Abstract
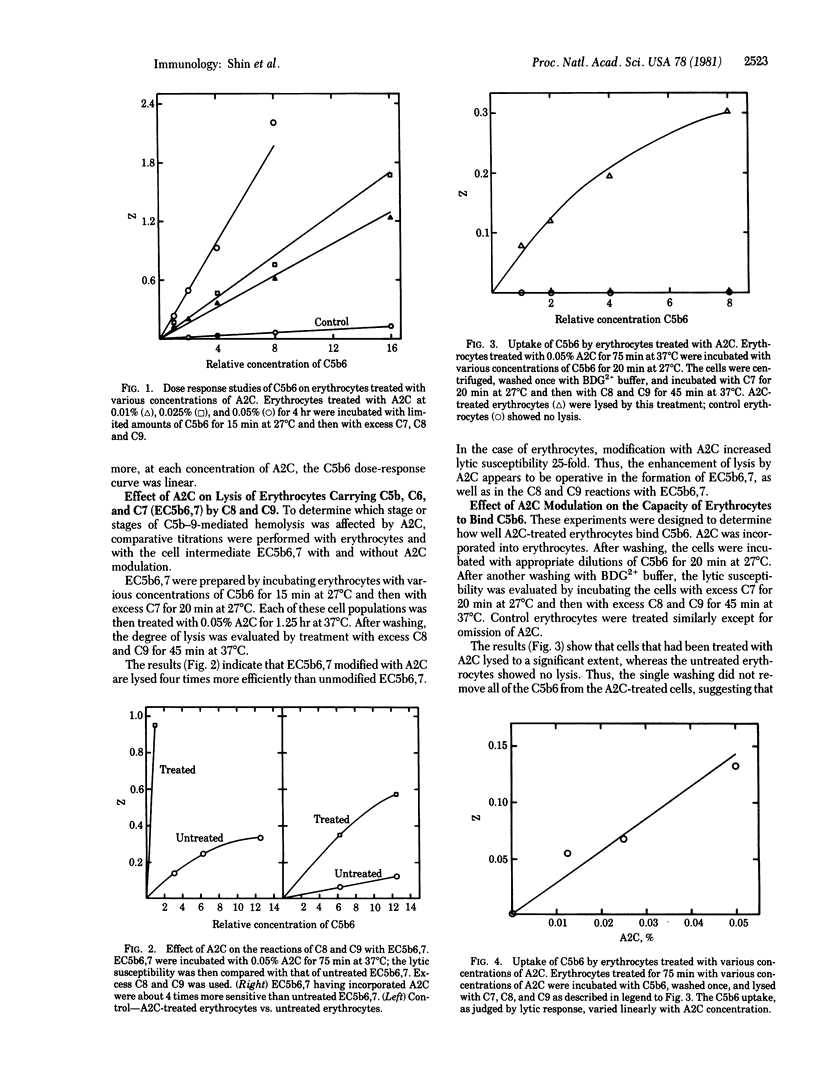
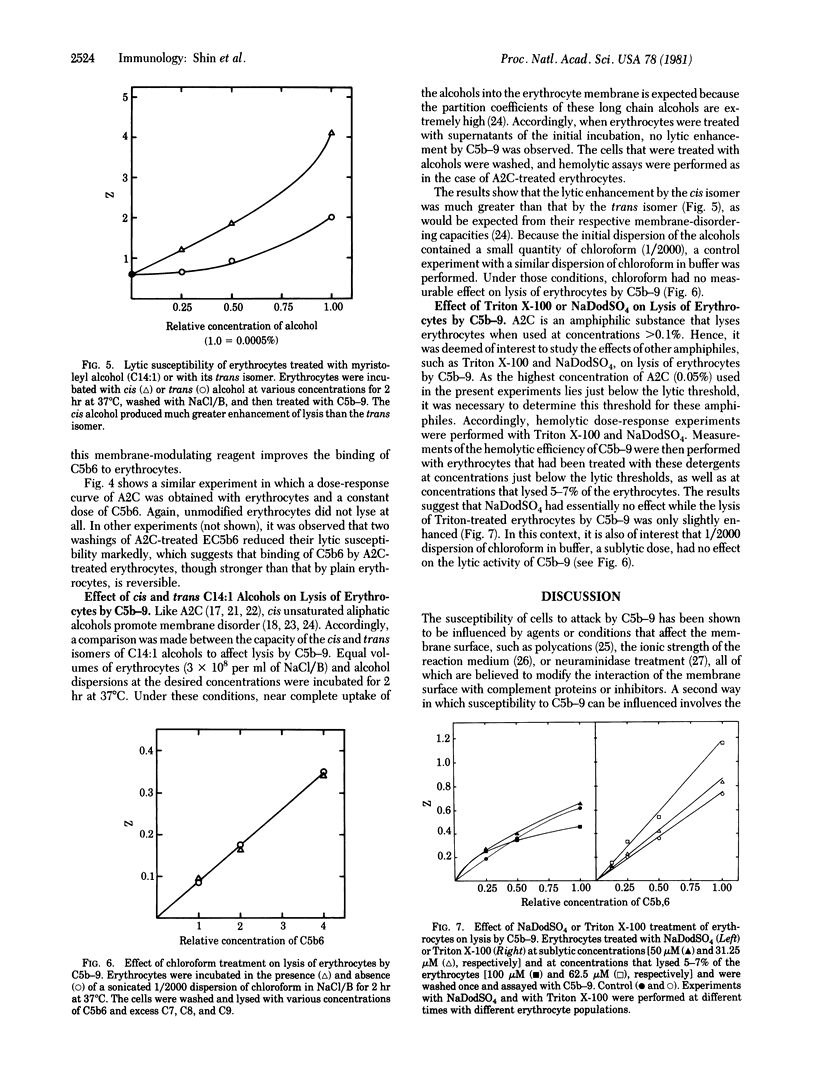
To evaluate the effect of membrane lipid acyl-chain packing on the efficiency of cell lysis by complement, we have studied membrane modulation by 2-(2-methoxy)-ethoxyethyl-8-(cis-2-n-octylcyclopropyl)-octanoate (A2C) and by myristoleyl alcohol, the cis isomer of a C14:1 aliphatic alcohol. These substances are known to increase the membrane lipid disorder by virtue of the bend in their acyl chains, which is believed to loosen the phospholipid acyl-chain packing. We have found that both of these compounds markedly enhance the lysis of erythrocytes by the terminal complement proteins C5b-9. The enhancing effect by A2C is operative in the formation of erythrocytes carrying complement components C5b, C6, and C7, as well as in the subsequent reactions with complement components C8 and C9. We have also found that A2C-treated erythrocytes bind C5b6 to a measurable extent, whereas untreated erythrocytes do not. We attribute this to a shift in the partition equilibrium of C5b6 toward membrane association, which would improve lytic efficiency. The increase of membrane lipid disorder by these agents would also be expected to increase insertion of hydrophobic peptides from C7, C8, and C9, with consequent gain in lytic efficiency. Treatment of erythrocytes with sublytic doses of NaDodSO4, or Triton X-100 did not enhance lysis by C5b-9 appreciably, suggesting that enhancement of lysis by C5b-9 is not a general property of amphiphiles.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Lint T. F., Siegel J., Kies M. W., Gewurz H. Potentiation of C56-initiated lysis by leucocyte cationic proteins, myelin basic proteins and lysine-rich histones. Immunology. 1976 Apr;30(4):467–473. [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Bjerrum O. J., Rother U., Knüfermann H., Wallach D. F. Immunochemical analyses of membrane-bound complement. Detection of the terminal complement complex and its similarity to "intrinsic" erythrocyte membrane proteins. Biochim Biophys Acta. 1975 Sep 16;406(1):21–35. doi: 10.1016/0005-2736(75)90039-5. [DOI] [PubMed] [Google Scholar]
- Bjerrum O. J., Bhakdi S. Demonstration of binding of triton X-100 to amphiphilic proteins in crossed immunoelectrophoresis. FEBS Lett. 1977 Sep 1;81(1):151–156. doi: 10.1016/0014-5793(77)80949-6. [DOI] [PubMed] [Google Scholar]
- Boyle M. D., Gee A. P., Borsos T. Studies on the terminal stages of immune hemolysis. VI. Osmotic blockers of differing Stokes' radii detect complement-induced transmembrane channels of differing size. J Immunol. 1979 Jul;123(1):77–82. [PubMed] [Google Scholar]
- Dahl J. S., Dahl C. E., Levine R. P. Role of lipid fatty acyl composition and membrane fluidity in the resistance of Acholeplasma laidlawii to complement-mediated killing. J Immunol. 1979 Jul;123(1):104–108. [PubMed] [Google Scholar]
- Giavedoni E. B., Chow Y. M., Dalmasso A. P. The functional size of the primary complement lesion in resealed erythrocyte membrane ghosts. J Immunol. 1979 Jan;122(1):240–245. [PubMed] [Google Scholar]
- Goldman J. N., Ruddy S., Austen K. F. Reaction mechanisms of nascent C567 (reactive lysis). I. Reaction characteristics for production of EC567 and lysis by C8 and C9. J Immunol. 1972 Aug;109(2):353–359. [PubMed] [Google Scholar]
- Hammer C. H., Abramovitz A. S., Mayer M. M. A new activity of complement component C3: cell-bound C3b potentiates lysis of erythrocytes by C5b,6 and terminal components. J Immunol. 1976 Sep;117(3):830–834. [PubMed] [Google Scholar]
- Hammer C. H., Nicholson A., Mayer M. M. On the mechanism of cytolysis by complement: evidence on insertion of C5b and C7 subunits of the C5b,6,7 complex into phospholipid bilayers of erythrocyte membranes. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5076–5080. doi: 10.1073/pnas.72.12.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer C. H., Shin M. L., Abramovitz A. S., Mayer M. M. On the mechanism of cell membrane damage by complement: evidence on insertion of polypeptide chains from C8 and C9 into the lipid bilayer of erythrocytes. J Immunol. 1977 Jul;119(1):1–8. [PubMed] [Google Scholar]
- Jain M. K., Gleeson J., Upreti A., Upreti G. C. Intrinsic perturbing ability of alkanols in lipid bilayers. Biochim Biophys Acta. 1978 May 4;509(1):1–8. doi: 10.1016/0005-2736(78)90002-0. [DOI] [PubMed] [Google Scholar]
- Kato K., Bito Y. Relationship between bactericidal action of complement and fluidity of cellular membranes. Infect Immun. 1978 Jan;19(1):12–17. doi: 10.1128/iai.19.1.12-17.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. E., Spector A. A. Effect of specific fatty acyl enrichments on membrane physical properties detected with a spin label probe. J Biol Chem. 1978 Sep 25;253(18):6493–6501. [PubMed] [Google Scholar]
- King M. E., Stavens B. W., Spector A. A. Diet-induced changes in plasma membrane fatty acid composition affect physical properties detected with a spin-label probe. Biochemistry. 1977 Nov 29;16(24):5280–5285. doi: 10.1021/bi00643a018. [DOI] [PubMed] [Google Scholar]
- Kosower E. M., Kosower N. S., Faltin Z., Diver A., Saltoun G., Frensdorff A. Membrane mobility agents. A new class of biologically active molecules. Biochim Biophys Acta. 1974 Sep 6;363(2):261–266. doi: 10.1016/0005-2736(74)90065-0. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Zipser Y., Kosower E. M. Membrane mobility agents: alteration of human red blood cell membrane properties. Arch Biochem Biophys. 1980 Aug;203(1):325–331. doi: 10.1016/0003-9861(80)90183-6. [DOI] [PubMed] [Google Scholar]
- Lauf P. K. Immunological and physiological characteristics of the rapid immune hemolysis of neuraminidase-treated sheep red cells produced by fresh guinea pig serum. J Exp Med. 1975 Oct 1;142(4):974–988. doi: 10.1084/jem.142.4.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig S., Pluznik D. H., Kosower N. S., Kosower E. M. Membrane mobility agent alters the consequences of lectin-cell interaction in a malignant cell membrane. Biochim Biophys Acta. 1975 Sep 2;401(3):458–467. doi: 10.1016/0005-2736(75)90243-6. [DOI] [PubMed] [Google Scholar]
- Michaels D. W., Abramovitz A. S., Hammer C. H., Mayer M. M. Increased ion permeability of planar lipid bilayer membranes after treatment with the C5b-9 cytolytic attack mechanism of complement. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2852–2856. doi: 10.1073/pnas.73.8.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Biesecker G., Müller-Eberhard H. J. Membrane attack complex of complement: generation of high-affinity phospholipid binding sites by fusion of five hydrophilic plasma proteins. Proc Natl Acad Sci U S A. 1979 Feb;76(2):897–901. doi: 10.1073/pnas.76.2.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm L. E., Mayer M. M. Life-span and size of the trans-membrane channel formed by large doses of complement. J Immunol. 1980 May;124(5):2281–2287. [PubMed] [Google Scholar]
- Schlager S. I., Ohanian S. H. Modulation of tumor cell susceptibility to humoral immune killing through chemical and physical manipulation of cellular lipid and fatty acid composition. J Immunol. 1980 Sep;125(3):1196–1200. [PubMed] [Google Scholar]
- Shin M. L., Paznekas W. A., Abramovitz A. S., Mayer M. M. On the mechanism of membrane damage by C: exposure of hydrophobic sites on activated C proteins. J Immunol. 1977 Oct;119(4):1358–1364. [PubMed] [Google Scholar]
- Shin M. L., Paznekas W. A., Mayer M. M. On the mechanism of membrane damage by complement: the effect of length and unsaturation of the acyl chains in liposomal bilayers and the effect of cholesterol concentration in sheep erythrocyte and liposomal membranes. J Immunol. 1978 Jun;120(6):1996–2002. [PubMed] [Google Scholar]
- Sims P. J., Lauf P. K. Steady-state analysis of tracer exchange across the C5b-9 complement lesion in a biological membrane. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5669–5673. doi: 10.1073/pnas.75.11.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura N., Shimada A. The ninth component of guinea-pig complement. Isolation and identification as an alpha 2-globulin. Immunology. 1971 Mar;20(3):415–425. [PMC free article] [PubMed] [Google Scholar]
- Upreti G. C., Jain M. K. Effect of the state of phosphatidylcholine on the rate of its hydrolysis by phospholipase A2 (bee venom). Arch Biochem Biophys. 1978 Jun;188(2):364–375. doi: 10.1016/s0003-9861(78)80021-6. [DOI] [PubMed] [Google Scholar]
- Upreti G. C., Rainier S., Jain M. K. Intrinsic differences in the perturbing ability of alkanols in bilayer: action of phospholipase A2 on the alkanol-modified phospholipid bilayer. J Membr Biol. 1980 Jul 15;55(2):97–112. doi: 10.1007/BF01871152. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. I., Gewurz G. The complex of C5b and C6: isolation, characterization, and identification of a modified form of C5b consisting of three polypeptide chains. J Immunol. 1978 Jun;120(6):2008–2015. [PubMed] [Google Scholar]


