Abstract
Immune cell development and function must be tightly regulated through cell surface receptors to ensure proper responses to pathogen and tolerance to self. In T cells, the signal from the T-cell receptor is essential for T-cell maturation, homeostasis, and activation. In mast cells, the high-affinity receptor for IgE transduces signal that promotes mast cell survival and induces mast cell activation. In dendritic cells and macrophages, the toll-like receptors recognize microbial pathogens and play critical roles for both innate and adaptive immunity against pathogens. Our research explores how signaling from these receptors is transduced and regulated to better understand these immune cells. Our recent studies have revealed diacylglycerol kinases and TSC1/2-mTOR as critical signaling molecules/regulators in T cells, mast cells, dendritic cells, and macrophages.
Keywords: T-cell receptor, T-cell development, Anergy, Regulatory T cells, Dendritic cell (DC), Macrophages, Mast cells, Toll-like receptor (TLR), FcεRI, Diacylglycerol kinase (DGK), Mammalian target of rapamycin (mTOR), Tuberous sclerosis 1
Regulating T-cell receptor signaling for T-cell development and function
During T-cell maturation in the thymus, a functional T-cell receptor (TCR) is generated through proper V(D)J recombination [1]. Proper TCR signaling is critical for T-cell maturation and survival. Absence of the pre-TCR or the TCR signal results in developmental blockage at the CD4−CD8− double negative (DN) stage and CD4+CD8+ double positive (DP) stage, respectively [2–4]. In addition, the strength of TCR signal influences the outcome of thymic selections. DP thymocytes expressing TCRs with high affinities to self-peptide MHC complexes are negatively selected due to programmed cell death. Negative selection deletes highly self-reactive T cells to establish central tolerance. Thymocytes express TCRs with low and moderate affinities to self-peptide-MHC complexes are positively selected to mature [5]. Proper thymic selection is critical for generating a repertoire T cells for effective immune responses against foreign antigens and, at the same time, self-tolerant. Furthermore, specialized TCRs such as the γδTCR and the invariant Vα14 Jα18 TCR in mouse may transduce signals different from the conventional αβTCR to direct the differentiation of precursors to distinct T-cell lineages [6]. In the periphery, tonic T-cell receptor signal maintains normal T-cell homeostasis [7], but does not cause full T-cell activation. In response to pathogen infection, TCR signal triggers naïve T-cell activation after recognizing foreign peptides presented by dendritic cells and other antigen-presenting cells (APCs). During early T-cell activation and further differentiation to effector T cells, T cells undergo drastic but characteristic changes, including enlargement of cell sizes, entry into cell cycle, synthesis of effector molecules including cytokines and granzymes, altered cell surface molecules, and trafficking between lymph nodes and tissues to mount effective immune responses. Abnormal TCR signaling can cause severe consequences. Defects in TCR signaling can result in impairment of immune function due to failure to generate T cells or insufficient activation of T cells [8]. It can also result in autoimmunity due to impairment of negative selection and shift of T-cell repertoire. Abnormally elevated TCR signaling can lead to autoimmunity due to uncontrolled T-cell activation [9]. While it is becoming clear that proper TCR signaling is essential for normal T-cell development and function, the mechanisms that regulate TCR signaling remain poorly understood.
T-cell receptor signaling
An outline of signal transduction from the TCR has been illustrated through the studies by many laboratories in the last 20 years (Fig. 1). Following TCR engagement, the Src family tyrosine kinase Lck is activated following dephosphorylation by Csk [10]. Lck phosphorylates the ITAMs on CD3, enzymes, and adaptors, leading to the activation and recruitment of Zap70 to the TCR ζ chain and subsequent formation of multi-molecule signaling complexes that are nucleated by LAT and SLP76 [11–14]. These proximal signaling events lead to the activation of PLCγ1 [15]. Activated PLCγ1 hydrolyzes phosphatidylinositol 4,5-bisphosphate [PIP2] to generate two important second messengers, diacylglycerol (DAG) and inositol 1,4,5-trisphosphates (IP3), that activate multiple downstream signaling cascades [16]. IP3 binds to its receptor in the endoplasmic reticulum (ER) to trigger the release of Ca++ from the ER to the cytosol. Depletion of Ca++ in the ER induces STIM1 conformation change and oligomerization, which further recruits Orai protein to open CRAC channels and Ca++ influx. Ca++ binds to calmodulin and triggers the activation of calcineurin, which dephosphorylates NFAT. Dephosphorylated NFAT translocates the nuclei to induce transcription [17]. NFAT participates in transcription of genes involved in T-cell activation and T-cell tolerance. DAG associates with and activates multiple effector molecules, including the classic type protein kinase Cs (PKCs), the novel type PKCs, protein kinases D (PKDs), RasGRPs, Munc13 s, and chimearins [18–20]. In T cells, DAG binds to PKCθ to induce its translocation to immune synapse and activation [21]. PKCθ phosphorylates Carma1 to allow recruitment of Bcl10 and Malt1 to lipid raft. Both the Carma1/Bcl10/Malt1 complex and PKCθ associate with the IKK complex to induce its activation [22]. Activated IKK complex phosphorylates IκB to trigger its ubiquitination and degradation, allowing nuclear translocation of NFκB to activate transcription [23]. In addition, DAG associates with RasGRP1 to activate Ras directly or by priming Sos for guanyl nucleotide-releasing activity [24]. GTP-bound Ras further activates the Raf-Mek1/2-Erk1/2-AP1 pathway. PKCθ also promotes TCR-induced Ras-Erk1/2 pathway by phosphorylating RasGRP1 at threonine 184 to increase its activity [25]. Numerous studies have demonstrated that both PKCθ- and RasGRP1-mediated signaling pathways play critical roles in T-cell development and peripheral T-cell function [26]. Given the importance of DAG-mediated signaling for T cells, it is important to determine how DAG is regulated and the importance for tight control of DAG concentration in T cells. Dysregulated DAG signaling can be detrimental, which is suggested by the action of phorbol esters, functional analogues of DAG, which can cause maximal T-cell activation and are carcinogens.
Fig. 1.
Schematic illustration of T-cell receptor signaling (see text for details)
Diacylglycerol kinases as critical regulators for T cells
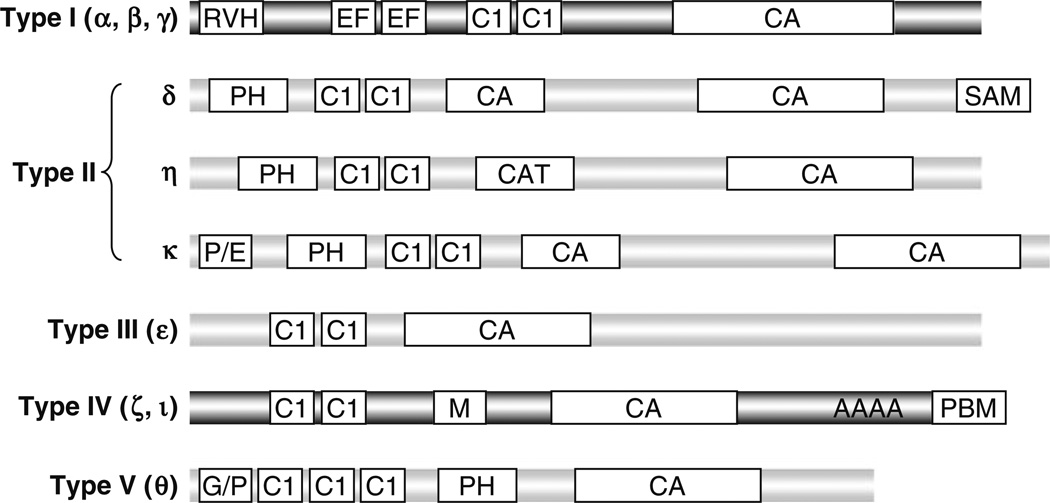
Diaclyglycerol kinases (DGKs) are evolutionarily conserved enzymes that catalyze the phosphorylation of DAG to generate phosphatidic acid (PA). Similar to DAG, PA is also a second messenger implicated as a mediator of the mitogenic action of various growth factors and hormones in several types of mammalian cells [27]. PA associates with and regulates many effector molecules, such as SHP-1 [28], mTOR [29], PDE4 (cAMP-specific phosphodiesterase) [30], PP-1 (protein phosphatase-1) [31], PI5 K (type I phosphatidylinositol 4-phosphate 5-kinase) [32], Sos [33], and p47phox [34]. DGKs have been hypothesized to play important roles in receptor signaling and cellular function by regulating DAG and PA concentration. However, the physiological functions of DGKs have been poorly understood. Each of the ten mammalian DGKs contains a kinase domain and at least two cysteine-rich C1 domains that are homologous to the DAG/phorbol ester-binding domain of protein kinase Cs (PKCs). In addition, DGK isoforms also contain distinct structural motifs that are used to define mammalian DGKs into five types [19, 35, 36] (Fig. 2). Most DGK isoforms do not have obvious preference for specific acyl chains of DAG [37]. DGKs may associate with proteins or lipids through these distinct structural motifs to control their subcellular localizations and to perform specific functions. Most DGK isoforms are expressed in multiple tissues, particularly in the hematopoietic system and the neuronal system. Within one tissue, multiple DGK isoforms may be present. Transcripts of at least five DGK isoforms can be detected in T cells, macrophages, and mast cells (our unpublished data).
Fig. 2.
Structural features of mammalian DGKs. Mammalian DGKs are divided into five types based on their structural features. CA catalytic domain, C1 cysteine-rich diacylglycerol/phorbol ester-binding domain, RVH recoverin homology domain, EF EFhand, PH pleckstrin homology domain, SAM sterile alpha motif domain, PBM PDZ domain-binding motif, M myristoylated alanine-rich C-kinase substrate (MARCKS) motif, AAAA ankyrin-repeats, G/P glycine/proline-rich region
Synergistic and crucial role of DGKα and ζ for T-cell development
The facts that defects in generating DAG or in DAG effectors cause abnormal T-cell development suggest the importance of DAG-mediated signaling. PLCγ1 deficiency in T cells results in the impairment of both positive and negative selection [38]. The RasGRP1-Ras-Erk1/2 pathway is crucial for positive selection of conventional αβ T cells but appears to be dispensable for innate CD8 T cell or Treg development [26, 39]. While the PKCθ-Carma1/Bcl10-IKK-NFκB pathway is not essential for positive selection of conventional αβ T cells [22], it plays important roles for iNKT cell and Treg generation [40]. These findings have led us to hypothesize that DAG-mediated signaling must be tightly controlled for proper T-cell maturation.
To test our hypothesis, we have generated and analyzed mice deficient of DGKα, ζ, or both. In the absence of either DGKα or ζ, T-cell numbers in the thymus and peripheral lymphoid organs are not obviously altered [41, 42]. However, absence of both DGKα and ζ (DGKαζDKO) results in a severe decrease in CD4+CD8− and CD4−CD8+ SP thymocytes. Positive selection but not negative selection is impaired in DGKαζDKO mice [43]. These data provide the first evidence that two DGK isoforms synergistically control a biological process. In CD4+CD8+ DP thymocytes of DGKαζDKO mice, TCR stimulation induces elevated DAG concentration and increased Ras and Erk1/2 activation as well as activation of the PKCθ-IKK-NFκB pathway. During in vitro thymic organ culture of DGKαζDKO thymi, addition of PA increases CD4 and CD8 SP thymocytes, revealing that DGK-derived PA is important for DP thymocyte maturation to the SP stage [43]. Thus, DGKs may function as a signal switch during T-cell development by turning off DAG-mediated signaling and at the same time turning on PA-mediated signaling. Further studies should determine how DGK-derived PA exerts its function in developing thymocytes.
In addition to promoting positive selection of conventional α/β T cells, DGKα and ζ also synergistically promote the development of the invariant Vα14-Jα18 NKT (iNKT) cell development. In the absence of either DGKα or ζ, iNKT cell numbers are not obviously abnormal compared to WT mice. iNKT cell numbers are drastically decreased in the thymus, spleen, and liver of DGKαζDKO mice. In contrast to conventional α/β T cells and iNKT cells, regulatory T-cell generation, γ/δ T-cell development as well as β-selection appears not inhibited by DGKα and ζ deficiency, suggesting that DGKα and ζ is differentially required for the development of these distinct T-cell lineages or developmental checkpoints (reference 43 and unpublished data). It would be important to determine further whether DGK activity does not participate for these developmental events or DGK isoforms other than DGKα and ζ may be involved.
Regulation of T-cell activation and tolerance by DGKs
T-cell-mediated immune response is critical for host defense against microbial infection and for tumor surveillance. However, T-cell function also needs to be tightly regulated to ensure immune responses are properly controlled. Dysregulated T-cell responses can be detrimental to the host [44]. Similar to developing T cells in the thymus, DAG-mediated signaling is critical for T-cell activation. Inhibition of DAG-mediated signaling pathways results in the impairment of T-cell activation and effector T-cell function [45]. In contrast, treatment of T cells with phorbol esters induces maximal T-cell activation. To determine the role of DGKs in T-cell activation, we first used a gain-of-function approach by overexpression of DGKα or ζ in T cell lines. Enhanced DGK function can potently inhibit TCR-induced Ras-Erk1/2-AP1 activation [46]. Gajewski’s and Merida’s groups have revealed that increased DGK activities inhibit the recruitment of RasGRP1 to the immune synapse or cytoplasmic membrane [47, 48]. Complementary to these gain-of-function data, studies in mice deficient of either DGK α or ζ result in hyperresponsive T cells in response to TCR stimulation, correlating with decreased conversion of DAG to PA and enhanced activation of the Ras-Erk1/2-AP1 pathway [41, 42]. Although DGKα or ζ-deficient T cells are hyper-proliferative in response to TCR stimulation, they remain in a naïve state in vivo. DGKα- or ζ-deficient mice also do not manifest obvious autoimmune diseases. However, DGKαζ DKO T cells display spontaneous activation phenotypes in vivo and in vitro. They express high levels of T-cell activation markers, display effector/memory T-cell phenotype, and readily produce cytokines. DGKαζDKO T cells proliferate more vigorously than WT T cells in response to TCR stimulation and even proliferate in vitro without TCR stimulation (our unpublished data). Together, these observations establish that DGKα and ζ are physiological inhibitors of TCR signaling and T-cell activation and synergistically control normal T-cell homeostasis.
While negative selection depletes majority of self-reactive T cells, this process is not complete. Some self-reactive T cells escape negative selection in the thymus and populate the periphery. Peripheral tolerance mechanisms ensure these self-reactive T cells from causing unnecessary tissue damage. One of the peripheral T-cell tolerance mechanisms is the induction of anergy. For naïve T cells to become fully activated, both TCR signaling and a costimulatory signal, such as CD28, are required [49, 50]. In the absence of costimulatory signaling, TCR signal itself can induce T cells into an anergic state [51–54]. In addition, anergic T cells can be induced by stimulation with partial agonist peptides in the presence of co-stimulation [55], or by treatment with the Ca++ ionophore ionomycin [51, 56–58]. Anergic T cells do not respond to antigen restimulation in the presence of appropriate co-stimulation. They do not make IL-2 or divide following re-stimulation, even though they express all the necessary receptors [44, 59, 60].
While the mechanisms involved in anergy induction may vary among different models, anergic T cells usually manifest impaired activation of the RasGRP1-Ras-Erk1/2 pathway but normal Ca++ influx and NFAT activation following TCR stimulation [61–63]. Treatment of T cells with ionomycin induces Ca++ influx, leading to activation of NFAT and anergy induction by promoting transcription of anergy-promoting molecules, such as FasL, Erg2/3 (early growth response 2/3), and several E3 ubiquitin ligases (Cbl-b, Itch, and Grail) [56, 58, 64–66]. While ionomycin alone induces T-cell anergy, ionomycin plus a DAG analogue, such as PMA, induces full T-cell activation. Since DAG and IP3 are produced by PLCγ1 at an equal molar ratio following TCR engagement, it has been hypothesized that DGK activity may contribute to T-cell anergy selectively terminating DAG-mediated signaling. In supporting this hypothesis, DGKα and ζ are expressed in high levels in naïve and anergic T cells but down-regulated in activated T cells. In the absence of either DGKα or ζ, T cells display resistance to anergy induction in both in vitro and in vivo, supporting an important role of DGK activity for T-cell anergy [42].
TSC1/2-mTOR signaling in T cells
The mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase that integrates numerous environmental stimuli including growth factors, nutrients, and stress-activated signals to regulate cell metabolism, survival, growth, and proliferation [67]. mTOR associates with different proteins to form two signaling complexes, mTOR complexes 1 and 2 (mTORC1 and mTORC2) with different sensitivity to rapamycin and signaling properties [68]. mTORC1 is sensitive to rapamycin inhibition, while mTORC2 is resistant to acute rapamycin treatment. mTORC1 phosphorylates and activates the 70 kDa ribosomal S6 kinase (S6K1) and the translational repressor 4 elongation factor binding protein 1 (4E-BP1) [69, 70] to promote cell growth and proliferation. Activated S6K1 phosphorylates S6 and other translational regulators such as eIF2 kinase and eIF-4B to regulate the translation initiation [71, 72] and ribosomal biogenesis. Phosphorylation of 4E-BP1 releases eukaryotic initiation factor 4E (eIF4E) to promote the recruitment of ribosome machinery in protein translation [73]. The mTORC2 phosphorylates Akt at serine 473 to increase Akt activity that further promotes nutrient uptake and cell survival [74, 75].
Both genetic and pharmacological studies have demonstrated that mTOR plays crucial roles during T-cell activation, anergy, lineage commitment, and other immune responses [76]. A decrease in mTOR signaling due to chemical inhibition or genetic manipulation has been associated with T-cell anergy [77], induction of regulatory T cells [78–82], impairment of effector T-cell generation [82, 83], and, surprisingly, enhanced memory T-cell responses to microbial pathogens [84]. In addition, mTOR has been shown to play an important role in T-cell trafficking in vivo by regulating the expression of CCR7 [85].
While it is becoming clear that mTOR plays critical roles in T cells and that TCR stimulation induces the mTOR activity [86], how TCR signaling leads to mTOR activation and the importance of tight control of mTOR signaling in T cells have been not fully understood. To investigate signaling pathways that are important for mTOR activation, we utilized genetically manipulated mice in the RasGRP1-Ras-Erk1/2 pathway. In the absence of RasGRP1, T-cell development is severely blocked at the CD4+CD8+ DP stage. We have found that in RasGRP1−/− thymocytes, TCR-induced activation of mTORC1 and mTORC2 is impaired. In contrast, increased Ras activity results in elevated activation of both mTORC1 and mTORC2 in thymocytes. Further manipulation of Mek1/2 activity in thymocytes with chemical inhibitors and in T cell lines with constitutively active and dominant negative Mek1/2 reveals that Mek1/2 activity is critical for TCR-induced mTORC1 and mTORC2 activity (Gorentla and Zhong, unpublished data). Together, these observations demonstrate that the RasGRP1-Ras-Mek1/2-Erk1/2 pathway is critical for TCR-induced mTOR activation. Consistent with these observations, we have found that DGKα and ζ deficiency causes enhanced mTOR activation that can be inhibited by Mek1/2 inhibitors (Gorentla and Zhong, unpublished data). It has been reported that PA can associate with mTOR to promote mTOR signaling. Both PLD- and DGKζ-derived PA have been shown to participate in mTOR activation in cell line models [29, 87]. At present, it is unclear how absence of DGK-derived PA may affect TCR-induced mTOR signaling. However, out data point to a dominant inhibitory role of DGKα and ζ in TCR-induced mTOR activation by negative control of DAG-mediated activation of the RasGRP1-Ras-Erk1/2 pathway. As the RasGRP1-Ras-Erk1/2 pathway also controls Rsk1 activation and AP1 activity, it remains to determine how this pathway may affect T-cell biology through the mTOR signaling.
Since the Ras-Mek1/2-Erk1/2 pathway controls multiple effector molecules besides mTOR, neither the DGKαζDKO nor the caKRas model would provide clear information with regard to how dysregulated mTOR signaling may affect T cells. To selectively manipulate mTOR signaling, we have decided to utilize a conditional TSC1-deficient mouse model. mTORC1 is activated by GTP-bound RheB (the Ras homologue enriched in brain). RheB is negatively regulated by an upstream Tuberous sclerosis complex 1/2 (TSC1/2), a bipartite protein complex of Hamartin (TSC1) and Tuberin (TSC2) [88, 89]. TSC1 stabilizes TSC2 to prevent its ubiquitination and degradation. TSC2 inhibits RheB through its GAP activity and thus functions as negative regulator of mTORC1 signaling. In cell line models, the PI3 K-Akt pathway has been shown to activate mTORC1 signaling through phosphorylation of TSC2 [90–92]. Phosphorylation of TSC2 triggers the dissociation of TSC2 from TSC complex, leading to the activation of mTORC1 signaling via Rheb [93]. To investigate the role of TSC1 in T cells, we have conditionally deleted TSC1 out in T cells. In TSC1-deficient T cells, TSC2 protein is hardly detectable, indicating critical role of TSC1 for TSC2 stability in T cells and a virtual TSC1/TSC2 double deficiency in TSC1 knockout T cells. In the absence of TSC1, TCR-induced mTORC1 activity is elevated and T-cell sizes are increased. However, mTORC2 and Akt activities are decreased, leading to increased T-cell death and decreased T-cell numbers in peripheral lymphoid organs (O’Brien and Zhong, unpublished data). Thus, TSC1 differentially controls mTORC1 and mTORC2 signaling in T cells and plays important roles in maintaining normal homeostasis of T cells.
Regulation of TLR signaling and innate immunity by DGKs and TSC1/2 mTOR signaling
Adaptive immune responses are tightly regulated by innate immune cells. TLRs are a family of evolutionarily conserved receptors that recognize specific microbial PAMPs and constitute a major mechanism to respond to microbial infection by the hosts [23, 24, 94, 95]. Although diverse in specificities of ligand recognition, TLRs appear to share several common intracellular signaling pathways. A MyD88-dependent pathway is utilized by most TLRs [96]. This pathway is initiated after association of MyD88 with the TLRs during microbial recognition. MyD88 in turn recruits IRAK1 and 4 (IL-1R-associated kinase 1 and 4), TRAF6 (TNF receptor-associated factor 6), and other signaling molecules to the cytoplasm membrane, leading to the activation of IKKα/β/γ (IκB kinase) complex [97–99]. IKKα/β/γ phosphorylates IκB, causing degradation and nuclear translocation of NFκB to induce expression of its target genes such as IL-12 and TNFα [100]. In addition, MyD88-mediated proximal signaling events also lead to the activation of JNK, p38, and Erk1/2 MAPKs through Tak1 [101]. JNK and p38 activities are important for proinflammatory cytokine production [102–104]. The TRIF-dependent pathway is mediated by two TIR-domain containing adaptor molecules TRIF and TRAM [105]. This pathway is utilized by TLR3 and TLR4 [106, 107], leading to phosphorylation and activation of IRF3 (IFN-regulatory factor 3) [108]. IRF3 activates transcription of type I IFNs [109]. The class IA PI3 Ks can negatively control TLR-induced responses. DCs deficient of the p85α regulatory subunit of the class IA PI3 Ks express a high level of IL-12 following stimulation of TLR2, 4, 5, and 9. Mice deficient in p85α in BALB/c background become resistant to Leishmania major infection due to enhanced Th1 responses [110].
While DGKζ-deficient T cells are hyperresponsive to TCR stimulation, we were surprised that DGKζ-deficient mice are more susceptible to Toxoplasma gondii infection than WT control mice [111]. It has been well established that host resistance to T. gondii is dependent on Th1 immune response and IFNγ. Dendritic cells play critical role in generating effective adaptive immune response against T. gondii through TLR-mediated recognition of the pathogen and subsequent production of IL12. As there is no intrinsic defect of DGKζ-deficient T cells to differentiate to Th1 lineage, we investigated whether DGKζ is involved in TLR-mediated innate immunity and found that TLR-induced production of proinflammatory cytokines such as IL12 by DGKζ-deficient DCs and BMMϕ is diminished. In DGKζ−/− bone marrow-derived macrophages (BMMϕ), PI3 K and Akt activation is enhanced following LPS stimulation. Importantly, treatment of DGKζ−/− BMMϕ with a PI3 K inhibitor or phosphatidic acid can restore TLR-induced IL12 production, indicating that DGKζ inhibits PI3 K/Akt signaling to promote TLR-induced proinflammatory cytokine production. It would be of interest to further determine whether PA exerts its effect by regulating PI3 K activity or through other mechanisms. These observations not only establish a critical role of DGKζ in TLR-mediated innate immune responses but also emphasize the importance of innate immune responses in the control of adaptive immunity as enhanced activation of DGKζ−/− T cells is not sufficient to confer resistance to T. gondii.
Growing evidence has also implicated mTOR in innate immune responses. Rapamycin treatment blocks TLR9-MyD88 interaction and IRF7 phosphorylation in plasmacytoid dendritic cells (pDCs) following CpG stimulation, leading to the inhibition of type I IFNs production and impaired antiviral immune responses in vivo. Concordantly, 4E-BP1 and 4E-BP2 double-deficient MEF cells express high levels of IRF7 and type I IFNs, rendering resistance to viral infection [112]. However, rapamycin treatment has also been found to have either inhibitory or stimulating effects on proinflammatory cytokine and IL-10 production in human dendritic cells and monocytes [113, 114]. Using TSC1 conditional knockout mice, we have found that TSC1 plays critical roles in mTOR signaling in dendritic cells and macrophages and controls TLR-induced proinflammatory cytokine production (Pan and Zhong, unpublished data). The study establishes a critical role of TSC1 in TLR-mediated innate immune responses.
Diacylglycerol kinases and TSC1/2-mTOR signaling in mast cells
Mast cells are the central effectors in immune pathogenesis of asthma and other allergic disorders and play important roles in host defense against helminth infection [115–119]. The high-affinity receptor for IgE FcεRI plays critical role for mast cell activation by inducing mast cell degranulation, synthesis and release of lipid mediators, and transcriptional activation and secretion of cytokines [120]. The paradigm of FcεRI signaling mimics TCR signaling in many aspects, such as activation of the Src family members Lyn and Fyn, the tyrosine kinase Syk, the Tec family kinase Btk, and P I3Ks [121–130], and the formation of multi-molecular signaling complexes by adaptor molecules such as LAT and SLP76. These proximal events lead to the activation of PLCγ [131–134], PKCs [135], MAPKs [136, 137], and mTOR [138, 139]. Similar to T cells, DAG is generated following FcεRI engagement and controls mast cell function by activating multiple signaling cascades. DAG activates PKCs and RasGRP1 that are important for mast cell degranulation and cytokine production [140–148]. Both in vitro and in vivo evidence has suggested a critical role of DAG in regulating mast cell function as defects in DAG generation or in DAG-mediated signaling pathways greatly impact mast cell function [142, 149–151].
Mast cells express at least five DGK isoforms at the mRNA levels (our unpublished data). FcεRI stimulation induces DAG phosphorylation and PA production in mast cells. In the absence of DGKζ, FcεRI-induced PA production is decreased, indicating that DGKζ is involved in DAG conversion to PA. While DGKζ deficiency appears to be dispensable for mast cell generation in vivo or differentiation from bone marrow progenitor cells in vitro, DGKζ differentially regulates mast cell degranulation and cytokine production. DGKζ-deficient BMMCs produce elevated amount of IL-6 following FcεRI stimulation. However, their degranulation is impaired when compared to WT BMMCs in vitro. DGKζ-deficient mice display diminished anaphylactic responses in vivo. DGKζ−/− BMMCs show enhanced Erk1/2 and PKCβ activation but decreased PLCγ phosphorylation and Ca influx, which may contribute to the differential effects DGKζ deficiency on cytokine production and degranulation in mast cells [111].
The role of TSC1/2-mTOR signaling in mast cells has been much less clear. A few studies have suggested potential roles of mTOR in mast cells. FcεRI stimulation induces phosphorylation of mTOR, S6K1, and 4E-BP1 that is dependent on PI3 K activity. Rapamycin inhibits FcεRI-induced S6K1 and 4E-BP1 phosphorylation and cytokine production without affecting degranulation [138, 139]. We have found that FcεRI stimulation induces both mTORC1 and mTORC2 activation that are differentially regulated by TSC1 (Shin and Zhong, unpublished data).
Summary
In summary, recent studies have revealed that DGKs and tight regulation of mTOR signaling are important for proper immune cell development and function. Mice with altered DGKs and the TSC1/2-mTOR pathway should provide important model systems to investigate the regulation of innate and adaptive cell functions and to develop new strategies to modulate immune cell function for the treatment of autoimmune and allergic diseases and for cancer immunotherapy.
Acknowledgments
The research is supported by grants from the American Heart Association, the American Cancer Society, NIH (R01-AI079088, R01-AI076357, R21-AI079873), and the Food Allergy and Anaphylaxis Network to Xiao-Ping Zhong.
Contributor Information
Xiao-Ping Zhong, Email: zhong001@mc.duke.edu, Department of Pediatrics, Duke University Medical Center, Durham, NC 27710, USA; Department of Immunology, Duke University Medical Center, Durham, NC 27710, USA; Department of Pediatrics-Allergy and Immunology, Duke University Medical Center, Box 2644, Durham, NC 27710, USA.
Jinwook Shin, Department of Pediatrics, Duke University Medical Center, Durham, NC 27710, USA.
Balachandra K. Gorentla, Department of Pediatrics, Duke University Medical Center, Durham, NC 27710, USA
Tommy O’Brien, Department of Pediatrics, Duke University Medical Center, Durham, NC 27710, USA; Department of Immunology, Duke University Medical Center, Durham, NC 27710, USA.
Sruti Srivatsan, Department of Pediatrics, Duke University Medical Center, Durham, NC 27710, USA; Department of Immunology, Duke University Medical Center, Durham, NC 27710, USA.
Li Xu, Department of Pediatrics, Duke University Medical Center, Durham, NC 27710, USA.
Yong Chen, Department of Pediatrics, Duke University Medical Center, Durham, NC 27710, USA.
Danli Xie, Department of Pediatrics, Duke University Medical Center, Durham, NC 27710, USA.
Hongjie Pan, Department of Pediatrics, Duke University Medical Center, Durham, NC 27710, USA.
References
- 1.Krangel MS. Mechanics of T cell receptor gene rearrangement. Curr Opin Immunol. 2009;21:133–139. doi: 10.1016/j.coi.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haks MC, Oosterwegel MA, Blom B, Spits HM, Kruisbeek AM. Cell-fate decisions in early T cell development: regulation by cytokine receptors and the pre-TCR. Semin Immunol. 1999;11:23–37. doi: 10.1006/smim.1998.0153. [DOI] [PubMed] [Google Scholar]
- 3.Moroy T, Karsunky H. Regulation of pre-T-cell development. Cell Mol Life Sci. 2000;57:957–975. doi: 10.1007/PL00000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 5.Takahama Y, Nitta T, Mat Ripen A, Nitta S, Murata S, Tanaka K. Role of thymic cortex-specific self-peptides in positive selection of T cells. Semin Immunol. 2010;22:287–293. doi: 10.1016/j.smim.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Hayes SM, Love PE. Strength of signal: a fundamental mechanism for cell fate specification. Immunol Rev. 2006;209:170–175. doi: 10.1111/j.0105-2896.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 7.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Elder ME, et al. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 1994;264:1596–1599. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- 9.Bachmaier K, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 10.Zikherman J, et al. CD45-Csk phosphatase-kinase titration uncouples basal and inducible T cell receptor signaling during thymic development. Immunity. 2010;32:342–354. doi: 10.1016/j.immuni.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guy CS, Vignali DA. Organization of proximal signal initiation at the TCR:CD3 complex. Immunol Rev. 2009;232:7–21. doi: 10.1111/j.1600-065X.2009.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, et al. ZAP-70: an essential kinase in T-cell signaling. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a002279. a002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu Rev Immunol. 2002;20:371–394. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 14.Koretzky GA, Abtahian F, Silverman MA. SLP76 and SLP65: complex regulation of signalling in lymphocytes and beyond. Nat Rev Immunol. 2006;6:67–78. doi: 10.1038/nri1750. [DOI] [PubMed] [Google Scholar]
- 15.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imboden JB, Stobo JD. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985;161:446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong XP, Guo R, Zhou H, Liu C, Wan CK. Diacylglycerol kinases in immune cell function and self-tolerance. Immunol Rev. 2008;224:249–264. doi: 10.1111/j.1600-065X.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008;409:1–18. doi: 10.1042/BJ20071040. [DOI] [PubMed] [Google Scholar]
- 20.Topham MK, Epand RM. Mammalian diacylglycerol kinases: molecular interactions and biological functions of selected isoforms. Biochim Biophys Acta. 2009;1790:416–424. doi: 10.1016/j.bbagen.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isakov N, Altman A. Protein kinase C θ in T cell activation. Annu Rev Immunol. 2002;20:761–794. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- 22.Lee KY, D’Acquisto F, Hayden MS, Shim JH, Ghosh S. PDK1 nucleates T cell receptor-induced signaling complex for NF-κB activation. Science. 2005;308:114–118. doi: 10.1126/science.1107107. [DOI] [PubMed] [Google Scholar]
- 23.Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 24.Roose JP, Mollenauer M, Ho M, Kurosaki T, Weiss A. Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Mol Cell Biol. 2007;27:2732–2745. doi: 10.1128/MCB.01882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roose JP, Mollenauer M, Gupta VA, Stone J, Weiss A. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol Cell Biol. 2005;25:4426–4441. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dower NA, et al. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 27.English D, Cui Y, Caberlotto L. Messenger functions of phosphatidic acid. Chem Phys Lipids. 1996;80:117–132. doi: 10.1016/0009-3084(96)02549-2. [DOI] [PubMed] [Google Scholar]
- 28.Frank C, Keilhack H, Opitz F, Zschornig O, Bohmer FD. Binding of phosphatidic acid to the protein-tyrosine phosphatase SHP-1 as a basis for activity modulation. Biochemistry. 1999;38:11993–12002. doi: 10.1021/bi982586w. [DOI] [PubMed] [Google Scholar]
- 29.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 30.Grange M, et al. The cAMP-specific phosphodiesterase PDE4D3 is regulated by phosphatidic acid binding. Consequences for cAMP signaling pathway and characterization of a phosphatidic acid binding site. J Biol Chem. 2000;275:33379–33387. doi: 10.1074/jbc.M006329200. [DOI] [PubMed] [Google Scholar]
- 31.Jones JA, Hannun YA. Tight binding inhibition of protein phosphatase-1 by phosphatidic acid. Specificity of inhibition by the phospholipid. J Biol Chem. 2002;277:15530–15538. doi: 10.1074/jbc.M111555200. [DOI] [PubMed] [Google Scholar]
- 32.Luo B, Prescott SM, Topham MK. Diacylglycerol kinase ζ regulates phosphatidylinositol 4-phosphate 5-kinase I α by a novel mechanism. Cell Signal. 2004;16:891–897. doi: 10.1016/j.cellsig.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Zhao C, Du G, Skowronek K, Frohman MA, Bar-Sagi D. Phospholipase D2-generated phosphatidic acid couples EGFR stimulation to Ras activation by Sos. Nat Cell Biol. 2007;9:706–712. doi: 10.1038/ncb1594. [DOI] [PubMed] [Google Scholar]
- 34.Karathanassis D, et al. Binding of the PX domain of p47phox to phosphatidylinositol 3, 4-bisphosphate and phosphatidic acid is masked by an intramolecular interaction. EMBO J. 2002;21:5057–5068. doi: 10.1093/emboj/cdf519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo B, Regier DS, Prescott SM, Topham MK. Diacylglycerol kinases. Cell Signal. 2004;16:983–989. doi: 10.1016/j.cellsig.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Sakane F, Imai S, Kai M, Yasuda S, Kanoh H. Diacylglycerol kinases: Why so many of them? Biochim Biophys Acta. 2007;1771:793–806. doi: 10.1016/j.bbalip.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Tang W, Bunting M, Zimmerman GA, McIntyre TM, Prescott SM. Molecular cloning of a novel human diacylglycerol kinase highly selective for arachidonate-containing substrates. J Appl Biol Chem. 1996;271:10237–10241. [PubMed] [Google Scholar]
- 38.Fu G, et al. Phospholipase C γ1 is essential for T cell development, activation, and tolerance. J Exp Med. 2010;207:309–318. doi: 10.1084/jem.20090880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Priatel JJ, et al. RasGRP1 regulates antigen-induced developmental programming by naive CD8 T cells. J Immunol. 2010;184:666–676. doi: 10.4049/jimmunol.0803521. [DOI] [PubMed] [Google Scholar]
- 40.Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 41.Zhong XP, et al. Enhanced T cell responses due to diacylglycerol kinase ζ deficiency. Nat Immunol. 2003;4:882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- 42.Olenchock BA, et al. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 43.Guo R, et al. Synergistic control of T cell development and tumor suppression by diacylglycerol kinase α and ζ. Proc Natl Acad Sci USA. 2008;105:11909–11914. doi: 10.1073/pnas.0711856105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 45.Flores I, Casaseca T, Martinez AC, Kanoh H, Merida I. Phosphatidic acid generation through interleukin 2 (IL-2)-induced alpha-diacylglycerol kinase activation is an essential step in IL-2-mediated lymphocyte proliferation. J Biol Chem. 1996;271:10334–10340. doi: 10.1074/jbc.271.17.10334. [DOI] [PubMed] [Google Scholar]
- 46.Zhong XP, Hainey EA, Olenchock BA, Zhao H, Topham MK, Koretzky GA. Regulation of T cell receptor-induced activation of the Ras-ERK pathway by diacylglycerol kinase zeta. J Biol Chem. 2002;277:31089–31098. doi: 10.1074/jbc.M203818200. [DOI] [PubMed] [Google Scholar]
- 47.Zha Y, et al. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat Immunol. 2006;7:1166–1173. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- 48.Sanjuan MA, et al. T cell activation in vivo targets diacylglycerol kinase α to the membrane: a novel mechanism for Ras attenuation. J Immunol. 2003;170:2877–2883. doi: 10.4049/jimmunol.170.6.2877. [DOI] [PubMed] [Google Scholar]
- 49.Green JM, Karpitskiy V, Kimzey SL, Shaw AS. Coordinate regulation of T cell activation by CD2 and CD28. J Immunol. 2000;164:3591–3595. doi: 10.4049/jimmunol.164.7.3591. [DOI] [PubMed] [Google Scholar]
- 50.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 51.Jenkins MK, Pardoll DM, Mizuguchi J, Chused TM, Schwartz RH. Molecular events in the induction of a nonresponsive state in interleukin 2-producing helper T-lymphocyte clones. Proc Natl Acad Sci USA. 1987;84:5409–5413. doi: 10.1073/pnas.84.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quill H, Schwartz RH. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987;138:3704–3712. [PubMed] [Google Scholar]
- 53.Boussiotis VA, Freeman GJ, Gray G, Gribben J, Nadler LM. B7 but not intercellular adhesion molecule-1 costimulation prevents the induction of human alloantigen-specific tolerance. J Exp Med. 1993;178:1753–1763. doi: 10.1084/jem.178.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wells AD, Walsh MC, Sankaran D, Turka LA. T cell effector function and anergy avoidance are quantitatively linked to cell division. J Immunol. 2000;165:2432–2443. doi: 10.4049/jimmunol.165.5.2432. [DOI] [PubMed] [Google Scholar]
- 55.Sloan-Lancaster J, Evavold BD, Allen PM. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993;363:156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 56.Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 57.Heissmeyer V, et al. A molecular dissection of lymphocyte unresponsiveness induced by sustained calcium signalling. Novartis Found Symp. 2005;267:165–174. discussion 74–9. [PubMed] [Google Scholar]
- 58.Heissmeyer V, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 59.Choi S, Schwartz RH. Molecular mechanisms for adaptive tolerance and other T cell anergy models. Semin Immunol. 2007;19:140–152. doi: 10.1016/j.smim.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wells AD, Walsh MC, Bluestone JA, Turka LA. Signaling through CD28 and CTLA-4 controls two distinct forms of T cell anergy. J Clin Invest. 2001;108:895–903. doi: 10.1172/JCI13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang SM, Beverly B, Tran AC, Brorson K, Schwartz RH, Lenardo MJ. Transactivation by AP-1 is a molecular target of T cell clonal anergy. Science. 1992;257:1134–1138. doi: 10.1126/science.257.5073.1134. [DOI] [PubMed] [Google Scholar]
- 62.Li W, Whaley CD, Mondino A, Mueller DL. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 63.Fields PE, Gajewski TF, Fitch FW. Blocked Ras activation in anergic CD4+ T cells. Science. 1996;271:1276–1278. doi: 10.1126/science.271.5253.1276. [DOI] [PubMed] [Google Scholar]
- 64.Rengarajan J, et al. Sequential involvement of NFAT and Egr transcription factors in FasL regulation. Immunity. 2000;12:293–300. doi: 10.1016/s1074-7613(00)80182-x. [DOI] [PubMed] [Google Scholar]
- 65.Safford M, et al. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 66.Harris JE, et al. Early growth response gene-2, a zinc-finger transcription factor, is required for full induction of clonal anergy in CD4+ T cells. J Immunol. 2004;173:7331–7338. doi: 10.4049/jimmunol.173.12.7331. [DOI] [PubMed] [Google Scholar]
- 67.Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim DH, Sabatini DM. Raptor and mTOR: subunits of a nutrient-sensitive complex. Curr Top Microbiol Immunol. 2004;279:259–270. doi: 10.1007/978-3-642-18930-2_15. [DOI] [PubMed] [Google Scholar]
- 69.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 71.Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J. 2001;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raught B, et al. Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J. 2004;23:1761–1769. doi: 10.1038/sj.emboj.7600193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- 74.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 76.Mondino A, Mueller DL. mTOR at the crossroads of T cell proliferation and tolerance. Semin Immunol. 2007;19:162–172. doi: 10.1016/j.smim.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng Y, et al. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 78.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 79.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 80.Valmori D, et al. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J Immunol. 2006;177:944–949. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]
- 81.Kang J, Huddleston SJ, Fraser JM, Khoruts A. De novo induction of antigen-specific CD4+ CD25+Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J Leukoc Biol. 2008;83:1230–1239. doi: 10.1189/jlb.1207851. [DOI] [PubMed] [Google Scholar]
- 82.Delgoffe GM, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee K, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sinclair LV, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Salmond RJ, Emery J, Okkenhaug K, Zamoyska R. MAPK, phosphatidylinositol 3-kinase, and mammalian target of rapamycin pathways converge at the level of ribosomal protein S6 phosphorylation to control metabolic signaling in CD8 T cells. J Immunol (Baltimore, Md : 1950) 2009;183:7388–7397. doi: 10.4049/jimmunol.0902294. [DOI] [PubMed] [Google Scholar]
- 87.Avila-Flores A, Santos T, Rincon E, Merida I. Modulation of the mammalian target of rapamycin pathway by diacylglycerol kinase-produced phosphatidic acid. J Biol Chem. 2005;280:10091–10099. doi: 10.1074/jbc.M412296200. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 89.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 91.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 92.Cai SL, et al. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol. 2006;173:279–289. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 94.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 95.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 96.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 97.Suzuki N, Suzuki S, Yeh WC. IRAK-4 as the central TIR signaling mediator in innate immunity. Trends Immunol. 2002;23:503–506. doi: 10.1016/s1471-4906(02)02298-6. [DOI] [PubMed] [Google Scholar]
- 98.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 99.Dunne A, O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2003;2003:re3. doi: 10.1126/stke.2003.171.re3. [DOI] [PubMed] [Google Scholar]
- 100.Takaesu G, Surabhi RM, Park KJ, Ninomiya-Tsuji J, Matsumoto K, Gaynor RB. TAK1 is critical for IkappaB kinase-mediated activation of the NF-kappaB pathway. J Mol Biol. 2003;326:105–115. doi: 10.1016/s0022-2836(02)01404-3. [DOI] [PubMed] [Google Scholar]
- 101.Shibuya H, et al. TAB 1: an activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science. 1996;272:1179–1182. doi: 10.1126/science.272.5265.1179. [DOI] [PubMed] [Google Scholar]
- 102.Huang Q, et al. Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat Immunol. 2004;5:98–103. doi: 10.1038/ni1014. [DOI] [PubMed] [Google Scholar]
- 103.Dumitru CD, et al. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–1083. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 104.Papoutsopoulou S, et al. ABIN-2 is required for optimal activation of Erk MAP kinase in innate immune responses. Nat Immunol. 2006;7:606–615. doi: 10.1038/ni1334. [DOI] [PubMed] [Google Scholar]
- 105.Yamamoto M, et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 106.Yamamoto M, et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 107.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 108.Hoshino K, Kaisho T, Iwabe T, Takeuchi O, Akira S. Differential involvement of IFN-beta in Toll-like receptor-stimulated dendritic cell activation. Int Immunol. 2002;14:1225–1231. doi: 10.1093/intimm/dxf089. [DOI] [PubMed] [Google Scholar]
- 109.Yoneyama M, Suhara W, Fukuhara Y, Fukuda M, Nishida E, Fujita T. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 1998;17:1087–1095. doi: 10.1093/emboj/17.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fukao T, et al. PI3 K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 111.Liu CH, et al. Diacylglycerol kinase zeta regulates microbial recognition and host resistance to Toxoplasma gondii. J Exp Med. 2007;204:781–792. doi: 10.1084/jem.20061856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Colina R, et al. Translational control of the innate immune response through IRF-7. Nature. 2008;452:323–328. doi: 10.1038/nature06730. [DOI] [PubMed] [Google Scholar]
- 113.Weichhart T, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 114.Baker AK, Wang R, Mackman N, Luyendyk JP. Rapamycin enhances LPS induction of tissue factor and tumor necrosis factor-alpha expression in macrophages by reducing IL-10 expression. Mol Immunol. 2009;46:2249–2255. doi: 10.1016/j.molimm.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Galli SJ. Complexity and redundancy in the pathogenesis of asthma: reassessing the roles of mast cells and T cells. J Exp Med. 1997;186:343–347. doi: 10.1084/jem.186.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Benoist C, Mathis D. Mast cells in autoimmune disease. Nature. 2002;420:875–878. doi: 10.1038/nature01324. [DOI] [PubMed] [Google Scholar]
- 117.Frossi B, De Carli M, Pucillo C. The mast cell: an antenna of the microenvironment that directs the immune response. J Leukoc Biol. 2004;75:579–585. doi: 10.1189/jlb.0603275. [DOI] [PubMed] [Google Scholar]
- 118.Nadler MJ, Matthews SA, Turner H, Kinet JP. Signal transduction by the high-affinity immuno-globulin E receptor Fc epsilon RI: coupling form to function. Adv Immunol. 2000;76:325–355. doi: 10.1016/s0065-2776(01)76022-1. [DOI] [PubMed] [Google Scholar]
- 119.Wedemeyer J, Tsai M, Galli SJ. Roles of mast cells and basophils in innate and acquired immunity. Curr Opin Immunol. 2000;12:624–631. doi: 10.1016/s0952-7915(00)00154-0. [DOI] [PubMed] [Google Scholar]
- 120.Rivera J, et al. Macromolecular protein signaling complexes and mast cell responses: a view of the organization of IgE-dependent mast cell signaling. Mol Immunol. 2002;38:1253. doi: 10.1016/s0161-5890(02)00072-x. [DOI] [PubMed] [Google Scholar]
- 121.Zhang J, Berenstein E, Evans R, Siraganian R. Transfection of Syk protein tyrosine kinase reconstitutes high affinity IgE receptor-mediated degranulation in a Syk-negative variant of rat basophilic leukemia RBL-2H3 cells. J Exp Med. 1996;184:71–79. doi: 10.1084/jem.184.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Parravicini V, et al. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- 123.Hata D, et al. Involvement of Bruton’s tyrosine kinase in FcεRI-dependent mast cell degranulation and cytokine production. J Exp Med. 1998;187:1235–1247. doi: 10.1084/jem.187.8.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saitoh S, et al. LAT is essential for FcεRI-mediated mast cell activation. Immunity. 2000;12:525–535. doi: 10.1016/s1074-7613(00)80204-6. [DOI] [PubMed] [Google Scholar]
- 125.Pivniouk VI, Martin TR, Lu-Kuo JM, Katz HR, Oettgen HC, Geha RS. SLP-76 deficiency impairs signaling via the high-affinity IgE receptor in mast cells. J Clin Invest. 1999;103:1737–1743. [PMC free article] [PubMed] [Google Scholar]
- 126.Wu JN, Jordan MS, Silverman MA, Peterson EJ, Koretzky GA. Differential requirement for adapter proteins src homology 2 domain-containing leukocyte phosphoprotein of 76 kDa and Adhesion- and degranulation-promoting adapter protein FcεRI signaling and mast cell function. J Immunol. 2004;172:6768–6774. doi: 10.4049/jimmunol.172.11.6768. [DOI] [PubMed] [Google Scholar]
- 127.Gu H, et al. Essential role for Gab2 in the allergic response. Nature. 2001;412:186–190. doi: 10.1038/35084076. [DOI] [PubMed] [Google Scholar]
- 128.Xie Z-H, Ambudkar I, Siraganian RP. The adapter molecule Gab2 regulates FcεRI-mediated signal transduction in mast cells. J Immunol. 2002;168:4682–4691. doi: 10.4049/jimmunol.168.9.4682. [DOI] [PubMed] [Google Scholar]
- 129.Fukao T, Terauchi Y, Kadowaki T, Koyasu S. Role of phosphoinositide 3-kinase signaling in mast cells: new insights from knockout mouse studies. J Mol Med. 2003;81:524–535. doi: 10.1007/s00109-003-0475-2. [DOI] [PubMed] [Google Scholar]
- 130.Manetz TS, Gonzalez-Espinosa C, Arudchandran R, Xirasagar S, Tybulewicz V, Rivera J. Vav1 regulates phospholipase cgamma activation and calcium responses in mast cells. Mol Cell Biol. 2001;21:3763–3774. doi: 10.1128/MCB.21.11.3763-3774.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schneider H, Cohen-Dayag A, Pecht I. Tyrosine phosphorylation of phospholipase C γ1 couples the Fc epsilon receptor mediated signal to mast cells secretion. Int Immunol. 1992;4:447–453. doi: 10.1093/intimm/4.4.447. [DOI] [PubMed] [Google Scholar]
- 132.Li W, Deanin GG, Margolis B, Schlessinger J, Oliver JM. Fc epsilon R1-mediated tyrosine phosphorylation of multiple proteins, including phospholipase C γ 1 and the receptor βγ 2 complex, in RBL-2H3 rat basophilic leukemia cells. Mol Cell Biol. 1992;12:3176–3182. doi: 10.1128/mcb.12.7.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fukamachi H, Kawakami Y, Takei M, Ishizaka T, Ishizaka K, Kawakami T. Association of protein-tyrosine kinase with phospholipase C-γ 1 in bone marrow-derived mouse mast cells. Proc Natl Acad Sci USA. 1992;89:9524–9528. doi: 10.1073/pnas.89.20.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Atkinson TP, Kaliner MA, Hohman RJ. Phospholipase C-γ 1 is translocated to the membrane of rat basophilic leukemia cells in response to aggregation of IgE receptors. J Immunol. 1992;148:2194–2200. [PubMed] [Google Scholar]
- 135.Liu Y, Graham C, Parravicini V, Brown MJ, Rivera J, Shaw S. Protein kinase C θ is expressed in mast cells and is functionally involved in Fcepsilon receptor I signaling. J Leukoc Biol. 2001;69:831–840. [PubMed] [Google Scholar]
- 136.Tsai M, Chen RH, Tam SY, Blenis J, Galli SJ. Activation of MAP kinases, pp90rsk and pp70–S6 kinases in mouse mast cells by signaling through the c-kit receptor tyrosine kinase or FcεRI: rapamycin inhibits activation of pp70-S6 kinase and proliferation in mouse mast cells. Eur J Immunol. 1993;23:3286–3291. doi: 10.1002/eji.1830231234. [DOI] [PubMed] [Google Scholar]
- 137.Kimata M, Inagaki N, Kato T, Miura T, Serizawa I, Nagai H. Roles of mitogen-activated protein kinase pathways for mediator release from human cultured mast cells. Biochem Pharmacol. 2000;60:589–594. doi: 10.1016/s0006-2952(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 138.Kim MS, Kuehn HS, Metcalfe DD, Gilfillan AM. Activation and function of the mTORC1 pathway in mast cells. J Immunol. 2008;180:4586–4595. doi: 10.4049/jimmunol.180.7.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tsai M, Tam SY, Galli SJ. Distinct patterns of early response gene expression and proliferation in mouse mast cells stimulated by stem cell factor, interleukin-3, or IgE and antigen. Eur J Immunol. 1993;23:867–872. doi: 10.1002/eji.1830230415. [DOI] [PubMed] [Google Scholar]
- 140.Kim TD, Eddlestone GT, Mahmoud SF, Kuchtey J, Fewtrell C. Correlating Ca2+ Responses and Secretion in Individual RBL-2H3 Mucosal Mast Cells. J Biol Chem. 1997;272:31225–31229. doi: 10.1074/jbc.272.50.31225. [DOI] [PubMed] [Google Scholar]
- 141.Blank U, Rivera J. The ins and outs of IgE-dependent mast-cell exocytosis. Trend Immunol. 2004;25:266–273. doi: 10.1016/j.it.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 142.Nechushtan H, Leitges M, Cohen C, Kay G, Razin E. Inhibition of degranulation and interleukin-6 production in mast cells derived from mice deficient in protein kinase C β. Blood. 2000;95:1752–1757. [PubMed] [Google Scholar]
- 143.White JR, Zembryki D, Hanna N, Mong S. Differential inhibition of histamine release from mast cells by protein kinase C inhibitors: staurosporine and K-252a. Biochem Pharmacol. 1990;40:447–456. doi: 10.1016/0006-2952(90)90542-s. [DOI] [PubMed] [Google Scholar]
- 144.Ozawa K, et al. Ca(2+)-dependent and Ca(2+)-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells. Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J Biol Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- 145.Zhang C, Baumgartner RA, Yamada K, Beaven MA. Mitogen-activated protein (MAP) kinase regulates production of tumor necrosis factor-α and release of arachidonic acid in mast cells. Indications of communication between p38 AND p42 map kinases. J Biol Chem. 1997;272:13397–13402. doi: 10.1074/jbc.272.20.13397. [DOI] [PubMed] [Google Scholar]
- 146.Ishizuka T, et al. Mast cell tumor necrosis factor alpha production is regulated by MEK kinases. PNAS. 1997;94:6358–6363. doi: 10.1073/pnas.94.12.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lorentz A, Klopp I, Gebhardt T, Manns MP, Bischoff SC. Role of activator protein 1, nuclear factor-κB, and nuclear factor of activated T cells in IgE receptor-mediated cytokine expression in mature human mast cells. J Allergy Clin Immunol. 2003;111:1062–1068. doi: 10.1067/mai.2003.1342. [DOI] [PubMed] [Google Scholar]
- 148.Turner H, Cantrell DA. Distinct ras effector pathways are involved in fcepsilon R1 regulation of the transcriptional activity of Elk-1 and NFAT in mast cells. J Exp Med. 1997;185:43–54. doi: 10.1084/jem.185.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang D, et al. Phospholipase Cgamma2 is essential in the functions of B cell and several Fc receptors. Immunity. 2000;13:25–35. doi: 10.1016/s1074-7613(00)00005-4. [DOI] [PubMed] [Google Scholar]
- 150.Wen R, Jou S-T, Chen Y, Hoffmeyer A, Wang D. Phospholipase Cγ2 is essential for specific functions of FcεR and FcγR. J Immunol. 2002;169:6743–6752. doi: 10.4049/jimmunol.169.12.6743. [DOI] [PubMed] [Google Scholar]
- 151.Leitges M, et al. Protein Kinase C-δ Is a negative regulator of antigen-induced mast cell degranulation. Mol Cell Biol. 2002;22:3970–3980. doi: 10.1128/MCB.22.12.3970-3980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]