Abstract
Rho kinase (ROCK) belongs to the AGC (PKA/PKG/PKC) family of serine/threonine kinases and is a major downstream effector of the small GTPase RhoA. ROCK plays central roles in the organization of the actin cytoskeleton and is involved in a wide range of fundamental cellular functions such as contraction, adhesion, migration, proliferation and gene expression. Two ROCK isoforms, ROCK1 a n d ROCK2, are assumed to be functionally redundant, based largely on the major common activators, the high degree of homology within the kinase domain and studies from overexpression with kinase constructs a n d chemical inhibitors (e.g., Y27632 a n d fasudil), which inhibit both ROCK1 and ROCK2. Extensive experimental a n d clinical studies support a critical role for the RhoA/ROCK pathway in the vascular bed in the pathogenesis of cardiovascular diseases, in which increased ROCK activity mediates vascular smooth muscle cell hypercontraction, endothelial dysfunction, inflammatory cell recruitment and vascular remodeling. Recent experimental studies, using ROCK inhibitors or genetic mouse models, indicate that the RhoA/ROCK pathway in myocardium contributes to cardiac remodeling induced by ischemic injury or persistent hypertrophic stress, thereby leading to cardiac decompensation and heart failure. This article, based on recent molecular, cellular and animal studies, focuses on the current understanding of ROCK signaling in cardiovascular diseases and in the pathogenesis of heart failure.
Keywords: cardiovascular disease, heart, inhibitor, Rho kinase, ROCK
Rho-kinase (Rho-associated coiled-coil-containing protein kinase [ROCK]) is one of the best-characterized effectors of small GTPase RhoA and belongs to the AGC PKA/PKG/PKC) family of serine/threonine kinases [1-4]. As a major downstream effector of RhoA, ROCK promotes actin-myosin-mediated contractile force generation by phosphorylating a variety of downstream target proteins. The ROCK family consists of two members, ROCK1 (also called ROKb or p160ROCK) and ROCK2 (also known as ROKa), that share 65% overall identity and 92% identity in the kinase domain. Both kinases contain a catalytic kinase domain at the N-terminus, followed by a central coiled-coil domain, including a Rho-binding domain and a C-terminal pleckstrin-homology domain, with an internal cysteine-rich domain. In humans and mice, both ROCK1 and ROCK2 are ubiquitously expressed across tissues [3].
The Rho/ROCK family has been investigated intensively for its roles in cellular processes, as the majority of cellular activities are directly or indirectly regulated by Rho/ROCK protein activity, for reviews see [5-16]. The progress in both pharmacological study and translational research have led to the discovery that ROCK could be a potential therapeutic target in the treatment of diverse disorders, such as cardiovascular disorders, neurologic disorders, metabolic disorders and cancers [17-20]. In this article, we will focus on the current information derived from studies of cardiovascular-related diseases, mainly covering hypertension, atherosclerosis and heart failure, with a special emphasis on the myocardium and cardiomyocytes. Two relatively selective ROCK inhibitors, Y27632 [21] and fasudil [22], which target the ATP-dependent kinase domain of ROCK1 and ROCK2, will be discussed; specifically we will examine their application in dissecting the roles of ROCK in cellular signaling and in diverse pathological events of animal models. Recent findings derived from ROCK1 and ROCK2 knockout mouse models will also be covered.
Substrates, functions & signaling pathways Regulation of ROCK activity
ROCK1 and ROCK2 are downstream targets of the small GTP-binding protein RhoA, working as a mediator in the RhoA-dependent signalling pathway. Stimulation of tyrosine kinase and G-protein-coupled receptors leads to activation of RhoA via the recruitment and activation of guanine nucleotide exchange factors [23,24]. Activated RhoA directly interacts with the C-terminal portion of the coiled-coil domain of ROCK and induces a conformational change, leading to activation of the serine/threonine kinase toward selective substrates [1-4]. ROCK’s activity can also be modulated through interaction of the C-terminal pleckstrin-homology domain with lipid mediators such as arachidonic acid and sphingosylphosphorylcholine [25-27], autophosphorylation [28], mechanical stress and proteolytic cleavage of the inhibitory C-terminal domain [29-31].
ROCK & cytoskeleton dynamics
Both ROCK1 and ROCK2 are highly homologous and share more than 20 immediate downstream substrates [6-12]. The major downstream substrates of ROCK include the myosin binding subunit of myosin light chain (MLC) phosphatase (MYPT1) [32-34], MLC2 [32,35], LIM kinases [36-40], ezrin/radixin/moesin (ERM) [41] and adducin [42], thereby modulating actin cytoskeleton organization, stress fiber formation and smooth muscle cell contraction. The consensus amino acid sequences for phosphorylation on these substrates are R/KXS/T or R/KXXS/T [34,38]. However, these substrates can also be phosphorylated by other serine—threonine kinases such as MLC kinase, PKA, PKC and PKG [43,44].
The ROCK/MYPT1/MLC2 pathway is extensively described in smooth muscle cells, where it mediates calcium sensitization and thereby enhances and sustains contraction in the vascular bed. Identification of this pathway first connected this kinase family with cardiovascular diseases associated with abnormal smooth muscle contraction, such as cerebral vasospasm, hypertension and ischemic cardiac injury. On the other hand, ROCK/MYPT1/MLC and ROCK/LIMK/cofilin pathways are heavily involved in stress fiber formation. ROCK seems to induce and maintain stress fibers by increasing contractility via MLC phopshorylation and by stabilizing actin filaments through LIMK activation, resulting in cofilin phosphorylation and thereby inhibiting its actin-depolymerization activity. The most recent updated information about these pathways has been summarized in several recent reviews [7,9,12,45].
ROCK & SRF
The prominent effects of RhoA/ROCK on cytoskeletal dynamics not only control cell contraction, adhesion, morphology and motility, but also involve transcriptional regulation. For instance, serum response factor (SRF) activity is regulated by RhoA/ROCK signaling on actin polymerization [46-49]. Myocardin, myocardin-related transcription factor-a (MRTF-A), and MRTF-B constitute a SRF coactivator family and their activity depends on actin dynamics [48-51]. Association of MRTF-A with G-actin results in its sequestration in the cytoplasm and actin polymerization leads to MRTF-A translocation into the nucleus and SRF target gene activation [48,49].
The RhoA/ROCK/actin/MRTF-A/SRF pathway has been more fully described in muscle cells and cultured fibroblasts [52]. Recent studies have demonstrated the involvement of this pathway in cardiovascular diseases. RhoA/ROCK may regulate MRTF-A mediated myofibroblast activation in cardiac fibrosis; a recent study demonstrated that TGF-b1-induced nuclear accumulation of MRTF-A in a ROCK-dependent manner in cardiac fibroblasts, leading to the activation of SRF and collagen synthesis [53]. RhoA/ROCK may also be involved in hyperglycemia-induced cell growth and SRF-dependent gene expression in rat aortic smooth-muscle cells, with pitavastatin, a HMG-CoA inhibitor, inhibiting hyperglycemia-augmented reactions via inhibition of RhoA/ROCK pathway [54].
ROCK & phosphatase & tensin homologue
The important roles of ROCK1 and ROCK2 are not only involved in governing various mechanisms modulating cytoskeletal dynamics in response to extracellular signals, but are also implicated in regulating other functions independent of their effects on the actin cytoskeleton. For example, RhoA/ROCK/phosphatase and tensin homologue pathway has been demonstrated to regulate the phosphatidylinositol 3-kinase (PI-3K)/Akt pathway, which has important roles in a diverse range of biological processes, including cell survival [55-57]. The phosphorylation of phosphatase and tensin homologue by ROCK increases its stability and phosphatase activity, leading to the reduction of Akt phosphorylation [55-57].
ROCK & IRS
ROCK has been demonstrated to interact and phosphorylate insulin receptor substrate-1 (IRS-1) and thereby modulate insulin signaling. However, both in vitro and in vivo studies have yielded conflicting results about the effects of RhoA/ROCK/IRS pathway on insulin signaling. The majority of the reported studies favor a detrimental role of ROCK activation in insulin signaling. These studies suggest that ROCK phosphorylates IRS-1 and IRS-2 and impairs activation of PI-3K in rat vascular smooth muscle cells, H9c2 rat cardiac myoblasts and C2C12 mouse myoblasts [58-60]. In contrast, Furukawa etal. reported that ROCK-mediated phosphorylation of IRS-1 positively regulates insulin action by facilitating tyrosine phosphorylation of IRS-1 in 3T3-L1 adipocytes and L6 myotubes [61]. Although it was consistently reported that ROCK activation increases serine phosphorylation levels of IRS-1 (Ser307 and Ser632/635) and IRS-2 [58-61], the effects on insulin-stimulated tyrosine phosphorylation of IRS-1 and IRS-2 and on PI-3K activation appear to be complex and may be context dependent. In vivo, whether treatment with ROCK inhibitors improves or impairs insulin signaling is also context dependent. In supporting a negative effect of ROCK activation on insulin signaling, in obese Zucker rats treatment with fasudil, a ROCK inhibitor, improves insulin signaling and glucose tolerance [62]. In supporting a positive effect of ROCK activation on insulin signaling, acute treatment of ROCK inhibitor, Y27632, causes insulin resistance by reducing insulin-mediated glucose uptake in skeletal muscle [61], and global ROCK1 deficiency in mice causes insulin resistance in vivo, in part via reduced serine 632/635 phosphorylation of IRS-1 [63].
Isoform functions
While the two ROCK isoforms are very similar and are possibly somewhat redundant, a growing body of evidence indicates that they also have some unique functions. Recent studies with individual knockdowns of ROCK1 and ROCK2 using short interfering RNA (siRNA)-based gene silencing or genetic approach, have demonstrated that these two isoforms have nonredundant in vitro functions. For instance, although both ROCK1 and ROCK2 control assembly of the actin cytoskeleton and cell contractility via phosphorylation of MYPT1, the mechanism may vary between the two isoforms. Only ROCK2 binds directly to and phosphorylates MYPT1 [64], suggesting that intermediate proteins are involved in ROCK1 binding to MYPT1. Moreover, functional differences between ROCK1 and ROCK2 have been reported in fibroblasts [65-67], smooth muscle cells [64], endothelial cells [68-70], keratinocytes [71] and cancer cells [72]. Their functional differences could be explained by the facts that both isoforms are expressed at different levels and/or they have different interaction partners in individual cell types.
The in vivo functional similarity and differences of ROCK1 and ROCK2 have been demonstrated by mouse genetic studies during development and under pathological conditions [73]. ROCK1 [74-76] and ROCK2 [77-80] knockout mice both exhibit embryonic lethality depending upon the genetic background. ROCK1 or ROCK2 knockout in C57BL/6 genetic background can result in mice born with eyes open at birth and an omphalocele phenotype due to disorganization of actin filaments in the epithelial cells of the eyelids and of the umbilical ring [73-75,78]. Most of these mice die soon after birth due to an omphalocele with organs, such as liver and gut, protruding from the peritoneal cavity. For both genetic knockouts, the mice that survive past the perinatal period develop phenotypically normal and are fertile, supporting the idea that the two isoforms are mostly redundant [67].
ROCK in cardiomyocytes
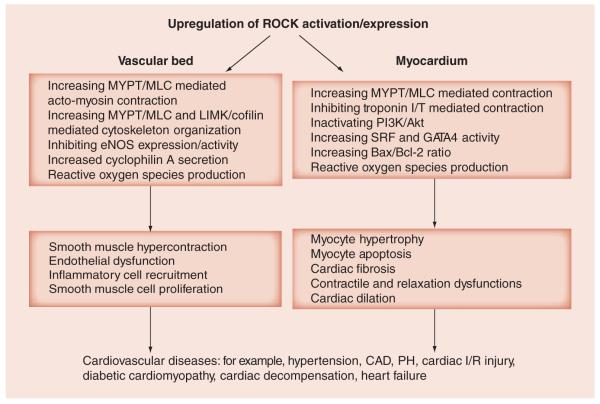
In the context of the myocardium, the role of the ROCK signaling pathway is less well understood than its role in the vasculature. Although cardiomyocytes express RhoA, ROCK1 and ROCK2, their key substrates remain largely unsolved (FIGURE 1).
Figure 1. Major molecular events and cell functions directly or indirectly affected by upregulation of ROCK activity and/or expression in the vascular bed and myocardium.
In the vascular bed, biochemical signaling pathways downstream of ROCK have been well described and their contribution to various cardiovascular diseases have been extensively documented. However, in the myocardium, the biochemical pathways downstream of ROCK and the related cellular mechanisms implicated in the pathogenesis of heart failure are much less well understood. CAD: Coronary artery disease; I/R: Ischemia/reperfusion; PH: Pulmonary hypertension; ROCK: Rho-associated coiled-coil-containing protein kinase.
ROCK & hypertrophy
In vitro experiments, extensively performed in cultured neonatal cardiomyocytes, have demonstrated that RhoA/ROCK signaling pathway mediates an induction of cardiomyocyte hypertrophy by GPCR agonists such as angiotensin II (Ang II), a-adrenergic agonists, and endothelin-1 [81-87]. The signaling pathways activated by RhoA/ROCK to promote cardiomyocyte hypertrophy are not well understood. The RhoA/ROCK pathway may be involved in myofiber assembly through effects on cytoskeleton organization, in hypertrophy gene expression in part through activating SRF transcriptional activity [8,83], and in the production of cytokines, including fibrogenic cytokines TGF-P2 and connective tissue growth factor [76], FGF-inducible 14-kDa protein (Fn14), a member of the TNF-receptor family [88], and inflammatory cytokine IL-18 [89].
ROCK & apoptosis
Cardiomyocyte apoptosis has been observed in major heart diseases, including cardiomyopathies, myocardial infarction, end-stage heart failure, arrhythmogenic right ventricular dysplasia and it is believed to play a crucial role in the development of heart failure [90-92]. Both proapoptotic and antiapoptotic roles of RhoA/ROCK have been extensively reported in a variety of in vitro and in vivo studies and most of them in noncardiomyocytes [10]. However, exactly how RhoA/ROCK regulates an apoptotic response is not completely understood in many instances, and is likely different depending on the cell type and the apoptotic stimulus. In cultured cardiomyocytes, acute activation of RhoA/ROCK (less than 24 h) inhibited apoptosis through the FAK/PI3K/Akt survival pathway, while more sustained activation of Rho/ROCK (48-72 h) induced apoptosis through activation of p53/Bax-mediated mitochondrial death pathway [93,94]. Our laboratory demonstrated the involvement of ROCK1 in cardiomyocyte apoptosis in hypertrophic hearts [56,95]. The antiapoptotic effects of ROCK1 deletion were found to be associated with enhanced ERK/MAPK and/or Akt activation [56,95], suggesting a role for ROCK1 in modulating the activity of these survival pathways under pathological conditions.
ROCK & contraction
The role of ROCK in cardiac contraction is unclear, although similar mechanisms as in smooth muscle cells have been proposed. ROCK was reported to mediate a1-adrenergic receptor agonist-stimulated contraction in the hearts through the MYPT/MLC pathway [96-98], thus increasing contraction. By contrast, phosphorylation of cardiac troponin I/T by ROCK resulted in impaired contraction [99].
Moreover, acute ROCK inhibition improved cardiac contraction in the diabetic heart [100], supporting a negative role for ROCK in cardiac contraction. Other potential roles of ROCK in regulating contraction include participating in the maturation of the myocardial contractile system [101,102]; mediating the contraction-induced suppression of sarcoendoplasmic reticulum Ca2+-ATPase2a expression [103].
ROCK inhibitors
Most of the studies to date have been performed using nonisoform selective ROCK inhibitors such as fasudil, Y-27632, or H-1152, all ofwhich target the ATP-dependent kinase domain of ROCK1 and ROCK2 [104,105]. These inhibitors also have possible nonselective effects [106,107] and at higher concentrations they also inhibit other serine/threonine kinases such as PKA and PKC [108]. When tested against a panel of 70 protein kinases, Y27632 (10 pM), fasudil (10 pM) and H-1152 (1 pM) inhibited 9, 13 and 12 protein kinases including ROCK2 by more than 50%, respectively [107].
Based on the overall promising studies showing beneficial effects of fasudil and Y27632 in a variety of animal disease models, considerable interest and effort have been devoted to the development of more potent and selective ROCK inhibitors (TABLE 1). One of these novel inhibitors of ROCK is SAR 407899 which is nonisoform selective; its potency was found to be three-times that of Y-27632 and eight-times that of fasudil, and has demonstrated antihypertensive effects [109]. Another novel inhibitor is azaindole-1, which is also an ATP-competitive inhibitor, modeled to bind to the catalytic domain of ROCK1. It has 30-fold the potency of Y27632 with a longer duration in the body and also has blood pressure lowering effects [110]. Two others inhibitors are GSK269962A and SB-772077-B, both competitively binding to the ROCK ATP pocket, they possess higher inhibitory potency than that of Y-27632 or fasudil [111,112]. Finally, a ROCK2 isoform-specific inhibitor, SLx-2119, which is also an ATP-competitive inhibitor, has recently been developed [113].
Table 1. Rho-associated coiled-coil-containing protein kinase inhibitors and potential therapeutic implications in cardiovascular diseases.
| Compound | Isoform specificity | Species | Therapeutic implication | Ref. |
|---|---|---|---|---|
| Fasudil | None; Ki 0.4 μM for ROCK |
Clinical trials; many animal models |
Hypertension, PH, CAD, diabetic cardiomyopathy, vasospasm, vasospastic angina, ischemic stroke, heart failure, erectile dysfunction, cardiac I/R injury, cardiac remodeling |
[18,118,127,138,157,169, 171,173,174,176–194] |
| Y-27632 | None; Ki 0.22 μM for ROCK1, Ki 0.30 μM for ROCK2 |
Rat, mice, human tissue |
PH, diabetic cardiomyopathy, atherosclerosis, vasospasm, MI, cardiac I/R injury, cardiac remodeling |
Rat: [130,156,159,168,170, 196–198]; Mice: [151,155,141]; Human tissue: [186,199] |
| SAR407899 | None; IC50 0.276 μM for ROCK1, IC50 0.102 μM for ROCK2; Ki 36 nM for ROCK2 |
Rat | Hypertension | [109] |
| Azaindole-1 | None; Ki 3.7nM for ROCK1, Ki 4.8 nM for ROCK2 |
Rat, dog | Hypertension | [110] |
| SLx-2119 | ROCK2; IC50 24 μM for ROCK1, IC50 0.105 μM for ROCK2 |
Human tissue | Cardiac remolding | [113] |
| GSK269962A | None; IC50 1.6 nM for ROCK1, IC50 4 nM for ROCK2 |
Rat | Hypertension | [111] |
| SB-772077-B | None; IC50 5.6 nM for ROCK1, IC50 6 nM for ROCK2 |
Rat | Hypertension, PH | [111,112] |
| GSK576371 | Unknown | Rat | POH | [172] |
CAD: Coronary artery disease; I/R: Ischemia/reperfusion injury; MI: Myocardial infarction; PH: Pulmonary hypertention; POH: Pressure overload-induced cardiac hypertrophy; Rho-associated coiled-coil-containing protein kinase.
In some studies, the beneficial effects of ROCK inhibition have been tested along with other treatments to enhance their effectiveness. One such case is using a combination of ROCK specific inhibitors in conjunction with statins [113]. Statins, which inhibit HMG-CoA reductase and block the synthesis of cholesterol, are clinical drugs for the treatment of hyperlipidemia to reduce the risk of adverse cardiovascular events. In addition to the cholesterol-lowering effects, statins have also been found to reduce ROCK expression and activity [114-117]. Another study has found that using ROCK inhibitors (fasudil) with another vasodilator agent (nitroglycerin) caused further dilations, beyond treatment with just nitroglycerin [118].
ROCK in cardiovascular diseases Hypertension
Arterial hypertension is a major risk factor for cardiovascular disease and one of the most common cardiovascular disorders. It is characterized by a high arterial pressure level, resulting from increased peripheral vascular resistance, attributable to increased vascular contractility and arterial wall remodeling. In addition, increased cardiac output, reduced renal sodium/water excretion and a distorted CNS for blood pressure regulation are key components of the pathogenesis of hypertension. Numerous factors, especially the renin-angiotensin-aldosterone system and reactive oxygen species (ROS) have been implicated in the pathophysiology of hypertension [119,120]. Ang II acts directly on vascular smooth muscle to cause vessel constriction and regulate vascular tone; it also alters renal sodium and water absorption via stimulating the synthesis and secretion of aldosterone. Ang II is also associated with cardiovascular remodeling through a process of promoting inflammation, hypertrophy and fibrosis [119,121,122]. On the other hand, cardiovascular cells produce ROS under various stresses such as pressure, stretch, hypoxia and Ang II, resulting in changes of the vascular redox state; these changes consequently activate specific signaling pathways leading to smooth muscle contraction and proliferation, induction of an inflammatory response and impairment of endothelium-dependent relaxation [120,123].
The role of ROCK signaling in arterial hypertension has been extensively studied using ROCK inhibitors such as Y27632 and fasudil [21,124-127]. Numerous studies have demonstrated that the Rho/ROCK pathway is increased in hypertensive animal models [124-126] and hypertensive patients [127]. Additional evidence for the importance of the RhoA/ROCK pathway for hypertension in humans comes from genetic studies, which demonstrates that ROCK polymorphism at amino acid position 431 [128] and a haplotype block consisting of 4 single-nucleotide polymorphisms within the ROCK2 allele [129] are associated with changes in systemic blood pressure. Importantly, RhoA/ROCK signaling is involved substantially in the vascular effects of oxidative stress [13,130] and various vasoactive factors, especially Ang II [125,131-133]. Enhanced smooth muscle contractility through an increase in MLC phosphorylation and impaired endothelial function through a decrease in nitric oxide (NO) production by stimulated RhoA/ROCK most likely contribute the hypertensive state [21,126,134]. ROCK activation also promotes inflammation and remodeling through inducing the expression of proinflammatory cytokines and adhesion molecules in endothelial and smooth muscle cells, including plasminogen activator inhibitor-1 [135,136] and monocyte chemoattractant protein-1 [137]; promoting ROS production through upregulation of NADPH oxidases [138]; and enhancing auto/paracrine growth mechanisms through facilitating the secretion of cyclophilin A from smooth muscle cells, which subsequently increases ROS production, inflammation and remodeling in the vascular bed [13]. In addition, ROCK could also regulate blood flow via direct effects on the CNS; the administration of ROCK inhibitors in the brainstem lowered blood pressure and reduced sympathetic nerve activity in hypertensive or heart failure animals [139-141].
In most vessel types, NO, produced after activation of endothelial NO synthase (eNOS), acts as an important vasodilator through activating soluble guanylate cyclases, thus stimulating the formation of cyclic GMP and the subsequent activation of cGMP-dependent protein kinase (PKG). The vasodilation induced by the NO/PKG pathway is therefore a crucial factor in maintaining vascular tone. Accumulating evidence indicates that there is extensive crosstalk between NO/PKG and RhoA/ROCK signaling in the vascular bed, resulting in enhanced contractility in the hypertensive state. The eNOS expression is negatively regulated by the RhoA/ROCK pathway, through decreasing eNOS mRNA stability [142-145]. RhoA/ROCK also negatively regulates eNOS phosphorylation and activity through the inhibition of the PI-3K/Akt pathway [146,147]. Conversely, the NO/PKG pathway negatively regulates RhoA/ROCK activation via phosphorylation and inhibition of RhoA [148].
The most recent updated information regarding ROCK in hypertension has been summarized in several recent reviews [7,9,45]. Limited studies using homozygous and heterozygous ROCK1 and ROCK2 knockout mice have been performed, to examine their contributions to the regulation of vascular functions. ROCK1 haploinsufficiency had no effect on Ang II-induced hypertension [75]. In addition, we observed that ROCK1−/− mice had normal blood pressure under baseline conditions [Wei et al, Unpublished Data]. ROCK1 appears to play a predominant role in vascular inflammation diseases [149]. Future studies with systemic and conditional deletion of ROCK1 and ROCK2 should allow genetic validation of ROCK as a crucial target for the treatment of hypertension.
Atherosclerosis
Atherosclerosis is characterized by progressive inflammation, lipid accumulation and arterial wall fibrosis, which leads to the build up of plaques, resulting in the diminishing function of smooth muscle contraction and endothelial relaxation. Multiple animal studies have demonstrated that ROCK is a critical contributor to many steps of the inflammatory atherosclerotic process and selective ROCK inhibitors lead to upregulation of eNOS, decreased vascular inflammation, and reduced atherosclerosis plaque formation [9,14,150].
Using a mouse model of accelerated atherosclerosis such as apolipoprotein E-deficient mice, increased ROCK-dependent smooth muscle contraction (without changes in ROCK expression) was observed in the aorta in the early stage of atherosclerosis [151]. During atherosclerosis lesion formation, ROCK activity, as indicated by ERM phosphorylation, which can be inhibited by Y27632 treatment, was increased in certain areas and cell types including endothelium, periadventitial adipocytes and macrophage foam cells, supporting a role of ROCK in the ERM phosphorylation-mediated macrophage infiltration and foam cell formation [152]. In addition, treatments with fasudil caused a decrease in arterial intima-medial thickness, maximum flow velocity and macrophage accumulation in the atherosclerosis lesions [153]. More support for a critical role of ROCK1 in the development of atherosclerosis comes from experiments using ROCK1−/− mice which have demonstrated that ROCK1 in bonemarrow-derived macrophages mediates macrophage foam cell formation and macrophage chemotaxis [154].
Ischemic injury
ROCK was found to have a role in cardiac ischemia/reperfusion (I/R) injuries, where blood flow is restricted or cut off and then is reintroduced into the area. I/R results in oxidative stress, mitochondrial dysfunction, inflammation and tissue damage. Increased RhoA/ROCK activity has been reported in I/R injuries [155,156]. A deleterious role of RhoA/ROCK signaling in I/R injury has been demonstrated in several in vivo models including mouse [155], rat [147,156,157] and swine [158]. In these models ROCK inhibition with fasudil or Y27632 resulted in reduced infarct size, less inflammation, reduced apoptosis and enhanced contractile function. The mechanisms by which RhoA/ROCK signaling contributes to I/R injuries include suppressing the reperfusion injury salvage kinase pathway, for example, PI-3K/Akt/eNOS signaling [147,156]; decreasing expression of the antiapoptotic Bcl-2 protein [155]; increasing mitochondria-nuclear translocation of apoptotic-inducing factor through the activation of c-Jun NH2-terminal kinase [157]; inducing inflammatory responses [147,156]; impairing energy production as ROCK inhibition with Y27632 during I/R injury resulted in increased lactate dehydrogenase and glyceraldehyde-3-phosphate dehydrogenase, normalization of creatine kinase levels and inhibition of ATP synthase degradation [159].
One specific application for the protective effects of ROCK inhibition against I/R injuries is in organ preservation during transplants. Using an isolated working rabbit heart model and a support rabbit, addition of the ROCK inhibitor fasudil to an organ preservation solution resulted in reduced MLC phosphorylation and increased eNOS expression associated with enhanced coronary blood flow and ventricular recovery, suggesting that the ROCK fasudil inhibitor could help prevent early myocardial dysfunction after transplantation [160].
Beneficial effects of ROCK inhibition by fasudil or Y27632 in ischemic preconditioning (IPC) have also been observed in several animal models [161-164], which have demonstrated reduced infarct size, oxidative stress and apoptosis. The activation of ROCK was reduced in IPC and activation of ERK-MAPK signaling by IPC was required to oppose ROCK activity [161].
The roles of RhoA/ROCK signaling in acute I/R injuries have not been investigated in ROCK1 or ROCK2 deficient mice yet. In a model for repetitive I/R injury, an increase in fibrosis but not in apoptosis was induced and ROCK1 deletion significantly reduced cardiac fibrosis through inhibiting cardiac fibroblast differentiation and activation derived from monocytic fibroblast precursors [165]. Further investigations should evaluate the function of each ROCK isoform in acute I/R injuries.
Pathological cardiac hypertrophy & heart failure
Pathological cardiac hypertrophy is defined by the augmentation of ventricular mass as a result of increased cardiomyocyte size induced by pathological stimuli such as hypertension, valvular insufficiency and stenosis, myocardial infarction or ischemia associated with coronary artery disease. The pathological cardiac hypertrophy has three basic phenotypical characteristics:
-
■
A change in gene-expression profiles from adult to a ‘fetal-like’ programs;
-
■
Histological alterations such as interstitial fibrosis, myocyte loss by apoptosis or necrosis and inadequate growth of the cardiac vasculature;
-
■
Contractile dysfunction (diastolic and/or systolic).
This type of hypertrophy is initially beneficial in overcoming adverse hemodynamic load in that it maintains cardiac output by increasing ventricular wall thickness and is thus recognized as an adaptive response [85,166,167]. However, persistent stress eventually leads to decompensated congestive heart failure, in which heart chambers become markedly enlarged and contractile function deteriorates [85,166,167]. There is considerable evidence that RhoA/ROCK signaling mediates a hypertrophic response [8]. In vivo studies using pharmacological inhibitors, Y27632 and fasudil, suggest an in vivo role for ROCK in the pathogenesis of cardiac hypertrophy and remodeling in a variety of animal models [138,168-174].
Recent genetic studies using ROCK1 deficient [76,95,175] and haploinsufficient mice [75] have demonstrated a critical role for this isoform in pathological remodeling and hypertrophic decompensation. Interestingly, partial or full ROCK1 deletion did not block the development of cardiomyocyte hypertrophy [75,76,95,175], but significantly reduced a number of structural and functional alterations attributable to pathological hypertrophic remodeling including cardiac fibrosis [75,76], cardiomyocyte apoptosis [56,95], cardiac dilation and contractile dysfunction [95,175].
The studies with ROCK1 deficient mice have revealed critical contributions of ROCK1 in the pathogenesis of heart failure. The roles for ROCK2 in cardiac hypertrophy and remodeling have not been tested yet. The finding that ROCK1 is not required for the development of cardiac hypertrophy, suggests that ROCK2 may play a dominant role in regulating hypertrophic response, or that the antihypertrophic effects of ROCK inhibitors [138,168-170] are not exclusively the result of ROCK inhibition. These observations suggest that ROCK1 and ROCK2 may have nonredundant functions in pathological hypertrophy: ROCK1 may be involved in cardiac fibrosis and apoptosis while ROCK2 may be involved in hypertrophy. Further studies are needed to determine the contribution of ROCK2 to cardiac hypertrophy, fibrosis, apoptosis and contraction.
Clinical implications
Despite the potential clinical importance of ROCK inhibition, fasudil is the only ROCK inhibitor approved for human use and was approved in Japan in 1995 for the prevention and treatment of cerebral vasospasm after surgery for subarachnoid hemorrhage [6,14,105]. Postmarketing surveillance studies have found that fasudil has exhibited no serious side effects [19].
Given the safety and effectiveness of fasudil in treating vasospasm after subarachnoid hemorrhage subarachnoid hemorrhage and extensive preclinical data in experimental model systems, small clinical trials have been carried out and have demonstrated some of the benefits of fasudil in cardiovascular diseases including essential hypertension [127], pulmonary hypertension [176-181], coronary artery disease [182], coronary artery spasm [118,183-186], aortic stiffness [187], heart failure associated vascular resistance and contraction [188], ischemic stroke [18], stable angina pectoris [189-192], chronic cerebral infarction [193] and kidney transplantation [194]. In addition, ROCK inhibition by statins may mediate their cholesterol-independent effects (pleiotropic effects) and contribute to the clinical benefits of statins in reducing cardiovascular events [114,195].
In these clinical studies, the underlying mechanism of the beneficial effects of fasudil has been attributable to the inhibition of ROCK in the vascular system resulting in the attenuation of smooth muscle hypercontraction, upregulation of eNOS expression and activity and reduction of inflammatory responses. However, the clinical effects of fasudil may also result from inhibition of other kinases given the possible nonselective effects of fasudil. With the development of more selective ROCK inhibitors and isoform selective inhibitors, further clinical studies will need to be performed to validate ROCK as the crucial target of fasudil in the treatment of cardiovascular diseases.
Conclusion
In conclusion, there is growing evidence that the RhoA/ROCK pathway plays an important pathophysiological role in cardiovascular diseases. Pharmacological ROCK inhibitors such as Y27632 and fasudil have proven to be remarkable tools in dissecting the roles of ROCK in cellular signaling and in animal disease models, including demonstrating that vascular tone is regulated by biochemically defined RhoA/ROCK pathways. The up-to-date progress in translational research supports the notion that ROCK is an important therapeutic target for the treatment of various cardiovascular diseases including hypertension, atherosclerosis, heart failure and ischemic damage. However, there are a number of questions that remain to be answered. While the effects of ROCK inhibitors in animal models of cardiac diseases may be known, the cellular site of their action, in particular, their action in cardiomyocytes, still remain largely unsolved (FIGURE 1). Although numerous studies have demonstrated the beneficial effects of ROCK inhibitors, whether these effects are mediated by inhibition of ROCK1, ROCK2 or both remains to be determined (TABLE 1). The generation and study of conditional knockout mice of ROCK1 and ROCK2 as well as the development of isoform-specific inhibitors would provide important insights into their physiopathological roles in cardiovascular diseases. Given the broad substrate specificity of fasudil, the evaluation of more selective ROCK inhibitors in human clinical trials will help to validate ROCK as the crucial target of fasudil’s action. Further research is needed to investigate the role of each ROCK isoform in the cardiovascular system and their values as drug targets.
Future perspective
Research in the RhoA/ROCK pathway has attracted much attention since the discovery of ROCK in 1996. A large body of knowledge on ROCK cellular functions, ROCK substrates, isoform functions and dynamic cross talks between RhoA/ROCK signaling with other signaling pathways has been rapidly accumulating. Most importantly, ROCK’s involvement in many cellular processes and its up-regulated activity in various cardiovascular disease pathologies make it a good target for inhibition. A significant amount of animal studies and human clinical trials with the application of ROCK inhibitors have demonstrated beneficial effects in the treatment of various cardiovascular diseases including arterial hypertension, atherosclerosis, I/R injuries, hypertrophic remodeling, cardiac dysfunction and heart failure. These studies strongly support the notion that ROCK is a promising therapeutic target in various disorders, especially in cardiovascular diseases. Currently, the inhibitors being used (fasudil and Y-27632) are nonisoform specific and could bind to other kinases at higher concentrations. In future years we expect to see more development and application of isoform-specific ROCK inhibitors in animal studies and clinical trials. In addition, we also expect to see more fundamental research with tissue-specific and conditional ROCK isoform knockout animal models. Determining the specific functions of the two isoforms and using that information combined with isoform specific inhibitors will generate new treatments for various cardiovascular diseases.
Executive summary.
-
■
Rho-associated coiled-coil-containing protein kinase (ROCK) has a major role in regulating actin cytoskeleton organization, stress fiber formation and smooth muscle cell contraction. ROCK’s action in cytoskeletal dynamics leads to a critical role in cell contraction, adhesion, morphology, motility and transcriptional regulation.
-
■
Two ROCK isoforms, ROCK1 and ROCK2, are assumed to be functionally redundant, based largely on the major common activators and substrates and the high degree homology within the kinase domain. Recent studies with individual knockdowns of ROCK1 and ROCK2 using short interfering RNA (siRNA)-based gene silencing or a genetic approach have demonstrated that these two isoforms have nonredundant in vitro and in vivo functions.
-
■
The current ROCK inhibitors (fasudil and Y-27632) are not isoform selective and can inhibit other kinases at high concentrations; thus new, more selective, inhibitors are being developed and tested. Fasudil is the only ROCK inhibitor approved for human use and was approved in Japan in 1995, for the prevention and treatment of cerebral vasospasm after surgery for subarachnoid hemorrhage.
-
■
Extensive experimental and clinical studies, performed with fasudil and Y-27632, have demonstrated beneficial effects and support the notion that ROCK is a promising therapeutic target for various disorders, especially cardiovascular disorders, in which increased ROCK activity mediates vascular smooth muscle cell hypercontraction, endothelial dysfunction, inflammatory cell recruitment and vascular and cardiac remodeling.
-
■
The role of the ROCK signaling pathway in the myocardium is less well understood than its role in the vasculature. Recent genetic studies indicate that ROCK isoforms may have distinct roles in cardiac remodeling: ROCK1 is not required for cardiac hypertrophy and may instead be involved in cardiac fibrosis and apoptosis.
Acknowledgments
Financial & competing interests disclosure
This work was supported by the Riley Children’s Foundation, the Indiana University Department of Pediatrics (Cardiology) and by NIH (NIH P01 HL085098 to Lei Wei). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Matsui T, Amano M, Yamamoto T, et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15(9):2208–2216. [PMC free article] [PubMed] [Google Scholar]
- 2.Ishizaki T, Maekawa M, Fujisawa K, et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15(8):1885–1893. [PMC free article] [PubMed] [Google Scholar]
- 3.Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBSLett. 1996;392(2):189–193. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- 4.Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK a is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol. Cell. Biol. 1996;16(10):5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116(2):167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 6.Hahmann C, Schroeter T. Rho-kinase inhibitors as therapeutics: from pan inhibition to isoform selectivity. Cell. Mol. Life Sci. 2010;67(2):171–177. doi: 10.1007/s00018-009-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loirand G, Pacaud P. The role of Rho protein signaling in hypertension. Nat. Rev. Cardiol. 2010;7(11):637–647. doi: 10.1038/nrcardio.2010.136. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto S, Del Re DP, Xiang SY, Zhao X, Florholmen G, Brown JH. Revisited and revised: is RhoA always a villain in cardiac pathophysiology? J. Cardiovas. Transl. Res. 2010;3(4):330–343. doi: 10.1007/s12265-010-9192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunes KP, Rigsby CS, Webb RC. RhoA/Rho-kinase and vascular diseases: what is the link? Cell. Mol. Life Sci. 2010;67(22):3823–3836. doi: 10.1007/s00018-010-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi J, Wei L. Rho kinase in the regulation of cell death and survival. Arch. Immunol. Ther. Exp. (Warsz) 2007;55(2):61–75. doi: 10.1007/s00005-007-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong M, Yan BP, Liao JK, Lam YY, Yip GW, Yu CM. Rho-kinase inhibition: a novel therapeutic target for the treatment of cardiovascular diseases. DrugDiscov. Today. 2010;15(15-16):622–629. doi: 10.1016/j.drudis.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67(9):545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satoh K, Fukumoto Y, Shimokawa H. Rho-kinase: important new therapeutic target in cardiovascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2011;301(2):H287–H296. doi: 10.1152/ajpheart.00327.2011. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q, Gensch C, Liao JK. Rho-associated coiled-coil-forming kinases (ROCKs): potential targets for the treatment of atherosclerosis and vascular disease. Trends Pharmacol. Sci. 2011;32(3):167–173. doi: 10.1016/j.tips.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connolly MJ, Aaronson PI. Key role of the RhoA/Rho kinase system in pulmonary hypertension. Pulm. Pharmacol. Ther. 2011;24(1):1–14. doi: 10.1016/j.pupt.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Pinterova M, Kunes J, Zicha J. Altered neural and vascular mechanisms in hypertension. Physiol. Res. 2011;60(3):381–402. doi: 10.33549/physiolres.932189. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki Y, Suzuki M, Hidaka H. The novel and specific Rho-kinase inhibitor (S)-(+)-2-methyl-1-[(4-methyl-5-isoquinoline)sulfonyl]-homopiperazine as a probing molecule for Rho-kinase-involved pathway. Pharmacol. Ther. 2002;93(2-3):225–232. doi: 10.1016/s0163-7258(02)00191-2. [DOI] [PubMed] [Google Scholar]
- 18.Shibuya M, Hirai S, Seto M, Satoh S, Ohtomo E. Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. J. Neurol. Sci. 2005;238(1-2):31–39. doi: 10.1016/j.jns.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y, Shibuya M, Satoh S, Sugimoto Y, Takakura K. A postmarketing surveillance study of fasudil treatment after aneurysmal subarachnoid hemorrhage. Surg. Neurol. 2007;68(2):126–131. doi: 10.1016/j.surneu.2006.10.037. ■ This study shows no serious adverse events in fasudil treated patients.
- 20.Zhao J, Zhou D, Guo J, et al. Effect of fasudil hydrochloride, a protein kinase inhibitor, on cerebral vasospasm and delayed cerebral ischemic symptoms after aneurysmal subarachnoid hemorrhage. Neurol. Med. Chir. (Tokyo) 2006;46(9):421–428. doi: 10.2176/nmc.46.421. [DOI] [PubMed] [Google Scholar]
- 21.Uehata M, Ishizaki T, Satoh H, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389(6654):990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 22.Asano T, Ikegaki I, Satoh S, et al. Mechanism of action of a novel antivasospasm drug, HA1077. J. Pharmacol. Exp. Ther. 1987;241(3):1033–1040. [PubMed] [Google Scholar]
- 23.Suzuki N, Hajicek N, Kozasa T. Regulation and physiological functions of G12/13-mediated signaling pathways. Neurosignals. 2009;17(1):55–70. doi: 10.1159/000186690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aittaleb M, Boguth CA, Tesmer JJ. Structure and function of heterotrimeric G protein-regulated Rho guanine nucleotide exchange factors. Mol. Pharmacol. 2010;77(2):111–125. doi: 10.1124/mol.109.061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu X, Gong MC, Jia T, Somlyo AV, Somlyo AP. The effects of the Rho-kinase inhibitor Y-27632 on arachidonic acid-, GTPgS-, and phorbol ester-induced Ca2+-sensitization of smooth muscle. FEBS Lett. 1998;440(1-2):183–187. doi: 10.1016/s0014-5793(98)01455-0. [DOI] [PubMed] [Google Scholar]
- 26.Feng J, Ito M, Kureishi Y, et al. Rho-associated kinase of chicken gizzard smooth muscle. J. Biol. Chem. 1999;274(6):3744–3752. doi: 10.1074/jbc.274.6.3744. [DOI] [PubMed] [Google Scholar]
- 27.Shirao S, Kashiwagi S, Sato M, et al. Sphingosylphosphorylcholine is a novel messenger for r-kinase-mediated Ca2+ sensitization in the bovine cerebral artery: unimportant role for protein kinase C. Circ. Res. 2002;91(2):112–119. doi: 10.1161/01.res.0000026057.13161.42. [DOI] [PubMed] [Google Scholar]
- 28.Doran JD, Liu X, Taslimi P, Saadat A, Fox T. New insights into the structure-function relationships of Rho-associated kinase: a thermodynamic and hydrodynamic study of the dimer-to-monomer transition and its kinetic implications. Biochem. J. 2004;384(Pt 2):255–262. doi: 10.1042/BJ20040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat. Cell. Biol. 2001;3(4):339–345. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- 30.Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat. Cell. Biol. 2001;3(4):346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- 31.Sebbagh M, Hamelin J, Bertoglio J, Solary E, Breard J. Direct cleavage of ROCK II by granzyme B induces target cell membrane blebbing in a caspase-independent manner. J. Exp. Med. 2005;201(3):465–471. doi: 10.1084/jem.20031877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amano M, Ito M, Kimura K, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271(34):20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 33.Kimura K, Ito M, Amano M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (r-kinase) Science. 1996;273(5272):245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 34.Kawano Y, Fukata Y, Oshiro N, et al. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J. Cell. Biol. 1999;147(5):1023–1038. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kureishi Y, Kobayashi S, Amano M, et al. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J. Biol. Chem. 1997;272(19):12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- 36.Maekawa M, Ishizaki T, Boku S, et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285(5429):895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 37.Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J. Biol. Chem. 2000;275(5):3577–3582. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- 38.Sumi T, Matsumoto K, Nakamura T. Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase. J. Biol. Chem. 2001;276(1):670–676. doi: 10.1074/jbc.M007074200. [DOI] [PubMed] [Google Scholar]
- 39.Amano T, Tanabe K, Eto T, Narumiya S, Mizuno K. LIM-kinase 2 induces formation of stress fibres, focal adhesions and membrane blebs, dependent on its activation by Rho-associated kinase-catalysed phosphorylation at threonine-505. Biochem. J. 2001;354(Pt 1):149–159. doi: 10.1042/0264-6021:3540149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin T, Zeng L, Liu Y, DeFea K, et al. Rho-ROCK-LIMK-cofilin pathway regulates shear stress activation of sterol regulatory element binding proteins. Circ. Res. 2003;92(12):1296–1304. doi: 10.1161/01.RES.0000078780.65824.8B. [DOI] [PubMed] [Google Scholar]
- 41.Matsui T, Maeda M, Doi Y, et al. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J. Cell. Biol. 1998;140(3):647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukata Y, Oshiro N, Kinoshita N, et al. Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility. J. Cell. 1999;145(2):347–361. doi: 10.1083/jcb.145.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartshorne DJ. Myosin phosphatase: subunits and interactions. Acta. Physiol. Scand. 1998;164(4):483–493. doi: 10.1046/j.1365-201X.1998.00447.x. [DOI] [PubMed] [Google Scholar]
- 44.Rikitake Y, Liao JK. ROCKs as therapeutic targets in cardiovascular diseases. Ex-pert Rev. Cardiovasc. Ther. 2005;3(3):441–451. doi: 10.1586/14779072.3.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wirth A. Rho kinase and hypertension. Biochim. Biophys. Acta. 2010;1802(12):1276–1284. doi: 10.1016/j.bbadis.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98(2):159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 47.Posern G, Treisman R. Actin’ together: serum response factor, its cofactors and the link to signal transduction. Trends Cell. Biol. 2006;16(11):588–596. doi: 10.1016/j.tcb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113(3):329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 49.Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SR cofactor MAL. Science. 2007;316(5832):1749–1752. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- 50.Wang D, Chang PS, Wang Z, et al. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105(7):851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 51.Wang DZ, Li S, Hockemeyer D, et al. Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc. Natl Acad. Sci. USA. 2002;99(23):14855–14860. doi: 10.1073/pnas.222561499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat. Rev. Mol. Cell. Biol. 2010;11(5):353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Small EM, Thatcher JE, Sutherland LB, et al. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ. Res. 2010;107(2):294–304. doi: 10.1161/CIRCRESAHA.110.223172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishiko K, Sakoda T, Akagami T, et al. Hyperglycemia induced cell growth and gene expression via the serum response element through RhoA and Rho-kinase in vascular smooth muscle cells. Prep. Biochem. Biotechnol. 2010;40(2):139–151. doi: 10.1080/10826060903558927. [DOI] [PubMed] [Google Scholar]
- 55.Li Z, Dong X, Wang Z, et al. Regulation of PTEN by Rho small GTPases. Nat. Cell. Biol. 2005;7(4):399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- 56.Chang J, Xie M, Shah VR, et al. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc. Natl Acad. Sci. USA. 2006;103(39):14495–14500. doi: 10.1073/pnas.0601911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vemula S, Shi JJ, Hanneman P, Wei L, Kapur R. ROCK1 functions as a suppressor of inflammatory cell migration by regulating PTEN phosphorylation and stability. Blood. 2010;115(9):1785–1796. doi: 10.1182/blood-2009-08-237222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Begum N, Sandu OA, Ito M, Lohmann SM, Smolenski A. Active Rho kinase (ROK-a) associates with insulin receptor substrate-1 and inhibits insulin signaling in vascular smooth muscle cells. J. Biol. Chem. 2002;277(8):6214–6222. doi: 10.1074/jbc.M110508200. [DOI] [PubMed] [Google Scholar]
- 59.Lim MJ, Choi KJ, Ding Y, et al. RhoA/Rho kinase blocks muscle differentiation via serine phosphorylation of insulin receptor substrate-1 and -2. Mol. Endocrinol. 2007;21(9):2282–2293. doi: 10.1210/me.2007-0114. [DOI] [PubMed] [Google Scholar]
- 60.Farah S, Agazie Y, Ohan N, Ngsee JK, Liu XJ. A Rho-associated protein kinase, ROKa, binds insulin receptor substrate-1 and modulates insulin signaling. J. Biol. Chem. 1998;273(8):4740–4746. doi: 10.1074/jbc.273.8.4740. [DOI] [PubMed] [Google Scholar]
- 61.Furukawa N, Ongusaha P, Jahng WJ, et al. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell. Metab. 2005;2(2):119–129. doi: 10.1016/j.cmet.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 62.Kanda T, Wakino S, Homma K, et al. Rho-kinase as a molecular target for insulin resistance and hypertension. FASEBJ. 2006;20(1):169–171. doi: 10.1096/fj.05-4197fje. [DOI] [PubMed] [Google Scholar]
- 63.Lee DH, Shi J, Jeoung NH, et al. Targeted disruption of ROCK1 causes insulin resistance in vivo. J. Biol. Chem. 2009;284(18):11776–11780. doi: 10.1074/jbc.C900014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Zheng XR, Riddick N, et al. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ. Res. 2009;104(4):531–540. doi: 10.1161/CIRCRESAHA.108.188524. ■■ This study highlights rho-associated coiled-coil-containing protein kinase (ROCK) isoform differences in smooth muscle cells and in their respective binding with the major substrate MYPT1.
- 65.Yoneda A, Multhaupt HA, Couchman JR. The Rho kinases I and II regulate different aspects of myosin II activity. J. Cell. Biol. 2005;170(3):443–453. doi: 10.1083/jcb.200412043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoneda A, Ushakov D, Multhaupt HA, Couchman JR. Fibronectin matrix assembly requires distinct contributions from Rho kinases I and -II. Mol. Biol. Cell. 2007;18(1):66–75. doi: 10.1091/mbc.E06-08-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noguchi M, Hosoda K, Fujikura J, et al. Genetic and pharmacological inhibition of Rho-associated kinase II enhances adipogenesis. J. Biol. Chem. 2007;282(40):29574–29583. doi: 10.1074/jbc.M705972200. [DOI] [PubMed] [Google Scholar]
- 68.Mong PY, Wang Q. Activation of Rho kinase isoforms in lung endothelial cells during inflammation. J. Immunol. 2009;182(4):2385–2394. doi: 10.4049/jimmunol.0802811. [DOI] [PubMed] [Google Scholar]
- 69.Bryan BA, Dennstedt E, Mitchell DC, et al. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J. 2010;24(9):3186–3195. doi: 10.1096/fj.09-145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shimada H, Rajagopalan LE. Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-kB p65. J. Biol. Chem. 2010;285(17):12536–12542. doi: 10.1074/jbc.M109.099630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lock FE, Hotchin NA. Distinct roles for ROCK1 and ROCK2 in the regulation of keratinocyte differentiation. PloS ONE. 2009;4(12):e8190. doi: 10.1371/journal.pone.0008190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inaba N, Ishizawa S, Kimura M, et al. Effect of inhibition of the ROCK isoform on RT2 malignant glioma cells. Anticancer Res. 2010;30(9):3509–3514. [PubMed] [Google Scholar]
- 73.Shi J, Zhang L, Wei L. Rho-kinase in development and heart failure: insights from genetic models. Pediatr. Cardiol. 2011;32(3):297–304. doi: 10.1007/s00246-011-9920-0. ■■ A recent review focusing on the genetic studies with ROCK1 or ROCK2 deficient mice.
- 74.Shimizu Y, Thumkeo D, Keel J, et al. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J. Cell. Biol. 2005;168(6):941–953. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rikitake Y, Oyama N, Wang CY, et al. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/− haploinsufficient mice. Circulation. 2005;112(19):2959–2965. doi: 10.1161/CIRCULATIONAHA.105.584623. ■ First study with heterozygous ROCK1-deficient mice showing an important role for ROCK1 in mediating cardiac fibrosis.
- 76.Zhang YM, Bo J, Taffet GE, et al. Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. FASEB J. 2006;20(7):916–925. doi: 10.1096/fj.05-5129com. ■ First study with homozygous ROCK1-deficient mice showing an important role for ROCK1 in mediating cardiac fibrosis.
- 77.Thumkeo D, Keel J, Ishizaki T, et al. Targeted disruption of the mouse Rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol. Cell. Biol. 2003;23(14):5043–5055. doi: 10.1128/MCB.23.14.5043-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thumkeo D, Shimizu Y, Sakamoto S, Yamada S, Narumiya S. ROCK-I and ROCK-II cooperatively regulate closure of eyelid and ventral body wall in mouse embryo. Genes Cells. 2005;10(8):825–834. doi: 10.1111/j.1365-2443.2005.00882.x. [DOI] [PubMed] [Google Scholar]
- 79.Duffy P, Schmandke A, Schmandke A, et al. Rho-associated kinase II (ROCKII) limits axonal growth after trauma within the adult mouse spinal cord. J. Neurosci. 2009;29(48):15266–15276. doi: 10.1523/JNEUROSCI.4650-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou Z, Meng Y, Asrar S, Todorovski Z, Jia Z. A critical role of Rho-kinase ROCK2 in the regulation of spine and synaptic function. Neuropharmacology. 2009;56(1):81–89. doi: 10.1016/j.neuropharm.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 81.Hoshijima M, Sah VP, Wang Y, Chien KR, Brown JH. The low molecular weight GTPase Rho regulates myofibril formation and organization in neonatal rat ventricular myocytes. Involvement of Rho kinase. J. Biol. Chem. 1998;273(13):7725–7730. doi: 10.1074/jbc.273.13.7725. [DOI] [PubMed] [Google Scholar]
- 82.Kuwahara K, Saito Y, Nakagawa O, et al. The effects of the selective ROCK inhibitor, Y27632, on ET-1-induced hypertrophic response in neonatal rat cardiac myocytes - possible involvement of Rho/ROCK pathway in cardiac muscle cell hypertrophy. FEBS Lett. 1999;452(3):314–318. doi: 10.1016/s0014-5793(99)00680-8. [DOI] [PubMed] [Google Scholar]
- 83.Wei L. Lysophospholipid signaling in cardiac myocyte hypertrophy. J. Mol. Cell. Cardiol. 2004;36(4):465–468. doi: 10.1016/j.yjmcc.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 84.Yanazume T, Hasegawa K, Wada H, et al. Rho/ROCK pathway contributes to the activation of extracellular signal-regulated kinase/GATA-4 during myocardial cell hypertrophy. J. Biol. Chem. 2002;277(10):8618–8625. doi: 10.1074/jbc.M107924200. [DOI] [PubMed] [Google Scholar]
- 85.Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ. Res. 2006;98(6):730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- 86.Ye Y, Hu SJ, Li L. Inhibition of farnesylpyrophosphate synthase prevents angiotensin II-induced hypertrophic responses in rat neonatal cardiomyocytes: involvement of the RhoA/Rho kinase pathway. FEBS Lett. 2009;583(18):2997–3003. doi: 10.1016/j.febslet.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 87.Hunter JC, Zeidan A, Javadov S, Kilic A, Rajapurohitam V, Karmazyn M. Nitric oxide inhibits endothelin-1-induced neonatal cardiomyocyte hypertrophy via a RhoA-ROCK-dependent pathway. J. Mol. Cell. Cardiol. 2009;47(6):810–818. doi: 10.1016/j.yjmcc.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 88.Chorianopoulos E, Heger T, Lutz M, et al. FGF-inducible 14-kDa protein (Fn14) is regulated via the RhoA/ROCK kinase pathway in cardiomyocytes and mediates nuclear factor-kB activation by TWEAK. Basic Res. Cardiol. 2010;105(2):301–313. doi: 10.1007/s00395-009-0046-y. [DOI] [PubMed] [Google Scholar]
- 89.Doi T, Sakoda T, Akagami T, et al. Aldosterone induces interleukin-18 through endothelin-1, angiotensin II, Rho/Rho-kinase, and PPARs in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2008;295(3):H1279–H1287. doi: 10.1152/ajpheart.00148.2008. [DOI] [PubMed] [Google Scholar]
- 90.Lee Y, Gustafsson AB. Role of apoptosis in cardiovascular disease. Apoptosis. 2009;14(4):536–548. doi: 10.1007/s10495-008-0302-x. [DOI] [PubMed] [Google Scholar]
- 91.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Ann. Rev. Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- 92.Dorn GW., 2nd Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc. Res. 2009;81(3):465–473. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Del Re DP, Miyamoto S, Brown JH. RhoA/Rho kinase up-regulate Bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. J. Biol. Chem. 2007;282(11):8069–8078. doi: 10.1074/jbc.M604298200. [DOI] [PubMed] [Google Scholar]
- 94.Del Re DP, Miyamoto S, Brown JH. Focal adhesion kinase as a RhoA-activable signaling scaffold mediating Akt activation and cardiomyocyte protection. J. Biol. Chem. 2008;283(51):35622–35629. doi: 10.1074/jbc.M804036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shi J, Zhang YW, Yang Y, Zhang L, Wei L. ROCK1 plays an essential role in the transition from cardiac hypertrophy to failure in mice. J. Mol. Cell. Cardiol. 2010;49(5):819–828. doi: 10.1016/j.yjmcc.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grimm M, Haas P, Willipinski-Stapelfeldt B, et al. Key role of myosin light chain (MLC) kinase-mediated MLC2a phosphorylation in the a 1-adrenergic positive inotropic effect in human atrium. Cardiovasc. Res. 2005;65(1):211–220. doi: 10.1016/j.cardiores.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 97.Rajashree R, Blunt BC, Hofmann PA. Modulation of myosin phosphatase targeting subunit and protein phosphatase 1 in the heart. Am. J. Physiol. Heart Circ. Physiol. 2005;289(4):H1736–H1743. doi: 10.1152/ajpheart.00318.2004. [DOI] [PubMed] [Google Scholar]
- 98.Davis JS, Hassanzadeh S, Winitsky S, et al. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell. 2001;107(5):631–641. doi: 10.1016/s0092-8674(01)00586-4. [DOI] [PubMed] [Google Scholar]
- 99.Vahebi S, Kobayashi T, Warren CM, de Tombe PP, Solaro RJ. Functional effects of Rho-kinase-dependent phosphorylation of specific sites on cardiac troponin. Circ. Res. 2005;96(7):740–747. doi: 10.1161/01.RES.0000162457.56568.7d. [DOI] [PubMed] [Google Scholar]
- 100.Lin G, Craig GP, Zhang L, et al. Acute inhibition of Rho-kinase improves cardiac contractile function in streptozotocin-diabetic rats. Cardiovasc. Res. 2007;75(1):51–58. doi: 10.1016/j.cardiores.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 101.Stepanova OV, Chadin AV, Masiutin AG, et al. Rho-associated protein kinase is involved in establishing the contractile phenotype of cardiomyocytes. Biofizika. 2010;55(5):880–885. [PubMed] [Google Scholar]
- 102.Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys. J. 2008;95(7):3479–3487. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vlasblom R, Muller A, Beckers CM, et al. RhoA-ROCK signaling is involved in contraction-mediated inhibition of SERCA2a expression in cardiomyocytes. Pflugers. Arch. 2009;458(4):785–793. doi: 10.1007/s00424-009-0659-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Narumiya S, Ishizaki T, Uehata M. Use and properties of ROCK-specific inhibitor Y-27632. Methods Enzymol. 2000;325:273–284. doi: 10.1016/s0076-6879(00)25449-9. [DOI] [PubMed] [Google Scholar]
- 105.Olson MF. Applications for ROCK kinase inhibition. Curr. Opin. Cell. Biol. 2008;20(2):242–248. doi: 10.1016/j.ceb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351(Pt 1):95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 2007;408(3):297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J. Cardiovasc. Pharmacol. 2007;50(1):17–24. doi: 10.1097/FJC.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lohn M, Plettenburg O, Ivashchenko Y, et al. Pharmacological characterization of SAR407899, a novel Rho-kinase inhibitor. Hypertension. 2009;54(3):676–683. doi: 10.1161/HYPERTENSIONAHA.109.134353. [DOI] [PubMed] [Google Scholar]
- 110.Kast R, Schirok H, Figueroa-Perez S, et al. Cardiovascular effects of a novel potent and highly selective azaindole-based inhibitor of Rho-kinase. Br. J. Pharmacol. 2007;152(7):1070–1080. doi: 10.1038/sj.bjp.0707484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Doe C, Bentley R, Behm DJ, et al. Novel Rho kinase inhibitors with anti-inflammatory and vasodilatory activities. J. Pharmacol. Exp. Ther. 2007;320(1):89–98. doi: 10.1124/jpet.106.110635. [DOI] [PubMed] [Google Scholar]
- 112.Dhaliwal JS, Badejo AM, Jr, Casey DB, Murthy SN, Kadowitz PJ. Analysis of pulmonary vasodilator responses to SB-772077-B [4-(7-((3-amino-1-pyrrolidinyl) carbonyl)-1-ethyl-1H-imidazo(4,5-c) pyridin-2-yl)-1,2,5-oxadiazol-3-amine], a novel aminofurazan-based Rho kinase inhibitor. J. Pharmacol. Exp. Ther. 2009;330(1):334–341. doi: 10.1124/jpet.109.151449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boerma M, Fu Q, Wang J, et al. Comparative gene expression profiling in three primary human cell lines after treatment with a novel inhibitor of Rho kinase or atorvastatin. Blood Coagul. Fibrinolysis. 2008;19(7):709–718. doi: 10.1097/MBC.0b013e32830b2891. ■■ First study reporting the development of a ROCK2 specific inhibitor.
- 114.Rawlings R, Nohria A, Liu PY, et al. Comparison of effects of rosuvastatin (10 mg) versus atorvastatin (40 mg) on Rho kinase activity in caucasian men with a previous atherosclerotic event. Am. J. Cardiol. 2009;103(4):437–441. doi: 10.1016/j.amjcard.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kobayashi N, Takeshima H, Fukushima H, et al. Cardioprotective effects of pitavastatin on cardiac performance and remodeling in failing rat hearts. Am. J. Hypertens. 2009;22(2):176–182. doi: 10.1038/ajh.2008.333. [DOI] [PubMed] [Google Scholar]
- 116.Kobayashi N, Ohno T, Yoshida K, et al. Cardioprotective mechanism of telmisartan via PPAR-g-eNOS pathway in dahl salt-sensitive hypertensive rats. Am. J. Hypertens. 2008;21(5):576–581. doi: 10.1038/ajh.2008.27. [DOI] [PubMed] [Google Scholar]
- 117.Zhou Q, Liao JK. Rho kinase: an important mediator of atherosclerosis and vascular disease. Curr. Pharm. Des. 2009;15(27):3108–3115. doi: 10.2174/138161209789057986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Otsuka T, Ibuki C, Suzuki T, et al. Administration of the Rho-kinase inhibitor, fasudil, following nitroglycerin additionally dilates the site of coronary spasm in patients with vasospastic angina. Coron. Artery Dis. 2008;19(2):105–110. doi: 10.1097/MCA.0b013e3282f3420c. [DOI] [PubMed] [Google Scholar]
- 119.Weir MR, Dzau VJ. The renin-angiotensin-aldosterone system: a specific target for hypertension management. Am. J. Hypertens. 1999;12(12 Pt 3):205S–213S. doi: 10.1016/s0895-7061(99)00103-x. [DOI] [PubMed] [Google Scholar]
- 120.Schulz E, Gori T, Munzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011;34(6):665–673. doi: 10.1038/hr.2011.39. [DOI] [PubMed] [Google Scholar]
- 121.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 2000;52(4):639–672. [PubMed] [Google Scholar]
- 122.de Cavanagh EM, Ferder M, Inserra F, Ferder L. Angiotensin II, mitochondria, cytoskeletal, and extracellular matrix connections: an integrating viewpoint. Am. J. Physiol. Heart Circ. Physiol. 2009;296(3):H550–H558. doi: 10.1152/ajpheart.01176.2008. [DOI] [PubMed] [Google Scholar]
- 123.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 2000;86(5):494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 124.Mukai Y, Shimokawa H, Matoba T, et al. Involvement of Rho-kinase in hypertensive vascular disease: a novel therapeutic target in hypertension. FASEB J. 2001;15(6):1062–1064. doi: 10.1096/fj.00-0735fje. [DOI] [PubMed] [Google Scholar]
- 125.Moriki N, Ito M, Seko T, et al. RhoA activation in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. Hypertens. Res. 2004;27(4):263–270. doi: 10.1291/hypres.27.263. [DOI] [PubMed] [Google Scholar]
- 126.Seko T, Ito M, Kureishi Y, et al. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ. Res. 2003;92(4):411–418. doi: 10.1161/01.RES.0000059987.90200.44. [DOI] [PubMed] [Google Scholar]
- 127.Masumoto A, Hirooka Y, Shimokawa H, Hironaga K, Setoguchi S, Takeshita A. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension. 2001;38(6):1307–1310. doi: 10.1161/hy1201.096541. [DOI] [PubMed] [Google Scholar]
- 128.Seasholtz TM, Wessel J, Rao F, et al. Rho kinase polymorphism influences blood pressure and systemic vascular resistance in human twins: role of heredity. Hypertension. 2006;47(5):937–947. doi: 10.1161/01.HYP.0000217364.45622.f0. [DOI] [PubMed] [Google Scholar]
- 129.Rankinen T, Church T, Rice T, Markward N, Blair SN, Bouchard C. A major haplotype block at the Rho-associated kinase 2 locus is associated with a lower risk of hypertension in a recessive manner: the HYPGENE study. Hypertens. Res. 2008;31(8):1651–1657. doi: 10.1291/hypres.31.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sun Q, Yue P, Ying Z, et al. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler. Thromb. Vasc. Biol. 2008;28(10):1760–1766. doi: 10.1161/ATVBAHA.108.166967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guilluy C, Bregeon J, Toumaniantz G, et al. The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat. Med. 2010;16(2):183–190. doi: 10.1038/nm.2079. [DOI] [PubMed] [Google Scholar]
- 132.Wirth A, Benyo Z, Lukasova M, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat. Med. 2008;14(1):64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 133.Kataoka C, Egashira K, Inoue S, et al. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 2002;39(2):245–250. doi: 10.1161/hy0202.103271. [DOI] [PubMed] [Google Scholar]
- 134.Hassona MD, Abouelnaga ZA, Elnakish MT, et al. Vascular hypertrophy-associated hypertension of profilin1 transgenic mouse model leads to functional remodeling of peripheral arteries. Am. J. Physiol. Heart Circ. Physiol. 2010;298(6):H2112–H2120. doi: 10.1152/ajpheart.00016.2010. [DOI] [PubMed] [Google Scholar]
- 135.Takeda K, Ichiki T, Tokunou T, et al. Critical role of Rho-kinase and MEK/ERK pathways for angiotensin II-induced plasminogen activator inhibitor type-1 gene expression. Arterioscler. Thromb. Vasc. Biol. 2001;21(5):868–873. doi: 10.1161/01.atv.21.5.868. [DOI] [PubMed] [Google Scholar]
- 136.Rikitake Y, Liao JK. Rho-kinase mediates hyperglycemia-induced plasminogen activator inhibitor-1 expression in vascular endothelial cells. Circulation. 2005;111(24):3261–3268. doi: 10.1161/CIRCULATIONAHA.105.534024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Funakoshi Y, Ichiki T, Shimokawa H, et al. Rho-kinase mediates angiotensin II-induced monocyte chemoattractant protein-1 expression in rat vascular smooth muscle cells. Hypertension. 2001;38(1):100–104. doi: 10.1161/01.hyp.38.1.100. [DOI] [PubMed] [Google Scholar]
- 138.Higashi M, Shimokawa H, Hattori T, et al. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ. Res. 2003;93(8):767–775. doi: 10.1161/01.RES.0000096650.91688.28. [DOI] [PubMed] [Google Scholar]
- 139.Ito K, Hirooka Y, Sakai K, et al. Rho/Rho-kinase pathway in brain stem contributes to blood pressure regulation via sympathetic nervous system: possible involvement in neural mechanisms of hypertension. Circ. Res. 2003;92(12):1337–1343. doi: 10.1161/01.RES.0000079941.59846.D4. [DOI] [PubMed] [Google Scholar]
- 140.Ito K, Hirooka Y, Kishi T, et al. Rho/Rho-kinase pathway in the brainstem contributes to hypertension caused by chronic nitric oxide synthase inhibition. Hypertension. 2004;43(2):156–162. doi: 10.1161/01.HYP.0000114602.82140.a4. [DOI] [PubMed] [Google Scholar]
- 141.Ito K, Kimura Y, Hirooka Y, Sagara Y, Sunagawa K. Activation of Rho-kinase in the brainstem enhances sympathetic drive in mice with heart failure. Auton. Neurosci. 2008;142(1-2):77–81. doi: 10.1016/j.autneu.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 142.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J. Biol. Chem. 1998;273(37):24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 143.Eto M, Barandier C, Rathgeb L, et al. Thrombin suppresses endothelial nitric oxide synthase and upregulates endothelin-converting enzyme-1 expression by distinct pathways: role of Rho/ROCK and mitogen-activated protein kinase. Circ. Res. 2001;89(7):583–590. doi: 10.1161/hh1901.097084. [DOI] [PubMed] [Google Scholar]
- 144.Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106(1):57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 145.Rikitake Y, Kim HH, Huang Z, et al. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36(10):2251–2227. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ming XF, Viswambharan H, Barandier C, et al. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol. Cell. Biol. 2002;22(24):8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wolfrum S, Dendorfer A, Rikitake Y, et al. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler. Thromb. Vasc. Biol. 2004;24(10):1842–1847. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, et al. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J. Biol. Chem. 2000;275(28):21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- 149.Noma K, Rikitake Y, Oyama N, et al. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J. Clin. Invest. 2008;118(5):1632–1644. doi: 10.1172/JCI29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dong M, Yan BP, Yu CM. Current status of Rho-associated kinases (ROCKs) in coronary atherosclerosis and vasospasm. Cardiovasc. Hematol. Agents Med. Chem. 2009;7(4):322–330. doi: 10.2174/187152509789541891. [DOI] [PubMed] [Google Scholar]
- 151.Mori-Kawabe M, Tsushima H, Fujimoto S, Tada T, Ito J. Role of Rho/Rho-kinase and NO/cGMP signaling pathways in vascular function prior to atherosclerosis. J. Atheroscler. Thromb. 2009;16(6):722–732. doi: 10.5551/jat.1875. [DOI] [PubMed] [Google Scholar]
- 152.Rekhter M, Chandrasekhar K, Gifford-Moore D, et al. Immunohistochemical analysis of target proteins of Rho-kinase in a mouse model of accelerated atherosclerosis. Exp. Clin. Cardiol. 2007;12(4):169–174. [PMC free article] [PubMed] [Google Scholar]
- 153.Wu DJ, Xu JZ, Wu YJ, et al. Effects of fasudil on early atherosclerotic plaque formation and established lesion progression in apolipoprotein E-knockout mice. Atherosclerosis. 2009;207(1):68–73. doi: 10.1016/j.atherosclerosis.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 154.Wang HW, Liu PY, Oyama N, et al. Deficiency of ROCK1 in bone marrow-derived cells protects against atherosclerosis in LDLR−/− mice. FASEBJ. 2008;22(10):3561–3570. doi: 10.1096/fj.08-108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bao W, Hu E, Tao L, et al. Inhibition of Rho-kinase protects the heart against ischemia/reperfusion injury. Cardiovasc. Res. 2004;61(3):548–558. doi: 10.1016/j.cardiores.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 156.Hamid SA, Bower HS, Baxter GF. Rho kinase activation plays a major role as a mediator of irreversible injury in reperfused myocardium. Am. J. Physiol. Heart Circ. Physiol. 2007;292(6):H2598–H2606. doi: 10.1152/ajpheart.01393.2006. [DOI] [PubMed] [Google Scholar]
- 157.Zhang J, Li XX, Bian HJ, Liu XB, Ji XP, Zhang Y. Inhibition of the activity of Rho-kinase reduces cardiomyocyte apoptosis in heart ischemia/reperfusion via suppressing JNK-mediated AIF translocation. Clin. Chim. Acta. 2009;401(1-2):76–80. doi: 10.1016/j.cca.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 158.Shibata I, Yoshitomi O, Use T, et al. Administration of the Rho-kinase inhibitor fasudil before ischemia or just after reperfusion, but not 30 min after reperfusion, protects the stunned myocardium in swine. Cardiovasc. Drugs Ther. 2008;22(4):293–298. doi: 10.1007/s10557-008-6106-y. [DOI] [PubMed] [Google Scholar]
- 159.Cadete VJ, Sawicka J, Polewicz D, Doroszko A, Wozniak M, Sawicki G. Effect of the Rho kinase inhibitor Y-27632 on the proteome of hearts with ischemia-reperfusion injury. Proteomics. 2010;10(24):4377–4385. doi: 10.1002/pmic.201000393. [DOI] [PubMed] [Google Scholar]
- 160.Kobayashi M, Tanoue Y, Eto M, et al. A Rho-kinase inhibitor improves cardiac function after 24-hour heart preservation. J. Thorac. Cardiovasc. Surg. 2008;136(6):1586–1592. doi: 10.1016/j.jtcvs.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 161.Zhang J, Bian HJ, Li XX, et al. ERK-MAPK signaling opposes Rho-kinase to reduce cardiomyocyte apoptosis in heart ischemic preconditioning. Mol. Med. 2010;16(7-8):307–31. doi: 10.2119/molmed.2009.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhao JL, Yang YJ, Pei WD, Sun YH, You SJ, Gao RL. Remote periconditioning reduces myocardial no-reflow by the activation of K ATP channel via inhibition of Rho-kinase. Int. J. Cardiol. 2009;133(2):179–184. doi: 10.1016/j.ijcard.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 163.Sakamoto K, Nakahara T, Ishii K. Rho-Rho kinase pathway is involved in the protective effect of early ischemic preconditioning in th rat heart. Biol. Pharm. Bull. 2011;34(1):156–159. doi: 10.1248/bpb.34.156. [DOI] [PubMed] [Google Scholar]
- 164.Demiryurek S, Kara AF, Celik A, Babul A, Tarakcioglu M, Demiryurek AT. Effects of fasudil, a Rho-kinase inhibitor, on myocardial preconditioning in anesthetized rats. Eur. J. Pharmacol. 2005;527(1-3):129–140. doi: 10.1016/j.ejphar.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 165.Haudek SB, Gupta D, Dewald O, et al. Rho kinase-1 mediates cardiac fibrosis by regulating fibroblast precursor cell differentiation. Cardiovasc. Res. 2009;83(3):511–518. doi: 10.1093/cvr/cvp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signallin pathways. Nat. Rev. Mol. Cell. Biol. 2006;7(8):589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 167.Hill JA, Olson EN. Cardiac plasticity. N. Engl. J. Med. 2008;358(13):1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 168.Satoh S, Ueda Y, Koyanagi M, et al. Chronic inhibition of Rho kinase blunts the process of left ventricular hypertrophy leading to cardiac contractile dysfunction in hypertension-induced heart failure. J. Mol. Cell. Cardiol. 2003;35(1):59–70. doi: 10.1016/s0022-2828(02)00278-x. [DOI] [PubMed] [Google Scholar]
- 169.Hattori T, Shimokawa H, Higashi M, et al. Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation. 2004;109(18):2234–2239. doi: 10.1161/01.CIR.0000127939.16111.58. [DOI] [PubMed] [Google Scholar]
- 170.Kobayashi N, Horinaka S, Mita S, et al. Critical role of Rho-kinase pathway for cardiac performance and remodeling in failing rat hearts. Cardiovasc. Res. 2002;55(4):757–767. doi: 10.1016/s0008-6363(02)00457-1. [DOI] [PubMed] [Google Scholar]
- 171.Fukui S, Fukumoto Y, Suzuki J, et al. Long-term inhibition of Rho-kinase ameliorates diastolic heart failure in hypertensive rats. J. Cardiovasc. Pharmacol. 2008;51(3):317–326. doi: 10.1097/FJC.0b013e31816533b7. [DOI] [PubMed] [Google Scholar]
- 172.Phrommintikul A, Tran L, Kompa A, et al. Effects of a Rho kinase inhibitor on pressure overload induced cardiac hypertrophy and associated diastolic dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2008;294(4):H1804–H1814. doi: 10.1152/ajpheart.01078.2007. [DOI] [PubMed] [Google Scholar]
- 173.Kagiyama S, Matsumura K, Goto K, Otsubo T, Iida M. Role of Rho kinase and oxidative stress in cardiac fibrosis induced by aldosterone and salt in angiotensin type 1a receptor knockout mice. Regul. Pept. 2010;160(1-3):133–139. doi: 10.1016/j.regpep.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 174.Ishimaru K, Ueno H, Kagitani S, Takabayashi D, Takata M, Inoue H. Fasudil attenuates myocardial fibrosis in association with inhibition of monocyte/macrophage infiltration in the heart of DOCA/salt hypertensive rats. J. Cardiovasc. Pharmacol. 2007;50(2):187–194. doi: 10.1097/FJC.0b013e318064f150. [DOI] [PubMed] [Google Scholar]
- 175.Shi J, Zhang YW, Summers LJ, Dorn GW, 2nd, Wei L. Disruption of ROCK1 gene attenuates cardiac dilation and improves contractile function in pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2008;44(3):551–560. doi: 10.1016/j.yjmcc.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fukumoto Y, Matoba T, Ito A, et al. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart. 2005;91(3):391–392. doi: 10.1136/hrt.2003.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ishikura K, Yamada N, Ito M, et al. Beneficial acute effects of Rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ. J. 2006;70(2):174–178. doi: 10.1253/circj.70.174. [DOI] [PubMed] [Google Scholar]
- 178.Guilluy C, Eddahibi S, Agard C, et al. RhoA and Rho kinase activation in human pulmonary hypertension: role of 5-HT signaling. Am. J. Respir. Crit. Care Med. 2009;179(12):1151–1158. doi: 10.1164/rccm.200805-691OC. [DOI] [PubMed] [Google Scholar]
- 179.Doe Z, Fukumoto Y, Takaki A, et al. Evidence for Rho-kinase activation in patients with pulmonary arterial hypertension. Circ. J. 2009;73(9):1731–1739. doi: 10.1253/circj.cj-09-0135. [DOI] [PubMed] [Google Scholar]
- 180.Fujita H, Fukumoto Y, Saji K, et al. Acute vasodilator effects of inhaled fasudil, a specific Rho-kinase inhibitor, in patients with pulmonary arterial hypertension. Heart Vessels. 2010;25(2):144–149. doi: 10.1007/s00380-009-1176-8. [DOI] [PubMed] [Google Scholar]
- 181.Li F, Xia W, Yuan S, Sun R. Acute inhibition of Rho-kinase attenuates pulmonary hypertension in patients with congenital heart disease. Pediatr. Cardiol. 2009;30(3):363–366. doi: 10.1007/s00246-008-9315-z. [DOI] [PubMed] [Google Scholar]
- 182.Nohria A, Grunert ME, Rikitake Y, et al. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ. Res. 2006;99(12):1426–1432. doi: 10.1161/01.RES.0000251668.39526.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105(13):1545–1547. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- 184.Mohri M, Shimokawa H, Hirakawa Y, Masumoto A, Takeshita A. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J. Am. Coll. Cardiol. 2003;41(1):15–19. doi: 10.1016/s0735-1097(02)02632-3. [DOI] [PubMed] [Google Scholar]
- 185.Inokuchi K, Ito A, Fukumoto Y, et al. Usefulness of fasudil, a Rho-kinase inhibitor, to treat intractable severe coronary spasm after coronary artery bypass surgery. J. Cardiovasc. Pharmacol. 2004;44(3):275–277. doi: 10.1097/01.fjc.0000134775.76636.3f. [DOI] [PubMed] [Google Scholar]
- 186.Disli OM, Ozdemir E, Berkan O, Bagcivan I, Durmus N, Parlak A. Rho-kinase inhibitors Y-27632 and fasudil prevent agonist-induced vasospasm in human radial artery. Can. J. Physiol. Pharmacol. 2009;87(8):595–601. doi: 10.1139/y09-043. [DOI] [PubMed] [Google Scholar]
- 187.Noma K, Goto C, Nishioka K, et al. Roles of Rho-associated kinase and oxidative stress in the pathogenesis of aortic stiffness. J. Am. Coll. Cardiol. 2007;49(6):698–705. doi: 10.1016/j.jacc.2006.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kishi T, Hirooka Y, Masumoto A, et al. Rho-kinase inhibitor improves increased vascular resistance and impaired vasodilation of the forearm in patients with heart failure. Circulation. 2005;111(21):2741–2747. doi: 10.1161/CIRCULATIONAHA.104.510248. [DOI] [PubMed] [Google Scholar]
- 189.Shimokawa H, Hiramori K, Iinuma H, et al. Anti-anginal effect of fasudil, a Rho-kinase inhibitor, in patients with stable effort angina: a multicenter study. J. Cardiovasc. Pharmacol. 2002;40(5):751–761. doi: 10.1097/00005344-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 190.Vicari RM, Chaitman B, Keefe D, et al. Efficacy and safety of fasudil in patients with stable angina: a double-blind, placebo-controlled, Phase 2 trial. J. Am. Coll. Cardiol. 2005;46(10):1803–1811. doi: 10.1016/j.jacc.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 191.Fukumoto Y, Mohri M, Inokuchi K, et al. Anti-ischemic effects of fasudil, a specific Rho-kinase inhibitor, in patients with stable effort angina. J. Cardiovasc. Pharmacol. 2007;49(3):117–121. doi: 10.1097/FJC.0b013e31802ef532. [DOI] [PubMed] [Google Scholar]
- 192.Otsuka T, Ibuki C, Suzuki T, et al. Vasodilatory effect of subsequent administration of fasudil, a Rho-kinase inhibitor, surpasses that of nitroglycerin at the concentric coronary stenosis in patients with stable angina pectoris. Circ. J. 2006;70(4):402–408. doi: 10.1253/circj.70.402. [DOI] [PubMed] [Google Scholar]
- 193.Nagata K, Kondoh Y, Satoh Y, et al. Effects of fasudil hydrochloride on cerebral blood flow in patients with chronic cerebral infarction. Clin. Neuropharmacol. 1993;16(6):501–510. doi: 10.1097/00002826-199312000-00003. [DOI] [PubMed] [Google Scholar]
- 194.Bussemaker E, Herbrig K, Pistrosch F, Palm C, Passauer J. Role of Rho-kinase in the regulation of vascular tone in hypertensive renal transplant recipients. Atherosclerosis. 2009;207(2):567–572. doi: 10.1016/j.atherosclerosis.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 195.Nohria A, Prsic A, Liu PY, et al. Statins inhibit Rho kinase activity in patients with atherosclerosis. Atherosclerosis. 2009;205(2):517–521. doi: 10.1016/j.atherosclerosis.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chan CK, Mak JC, Man RY, Vanhoutte PM. Rho kinase inhibitors prevent endothelium-dependent contractions in the rat aorta. J. Pharmacol. Exp. Ther. 2009;329(2):820–826. doi: 10.1124/jpet.108.148247. [DOI] [PubMed] [Google Scholar]
- 197.Casey DB, Badejo AM, Dhaliwal JS, et al. Analysis of responses to the Rho-kinase inhibitor Y-27632 in the pulmonary and systemic vascular bed of the rat. Am. J. Physiol. Heart Circ. Physiol. 2010;299(1):H184–H192. doi: 10.1152/ajpheart.00181.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xu EZ, Kantores C, Ivanovska J, et al. Rescue treatment with a Rho-kinase inhibitor normalizes right ventricular function and reverses remodeling in juvenile rats with chronic pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 2010;299(6):H1854–H1864. doi: 10.1152/ajpheart.00595.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.van der Heijden M, Versteilen AM, Sipkema P, van Nieuw Amerongen GP, Musters RJ, Groeneveld AB. Rho-kinase-dependent F-actin rearrangement is involved in the inhibition of PI3-kinase/Akt during ischemia-reperfusion-induced endothelial cell apoptosis. Apoptosis. 2008;13(3):404–412. doi: 10.1007/s10495-007-0173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]