Abstract
Primary Sjögren's syndrome (pSS) is a chronic autoimmune disease characterized by a progressive lymphocytic infiltration of the exocrine glands with varying degrees of systemic involvement. Chronic inflammation compromises the glands’ function that leads to dry symptoms in the mouth/eyes. Renal involvement is a well recognized extraglandular manifestation of pSS. Metabolic bone disease (MBD), however, rarely occurs as the primary manifestation of a renal tubule disorder due to pSS. To the best of our knowledge there are only 6 reported cases of metabolic bone disease as the primary manifestation of pSS to date. Four of these had distal renal tubular acidosis (RTA), and 2 had a combined picture of distal and proximal tubular dysfunction. We herein present our experience of 3 cases who presented to us with a clinical picture suggestive of MBD. While investigating these patients, we found evidence of RTA, which was found to be secondary to pSS.
Keywords: Interstitial nephritis, metabolic bone disease, osteomalacia, renal tubular acidosis, Sjögren's syndrome
INTRODUCTION
Sjögren's syndrome is a slowly progressing autoimmune disease characterized by lymphocytic infiltration of the exocrine glands, mainly the lacrimal and salivary glands, resulting in their impaired secretory function. Simultaneously, systemic involvement and symptoms of cutaneous, respiratory, renal, hepatic, neurologic, and vascular systems often occur.[1] This syndrome can present either alone (as primary Sjögren's syndrome) or in the context of an underlying connective tissue disease (as secondary Sjögren's syndrome).[2] Renal involvement is a well recognized extra glandular manifestation of primary Sjögren's syndrome (pSS). Most common manifestations are related to tubular dysfunction, resulting from chronic interstitial nephritis, which can manifest as distal renal tubular acidosis (RTA), proximal RTA, tubular proteinuria, or nephrogenic diabetes insipidus.[3,4] Hypokalemic periodic paralysis, urolithiasis, or osteomalacia are uncommon renal manifestations of pSS.[1] Metabolic bone disease (MBD) rarely occurs as the first manifestation of a renal tubule disorder due to pSS. We herein present three cases who presented to us with a clinical picture of metabolic bone disease, secondary to pSS.
CASE REPORTS
Case 1
A 45-year-old married woman presented with lower limb and lower back pain since 7 years, progressive proximal muscle weakness of lower limbs for the past 3 years and increasing difficulty during walking for the past 3 years. She developed proximal muscle weakness of upper limbs since the last 1 year and was incapacitated and confined to bed for last 3 months. She also gave history of recurrent oral ulcers and gritty sensation of eyes for past 6 months. There was no history of fever, joint pain, skin rash, photosensitivity, or parotid swelling. There was no family history of a similar illness. The lady was emaciated, bed bound with contractures of both knees. There was a generalized atrophy of muscles in all extremities (proximal > distal). Power in the proximal muscle group of the lower limb was 2/5 and in the distal muscles was 4/5. In upper limb power in the proximal muscles was 3/5 and in the distal muscles was 4/5. The sensory examination was normal. Also noticed were multiple dental caries. Her investigations are summarized in Table 1.
Table 1.
Laboratory parameter of 3 patients
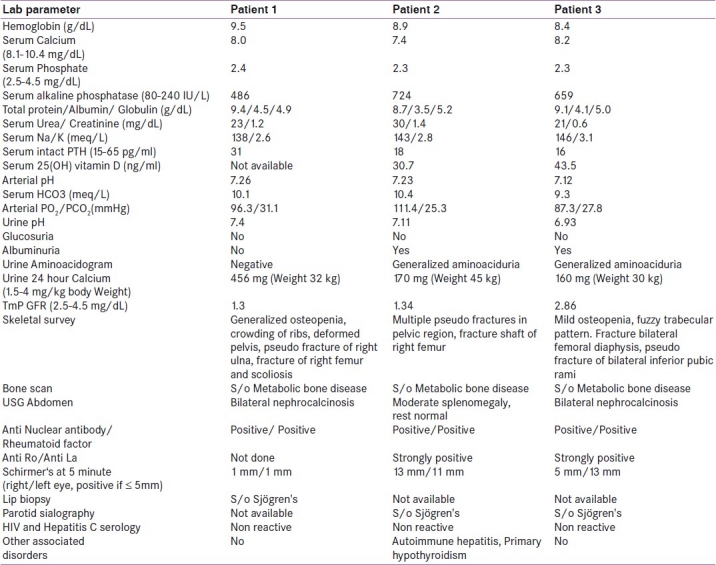
In view of history and investigations, possibility of MBD secondary to distal RTA was considered. While investigating for the etiology of RTA, we found that she had a positive anti nuclear antibody (ANA) and rheumatoid factor (RF), serum C3 level was normal and anti-dsDNA was negative. The mucosal biopsy from her lower lip was consistent with Sjögren's syndrome.
Case 2
A 35-year-old married man, presented with pain in lower limbs for the past 2½ years and progressive proximal muscle weakness of the lower limbs with increasing difficulty during walking for the past 2 years. He denied any history of weakness in the upper limbs. He was treated with oral calcium and vitamin D for approximately 6 months but it did not lead to any clinical improvement. There was no history of fever, joint pain, skin rash, photosensitivity or parotid swelling. There was no family history of a similar illness. On examination, patient had pallor, wasting of both thighs and proximal muscle weakness of bilateral lower limbs. He had poor dental hygiene, multiple dental caries and loss of multiple teeth. His investigations are tabulated in Table 1.
A diagnosis of metabolic bone disease secondary to distal RTA along with proximal tubular dysfunction was made in view of an alkaline urinary pH (>5.5) in the setting of metabolic acidosis and low TmP-GFR, generalized aminoaciduria and albuminuria. The ANA and rheumatoid factor were positive while anti-dsDNA and serum C3 levels were normal. Both anti Ro and anti La antibodies were strongly positive. Bilateral parotid sialography showed diffuse globular sialeactasis of right parotid gland with mild pruning of peripheral ductal system in the left parotid gland, features compatible with pSS. He also had associated features of autoimmune hepatitis and primary hypothyroidism.
Case 3
A 20-year-old unmarried girl was admitted with primary complaints of lower back pain, progressive proximal muscle weakness of lower limbs for the past 4 ½ years and bony pain in both thighs and in pelvic region for the past 4 years. Four years ago she had one episode of sudden onset flaccid weakness of all four limbs associated with neck muscle weakness. At that time, there was no history of seizures, change in sensorium, and bladder or bowel involvement. She recovered completely within 24 hours after treatment with intravenous fluids. Her symptoms, however, progressively worsened and for the past 2 years, she has started having difficulty in walking. She denied any history of weakness or bony pain in the upper limbs. She was treated with oral calcium and injectable vitamin D without any relief of her symptoms. In January 2010, she developed spontaneous pain in her right thigh without any history of trauma and was diagnosed to have a fracture shaft of right femur and was managed conservatively. In the 1st week of July 2010, she developed another fracture of the left femur diaphysis after trivial trauma. At this juncture she consulted an orthopedic surgeon, who suspected MBD and referred her to the Endocrinology department for further investigations. She gave a history of grittiness and foreign body sensation in both eyes and also history of dryness of mouth for the past 2-3 years. She had achieved menarche at age of 15 years and gave a history of regular menstrual periods. There was no family history of a similar illness. On examination she was found to be short statured. Her supine length was 140 cm (<5th centile on K. N. Agarwal growth chart), against target height of 157 cm (50th centile on K. N. Agarwal growth chart). Muscle power in the upper limbs was normal, while proximal muscle power in lower limbs was 4/5, with normal distal power. Sensory examination was normal. Investigations are summarized in Table 1. Her bone scan and radiograph pelvis are shown in Figures 1a and b.
Figure 1.
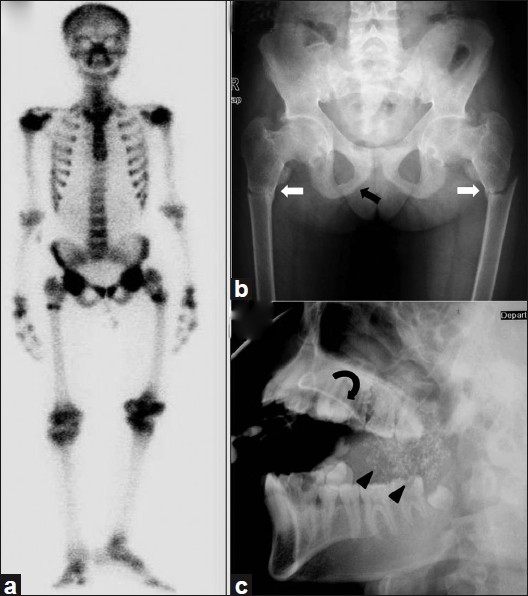
(a) Bone scan image shows diffuse increased tracer uptake in the entire skeleton with focal increased uptake in multiple ribs, bilateral upper third of femur and humeral head. (b) Radiograph pelvis anteroposterior view shows reduced bone density with fuzzy trabecular pattern and fracture of bilateral femoral diaphysis (white arrow). Also note the presence of pseudofracture involving bilateral inferior pubic rami (black arrow) and fuzzy outline of pubic symphysis. (c) Sialogram right parotid gland lateral oblique image demonstrates punctate sialeactasis (arrowheads). Main duct is normal (curved arrows)
A provisional diagnosis of MBD secondary to distal RTA was made. There was also evidence of proximal tubular involvement as suggested by generalized aminoaciduria and albuminuria. Further investigations were carried out to find out the etiology of RTA. The significant findings on working up for etiology were a positive rheumatoid factor and ANA. Schirmer's test was 5 mm in right eye and 13 mm in left eye. Both anti Ro and anti La antibodies were strongly positive. Sialogram parotid gland demonstrated bilateral symmetrical diffuse punctate sialeactasis, suggestive of Sjögren's syndrome [Figure 1c].
DISCUSSION
Primary Sjögren's syndrome (pSS) is a disease of exocrine glands presenting with manifestations related to dry eyes and dry mouth. Non-exocrine organ systems may also be involved, including skin, lung, gastrointestinal tract, central and peripheral nervous system, muscular skeletal apparatus, and the kidney.[1,5] The reported rate of renal involvement in pSS in literature is variable ranging from 4.2% to 50%.[4] This variation results from lack of uniform criteria for diagnosis of pSS, different criteria for assessment of renal involvement and inclusion of both primary and secondary forms of Sjögren's syndrome together in some studies, making it difficult to understand whether renal involvement is due to Sjögren's syndrome per se or due to associated disorders. The spectrum of renal disease includes interstitial nephritis which can manifest as distal RTA, proximal RTA, tubular proteinuria, nephrogenic diabetes insipidus; glomerular diseases or renal failure.[3] The most common manifestations are related to tubular dysfunction which results from chronic interstitial nephritis.[3,4]
Although the incidence of osteomalacia in pSS patients with RTA has been reported to range from 25 to 45%,[6,7] but to best of our knowledge, only 6 cases have been reported of metabolic bone disease as a primary manifestation in pSS to date[8–13] [Table 2]. Four of these patients had distal RTA while the other two cases had a combined picture of distal and proximal tubular dysfunction. In proximal RTA, renal phosphate loss is the principal contributing factor to osteomalacia, while in distal RTA, a combination of acidosis and hypophosphatemia are implicated, and coexisting vitamin D deficiency may be an aggravating factor.[14] Osteomalacia has not been described in absence of RTA in pSS.[14]
Table 2.
Clinical profile of patients manifested primarily with metabolic bone disease in pSS previously
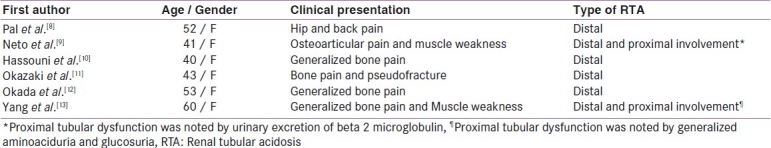
Here, we present a report of three cases that presented to us with a clinical picture of MBD and on further evaluation were found to have evidence of RTA, which on further work up was found secondary to pSS. All three patients had evidence of distal RTA, while patient 2 and 3 also had involvement of proximal tubules. Although the bicarbonate loading test was not done in our patients 2 and 3, the generalized aminoaciduria and albuminuria in both these patients favored at least some proximal tubular involvement. Fanconi's syndrome is a rare kidney manifestation in Sjögren's syndrome. To the best of our knowledge, only ten case of Fanconi's syndrome in pSS have been published to date.[15–22] It may be complete including proximal tubular acidosis or incomplete with the absence of glucosuria and intact TmHCO3. Contrasting with the usual preservation of distal tubular function in adult Fanconi's syndrome, it may exhibit at least one distal tubular abnormality when it is associated with pSS. Although Fanconi's syndrome is a well-known cause of osteomalacia,[23] we could find only 2 case reports of metabolic bone disease as a presenting manifestation in pSS in association with proximal tubular dysfunction[9,13] [Table 2]. In one of these cases, proximal tubule dysfunction was noted by urinary excretion of beta 2 microglobulin and in another case by diffuse aminoaciduria and glucosuria. Both these patients also showed characteristics of distal tubular dysfunction, such as alkalized urine in presence of systemic acidosis as shown in our patients.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Fox IR. Sjögren's syndrome. Lancet. 2005;366:321–31. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 2.Mavragani CP, Moutsopoulos NM, Moutsopoulos HM. The management of Sjögren's syndrome. Nat Clin Pract Rheumatol. 2006;5:252–61. doi: 10.1038/ncprheum0165. [DOI] [PubMed] [Google Scholar]
- 3.Goules A, Masouridi S, Tzioufas AG, Ioannidis JP, Skopouli FN, Moutsopoulos HM. Clinically significant and biopsy-documented renal involvement in primary Sjögren's syndrome. Medicine (Baltimore) 2000;79:241–9. doi: 10.1097/00005792-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Bossini N, Savoldi S, Franceschini F, Mombelloni S, Baronio M, Cavazzana I, et al. Clinical and morphological features of kidney involvement in primary Sjögren's syndrome. Nephrol Dial Transplant. 2001;16:2328–36. doi: 10.1093/ndt/16.12.2328. [DOI] [PubMed] [Google Scholar]
- 5.Manthorpe R, Asmussen K, Oxholm P. Primary Sjögren's syndrome: Diagnostic criteria, clinical features and disease activity. J Rheumatol Suppl. 1997;50:8–11. [PubMed] [Google Scholar]
- 6.Richards P, Chamberlain MJ, Wrong OM. Treatment of osteomalacia of renal tubular acidosis by sodium bicarbonate alone. Lancet. 1972;2:994–7. doi: 10.1016/s0140-6736(72)92405-1. [DOI] [PubMed] [Google Scholar]
- 7.Elkinton JR. Renal acidosis: Diagnosis and treatment. Med Clin North Am. 1963;47:935–58. [Google Scholar]
- 8.Pal B, Griffiths ID. Primary Sjögren's syndrome presenting as osteomalacia secondary to renal tubular acidosis. Br J Clin Pract. 1998;42:436–8. [PubMed] [Google Scholar]
- 9.Monte Neto JT, Sesso R, Kirsztajn GM, Da Silva LC, De Carvalho AB, Pereira AB. Osteomalacia secondary to renal tubular acidosis in a patient with primary Sjögren's syndrome. Clin Exp Rheumatol. 1991;9:625–7. [PubMed] [Google Scholar]
- 10.Hajjaj-Hassouni N, Guedira N, Lazrak N, Hassouni F, Filali A, Mansouri A, et al. Osteomalacia as a presenting manifestation of Sjögren's syndrome. Rev Rhum Engl Ed. 1995;62:529–32. [PubMed] [Google Scholar]
- 11.Okazaki H, Muto S, Kanai N, Shimizu H, Masuyama J, Minato N, et al. A case of primary Sjögren's syndrome presenting as osteomalacia secondary to renal tubular acidosis. Ryumachi. 1991;31:45–53. [PubMed] [Google Scholar]
- 12.Okada M, Suzuki K, Hidaka T, Shinohara T, Kataharada K, Matsumoto M, et al. Rapid improvement of osteomalacia by treatment in case with sjögren's syndrome, rheumatoid arthritis and renal tubular acidosis type 1. Intern Med. 2001;40:829–32. doi: 10.2169/internalmedicine.40.829. [DOI] [PubMed] [Google Scholar]
- 13.Yang YS, Peng CH, Sia SK, Huang CN. Acquired hypophosphatemia osteomalacia associated with Fanconi's syndrome in Sjögren's syndrome. Rheumatol Int. 2007;27:593–7. doi: 10.1007/s00296-006-0257-6. [DOI] [PubMed] [Google Scholar]
- 14.Fulop M, Mackay M. Renal tubular acidosis, Sjögren's syndrome, and bone disease. Arch Intern Med. 2004;164:905–9. doi: 10.1001/archinte.164.8.905. [DOI] [PubMed] [Google Scholar]
- 15.Bridoux F, Kyndt X, Abou-Ayache R, Mougenot B, Baillet S, Bauwens M, et al. Proximal tubular dysfunction in primary Sjögren's syndrome: A clinicopathological study of 2 cases. Clin Nephrol. 2004;61:434–9. doi: 10.5414/cnp61434. [DOI] [PubMed] [Google Scholar]
- 16.Shearn MA, Tu WH. Nephrogenic diabetes insipidus and other defect of renal tubular function in Sjögren's syndrome. Am J Med. 1965;39:312–8. doi: 10.1016/0002-9343(65)90057-4. [DOI] [PubMed] [Google Scholar]
- 17.Delplace M. Renal manifestations of Sjögren's syndrome.Review of the literature starting with a case. Sem Hosp. 1983;59:1693–8. [PubMed] [Google Scholar]
- 18.Walker BR, Alexander F, Tannenbaum PJ. Fanconi's syndrome with renal tubular acidosis and light chain proteinuria. Nephron. 1971;8:103–7. doi: 10.1159/000179912. [DOI] [PubMed] [Google Scholar]
- 19.Morris RC, Sebastian A, Morris E, Ueki I. Hypergammaglobulinemic renal tubular acidosis: A spectrum of physiological disturbances. J Clin Invest. 1968;47:70. [Google Scholar]
- 20.Kamn DE, Fischer MS. Proximal renal tubular acidosis and the Fanconi's syndrome in a patient with hypergammaglobulinemia. Nephron. 1972;9:208–19. doi: 10.1159/000180152. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura H, Kita J, Kawakami A, Yamasaki S, Ida H, Sakamoto N, et al. Multiple bone fracture due to Fanconi's syndrome in primary Sjögren's syndrome complicated with organizing pneumonia. Rheumatol Int. 2009;30:265–7. doi: 10.1007/s00296-009-0924-5. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi T, Muto S, Nemoto J, Miyata Y, Ishiharajima S, Hironaka M, et al. Fanconi's syndrome and distal (Type 1) renal tubular acidosis in a patient with primary Sjögren's syndrome with monoclonal gammopathy of undetermined significance. Clin Nephrol. 2006;65:427–32. doi: 10.5414/cnp65427. [DOI] [PubMed] [Google Scholar]
- 23.Clarke BL, Wynne AG, Wilson DM, Fitzpatrick LA. Osteomalacia associated with adult Fanconi's syndrome: Clinical and diagnostic features. Clin Endocrinol (Oxf) 1995;43:479–90. doi: 10.1111/j.1365-2265.1995.tb02621.x. [DOI] [PubMed] [Google Scholar]


