Abstract
A 29-year-old gentleman presented with episodic features suggestive of Cushing's syndrome. He was evaluated and diagnosed with ectopic Adrenocorticotropic hormone (ACTH)–dependent Cushing's syndrome due to a thymic tumor. The thymic lesion was excised and histopathology confirmed thymic carcinoma with neuroendocrine differentiation, with local, perineural, and vascular invasion. The postoperative problems and further treatment options have been discussed in this case report.
Keywords: Ectopic Cushing's syndrome, thymic carcinoma, 68Ga-DOTANOC PET
INTRODUCTION
Ectopic ACTH secreting tumors constitute 15% of Cushing's syndrome, usually presenting with rapid onset and progression of the disease. Lung carcinomas, and bronchial, thymic, and pancreatic carcinoids are the most common tumors associated with this disorder.[1]
CASE REPORT
A 29-year-old gentleman presented with recurrent episodes of acne, facial and limb edema, muscle weakness, and hypertension over four years, with periods of remission in between. The initial episodes occurred once a year, lasting for about 10 days. However, six months prior to presentation to our institution, the episodes occurred every month. On examination, he had a rounded plethoric face with hyperpigmented striae and acne with multiple pigmented scars. Blood pressure was 160 / 90 mm Hg and proximal myopathy was present. His serum cortisol was elevated, and was not suppressed with 8 mg dexamethasone. Plasma ACTH was elevated and serum potassium was normal [Table 1]. Pituitary magnetic resonance imaging (MRI) showed a 3.5 mm lesion, suggestive of a Rathke's cleft cyst. The computed tomography (CT) scan of the thorax and abdomen showed a 32 × 22 mm thymic mass [Figure 1] and bilaterally enlarged adrenals.
Table 1.
Biochemical parameters
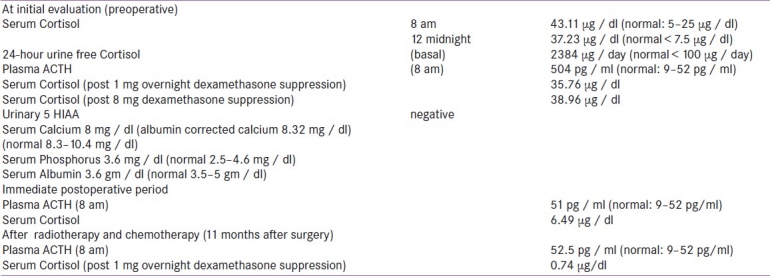
Figure 1.
CT Thorax showing a 32 × 22 mm rounded soft tissue density lesion in the region of thymus
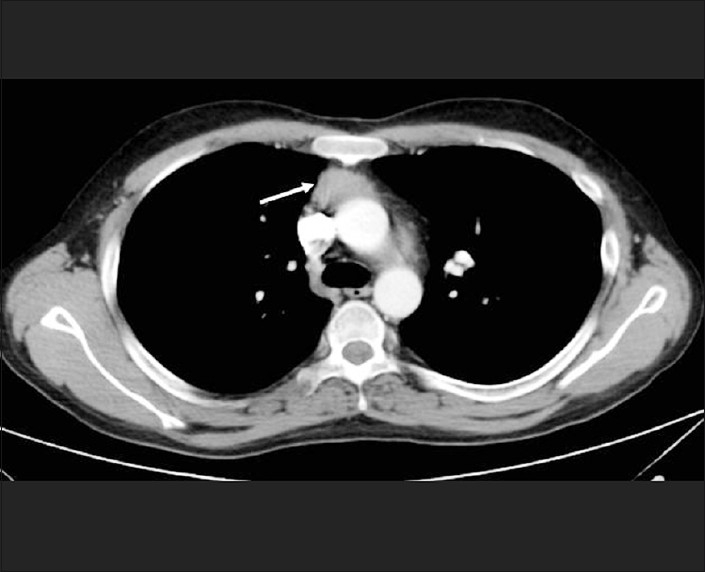
The biochemical tests and imaging features were consistent with ectopic ACTH-dependent Cushing's syndrome, due to a thymic carcinoid. His serum calcium was normal, no pituitary tumor was identified on MRI, the CT abdomen showed normal pancreas, and MEN 1 was excluded. He underwent a total thymectomy. Per-operatively there was a 35 mm diameter hard mass in the right lobe of the thymus infiltrating the superior vena cava, innominate vein, and the pericardium. The tumor was excised along with the infiltrated portions of the pericardium and the walls of the veins, and a primary venous reconstruction was performed. Histopathology was consistent with thymic carcinoma, with neuroendocrine differentiation [Figure 2]. The tumor was composed of lobules and nests of cells demarcated by fibrovascular septae. The lobules contained polygonal cells, with round-to-oval vesicular nuclei, prominent nucleoli, and moderate amounts of pale-to-eosinophilic cytoplasm. The mitotic rate was 5-10 / HPF. There were foci of necrosis and the tumor was infiltrating the surrounding fibroadipose tissue with perineural and vascular invasion. The tumor cells showed diffuse cytoplasmic positivity for neuron specific enolase, synaptophysin and focal positivity for chromogranin and CD117. The tumor cells were negative for calcitonin, CD5, and low molecular weight cytokeratin. The term atypical carcinoid was used in the past to describe thymic neuroendocrine carcinoma grade 2. Our patient's thymic carcinoma had no histological features of a carcinoid, and hence, was labeled as a thymic carcinoma. Neuroendocrine differentiation was identified only on immunostaining.[2]
Figure 2.
Thymic carcinoma with neuroendocrine differentiation: lobules of tumor cells separated by fibrous septae with melanin-laden neoplastic cells and melanophages in the stroma (H and E, ×200)
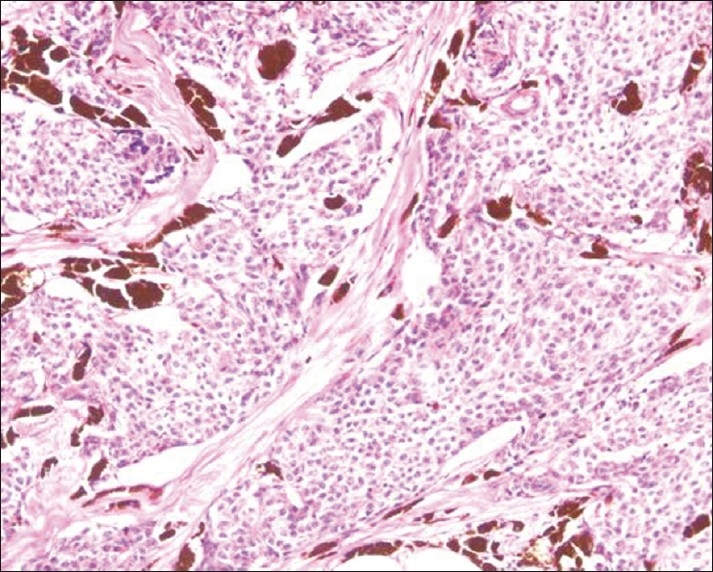
He developed postoperative hypotension, with a drop in the serum cortisol to 6.49 μg / dl and ACTH 51 pg / ml, and was initiated on glucocorticoid replacement. Two weeks after the surgery he developed thrombosis of the left brachiocephalic and subclavian veins and was treated with anticoagulation. His cushingoid features resolved completely in six weeks. A CT scan of the thorax, repeated a month after thymectomy, showed no residual lesion. He underwent local radiotherapy and five cycles of chemotherapy with carboplatin and etoposide. A fluorodeoxyglucose positron emission tomography (FDGPET)-CT scan after completion of chemotherapy (11 months after surgery) showed a recurrence at the operated site measuring 42.2 × 8.9 mm [Figure 3]. A 68Ga-DOTANOC PET scan revealed only a faint uptake in the region of the thymus, and hence, a decision was made for him to be followed up conservatively, rather than offer isotope ablation. A second surgery was also not considered in view of postoperative venous thrombosis and post-radiotherapeutic changes. His ACTH levels have remained normal, with suppressed serum cortisol following dexamethasone suppression [Table 1].
Figure 3.
CT Scan of the thorax after radiotherapy and chemotherapy, showing recurrence of thymic carcinoma
DISCUSSION
Thymic carcinomas are rare but aggressive tumors[3] with peak incidence between the second to fourth decades of life.[4] They may present with symptoms due to mass effect or with endocrinopathy. Cushing's syndrome is the most common endocrine manifestation of these tumors.[3] Intermittent ACTH secretion with a cyclical clinical presentation has also been described with these tumors, similar to the presentation in our patient, although it has not been biochemically documented in this case.[4] Radical surgical excision is the treatment of choice.[3] The role of adjuvant chemotherapy and radiotherapy are controversial.[4] These tumors express somatostatin receptors, and hence, 90Y-DOTA0Tyr3 octreotide and 177Lu-DOTATATE therapies are useful in halting the progression, in case the PET–CT shows significant isotope uptake.[5,6] Response to therapy is dependent on the intensity of the uptake.[6] The prognosis is guarded with a five-year survival rate of 27%. Histological grade, tumor extension, incomplete resectability, and distant metastasis predict a poor prognosis.[7]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Stewart PM. 11th ed. Philadelphia: Elsevier Publishers; 2008. The Adrenal cortex: In Williams textbook of Endocrinology; pp. 445–503. [Google Scholar]
- 2.Marx A, Shimosato Y, Kuo TT, Chan JK, Travis WD, Wick MR. Thymic Neuroendocrine Tumours. In: Travis WD, Brambilla E, Konrad H, Müller-Hermelink HK, Harris KC, editors. Pathology and genetics of tumors of the lung, pleura, thymus and heart. Lyon: IARC Press; 2004. pp. 188–95. [Google Scholar]
- 3.Filosso PL, Actis Dato GM, Ruffini E, Bretti S, Ozzello F, Mancuso M. Multidisciplinary treatment of advanced thymic neuroendocrine carcinoma (carcinoid): Report of a successful case and review of literature. J Thorac Cardiovasc Surg. 2004;127:1215–9. doi: 10.1016/j.jtcvs.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 4.de Perrot M, Spiliopoulos A, Fischer S, Totsch M, Keshavjee S. Neuroendocrine carcinoma (carcinoid) of the thymus associated with Cushing's syndrome. Ann Thorac Surg. 2002;73:675–81. doi: 10.1016/s0003-4975(01)02713-8. [DOI] [PubMed] [Google Scholar]
- 5.Valkema R, Pauwels S, Kvols LK, Barone R, Jamar F, Bakker WH, et al. Survival and Response after peptide receptor radionuclide therapy with [90Y-DOTA0,Tyr3]octreotide in patients with advanced gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med. 2006;36:147–56. doi: 10.1053/j.semnuclmed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Van Essen M, Krenning EP, Bakker WH, de Herder WW, van Aken MO, Kwekkeboom DJ. Peptide receptor radionuclide therapy with 177Lu-octreotate in patients with foregut carcinoid tumours of bronchial, gastric and thymic origin. Eur J Nucl Med Mol Imaging. 2007;34:1219–27. doi: 10.1007/s00259-006-0355-4. [DOI] [PubMed] [Google Scholar]
- 7.Chaer R, Massad MG, Evans A, Snow NJ, Geha AS. Primary neuroendocrine tumors of the thymus. Ann Thorac Surg. 2002;74:1733–40. doi: 10.1016/s0003-4975(02)03547-6. [DOI] [PubMed] [Google Scholar]


