Endocrinol Metab. 2003;88:1559–64. doi: 10.1210/jc.2002-021600. [DOI] [PubMed] [Google Scholar]
- 60.Hadigan C, Rabe J, Meininger G, Aliabadi N, Breu J, Grinspoon S. Inhibition of lipolysis improves insulin sensitivity in protease inhibitor-treated HIV-infected men with fat redistribution. Am J Clin Nutr. 2003;77:490–4. doi: 10.1093/ajcn/77.2.490. [DOI] [PubMed] [Google Scholar]
- 61.Gan SK, Samaras K, Thompson CH, Kraegen EW, Carr A, Cooper DA, et al. Altered myocellular and abdominal fat partitioning predict disturbance in insulin action in HIV protease inhibitor-related lipodystrophy. Diabetes. 2002;51:3163–9. doi: 10.2337/diabetes.51.11.3163. [DOI] [PubMed] [Google Scholar]
- 62.Ratner Kaufman F, Gertner JM, Sleeper LA, Donfield SM. Growth hormone secretion in HIV-positive versus HIV-negative hemophilic males with abnormal growth and pubertal development.The Hemophilia Growth and Development Study. J Acquir Immune Defic Syndrome Hum Retrovirol. 1997;15:137–44. doi: 10.1097/00042560-199706010-00007. [DOI] [PubMed] [Google Scholar]
- 63.Pinto G, Blanche S, Thiriet I, Souberbielle JC, Goulet O, Brauner R. Growth hormone treatment of children with human immunodeficiency virus-associated growth failure. Eur J Pediatr. 2000;159:937–8. doi: 10.1007/pl00008378. [DOI] [PubMed] [Google Scholar]
- 64.Rondanelli M, Caselli D, Arico M, Maccabruni A, Magnani B, Bacchella L, et al. Insulin-like growth factor 1 (IGF-1) and IGF-binding protein 3 response to growth hormone is impaired in HIV-infected children. AIDS Res Hum Retroviruses. 2002;18:331–9. doi: 10.1089/088922202753519106. [DOI] [PubMed] [Google Scholar]
- 65.Vigano A, Saresella M, Trabattoni D, Giacomet V, di Natale B, Merlo M, et al. Growth hormone in T-lymphocyte thymic and post thymic development: A study in HIV-infected children. J Pediatr. 2004;145:542–8. doi: 10.1016/j.jpeds.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 66.Vigano A, Mora S, Brambilla P, Schneider L, Merlo M, Monti LD, et al. Impaired growth hormone secretion correlates with visceral adiposity in highly active antiretroviral treated HIV-infected adolescents. AIDS. 2003;17:1435–41. doi: 10.1097/00002030-200307040-00003. [DOI] [PubMed] [Google Scholar]
- 67.Grinspoon S, Baum H, Lee K, Anderson E, Herzog D, Klibanski A. Effects of short term recombinant human insulin-like growth factor-1 administration on bone turn over in osteopenic women with anorexia nervosa. J Clin Endocrinol Metab. 1996;81:3864–70. doi: 10.1210/jcem.81.11.8923830. [DOI] [PubMed] [Google Scholar]
- 68.Coodley GO, Loveless MO, Nelson HD. Endocrine function in the HIV Wasting syndrome. J AIDS. 1994;7:46–51. [PubMed] [Google Scholar]
- 69.Koutkia P, Meininger G, Canavan B, Breu J, Grinspoon S. Metabolic regulation of growth hormone by free fatty acids, somatostatin, and ghrelin in HIV-lipodystrophy. Am J Physiol Endocrinol Metab. 2005;90:32–8. doi: 10.1152/ajpendo.00335.2003. [DOI] [PubMed] [Google Scholar]
- 70.Koutkia P, Cananvan B, Breu J, Torriani M, Kissko J, Grinspoon S. Growth hormone-releasing hormone in HIV-infected men with lipodystrophy: A randomized, controlled trial. JAMA. 2004;292:210–8. doi: 10.1001/jama.292.2.210. [DOI] [PubMed] [Google Scholar]
- 71.Tang WW, Kaptein E, Feinstein EI, Massry SG. Hyponatremia in hospitalized patients with acquired immunodeficiency syndrome (AIDS) and the AIDS-related complex. Am J Med. 1993;94:169–74. doi: 10.1016/0002-9343(93)90179-s. [DOI] [PubMed] [Google Scholar]
- 72.Kalin MF, Poretsky L, Seres DS, Zumoff B. Hyporeninemic hypoaldosteronism associated acquired immunodeficiency syndrome. Am J Med. 1987;82:1035–8. doi: 10.1016/0002-9343(87)90171-9. [DOI] [PubMed] [Google Scholar]
- 73.Choi MJ, Fernandez PC, Patnaik A, Coupaye-Gerard B, D’Andrea D, Szerlip H, et al. Brief report: Trimethoprim-induced hyperkalemia in a patient with AIDS. Ann Intern Med. 1993;328:703–6. doi: 10.1056/NEJM199303113281006. [DOI] [PubMed] [Google Scholar]
- 74.Tebas P, Powderly WG, Claxton S, Marin D, Tantisiriwat W, Teitelbaum SL, et al. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS. 2000;14:F63–7. doi: 10.1097/00002030-200003100-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amiel C, Ostertag A, Slama L, Baudoin C, N’Guyen T, Lajeunie E, et al. BMD is reduced in HIV-infected men irrespective of treatment. J Bone Miner Res. 2004;19:402–9. doi: 10.1359/JBMR.0301246. [DOI] [PubMed] [Google Scholar]
- 76.Dolan SE, Huang JS, Killilea KM, Sullivan MP, Aliabadi N, Grinspoon S. Reduced bone density in HIV-infected women. AIDS. 2004;18:475–83. doi: 10.1097/00002030-200402200-00014. [DOI] [PubMed] [Google Scholar]
- 77.Koutkia P, Canavan B, Breu J, Grinspoon S. Effects of growth hormone-releasing hormone on bone turnover in human immunodeficiency virus-infected men with fat accumulation. J Clin Endocrinol Metab. 2005;90:2154–60. doi: 10.1210/jc.2004-1466. [DOI] [PubMed] [Google Scholar]
- 78.Mora S, Zamproni I, Beccio S, Bianchi R, Giacomet V, Viganò A. Longitudinal changes of bone mineral density and metabolism in antiretroviral-treated human immunodeficiency virus-infected children. J Clin Endocrinol Metab. 2004;89:24–8. doi: 10.1210/jc.2003-030767. [DOI] [PubMed] [Google Scholar]
- 79.Stagi S, Bindi G, Galluzzi F, Galli L, Salti R, de Martino M. Changed bone status in human immunodeficiency virus type 1 (HIV-1) perinatally infected children is related to low serum free IGF-1. Clin Endocrinol (Oxf) 2004;61:692–9. doi: 10.1111/j.1365-2265.2004.02150.x. [DOI] [PubMed] [Google Scholar]
- 80.Mondy K, Powerly WG, Claxton SA, Yarasheski KH, Royal M, Stoneman JS, et al. Alendronate, vitamin D, and calcium for the treatment of osteopenia/osteoporosis associated with HIV infection. J Acquir Immune Defic Syndr. 2005;38:426–31. doi: 10.1097/01.qai.0000145352.04440.1e. [DOI] [PubMed] [Google Scholar]
- 81.Fairfield WP, Finkelstein JS, Klibanski A, Grinspoon SK. Osteopenic in eugonadal men with acquired immune deficiency syndrome wasting syndrome. J Clin Endocrinol Metab. 2001;86:2020–6. doi: 10.1210/jcem.86.5.7515. [DOI] [PubMed] [Google Scholar]
- 82.Kuehn EW, Anders HJ, Bogner JR, Obermaier J, Goebel FD, Schlöndorff D. Hypocalcemia in HIV infection and AIDS. J Intern Med. 1999;245:69–73. doi: 10.1046/j.1365-2796.1999.00407.x. [DOI] [PubMed] [Google Scholar]
- 83.Wanke CA, Silva M, Knox TA, Forrester J, Speigelman D, Gorbach SL. Weight loss and wasting remain common complications in individuals infected with HIV in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;31:803–5. doi: 10.1086/314027. [DOI] [PubMed] [Google Scholar]
- 84.Thiebaut R, Malvy D, Marimoutou C, Davis F. Anthropometric indices as predictors of survival in AIDS adults. Aquitaine Cohort, France, 1985-1997. Group d’Epidemiologie Clinique du Sida en Aquitaine (GECSA) Eur J Epidemiol. 2000;16:633–9. doi: 10.1023/a:1007696530440. [DOI] [PubMed] [Google Scholar]
- 85.Macallan DE, Noble C, Baldwin C, Jebb SA, Prentice AM, Coward WA, et al. Energy expenditure and wasting in human immunodeficiency virus infection. N Engl J Med. 1995;333:83–8. doi: 10.1056/NEJM199507133330202. [DOI] [PubMed] [Google Scholar]
- 86.Makonkawkeyoon S, Limson-Pobre RN, Moreira AL, Schauf V, Kaplan G. Thalidomide inhibits the replication of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:5974–8. doi: 10.1073/pnas.90.13.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lunardi-Iskandar Y, Bryant JL, Zeman RA, Lam VH, Samaniego F, Besnier JM, et al. Tumorigenesis and metastasis of neoplastic Kaposi's sarcoma cell line in immunodeficient mice blocked by a human pregnancy hormone. Nature. 1995;375:64–8. doi: 10.1038/375064a0. [DOI] [PubMed] [Google Scholar]
- 88.Clark RH, Feleke G, Din M, Yasmin T, Singh G, Khan FA, et al. Nutritional treatment for acquired immunodeficiency virus-associated wasting using beta hydroxyl beta methylbutyrate, glutamine and arginine: A randomized, double-blind, placebo-controlled study. JPEN J Parenter Enteral Nutr. 2000;24:133–9. doi: 10.1177/0148607100024003133. [DOI] [PubMed] [Google Scholar]
- 89.Hellerstein MK, Wu K, McGrath M, Faix D, George D, Shackleton CH, et al. Effects of dietary omega-3 fatty acid supplementation in men with weight loss associated with the acquired immune deficiency syndrome: Relation to indices of cytokine production. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:258–70. doi: 10.1097/00042560-199603010-00006. [DOI] [PubMed] [Google Scholar]
- 90.Landman d, Sarai A, Sathe SS. Use of pentoxifylline therapy for patients with AIDS- related wasting: Pilot study. Clin Infect Dis. 1994;18:97–9. doi: 10.1093/clinids/18.1.97. [DOI] [PubMed] [Google Scholar]
- 91.Miller KK, Daly PA, Sentochnik D, Doweiko J, Samore M, Basgoz NO, et al. Pseudo-Cushing's syndrome in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;27:68–72. doi: 10.1086/514638. [DOI] [PubMed] [Google Scholar]
- 92.Friis-Moller N, Weber R, Reiss P, Thiébaut R, Kirk O, d’Arminio Monforte A, et al. Cardiovascular disease risk factors in HIV patients, association with antiretroviral therapy: Results from the DAD study. AIDS. 2003;17:1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 93.Hadigan C, Meigs JB, Wilson PW, D’Agostino RB, Davis B, Basgoz N, et al. Prediction of coronary heart disease risk in HIV-infected patients with fat redistribution. Clin Infect Dis. 2003;36:909–16. doi: 10.1086/368185. [DOI] [PubMed] [Google Scholar]
- 94.Dolan SE, Hadigan C, Killilea KM, Sullivan MP, Hemphill L, Lees RS, et al. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr. 2005;39:44–54. doi: 10.1097/01.qai.0000159323.59250.83. [DOI] [PubMed] [Google Scholar]
- 95.Bhasin S, Parker RA, Sattler F, Haubrich R, Alston B, Umbleja T, et al. Effects of testosterone supplementation on whole body and regional fat mass and distribution in human immunodeficiency virus-infected men with abdominal obesity. J Clin Endocrinol Metab. 2007;92:1049–57. doi: 10.1210/jc.2006-2060. [DOI] [PubMed] [Google Scholar]


