Abstract
Gestational diabetes mellitus (GDM) complicates a substantial number of pregnancies. There is consensus that in patients of GDM, excellent blood glucose control, with diet and, when necessary, oral hypoglycemics and insulin results in improved perinatal outcomes, and appreciably reduces the probability of serious neonatal morbidity compared with routine prenatal care. Goals of metabolic management of a pregnancy complicated with GDM have to balance the needs of a healthy pregnancy with the requirements to control glucose level. Medical nutrition therapy is the cornerstone of therapy for women with GDM. Surveillance with daily self-monitoring of blood glucose has been found to help guide management in a much better way than blood glucose checking in labs and clinics, which tends to be less frequent. Historically, insulin has been the therapeutic agent of choice for controlling hyperglycemia in pregnant women. However, difficulty in medication administration with multiple daily injections, potential for hypoglycemia, and increase in appetite and weight make this therapeutic option cumbersome for many pregnant patients. Use of oral hypogycemic agents (OHAs) in pregnancy has opened new vistas for GDM management. At present, there is a growing acceptance of glyburide (glibenclamide) use as the primary therapy for GDM. Glyburide and metformin have been found to be safe, effective and economical for the treatment of gestational diabetes. Insulin, however, still has an important role to play in GDM. GDM is a window of opportunity, which needs to be seized, for prevention of diabetes in future life. Goal of our educational programs should be not only to improve pregnancy outcomes but also to promote healthy lifestyle changes for the mother that will last long after delivery. Team effort on part of obstetricians and endocrinologists is required to make “the diabetes capital of the world” into “the diabetes care capital of the world”.
Keywords: Gestational diabetes mellitus, glyburide, insulin, medical nutrition therapy, metformin, oral hypoglycemics
INTRODUCTION
Gestational diabetes mellitus (GDM) complicates a substantial number of pregnancies. The overall incidence of 3–6% has steadily increased over time,[1,2] ranging from 2.2% in South America to 15% in the Indian subcontinent.[3] The new diagnostic criteria being put to use are all set to increase the incidence owing to increased detection. It remains a major cause of perinatal morbidity and mortality, as well as maternal morbidity. In a recent review,[4] the present day relevance of GDM and screening and diagnostic criteria for the same were discussed at length. It has also been reported that treatment for GDM has been subjected to the rigors of scientific studies for efficacy and safety, and it has proven its worthiness in this era of evidence-based medicine. Therefore, it is obligatory for all physicians caring for the pregnant women to screen, diagnose and treat them for gestational diabetes by whatsoever methods adopted. In the present review, we shall discuss issues related to non-insulin management of GDM including metabolic goals and surveillance, medical nutrition therapy (MNT) and oral hypoglycemic agents (OHA) in GDM. Role of insulin in GDM shall be discussed in the concluding part of the present review series on GDM. As obesity increases in this country, and our population becomes moresedentary and urbanized, the rate of GDM will rise. Although there has been controversy regarding which diagnostic standards to use for GDM, there is agreement that excellent blood glucose control, with diet and, when necessary, oral hypoglycemics and insulin results in improved perinatal outcomes. Finally, the goal of our educational programs should be not only to improve pregnancy outcomes but also to promote healthy lifestyle changes for the mother that will last long after delivery. As has been mentioned elsewhere although our nation has earned the dubious distinction of being “the diabetes capital of the world”, if we now do a good job of screening and managing GDM, we can lay claim to be the “diabetes care capital of world”.[4] Managing GDM is like primary prevention of the disease for the next generation, as it helps decrease the incidence of type 2 diabetes mellitus (DM) in the generation to come.
WHY MANAGE GDM?
To begin with, Crowther et al.,[5] in a randomized controlled trial (RCT), reported that treating GDM appreciably reduced the probability of serious neonatal morbidity compared with routine prenatal care. Treatment included individualized MNT, daily self-monitoring of blood glucose (SMBG), and insulin when needed. Maternal–fetal Medicine Units Network very recently conducted a randomized clinical trial for the treatment of mild gestational diabetes,[6] the results of which provided further compelling evidence that among women who have GDM and normal fasting glucose levels, treatment, as necessary, reduces rates of adverse pregnancy outcomes including perinatal mortality, neonatal hypoglycemia, neonatal hyperbilirubinemia, elevated cord blood C-peptide level, and birth trauma. It is also imperative to note that only 20% of treated Australian Carbohydrate Intolerance Study trial subjects and 8% of Maternal–fetal Medicine Units Network subjects required insulin, implying that lifestyle modification and dietary intervention will be effective in 80–90% of women with GDM.
METABOLIC MANAGEMENT DURING PREGNANCY: GOALS AND SURVEILLANCE
Maternal glycemia
It is important to have normative data when formulating therapeutic goals. Evidence presented at the Fifth International Workshop-Conference on GDM[7] gave some insights into this perspective. It was indicated that in non-diabetic pregnancies, the mean of peak postprandial glucose concentration approximates 110 ± 16 mg/dl (6.1 ± 0.9 mmol/l), which is coupled with a substantial intra- and inter-subject variation of the time to peak glucose excursion after starting the meal (range 45–120 min).[8] These normative values were found comparable to those found in a prospective study of ambulatory fingerstick capillary glucose monitoring in normal pregnant women.[9] Fasting capillary blood glucose values in the range of 90–99 mg/dl (5.0–5.5 mmol/l), 1-hour postprandial levels of <140 mg/dl (<7.8 mmol/l), and 2-hour postprandial blood glucose in ranges of <120–127 mg/dl (<6.7–7.1 mmol/l) were used as “upper boundary” treatment targets in clinical trials of GDM, and these trials achieved satisfactory clinical outcomes, including frequency of fetal macrosomia less than 11%, suggesting that the treatment targets were appropriate.[7] However, there are no data from controlled trials of lower versus higher targets or 1-hour versus 2-hour postprandial testing to identify ideal goals for the prevention of fetal risks. Apropos the way to monitor blood sugar levels, daily SMBG, using meters appears to be superior to less frequent monitoring in the clinics and labs for detection of glucose concentrations that may warrant intensification of therapy beyond individualized MNT.
Presently, there is consensus that the recommendations of the Fourth International Workshop-Conference on GDM[10] to maintain maternal capillary glucose concentrations at <96 mg/dl (<5.3 mmol/l) in the fasting state, <140 mg/dl (<7.8 mmol/l) at 1 hour, and <120 mg/dl (<6.7 mmol/l) 2 hours after starting the meal should be the treatment targets. It is worth emphasizing, however, that these recommendations do not consider glycemic values higher than those normally recorded in pregnancy, and they refer to glycemic levels associated with pregnancy outcome.[10] Although there is consensus that measuring postprandial glucose levels is more important than pre-prandial levels since the former correlates better with certain adverse neonatal events like malformations, macrosomia, hypoglycemia, and shoulder dystocia,[11,12] it has been debated as to whether glucose should be measured 1 or 2 hours after a meal. Continuous blood glucose monitoring using the continuous glucose monitoring system (CGMS) has recently shown that glucose peaks occur about 70 ± 13 min after meals in non-diabetic pregnant women and after about 90 min in diabetic women.[13] No differences in postprandial glycemic profile were noticed between breakfast, lunch and dinner. Leguizamon et al. in a review reported that patients’ management based on glucose levels measured an hour after meals led to better perinatal outcomes than when patients were managed based upon glucose level measurement 2 hours after meals, in terms of less neonatal hypoglycemia, less macrosomia and fewer cesarean deliveries.[14]
Langer et al. found higher macrosomia rates with mean blood glucose levels higher than 105 mg/dl, whereas there is also evidence that the risk of babies being small for their gestational age was high when the mean blood glucose levels dropped below 87 mg/dl.[15,16] Therefore, it implicates that mean blood glucose values should be kept between 87 mg/dl and 105 mg/dl [fasting plasma glucose (FPG) ~ 90 mg/dl and 2-hour postprandial plasma glucose ~ 120 mg/dl] in GDM patients so as to avoid these fetal complications.[7] Long back, Karlson and Kielmer[17] found an association between mean blood glucose levels and perinatal mortality, which was 4% in GDM women with mean plasma glucose levels below 100 mg/dl, 15% for levels between 100 mg/dl and 150 mg/dl, and 24% if it exceeded 150 mg/dl. The American Diabetic Association ADA's position statement[18] also suggests that fasting blood glucose levels higher than 105 mg/dl carries a greater risk of perinatal mortality in GDM patients.
As the science and medicine behind GDM evolves, the thresholds being used presently to prevent maternal and fetal complications of GDM may need to be reconsidered. There is already an ongoing debate in the light of recent findings in normal pregnant women monitoring their own blood glucose levels[19] or using a CGMS[20,21] and because of the results of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study.[22] HAPO study has addressed the risk of adverse outcomes associated with various degrees of maternal glucose intolerance less severe than overt diabetes.
Fetal biometric monitoring
Assessing the fetal response to maternal GDM by ultrasound measurement of fetal abdominal circumference (AC) starting in the second and early third trimesters and repeated every 2–4 weeks can provide useful information (in combination with maternal SMBG levels) to guide management decisions.[7] Evidence reviewed from RCTs indicates that modification of metabolic management based on fetal growth measurements may improve perinatal outcome or at least be equivalent to standard intensified management. Less intensified management may be endorsed with normal growth (fetal AC < 75th percentile for gestational age), although most authorities feel that some SMBG should be continued. Lower targets for glycemic control may be selected when size of the fetal abdomen is excessive, or pharmacological therapy can be added or intensified if a large AC is detected despite apparently good glycemic control. Consideration has to be given to the accuracy of the measurements of fetal size and maternal glucose measurements for this approach to be effective in clinical practice.
Other methods of surveillance
Urine ketone testing has been recommended by some in GDM patients with severe hyperglycemia, weight loss during treatment, or other concerns of possible “starvation ketosis.” However, the effectiveness of ketone monitoring, both in urine and blood, in improving fetal outcomes has not been proven and hence cannot be recommended as standard practice. Insufficient data are available to determine whether measurement of glycosylated hemoglobin or other circulating proteins is of value in the routine management of GDM. Issues such as depression, eating disorders, stress, anxiety and other psychosocial factors that can block effective response to prescribed treatment in women with GDM need to be looked into and appropriately managed.
MEDICAL NUTRITION THERAPY
All the recent international workshop-conferences on GDM[7,10,23–25] have recognized MNT as the cornerstone of therapy for women with GDM. Dietary intake is foundational to optimal pregnancy outcomes because nutritional quality and quantity have an important impact on the overall growth and development of the fetus. Specifically, the management of GDM entails calorie and nutrient restrictions and manipulations as a strategy to normalize blood glucose levels. Because MNT is the primary therapy for majority of women diagnosed with GDM,[26,27] the challenge for MNT in case of GDM is to balance the needs of a healthy pregnancy with the need to control glucose level. Despite the fact that trials have proven that almost up to 80–90% cases of GDM can be effectively managed with MNT, still relatively very little information is available to allow evidence-based recommendations regarding specific nutritional approaches such as total calories and nutrient distribution to the management of GDM.
MNT has been defined as a “carbohydrate-controlled meal plan that promotes adequate nutrition with appropriate weight gain, normoglycemia, and the absence of ketosis.”[28] To define “carbohydrate-controlled,” guidelines were developed for the following topics: total amount of carbohydrate, use of foods with sugar, carbohydrate distribution, breakfast-time carbohydrate, glycemic index, fiber, and artificial sweeteners. Guidelines were also developed for other aspects of nutrition therapy: individualizing weight gain and caloric needs; monitoring ketone levels; and determining protein, fat, and micronutrient needs. Studies[1,29] have shown that implementing nutrition therapy with SMBG and criteria for advancing treatment had a positive impact on maternal and infant outcomes.
The food plan should be designed preferably by a dietician so as to fulfill minimum nutrient requirements for pregnancy set by the Institute of Medicine (IOM)[30] and to achieve glycemic goals without inducing weight loss or excessive weight gain. MNT is a self-management therapy. Education, support, and follow-up are required to assist the woman to make lifestyle changes essential to successful nutrition therapy. Food plans must be flexible and the modifiable components can include calorie level; amount, distribution, and type of carbohydrate; and amount and type of physical activity. Outcome measures like weight changes, blood glucose and ketone levels, and review of food records must guide adjustments to the food plan. This implies the need for follow-up visits to successfully implement nutrition intervention, and the nutrition practice guidelines do suggest three visits.
Almost 20 years ago, IOM's Nutrition for Pregnancy publication provided the first weight-gain recommendations based on pre-pregnancy body mass index (BMI).[30] There is a strong correlation between infant birth weight and maternal pregravid BMI for women who begin pregnancy in the normal and underweight categories.[30,31] Women with normal BMI (19.8–26.0 kg/m2) have been recommended to gain a total of 11.4–15.9 kg; for the ones who are overweight (BMI 26.1–29.0 kg/m2), the weight-gain recommendation is 6.8–11.4 kg; whereas obese women with a BMI >29 kg/m2 are permitted weight gain only up till 7 kg. As the BMI increases, the correlation between infant size and maternal gain weakens to the point that there is no correlation between weight gain and infant size in obese women. Obese women, regardless of weight gain, produce larger infants.[32]
Recently, the American epidemic of obesity has been linked to weight gain in pregnancy.[33] Women often gain too much weight during pregnancy and retain excessive amounts of weight during the postpartum period. Moreover, women who are overweight or obese before pregnancy are more likely to gain more weight related to childbearing.[34] Retention of gestational weight gain may contribute to obesity and increase the future risk for chronic diseases, including but not limited to type 2 diabetes.
For women at high risk for excessive weight gain, interventions need to begin in the first trimester. Research on the pattern of maternal gestational weight gain shows that weight gained in the first trimester is more predictive of infant weight than the weight gained in the third trimester.[35] Calorie intake is also a factor influencing weight gain. The Dietary Reference Intakes (DRI) published in 2001 recommend an increase in calories for pregnancy: no increase in calories in the first trimester, an additional 340 kcal/day during the second trimester, and 452 kcal/day during the third trimester.[36] However, to determine the actual calorie intake is always difficult and mostly turns out to be imprecise and there is a new awareness that actual calorie intakes are generally underestimated.
The question now is “Do the IOM recommendations for weight gain and calorie intake apply to the woman with GDM?” There is no indication that normal-weight and underweight women with GDM should not follow the IOM weight-gain guidelines and calorie intake. But for the overweight and obese woman, there is no consensus regarding calorie and weight gain recommendations. Restricting calories has been a strategy for controlling weight gain, glucose levels, and avoiding macrosomia in infants of women with GDM. Severe calorie restriction, <1500 calories/day or 50% restriction, increases ketonuria and ketonemia. In a study, high levels of third-trimester β-hydroxybutyrate resulted in lowered mental developmental index scores and average Stanford–Binet scores,[37] and this has formed the basis to formulate recommendations to avoid severe calorie restriction leading to ketonemia/ketosis. Modest calorie restriction, 1600–1800 calories/day or a 33% reduction in intake, does not lead to ketosis but controls weight gain and glucose levels in obese women and has been more successful. Based on these data, ADA Clinical Practice Recommendations have suggested a 30–33% calorie restriction for obese women with GDM, while advising a minimum 1800 calorie level.[18,38]
To ensure that over-restriction of calories and nutrition does not occur, one can monitor urine ketones, food intake, and weight until there is confidence that the nutrient changes are appropriate. Training patients in “carbohydrate counting,” use of food records and testing postprandial fingerstick capillary blood glucose can also facilitate this goal. Record-keeping of food intake by the patient herself has been shown to be an effective tool to increase adherence to calorie control.[39] It also provides the caregiver with more specific information for assessment and counseling. As of now, calorie formulas frequently cited are 35–40 kcal/kg desirable body weight for underweight, 30–35 kcal/kg for normal BMI, and 25–30 kcal/kg for overweight women. However, as mentioned earlier also, there is a lack of adequate research to support these formulas.
The DRI report set a minimum level of 130 g/day for nonpregnant women and 175 g carbohydrate per day for pregnancy; this has an additional 33 g carbohydrate for fetal brain development and functioning. This new minimum recommendation provides an important basis for the level of carbohydrate restriction for women with GDM. Elevated glucose values, and in particular postprandial glucose elevations, are associated with adverse outcomes in GDM.[40] Carbohydrate is the main nutrient that affects postprandial glucose levels. Carbohydrate intake can be manipulated by controlling the total amount of carbohydrate, the distribution of carbohydrate over several meals and snacks, and the type of carbohydrate. In recent years, the glycemic index has received attention as a nutrition intervention to improve glucose control. Foods with a low glycemic index (<55) produce a lower postmeal glucose elevation, whereas those with a high glycemic index (>70) show higher postprandial values. A meta-analysis of studies using low-glycemic index diets in nonpregnant people with diabetes found an additional 0.4% lowering of glycated hemoglobin (HbA1c).[41] Research using the glycemic index in pregnancy has found that pregnancy does not change the glycemic index values of specific foods.[42] Using a low-glycemic index diet will, therefore, reduce the glucose level after eating. Clinicians reviewing postprandial glucose levels for women with GDM often observe that some foods do cause higher glucose values, even when total carbohydrate level is controlled. The glycemic index can help explain these glucose spikes and guide nutrition recommendations. Nutrition interventions for GDM should emphasize overall healthy food choices, portion control, and cooking practices that can be continued postpartum and may potentially help prevent later diabetes, obesity, cardiovascular disease and cancer.
High-fiber diets have also been evaluated in GDM. A pilot study in non-insulin requiring women with GDM demonstrated that high-fiber diets were not associated with a lowering of blood glucose levels.[43] Exercise is an obvious adjunct therapy to MNT for women with GDM. One study of the acute effect of exercise on glucose levels showed a 23 mg/dl (1.3 mmol/l) drop in glucose values at 30 min.[44] However, the safety of prescribed exercise for glucose management has been a concern. Planned physical activity limited to 30 min/day is recommended for all individuals capable of participating. Advising GDM patients to walk briskly or to do arm exercises while seated in a chair for at least 10 min after each meal accomplishes this goal. However, women should monitor fetal activity and blood glucose levels before and after exercise and should not overexert. Authors do encourage women who have been physically active before becoming pregnant to continue an active lifestyle.
NEED FOR ADDITIONAL CONCURRENT THERAPY
If a balance between nutrient needs and glucose control cannot be achieved, then concurrent medication therapy is needed to assist in reducing insulin resistance and supplementing insulin production to provide normoglycemia and improved pregnancy outcomes. Criteria for adding pharmacological therapy vary in research studies. In many studies, the criteria are one or more blood glucose values outside the target range within a designated time frame. Also, elevated fasting glucose values alone are the criteria for insulin or glyburide, as nutrition therapy primarily targets postprandial glucose levels. Additional criteria to consider are weight loss, positive ketone levels, and inadequate nutrient intake; however, blood glucose values alone are not enough to judge the need for additional therapy.
How long dietary measures alone should be attempted before starting pharmacological therapy depends on the patient's glycemic control and the gestational age when GDM is diagnosed. In the opinion of authors, it takes at least 2 weeks before one can say whether dietary measures alone will suffice or not. Uncontrolled GDM is an endocrine emergency, however, and immediate, aggressive management may be needed to ensure foetal and maternal well-being. Early onset of GDM will require pharmacological management more often than GDM which begins towards the end of third trimester.
ORAL HYPOGLYCEMIC AGENTS
Historically, insulin has been the therapeutic agent of choice for controlling hyperglycemia in pregnant women. It still is the treatment of choice in many situations, but the spectrum of insulin in GDM is not in the scope of present review and shall be discussed in detail elsewhere. However, difficulty in medication administration with multiple daily injections, potential for hypoglycemia, and increase in appetite and weight make this therapeutic option cumbersome for many pregnant patients.[45] Moreover, hypoglycemia occurs in approximately 71% of women who take insulin at some time during their pregnancy. In the following text, we shall deliberate upon OHAs and their usage in GDM, with the vision in place that the primary risks of all medications in pregnancy are affected by the transplacental passage, association with fetal anomalies, potential for maternal adverse effects; and after delivery of the newborn, the safety of the medications during breastfeeding. Glyburide and metformin are the two drugs which have opened new vistas for use of OHAs in the management of GDM.
Glyburide, also called glibenclamide, is a second-generation oral sulfonylurea. Glyburide acts by enhancing the release of insulin from the pancreatic beta cells in normal and type 2 DM patients. Pharmacologically, it is well absorbed, independent of food intake, following oral administration. It is metabolized by the liver and time to peak concentration is 2–3 hours with a half-life of 7–10 hours. The initial dose of glyburide is 2.5 mg once or twice a day and can be increased after titration with blood glucose values up to a maximum of 20 mg/day, but no more than 7.5 mg should be taken at a single time.[46,47] Glyburide should be taken at least 30 minutes, preferably 60 minutes, before meals so that the peak action covers the postprandial glucose surge. Because of its extended duration of action, glyburide taken from 10 p.m. to 11 p.m. is effective in lowering the FPG levels in the morning.
The use of oral drugs has become an attractive option in pregnancy. The ease and relatively infrequent administration of glyburide, as compared to insulin, finds favor with patients.. Thus, prescribing glyburide rather than insulin during pregnancy may increase patient compliance, satisfaction, and overall maternal and neonatal outcome. Unlike other sulfonylureas, there is substantial evidence demonstrating the lack of transplacental passage of glyburide to the fetus,[48,49] suggesting insignificant fetal exposure with this drug and its safety in pregnancy.[50] Possible explanations for such lack of placental transport include the extensive plasma protein binding and short elimination half-life.[51,52] From a clinical perspective, in the landmark study of glyburide versus insulin in GDM conducted by Langer, glyburide was not detected in the cord blood of any infant.[53] There have been many studies involving the use of glyburide in pregnancy. Lim in 1997 first reported that there was no difference in pregnancy outcomes of women using this drug for gestational diabetes[54] and claimed that most women with GDM can be managed effectively and safely with it. Langer in his study compared glyburide with insulin in the treatment of gestational diabetes.[53] The daily blood glucose concentrations and glycosylated hemoglobin values were similar between patients on glyburide and insulin. The failure rate was 4% in the glyburide patients, thus requiring the need to switch to insulin. There were no differences in the infants who were large for gestational age or with macrosomia, lung complications, hypoglycemia, admission to the neonatal intensive care unit, or fetal anomalies. Also, the cord insulin concentrations were similar between the groups. Glyburide was not detected in the cord serum of infants of mothers administered thedrug. Langer concluded that glyburide was a clinically effective alternative to insulin therapy in women with gestational diabetes. A cost analysis was then performed by Goetzl to compare the costs associated with glyburide versus insulin therapy.[55] Glyburide was found to be significantly less costly than insulin, with an average cost savings per patient of $165.84, almost 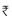 7500/-. Thus, glyburide is not only convenient and equally effective, but also more cost-effective than insulin in the treatment of gestational diabetes.
7500/-. Thus, glyburide is not only convenient and equally effective, but also more cost-effective than insulin in the treatment of gestational diabetes.
To assess the comparative effectiveness of glyburide versus insulin, Nicholson et al. did a meta-analysis of the RCTs of glyburide versus insulin in the treatment of gestational diabetes.[56] There have been three RCTs with a total of 478 participants, conducted in India, the United States, Brazil, Australia and New Zealand. There were no differences in the glycemic control of both fasting and 2-hour postprandial plasma glucose. There were similar rates of cesarean delivery and newborn birth weights between the groups. Some investigators reported a higher rate of maternal hypoglycemia in the women who received insulin (20%) compared with women receiving glyburide (4%),[53] although other investigators reported similar hypoglycemia rates.[57,58] In contrast, neonatal hyperglycemia was reported by some to be higher among those women who received glyburide (33%) compared with those receiving insulin (4%),[58] whereas others did not find such differences.[53] Dhulkotia et al. also did a systematic review and meta-analysis to compare OHAs versus insulin in the management of gestational diabetes and concluded that there are no differences in glycemic control or pregnancy outcomes when OHAs were compared with insulin.[59] They recommended that metformin and glyburide should be considered as safe alternatives to insulin.
Glyburide is a category C medication in pregnancy. Hypoglycemia may occur with all sulfonylureas, and glyburide is no exception. The incidence of hypoglycemia with glyburide ranges from 1 to 5%. The most common adverse effects are gastrointestinal (nausea, vomiting, dyspepsia) and dermatologic (pruritus, urticaria, erythema, and maculopapular eruptions). Elevations of liver function tests have been reported, but jaundice is rare.[46] The overall incidence of adverse effects ranges from 3.2 to 4.1%.
Identifying those women who might fail glyburide therapy in pregnancy is important when deciding medical therapy for the treatment of gestational diabetes. Conway, in an observational trial to examine factors predicting failure of glyburide treatment in gestational diabetes, found that among women with high FPG levels greater than or equal to 110 mg/dl, 24% failed to respond to glyburide.[60]
Studies focusing on the transfer of glyburide into the milk of lactating mothers have been performed.[61,62] There were no cases of neonatal hypoglycemia and it appears that breastfeeding is safe in women receiving glyburide.However, the drug has not been endorsed by any decision-making body for routine use in GDM.
Metformin is a biguanide that improves insulin sensitivity and reduces both fasting and postprandial plasma glucose. It functions by decreasing hepatic glucose output by inhibition of gluconeogenesis and enhances peripheral glucose uptake in the muscles and adipose tissues.[45] It also decreases intestinal glucose absorption and increases insulin sensitivity. It is metabolized by the CYP 450 pathway with a half-life of 6 hours and is excreted in urine. Metformin is available in 250 mg, 500 mg, 850 mg, and 1000 mg tablets in both regular-release and extended-release forms. The usual starting dose is usually 500–1000 mg/day, which can be increased gradually to a maximum dose of 2500 mg/day.
Metformin has been used by women throughout pregnancy. It was Coetzee and colleagues[63] who did the first studies on metformin during the 1970s. Women with insulin-independent diabetes were prospectively followed throughout gestation; 22 women received metformin compared with 42 women who received insulin. The perinatal mortality rate was same for both. There were no cases of maternal hypoglycemia or lactic acidosis with metformin. In addition, metformin use in the first trimester was not associated with congenital anomalies.[64] In a follow-up study, Coetzee was able to achieve glycemic control in women on metformin within 24 hours compared with 2–3 weeks for insulin.[65] In 2000, Hellmuth and colleagues[66] performed a cohort study of type 2 DM pregnant women on metformin versus glyburide versus insulin. Their findings suggest concern for the use of metformin due to the increased rate of preeclampsia (32% metformin vs. 7% glyburide vs. 10% insulin) and intrauterine fetal death (8% vs. 0% vs. 2.3%, respectively). However, this study has become controversial with critics claiming that women in the study were not well matched. Those women who received the metformin were morbidly obese and started the medication later in the pregnancy. Thus, the women were inherently at risk for adverse pregnancy outcomes unrelated to metformin.[45,67] In contrast, in a series of 90 women with polycystic ovarian syndrome (PCOS) who conceived on 1.5–2.55 g/day of metformin and continued metformin in pregnancy, treatment with metformin was not associated with preeclampsia in pregnancy (5.2% metformin vs. 3.6% no metformin).[68]
Others studies have been conducted primarily in women with PCOS treated for infertility. In an observation trial of 72 women who conceived on 2.55 g/day of metformin, there was a higher live birth rate in those who received metformin compared with those without metformin (75% vs. 34%). The spontaneous abortion rate was lower with metformin (17% vs. 62%). There were no cases of lactic acidosis, fetal anomalies, or maternal or neonatal hypoglycemia.[69] The neonates whose mothers received metformin were also followed prospectively, and they displayed normal weight and social and motor skills at 6 months. At 18 months, there were no differences in height, weight, motor, or social skills between the neonatal groups.[70]
A randomized, double-blinded controlled trial was performed on 18 women with PCOS who conceived on metformin compared with 22 women who received a placebo. Women who received metformin had a lower rate of pregnancy complications (0% metformin vs. 32% placebo) including preterm birth, sepsis, deep venous thrombosis, or adult respiratory distress syndrome. There were no differences in neonatal outcomes such as birth weight.[71] Another study involving PCOS women who conceived on metformin showed a lower rate of developing gestational diabetes later in pregnancy (3% metformin vs. 31% no metformin).[72]
An Australian study (Metformin in Gestational diabetes – MiG study) conducted by Rowan and colleagues included 751 women (371 received metformin, and 378 received insulin) who were randomized between 20 and 33 weeks of pregnancy.[73] The metformin failure rate was 7.4%, in which a second diabetic agent was needed to maintain controlled glucose levels. Although there was no difference in mean fasting blood glucose levels between groups, those on metformin had lower 2-hour postprandial glucose levels. There was no difference in the rate of preeclampsia. Infants of women randomized to metformin experienced a lower rate of hypoglycemia compared with insulin (insulin 8.1% vs. metformin 3.3%, P = 0.008). There was no difference in any other perinatal outcome.
Metformin is a category B medication in pregnancy. The risks from metformin in pregnancy include the potential for neonatal hypoglycemia. Metformin has been found to have a maternal-to-fetal transfer rate of 10–16%.[74,75] Neonatal hypoglycemia is always a concern postnatally. However, in several reports on the infants in the immediate neonatal period, there was no increase in the rate of neonatal hypoglycemia after delivery compared with women who received insulin. In those who did develop neonatal hypoglycemia, it was determined that this outcome was related to maternal hyperglycemia at the time of delivery.[63–65,72,76] There were no cases of neonatal lactic acidosis.
Metformin has been primarily used in two different groups of women during pregnancy: one is pregnant women with PCOS where it has been used during organogenesis in the first trimester and the second group is pregnant women with diabetes, both pregestational and gestational diabetes.[63–65,72,76] In all these reports, there was no increased risk of congenital anomalies compared with those who received insulin or the general population. Therefore, metformin is not considered to be teratogenic. Hypoglycemia related to metformin may occur in 0–21% of pregnant women.[77] The frequently observed gastrointestinal adverse effects include diarrhoea, flatulence, nausea, and vomiting, with the incidence ranging from 2 to 63%.[77,78] These are the reasons why metformin is started at a low dose and increased gradually.
In breastfed infants whose mothers are on metformin, the mean infant exposure to drug is less than 1% of the weight-normalized maternal dose, which is much below the 10% level of concern for breastfeeding.[79–81] Based on these findings, metformin use by breastfeeding mothers is absolutely safe.
Acarbose, an α-glucosidase inhibitor, is poorly absorbed from the gastrointestinal tract, and two preliminary studies have suggested efficacy in reducing postprandial glucose excursions in GDM, but with the expected high frequency of abdominal cramping. A small proportion of this drug may be absorbed systemically, and safety and potential transplacental passage have not been fully evaluated. Use of thiazolidinediones, glinides, and glucagon-like peptide 1 agonists during pregnancy is considered experimental. None of these drugs has been endorsed by any decision-making body for routine use in GDM.
CONCLUSION
GDM is a window of opportunity for prevention of diabetes in future life. The opportunity provided by GDM can be utilized only if optimal medical and obstetric care is provided to the antenatal patient with GDM.[82] Optimal management of GDM remains a challenge for the obstetricians and endocrinologists. MNT is the most common therapy which suffices for GDM, but when required, pharmacological treatment becomes necessary. Patients making difficult choices among therapeutic options often ask their physicians what they would do if they were in the same situation. It requires physician's caring for women's health to meaningfully shape therapeutic options and assist patients in making difficult decisions, and finally provide effective and quality health care. Providing effective and quality health care for women, in turn, requires a broad background of information and a wide spectrum of resources.
Much has changed in women's health care in the recent decades, and many of the changes have arisen from questioning and in some cases by radically changing many modalities of treatment which had previously been considered apt. This is indeed the only way for medicine to evolve.[83] From insulin to OHAs has been a great journeyin GDM, with use of OHAs in pregnancy opening newer, simpler vistas for GDM management. OHAs have reached the high tables in the management of GDM. Glyburide and metformin have been found to be safe, effective and economical for the treatment of gestational diabetes. They are also found to be safe for infants who are breastfed. More studies are needed to identify the risks and benefits of these, and other, oral hypoglycemic drugs, before they can be recommended as first line drugs for the treatment of pregestational and gestational diabetes. However, they can definitely be used in situations where insulin administration is not feasible or not accepted by the patient.
Optimal treatment of GDM requires a team effort on part of obstetricians and endocrinologists; Above all, what is required is “an active and alert clinician”. Let us join hands to manage the GDM effectively, not only for the present generation, but also for the generations to come. Let us give ourselves an opportunity to take pride in our medicare system by converting “the diabetes capital of the world” into “the diabetes care capital of the world”.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Jovanovic L, Pettitt DJ. Gestational diabetes mellitus. JAMA. 2001;286:2516–8. doi: 10.1001/jama.286.20.2516. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991-2000. Obstet Gynecol. 2004;103:526–33. doi: 10.1097/01.AOG.0000113623.18286.20. [DOI] [PubMed] [Google Scholar]
- 3.Beischer NA, Oats JN, Henry OA, Sheedy MT, Walstab JE. Incidence and severity of gestational diabetes mellitus according to country of birth in women living in Australia. Diabetes. 1991;40(Suppl 2):35–8. doi: 10.2337/diab.40.2.s35. [DOI] [PubMed] [Google Scholar]
- 4.Magon N. Gestational diabetes mellitus: Get, set, go - From diabetes capital of the world to diabetes care capital of the world. Indian J Endocrinol Metab. 2011;15:161–9. doi: 10.4103/2230-8210.83398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowther CA, Hiller FE, Moss JR, McPhee AJ, Jeffries WS, Robinson FS. Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 6.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–48. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the Fifth International Workshop- Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl 2):S251–60. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 8.Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol. 2004;191:949–53. doi: 10.1016/j.ajog.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 9.Paretti E, Mecacci F, Papini M, Cioni R, Carignani L, Mignosa M, et al. Third-trimester maternal glucose levels from diurnal profiles in nondiabetic pregnancies: Correlation with sonographic parameters of fetal growth. Diabetes Care. 2001;24:1319–23. doi: 10.2337/diacare.24.8.1319. [DOI] [PubMed] [Google Scholar]
- 10.Metzger BE, Coustan DR. The organizing committee: Summary and recommendations of the Fourth International Workshop- Conference on Gestational Diabetes Mellitus. Diabetes Care. 1998;21(Suppl 2):161–7. [PubMed] [Google Scholar]
- 11.Jovanovic-Peterson L, Peterson CM, Reed GF, Metzger BE, Mills JL, Knopp RH, et al. Maternal postprandial glucose levels and infant birth weight: Diabetes in Early Pregnancy Study: The National Institute of Child Health and Human Development –Diabetes in Early Pregnancy Study. Am J Obstet Gynecol. 1991;164:103–11. doi: 10.1016/0002-9378(91)90637-7. [DOI] [PubMed] [Google Scholar]
- 12.De Veciana M, Major CA, Morgan MA, Asrat T, Toohey JS, Lien JM, et al. Post prandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333:1237–41. doi: 10.1056/NEJM199511093331901. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Haroush A, Yogev Y, Chen R, Rosen B, Hod M, Langer O. The postprandial glucose profile in the diabetic pregnancies. Am J Obstet Gynecol. 2004;191:576–81. doi: 10.1016/j.ajog.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 14.Leguizamon G, Krupitzki H, Glujovsky D, Olivera Ravasi M, Reece EA. Blood glucose monitoring in gestational diabetes mellitus:1-versus 2-h blood glucose determinations. J Matern Fetal Neonatal Med. 2002;12:384–8. doi: 10.1080/jmf.12.6.384.388. [DOI] [PubMed] [Google Scholar]
- 15.Langer O, Mazze R. The relationship between large for gestational age infants and glycemic control in women with gestational diabetes. Am J Obstet Gynecol. 1988;159:1478–83. doi: 10.1016/0002-9378(88)90578-9. [DOI] [PubMed] [Google Scholar]
- 16.Langer O. Prevention of macrosomia. Baillieres Clin Obstet Gynaecol. 1991;5:333–47. doi: 10.1016/s0950-3552(05)80101-4. [DOI] [PubMed] [Google Scholar]
- 17.Karlson K, Kjellmer I. The outcome of diabetic pregnancies in relation to the mother's blood sugar level. Am J Obstet Gynaecol. 1972;112:213–20. doi: 10.1016/0002-9378(72)90118-4. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Gestational Diabetes Mellitus (Position Statement) Diabetes Care. 2004;27(Suppl 1):S88–90. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- 19.Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight non diabetic pregnant women. Am J Obstet Gynecol. 2004;191:949–53. doi: 10.1016/j.ajog.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 20.Siegmund T, Rad NT, Ritterath C, Siebert G, Henrich W, Buhling KJ. Longitudinal changes in the continuous glucose profile measured by the CGMS in healthy pregnant women and determination of cut-off values. Eur J Obstet Gynecol Reprod Biol. 2008;139:46–52. doi: 10.1016/j.ejogrb.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Langer O, Yogev Y, Xenakis EM, Brustman L. Overweight and obese in gestational diabetes: The impact on pregnancy outcome. Am J Obstet Gynecol. 2005;192:1768–76. doi: 10.1016/j.ajog.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 22.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. HAPO Study Cooperative Research Group.Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association. Summary and recommendations of the First International Conference-Workshop on Gestational Diabetes Mellitus. Diabetes Care. 1980;3:499–501. [PubMed] [Google Scholar]
- 24.Frenkel N. Summary and recommendations of the Second International Workshop- Conference on Gestational Diabetes. Diabetes. 1985;34(Suppl 2):S123–6. [Google Scholar]
- 25.Metzer BD. Summary and recommendations of the Third International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes. 1991;40(Suppl 2):S197–201. doi: 10.2337/diab.40.2.s197. [DOI] [PubMed] [Google Scholar]
- 26.Langer O. Maternal glycemic criteria for insulin therapy in gestational diabetes mellitus. Diabetes Care. 1998;21(Suppl 2):B91–8. [PubMed] [Google Scholar]
- 27.Gunderson EP. Gestational diabetes and nutritional recommendations. Curr Diab Rep. 2004;4:377–86. doi: 10.1007/s11892-004-0041-5. [DOI] [PubMed] [Google Scholar]
- 28.Chicago, IL: American Dietetic Association; 2001. Medical Nutrition Therapy, Evidence-Based Guides for Practice: Nutrition Practice Guidelines for Gestational Diabetes Mellitus (CDROM) [Google Scholar]
- 29.Reader D, Splett P, Gunderson E. Impact of gestational diabetes mellitus nutrition practice guidelines implemented by registered dietitians on pregnancy outcomes. J Am Diet Assoc. 2006;106:1426–33. doi: 10.1016/j.jada.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Washington, DC: Institute of Medicine, National Academy of Sciences; 1990. Food and Nutrition Board: Nutrition During Pregnancy. Part 1: Weight Gain. Part 2: Nutrient Supplements. [Google Scholar]
- 31.Eastman NJ. Weight relationship in pregnancy. Obstet Gynecol Surv. 1968;23:1003–25. [PubMed] [Google Scholar]
- 32.Abrams BF, Laros RK., Jr Prepregnancy weight, weight gain, and birth weight. Am J Obstet Gynecol. 1986;154:503–9. doi: 10.1016/0002-9378(86)90591-0. [DOI] [PubMed] [Google Scholar]
- 33.Gunderson EP, Abrams B. Epidemiology of gestational weight gain and the body weight changes after pregnancy. Epidemiol Rev. 2000;22:261–74. doi: 10.1093/oxfordjournals.epirev.a018038. [DOI] [PubMed] [Google Scholar]
- 34.Gunderson EP, Murtaugh MA, Lewis CE, Quesenberry CP, West DS, Sidney S. Excess gains in weight and waist circumference associated with childbearing: The Coronary Artery Risk Development in Young Adult Study (CARDIA) Int J Obes Relat Metab Disord. 2004;28:525–35. doi: 10.1038/sj.ijo.0802551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown JE, Murtaugh MA, Jacobs DR, Jr, Margellos HC. Variation in newborn size according to pregnancy weight change by trimester. Am J Clin Nutr. 2002;76:205–9. doi: 10.1093/ajcn/76.1.205. [DOI] [PubMed] [Google Scholar]
- 36.Washington, DC: National Academies Press; 2002. Food and Nutrition Board, Institute of Medicine: U.S. Dietary Reference Intakes: Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. [Google Scholar]
- 37.Rizzo T, Metzger BE, Burns WJ, Burns K. Correlations between antepartum maternal metabolism and child intelligence. N Engl J Med. 1991;325:911–6. doi: 10.1056/NEJM199109263251303. [DOI] [PubMed] [Google Scholar]
- 38.American Diabetes Association. Gestational diabetes (Position Statement) Diabetes Care. 2000;23(Suppl 1):S77–9. [PubMed] [Google Scholar]
- 39.Foyett JP. Management of obesity. Prim Care Rep. 2000;6:19. [Google Scholar]
- 40.De Veciana M, Major CA, Morgan MA, Asrat T, Toohey JS, Lien JM, et al. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333:1237–41. doi: 10.1056/NEJM199511093331901. [DOI] [PubMed] [Google Scholar]
- 41.Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: A meta-analysis of randomized controlled trials. Diabetes Care. 2003;26:2261–7. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- 42.Lock DR, Bar-Eyal A, Voet H, Madar Z. Glycemic indices of various foods given to pregnant diabetic subjects. Obstet Gynecol. 1988;71:180–3. [PubMed] [Google Scholar]
- 43.Reece EA, Hagay Z, Caseria D, Gay LJ, De Gennaro N. Do fiber-enriched diabetic diets have glucose lowering effects in pregnancy? Am J Pernit. 1993;10:272–4. doi: 10.1055/s-2007-994738. [DOI] [PubMed] [Google Scholar]
- 44.Avery MD, Walker AJ. Acute effect of exercise on blood glucose and insulin levels in women with gestational diabetes. J Matern Fetal Med. 2001;10:52–8. doi: 10.1080/714904296. [DOI] [PubMed] [Google Scholar]
- 45.Norman RJ, Wang JX, Hague W. Should we continue or stop insulin-sensitizing drugs during pregnancy? Curr Opin Obstet Gynecol. 2004;16:245–50. doi: 10.1097/00001703-200406000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Anjalakshi C, Balaji V, Balaji MS, Seshiah V. A prospective study comparing insulin and glibenclamide in gestational diabetes mellitus in Asian Indian women. Diabetes Res Clin Pract. 2007;76:474–5. doi: 10.1016/j.diabres.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 47.Merlob P, Levitt O, Stahl B. Oral antihyperglycemic agents during pregnancy and lactation: A review. Paediatr Drugs. 2002;4:755–60. doi: 10.2165/00128072-200204110-00007. [DOI] [PubMed] [Google Scholar]
- 48.Sivan E, Feldman B, Dolitzki M, Nevo N, Dekel N, Karasik A. Glyburide crosses the placenta in vivo in pregnant rats. Diabetologia. 1995;38:753–6. doi: 10.1007/s001250050348. [DOI] [PubMed] [Google Scholar]
- 49.Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807–12. doi: 10.1016/0002-9378(91)90421-m. [DOI] [PubMed] [Google Scholar]
- 50.Elliott BD, Schenker S, Langer O, Johnson R, Prihoda T. Comparative placental transport of oral hypoglycemic agents in humans: A model of human placental drug transfer. Am J Obstet Gynecol. 1994;171:653–60. doi: 10.1016/0002-9378(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 51.Koren G. Glyburide and fetal safety: Transplacental pharmacokinetic considerations. Reprod Toxicol. 2001;15:227–9. doi: 10.1016/s0890-6238(01)00122-8. [DOI] [PubMed] [Google Scholar]
- 52.Garcia-Bournissen F, Feig DS, Koren G. Maternal-fetal transport of hypoglycaemic drugs. Clin Pharmacokinet. 2003;42:303–13. doi: 10.2165/00003088-200342040-00001. [DOI] [PubMed] [Google Scholar]
- 53.Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343:1134–8. doi: 10.1056/NEJM200010193431601. [DOI] [PubMed] [Google Scholar]
- 54.Lim JM, Tayob Y, O’Brien PM, Shaw RW. A comparison between the pregnancy outcome of women with gestation diabetes treated with glibenclamide and those treated with insulin. Med J Malaysia. 1997;52:377–81. [PubMed] [Google Scholar]
- 55.Goetzl L, Wilkins I. Glyburide compared to insulin for the treatment of gestational diabetes mellitus: a cost analysis. J Perinatol. 2002;22:403–6. doi: 10.1038/sj.jp.7210759. [DOI] [PubMed] [Google Scholar]
- 56.Nicholson W, Bolen S, Witkop CT, Neale D, Wilson L, Bass E. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: A systematic review. Obstet Gynecol. 2009;113:193–205. doi: 10.1097/AOG.0b013e318190a459. [DOI] [PubMed] [Google Scholar]
- 57.Anjalakshi C, Balaji V, Balaji MS, Seshiah V. A prospective study comparing insulin and glibenclamide in gestational diabetes mellitus in Asian Indian women. Diabetes Res Clin Pract. 2007;76:474–5. doi: 10.1016/j.diabres.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 58.Bertini AM, Silva JC, Taborda W, Becker F, Lemos Bebber FR, Zucco Viesi JM, et al. Perinatal outcomes and the use of oral hypoglycemic agents. J Perinat Med. 2005;33:519–23. doi: 10.1515/JPM.2005.092. [DOI] [PubMed] [Google Scholar]
- 59.Dhulkotia JS, Ola B, Fraser R, Farrell T. Oral hypoglycemic agents vs insulin in management of gestational diabetes: A systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203(457):e1–9. doi: 10.1016/j.ajog.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 60.Conway D, Gonzales O, Skiver D. Use of glyburide for the treatment of gestational diabetes: The San Antonio experience. Obstet Gynecol Surv. 2004;59:491–3. doi: 10.1080/14767050310001650725. [DOI] [PubMed] [Google Scholar]
- 61.Feig DS, Briggs GG, Kraemer JM, Ambrose PJ, Moskovitz DN, Nageotte M, et al. Transfer of glyburide and glipizide into breast milk. Diabetes Care. 2005;28:1851–5. doi: 10.2337/diacare.28.8.1851. [DOI] [PubMed] [Google Scholar]
- 62.Glatstein MM, Djokanovic N, Garcia-Bournissen F, Finkelstein Y, Koren G. Use of hypoglycemic drugs during lactation. Can Fam Physician. 2009;55:371–3. [PMC free article] [PubMed] [Google Scholar]
- 63.Coetzee EJ, Jackson WP. Pregnancy in established non-insulin-dependent diabetics: A five-and-a-half year study at Groote Schuur Hospital. S Afr Med J. 1980;58:795–802. [PubMed] [Google Scholar]
- 64.Coetzee EJ, Jackson WP. Oral hypoglycaemics in the first trimester and fetal outcome. S Afr Med J. 1984;65:635–7. [PubMed] [Google Scholar]
- 65.Coetzee EJ, Jackson WP. The management of non-insulin-dependent diabetes during pregnancy. Diabetes Res Clin Pract. 1986;1:281–7. doi: 10.1016/s0168-8227(86)80036-5. [DOI] [PubMed] [Google Scholar]
- 66.Hellmuth E, Damm P, Molsted-Pedersen L. Oral hypoglycaemic agents in 118 diabetic pregnancies. Diabet Med. 2000;17:507–11. doi: 10.1046/j.1464-5491.2000.00314.x. [DOI] [PubMed] [Google Scholar]
- 67.Checa MA, Requena A, Salvador C, Tur R, Callejo J, Espinós JJ, et al. Reproductive Endocrinology Interest Group of the Spanish Society of Fertility.Insulin-sensitizing agents: Use in pregnancy and as therapy in polycystic ovary syndrome. Hum Reprod Update. 2005;11:375–90. doi: 10.1093/humupd/dmi015. [DOI] [PubMed] [Google Scholar]
- 68.Glueck CJ, Bornovali S, Pranikoff J, Goldenberg N, Dharashivkar S, Wang P. Metformin, preeclampsia, and pregnancy outcomes in women with polycystic ovary syndrome. Diabet Med. 2004;21:829–36. doi: 10.1111/j.1464-5491.2004.01251.x. [DOI] [PubMed] [Google Scholar]
- 69.Glueck CJ, Wang P, Goldenberg N, Sieve-Smith L. Pregnancy outcomes among women with polycystic ovary syndrome treated with metformin. Hum Reprod. 2002;17:2858–64. doi: 10.1093/humrep/17.11.2858. [DOI] [PubMed] [Google Scholar]
- 70.Glueck CJ, Goldenberg N, Pranikoff J, Loftspring M, Sieve L, Wang P. Height, weight, and motor-social development during the first 18 months of life in 126 infants born to 109 mothers with polycystic ovary syndrome who conceived on and continued metformin through pregnancy. Hum Reprod. 2004;19:1323–30. doi: 10.1093/humrep/deh263. [DOI] [PubMed] [Google Scholar]
- 71.Vanky E, Salvesen KA, Heimstad R, Fougner KJ, Romundstad P, Carlsen SM. Metformin reduces pregnancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: Results of a randomized study. Hum Reprod. 2004;19:1734–40. doi: 10.1093/humrep/deh347. [DOI] [PubMed] [Google Scholar]
- 72.Glueck CJ, Wang P, Kobayashi S, Phillips H, Sieve-Smith L. Metformin therapy throughout pregnancy reduces the development of gestational diabetes in women with polycystic ovary syndrome. Fertil Steril. 2002;77:520–5. doi: 10.1016/s0015-0282(01)03202-2. [DOI] [PubMed] [Google Scholar]
- 73.Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. MiG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003–15. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- 74.Kovo M, Haroutiunian S, Feldman N, Hoffman A, Glezerman M. Determination of metformin transfer across the human placenta using a dually perfused ex vivo placental cotyledon model. Eur J Obstet Gynecol Reprod Biol. 2008;136:29–33. doi: 10.1016/j.ejogrb.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 75.Nanovskaya TN, Nekhayeva IA, Patrikeeva SL, Hankins GD, Ahmed MS. Transfer of metformin across the dually perfused human placental lobule. Am J Obstet Gynecol. 2006;195:1081–5. doi: 10.1016/j.ajog.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 76.Glueck CJ, Goldenberg N, Streicher P, Wang P. Metformin and gestational diabetes. Curr Diab Rep. 2003;3:303–12. doi: 10.1007/s11892-003-0022-0. [DOI] [PubMed] [Google Scholar]
- 77.Bolen S, Feldman L, Vassy J, Wilson L, Yeh HC, Marinopoulos S, et al. Systematic review: Comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147:386–99. doi: 10.7326/0003-4819-147-6-200709180-00178. [DOI] [PubMed] [Google Scholar]
- 78.Asche CV, McAdam-Marx C, Shane-McWhorter L, Sheng X, Plauschinat CA. Association between oral antidiabetic use, adverse events, and outcomes in patients with type 2 diabetes. Diabetes Obes Metab. 2008;10:638–45. doi: 10.1111/j.1463-1326.2007.00758.x. [DOI] [PubMed] [Google Scholar]
- 79.Briggs GG, Ambrose PJ, Nageotte MP, Padilla G, Wan S. Excretion of metformin into breast milk and the effect on nursing infants. Obstet Gynecol. 2005;105:1437–41. doi: 10.1097/01.AOG.0000163249.65810.5b. [DOI] [PubMed] [Google Scholar]
- 80.Gardiner SJ, Kirkpatrick CM, Begg EJ, Zhang M, Moore MP, Saville DJ. Transfer of metformin into human milk. Clin Pharmacol Ther. 2003;73:71–7. doi: 10.1067/mcp.2003.9. [DOI] [PubMed] [Google Scholar]
- 81.Hale TW, Kristensen JH, Hackett LP, Kohan R, Ilett KF. Transfer of metformin into human milk. Diabetologia. 2002;45:1509–14. doi: 10.1007/s00125-002-0939-x. [DOI] [PubMed] [Google Scholar]
- 82.Kalra S, Malik S, John M. Gestational diabetes mellitus: A window of opportunity. Indian J Endocrinol Metab. 2011;15:149–51. doi: 10.4103/2230-8210.83395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Magon N. Patograph revisited. [Last accessed on 2011 Aug 16];Int J Clin Cases Investig. 2011 3:1–6. Available from: http://ijcci.info/pdf/aug11/Guest.pdf . [Google Scholar]


