The efficacy of chloroquine in the treatment of Plasmodium vivax malaria is declining on the Northwestern border of Thailand. This randomized controlled trial in 500 adults and children shows that dihydroartemisinin-piperaquine is a safe and effective alternative treatment.
Abstract
Background. Chloroquine (CQ) remains the treatment of choice for Plasmodium vivax malaria. Initially confined to parts of Indonesia and Papua, resistance of P. vivax to CQ seems to be spreading, and alternative treatments are required.
Methods. We conducted a randomized controlled study to compare the efficacy and the tolerability of CQ and dihydroartemisinin-piperaquine (DP) in 500 adults and children with acute vivax malaria on the Northwestern border of Thailand.
Results. Both drugs were well tolerated. Fever and parasite clearance times were slower in the CQ than in the DP group (P < .001). By day 28, recurrent infections had emerged in 18 of 207 CQ recipients compared with 5 of 230 treated with DP (relative risk, 4.0; 95% confidence interval [CI], 1.51–10.58; P = .0046). The cumulative risk of recurrence with P. vivax at 9 weeks was 79.1% (95% CI, 73.5%–84.8%) in patients treated with CQ compared with 54.9% (95% CI, 48.2%–61.6%) in those receiving DP (hazard ratio [HR], 2.27; 95% CI, 1.8–2.9; P < .001). Children <5 years old were at greater risk of recurrent P. vivax infection (74.4%; 95% CI, 63.2%–85.6%) than older patients (55.3% [95% CI, 50.2%–60.4%]; HR, 1.58 [95% CI, 1.1–2.2]; P = .005). In vitro susceptibility testing showed that 13% of the tested isolates had a CQ median inhibitory concentration >100 nmol/L, suggesting reduced susceptibility.
Conclusions. The efficacy of CQ in the treatment of P. vivax infections is declining on the Thai-Myanmar border. DP is an effective alternative treatment.
Clinical Trials Registration. ISRCTN87827353.
Chloroquine (CQ) remains the drug of choice for the prevention and treatment of infections caused by Plasmodium vivax in most endemic regions, although the decline in the efficacy of CQ may have been overlooked [1, 2]. CQ-resistant P. vivax was first described in 1989 in Papua New Guinea [3]. For several years, reports of reduced CQ efficacy were limited to the island of New Guinea [4–6]. The degree of CQ resistance in Papua, Indonesia and Papua New Guinea has now become so severe that local antimalarial treatment policies have been revised to adopt artemisinin combination therapy for both Plasmodium falciparum and P. vivax infections. More recently, CQ-resistant P. vivax has been documented across the Indonesian archipelago [1] and in Myanmar [7, 8], South Korea [9], Turkey [10], the Horn of Africa [11], and South America [12].
In Thailand, malaria remains a problem along the borders with Cambodia and Myanmar, where multidrug-resistant P. falciparum is endemic. In 1996, in camps for displaced persons of the Karen ethnic minority on the Northwestern border of Thailand, we found that CQ was a highly effective treatment for P. vivax malaria, with <3% of patients having a recurrence (relapse, reinfection or recrudescence) by day 28 [13]. However, in a study conducted 7 years later in neighboring Myanmar, almost 35% of treated patients had a parasite recurrence by day 28, raising the possibility that resistance was spreading along the Thai-Myanmar border [7]. In addition, in vitro sensitivity testing on fresh parasite isolates collected between 2003 and 2007 in the same region indicated a decreased susceptibility to CQ in some isolates [14]. Patients with CQ-resistant P. vivax can be treated effectively with the dihydroartemisinin-piperaquine combination (DP) as well as other artemisinin combination therapies [2, 5, 15]. To reassess the efficacy of CQ in the treatment of P. vivax infections on the Northwestern border of Thailand and study the efficacy of DP, we conducted a randomized controlled trial in patients with P. vivax monoinfections.
METHODS
Patients
The study took place in the Shoklo Malaria Research Unit clinics on the Thailand-Myanmar border, an area of low (entomologic inoculation rate, <1) and seasonal malaria transmission[16]. Patients >1 year old and with a body weight of ≥5 kg were eligible for enrollment if they had a microscopically confirmed monoinfection of P. vivax (parasitemia, ≥5/500 white blood cells), if they were febrile (axillary temperature, ≥37.5°C) or had a history of fever, and if they or their guardians gave fully informed consent. Patients were excluded if they had a known hypersensitivity to the study drugs; had intercurrent illness or were pregnant, lactating, or severely anemic (hematocrit, <20%); or had received mefloquine treatment in the previous 60 days or DP in the previous 3 months.
Randomization
Patients were allocated to the treatment arms based on a pregenerated randomization list in blocks of 20. The individual allocations were concealed in sealed envelopes and opened only after enrollment by the field team. Although patients and clinic workers were not blinded to the treatment arm after allocation, laboratory technicians were unaware of treatment allocation.
Drugs
Chloroquine (Government Pharmaceutical Organization, Thailand) was given once a day for 3 days, with a target dose of 25 mg base/kg with a glass of water. DP (Duocotexin; Holley People's Republic of China) was administered once a day for 3 days with milk, with a total target dose of 7 mg/kg for dihydroartemisinin and 55 mg/kg for piperaquine. All patients with a normal G6PD test result were offered a course of 14 days of primaquine (0.5 mg base/kg/d) at the end of the follow-up period. This delay was instituted because primaquine itself is active against asexual stages of P. vivax [17] and its administration earlier would have compromised the analysis of the efficacy of CQ and DP.
Enrollment Procedures
A case-record form was completed for all patients documenting symptoms before clinic attendance, concomitant illness, drug history and physical examination. Capillary blood was collected for malaria thick and thin films, hematocrit, and G6PD deficiency testing. Patients >1 year of age presenting with parasitemia of >100 P. vivax trophozoites/500 white blood cells were asked to provide 5 mL of venous blood for in vitro sensitivity testing.
Follow-up
Patients were seen daily from admission until they were afebrile and aparasitemic and then weekly until day 63. At each visit, temperature was measured and a symptom questionnaire was completed. Capillary blood was taken for malaria blood smear and hematocrit weekly. Any concomitant medication taken during the study period was recorded. Patients presenting to the clinic within the follow-up period with microscopically confirmed P. vivax had capillary blood sampling for measurement of CQ and desethylchloroquine plasma concentrations. Patients with P. falciparum infection diagnosed during the follow-up period were withdrawn from the study and treated with the standard 3-day treatment of mefloquine and artesunate.
Drug Measurements
CQ and desethylchloroquine plasma concentrations were measured in patients >10 years old at the time of recurrent malaria. Plasma drug concentrations were quantified using solid-phase extraction and high-performance liquid chromatography with UV detection [18]. The limit of detection was 1 ng/mL for both drugs.
Laboratory Methods
Malaria blood films were stained with Giemsa, and parasite counts were expressed as the number of parasites per 1000 red blood cells or, if the parasitemia was <1% packed red blood cells, as the number of parasites per 500 white blood cells. G6PD deficiency was assessed by the fluorescent spot test SQMMR500 (R&D Diagnostic). In vitro samples for drug susceptibility were processed and analyzed as described elsewhere [14].
Study End Points
The analysis of efficacy was conducted using a modified intention-to-treat analysis that included all patients who fulfilled the enrollment criteria. The primary end point was the overall risk of recurrence with P. vivax during the 63-day follow-up period. Secondary end points included the risk of reappearance of P. falciparum, the parasite and fever clearance times, and the adverse events reported by the patients.
Sample Size
The original sample size of 500 patients had 80% power and 95% confidence to detect a 15% difference in the primary end point, assuming a 50% recurrence rate in the CQ group, allowing for up to 15% loss to follow-up by day 63.
Statistical Analysis
Data were double entered into Microsoft Access software and analysis was performed using SPSS for Windows (version 16; SPSS). The Mann–Whitney U test was used for nonparametric comparisons, and Student t test or 1-way analysis of variance for parametric comparisons. Proportions were examined using χ2 with Yates' correction or by Fisher’s exact test. Efficacy end points were assessed by survival analysis, in which the cumulative risk of failure was calculated by the Kaplan–Meier product limit formula and compared by the Mantel–Haenszel log-rank test. In addition, treatments were compared and the hazard ratio (HR) calculated using a Cox proportional hazards model. Data were censored for patients who were unavailable for follow-up or, in the case of secondary end points, presented again with a different outcome and not regarded as treatment failures. Those patients with recurrent vomiting or adverse drug effects, which required early termination of treatment and the administration of rescue therapy, were regarded as therapeutic failures.
Ethical Approval
The study was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University (MUTM 2006-029), and the Oxford Tropical Research Ethics Committee, Oxford University (OXTREC 027-05). The trial was registered at ClinicalTrials.gov (ISRCTN87827353).
RESULTS
Recruitment and Baseline Characteristics
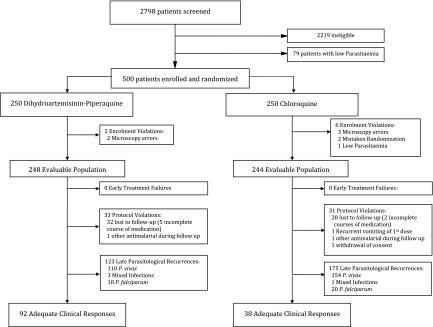
Between January 2007 and December 2008, 500 patients were enrolled in the study. Eight of these patients did not meet the inclusion criteria and were excluded from further analysis (Figure 1). The baseline characteristics of the 492 patients in the evaluable population (modified intention-to-treat analysis) are presented in Table 1; there were no differences in baseline characteristics between treatment arms. Follow-up to day 63 or day of failure (per-protocol analysis) was achieved in 86.7% of patients (215/248) treated with DP and 87.3% (213/244) given CQ (P = .95). Nonadherence to follow-up was more likely in adults (16%; 47/291) than in children (8.5%; 17/201; P = .01), but these patients otherwise had similar baseline characteristics as those who were adherent to follow-up. With the exception of a 3-year old boy who vomited CQ repeatedly, there were no other early treatment failures. In patients receiving a complete course of treatment, the median dose of CQ administered in the CQ arm was 24.8 mg base/kg (range, 21.3–29.4), and patients in the DP arm received median doses of 6.7 mg/kg (range, 5–10) of dihydroartemisinin and 53.3 mg/kg (range, 40–80) of piperaquine.
Figure 1.
Study profile.
Table 1.
Patient Characteristics at Baselinea
| Characteristic | DP treatment (n = 248) | CQ treatment (n = 244) |
| Asexual parasitemia, geometric mean (95% CI), μL−1 | 3776 (3111–4582) | 4406 (3637–5339) |
| Gametocytemia, geometric mean (95% CI), μL−1 | 214 (178–257) | 280 (236–333) |
| Gametocytemia present | 85 (210) | 84 (205) |
| Schizonts present at enrollment | 32 (79) | 30 (74) |
| Male sex | 70 (173) | 64 (155) |
| G6PD deficiency | 6.1 (15/247) | 5.3 (13) |
| Weight, median (range), kg | 45 (7–74) | 45 (9–61) |
| Age, median (range), years | 18 (1–57) | 18 (1–63) |
| Age, years | ||
| <5 | 14 (34) | 12 (30) |
| 5–14 | 27 (67) | 29 (70) |
| >14 | 59 (147) | 59 (144) |
| Fever >38.0°C | 42 (94/225) | 42 (97/229) |
| Duration of febrile illness, median (range), days | 2 (0–8) | 2 (0–5) |
| Hematocrit, mean (SD), % | 37.2 (5.0) | 37.6 (5.2) |
| Anemia (hematocrit <30%) | 4.5 (11/247) | 5.8 (14/242) |
| Splenomegaly | 14 (34/243) | 12 (29/237) |
| Hepatomegaly | 23 (57/247) | 26 (63) |
Abbreviations: CI, confidence interval; CQ, chloroquine; DP, dihydroartemisinin-piperaquine; SD, standard deviation.
Unless otherwise indicated, data represent % (No.) of patients, with denominators where these were smaller than the full sample.
Early Therapeutic Response
Within 24 hours after the start of treatment, 63.7% of patients (156/245) receiving DP had clearance of asexual parasitemia after, compared with only 21.1% (51/242) receiving CQ (odds ratio, 6.6; 95% confidence interval [CI], 4.3–10.0; P < .001). After 48 hours, these proportions had risen to 97.5% (237/243) and 84.1% (201/239), respectively (P < .001). Five patients treated with CQ were still parasitemic at 72 hours, and all but 1 had cleared by 96 hours. In the CQ but not in the DP group, patients with schizont stages present at enrollment were more likely to be parasitemic at 24 hours than were patients with only trophozoite asexual stages present (93% [69/74] vs 72.6% [122/168], respectively; odds ratio, 5.2; 95% CI, 1.9–15.7; P = .0005). Overall 84.3% of patients (415/492) had P. vivax gametocytemia evident on their admission blood film. By 48 hours, this proportion had fallen to 7.6% (18/238) in the CQ group and 1.3% (3/239) in the DP group (P = .001). Although all patients had a history of fever (median duration, 2 days) on admission to the study, only 42.1% (191/454) were febrile at enrollment. Fever clearance was significantly faster in patients receiving DP; 92.9% (183/197) were afebrile by day 2 compared with 82.2% (171/208) of those receiving CQ (P = .001).
Efficacy Analysis
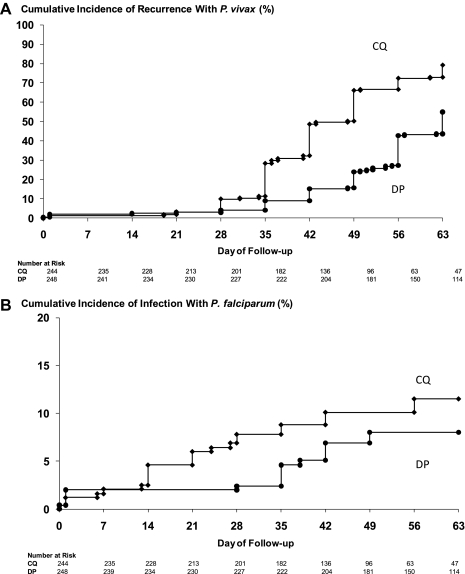
In the modified intention-to-treat analysis, the cumulative risk of recurrence with P. vivax at 9 weeks was 79.1% (95% CI, 73.5%–84.8%) in patients treated with CQ compared with 54.9% (95% CI, 48.2%–61.6%) in those receiving DP (HR, 2.27; 95% CI, 1.8–2.9; P < .001) (Table 2). This significant difference had already emerged by day 28 of follow-up and was confirmed at both day 28 and day 63 in the per-protocol population (Table 3 and Figure 2). By day 28, recurrent infections had emerged in 8.7% CQ recipients (18/207) compared with 2.2% (5/230) treated with DP (relative risk, 4.0; 95% CI, 1.51–10.58; P = .005). The median time to recurrence of P. vivax infection was 56 days (interquartile range [IQR], 42–56; range, 14–65) in the DP group compared with 42 days (IQR, 35–49; range 19–66) in the CQ group (P < .001). Falciparum malaria was also more common in the CQ group; by day 28, 1 patient in the DP group and 15 in the CQ group were parasitemic with P. falciparum (either alone or mixed with P. vivax) (HR, 3.1; 95% CI, 1.2–7.9; P = .015). These figures had increased to 13 and 21, respectively, by day 63, although this difference was no longer significant in the modified intention-to-treat analysis (Table 2). Recurrent malaria within 28 days (both P. vivax and P. falciparum) was therefore 6.11 (95% CI, 2.61–14.29) times more frequent in CQ recipients than in DP recipients.
Table 2.
Risk of Treatment Failure: Modified Intention-to-Treat Analysis
| Cumulative risk of recurrencea (95% CI), % |
|||
| Type of recurrence | DP treatment (n = 248) | CQ treatment (n = 244) | Hazard ratiob (95% CI) |
| Plasmodium vivax | |||
| Day 63c | 54.9 (48.2–61.6) | 79.1 (73.4–84.8) | 2.27 (1.8–2.9) (P < .001) |
| Day 28d | 4.1 (1.6–6.6) | 9.8 (5.9–13.7) | 2.3 (1.1–4.9) (P = .032) |
| Plasmodium falciparum | |||
| Day 63d | 8.0 (4.5–11.5) | 11.5 (6.6–16.4) | 1.5 (0.8–2.9) (P = .176) |
| Day 28d | 2.4 (0.4–4.4) | 7.8 (4.3–11.3) | 3.1 (1.2–7.9) (P =. 015) |
Abbreviations: CI, confidence interval; CQ, chloroquine; DP, dihydroartemisinin-piperaquine.
Cumulative risk calculated by Kaplan-Meier method. Patients with an incomplete course of treatment were included in the analysis conservatively as treatment failures; other patients with incomplete follow-up were censored on the last day of follow-up as nonfailures.
Hazard ratios were calculated using a Cox proportional hazards model.
Primary end point.
Secondary end point.
Figure 2.
A, Cumulative risk of treatment failure in patients with Plasmodium vivax parasitemia (P. vivax alone or mixed infections). Diamonds represent chloroquine (CQ); circles, dihydroartemisinin-piperaquine (DP). The overall difference between treatment groups at day 63 was significant (P < .001). B, Cumulative risk of treatment failure in patients with Plasmodium falciparum parasitemia (P. falciparum infection alone or mixed infections). The overall difference between treatment groups was significant at day 28 (P = .015) but not by day 63 (P = .176).
Table 3.
Risk of Treatment Failure: Per-Protocol Analysis
| Patients with recurrence, % (no.) |
|||
| Recurrence | DP treatment | CQ treatment | Odds ratio (95% CI) |
| Plasmodium vivax | |||
| Day 63a | 55.1 (113/205) | 80.3 (155/193) | 3.3 (2.1–5.3) (P < .001) |
| Day 28a | 2.2 (5/230) | 8.7 (18/207) | 4.3 (1.5–13.5) (P = .005) |
| Plasmodium falciparum | |||
| Day 63b | 10.1 (13/129) | 29.6 (21/71) | 3.8 (1.6–8.7) (P < .001) |
| Day 28b | 0.4 (1/228) | 7.0 (15/215) | 17.0 (2.3–349) (P < .001) |
Abbreviations: CI, confidence interval; CQ, chloroquine; DP, dihydroartemisinin-piperaquine.
Excludes all patients with protocol violations or incomplete follow-up due to P. falciparum parasitemia before day 61 (for day 63 risk) or 26 (for day 28 risk).
Excludes all patients with protocol violations or incomplete follow-up due to recurrent P. vivax parasitemia before day 61 (for day 63 risk) or 26 (for day 28 risk).
Cox regression analysis (modified intention-to-treat population) revealed 2 independent baseline factors predicting recurrent P. vivax infection: treatment with CQ (adjusted HR [AHR], 2.3; 95% CI, 1.8–2.9; P < .001) and young age (1–4 years) (AHR, 1.58; 95% CI, 1.1–2.2; P = .005 for comparison with older children and adults). In addition, patients treated with CQ who had delayed parasite clearance (still parasitemic on day 2), were at significantly greater risk of recurrent P. vivax infection by day 28 (23.5%; 95% CI, 9.2%–37.8%) compared with 6.5% (95% CI, 2.4%–10.6%) in patients whose initial parasitemia had cleared within 48 hours (P = .004). This difference remained significant after age and baseline parasitemia were controlled for (AHR, 4.2; 95% CI, 1.6–11.1; P = .004).
The minimum effective plasma concentration (MEC) of combined CQ plus desethylchloroquine considered effective against CQ-susceptible parasites is ∼12 ng/mL [1, 19]. Of 156 recurrences of P. vivax infection in the CQ group, plasma concentrations of CQ and desethylchloroquine were determined in 85 patients (54%). In the 5 patients with recurrence before day 28, these plasma concentrations at the time of failure were 4.59, 5.00, 5.00, 7.50, and 15.5 ng/mL. The patient with a plasma CQ-desethylchloroquine concentration of 15.5 ng/mL (higher than the MEC of 12 ng/mL) had treatment failure at day 21.
Symptoms and Tolerability
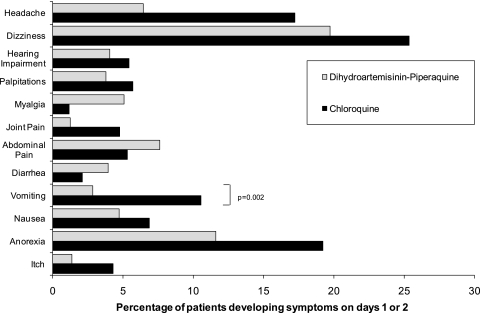
Early vomiting within the first hour of drug administration was recorded in 7.9% of patients treated with DP (19/241) and 7.1% of those treated with CQ (17/241) (P = .86). With the exception of the 3-year-old boy in the CQ group, all patients tolerated drug readministration (Figure 3). The only significant difference between the 2 treatment arms in the development of symptoms was a 4.0-fold (95% CI, 1.5–11.3-fold) increased risk of vomiting on days 1 and 2 associated with CQ; 10.5% of patients receiving CQ (22/209) vomited, compared with 2.8% (6/211) of those receiving DP (P = .003). The risk of diarrhea was 0.2% in both treatment arms assessed at day 7. No serious adverse event occurred during the study.
Figure 3.
Adverse events on day 1 or 2 in patients without symptoms at admission.
Parasite In Vitro Drug Susceptibility
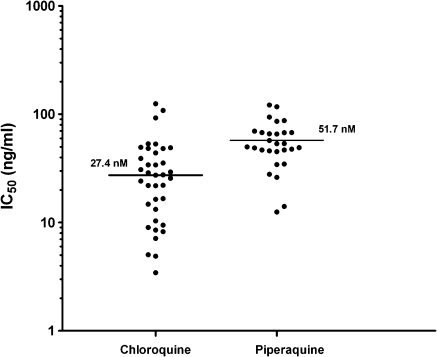
CQ susceptibility was assessed in 50 isolates before administration of antimalarial medication, of which 43 had a majority of ring stages present. Drug susceptibility could be assessed successfully in 86% of these isolates (37/43). The median inhibitory concentration (IC50) for CQ was 27.4 ng/mL (IQR, 11.8–46.1l; range, 3.4–124.8) and 51.7 ng/mL (IQR, 45.3–69.3; range, 12.5–121.7) for piperaquine (Figure 4). In total, 13.5% of isolates (5/37) had CQ IC50 values >50 ng/mL (∼100 nmol/L).
Figure 4.
Ex vivo drug susceptibility to chloroquine and piperaquine. IC50, median inhibitory concentration.
DISCUSSION
An estimated 2849 million persons are at risk of P. vivax infection [20], with between 76 and 391 million clinical cases per year. The efficacy of CQ, the drug used to treat vivax malaria for >50 years, has fallen in some areas, and resistance seems to be spreading. The assessment of treatment efficacy in P. vivax is more difficult than in P. falciparum, because there are no genotyping methods that reliably distinguish relapses from new infections, and long-term cultures of P. vivax cannot be maintained, thereby confining in vitro testing of drugs to assays on fresh isolates. This means that it is difficult to document unequivocal cases of treatment failure in areas where resistance is emerging, but any P. vivax infection that occurs within 28 days after the start of CQ treatment, whether recrudescence, relapse, or new infection, has grown through residual CQ concentrations in blood. If these concentrations are adequate, then the infection is resistant, by definition [21].
In this study we assessed the in vivo efficacy of CQ and compared it with that of DP, an artemisinin combination therapy with documented efficacy against CQ-resistant P. vivax [5, 22, 23]. More than 8% of patients treated with CQ had a recurrent infection within 28 days, and this rate rose to 22% in young children. Parasite and fever clearance times were both significantly faster after DP treatment. The artemisinin derivatives are the most rapidly acting drugs against P. vivax [17], so this difference is expected. The artemisinin component of DP contributes significantly to the initial therapeutic response but would not be expected to affect subsequent relapses or reinfections. Parasite clearance time was prolonged after CQ treatment, with 16% of patients still parasitemic at 48 hours and 2% at 76 hours. Delayed parasite clearance, as defined by parasitemia on day 2, increased the subsequent risk of recurrent P. vivax infection within 28 days by almost 4-fold after CQ treatment. Interestingly, patients who had schizonts in the peripheral blood on admission had significantly slower parasite clearance after CQ treatment. This presumably reflects uninterrupted schizogony and is consistent with in vitro observations showing marked reduction in activity of CQ against these stages [24]. As expected, children who have less natural immunity against malaria were more at risk of parasite recurrence independent of the above-mentioned risk factors. During follow-up, the risk of recurrence of P. vivax infection was significantly greater after CQ than after DP treatment (AHR, 2.3), a difference that was already apparent by day 28.
Recurrences can result from recrudescence, reinfection, or relapses from dormant liver stages, although we were unable to distinguish between these. Both CQ and DP have long elimination phases and therefore persistent blood concentrations, delaying the time to the first relapse. In areas where CQ sensitive parasites predominate, the prolonged posttreatment prophylaxis of CQ usually affords a minimum of 28 days without recurrence. In the current study the main difference in the parasitologic response between CQ and DP was evident within this period, with nearly 9% of patients treated with CQ having a recurrence by day 28. This figure is 3 times higher than in our previous study in 1996, but one-third of that reported from neighboring Myanmar [7]. In addition, in 1 of these early recurrences (day 21) the combined CQ plus desethylchloroquine plasma concentration (15.5 ng/mL) was higher than the MEC of 12 ng/mL, demonstrating recurrence through drug levels that would be expected to inhibit CQ-sensitive parasites [1, 19]. In the latest published study in the same area, primaquine was administered with CQ, which may have masked the emergence of recurrences by day 28 [25]. In the present study, the confounding effect of primaquine asexual stage activity was avoided by administering the drug at the end of the follow-up period, to focus solely on evaluating the main schizontocidal drugs. Associated in vitro results highlight the fact that whereas the IC50 values for CQ were generally low (median, 27.4 ng/mL), some isolates exhibited relatively high values. Drug susceptibility testing was performed only in a small proportion of patients, and hence our study was underpowered to assess the in vivo–ex vivo correlates. However, in the parasites that could be tested, 13% of isolates had reduced susceptibility to CQ (IC50 >100 nmol/L), in keeping with the overall 9% risk of recurrence within 28 days.
In conclusion our results suggest that CQ efficacy on the Northwestern border of Thailand is declining. DP is a suitable alternative in the treatment of P. vivax infections in this area, although further studies are needed to define how best to combine this treatment with appropriate antirelapse therapy (primaquine).
Notes
Acknowledgments.
We thank all the patients and staff at all the Shoklo Malaria Research Unit (SMRU) clinics who participated in this study.
Financial support.
Holley Pharm provided financial support for the study and donated the dihydroartemisinin-piperaquine but had no role in the planning, design, and conduct of the study, nor in the analysis and interpretation of results or writing of the manuscript. The SMRU is attached to the Faculty of Tropical Medicine, Mahidol University. This investigation was part of the Wellcome Trust Mahidol University Oxford Tropical Medicine Research Programme, supported by the Wellcome Trust of Great Britain.
Potential conflicts of interest.
All authors:No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions.
A. P. P., K. M. L., E. A., and F. N. conducted the trial; R. P. analyzed the data; B. R. and K. S., performed the in vitro tests; N. L. measured the drug concentrations; P. S., E. A., N. J. W., and F. N. conceived the protocol and supervised the study; all authors contributed to the interpretation of data and writing of the manuscript.
References
- 1.Baird JK. Chloroquine resistance in Plasmodium vivax. Antimicrob Agents Chemother. 2004;48:4075–83. doi: 10.1128/AAC.48.11.4075-4083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douglas NM, Anstey NM, Angus BJ, Nosten F, Price RN. Artemisinin combination therapy for vivax malaria. Lancet Infect Dis. 2010;10:405–16. doi: 10.1016/S1473-3099(10)70079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rieckmann KH, Davis DR, Hutton DC. Plasmodium vivax resistance to chloroquine? Lancet. 1989;2:1183–4. doi: 10.1016/s0140-6736(89)91792-3. [DOI] [PubMed] [Google Scholar]
- 4.Baird JK, Basri H, Purnomo, et al. Resistance to chloroquine by Plasmodium vivax in Irian Jaya, Indonesia. Am J Trop Med Hyg. 1991;44:547–52. doi: 10.4269/ajtmh.1991.44.547. [DOI] [PubMed] [Google Scholar]
- 5.Ratcliff A, Siswantoro H, Kenangalem E, et al. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet. 2007;369:757–65. doi: 10.1016/S0140-6736(07)60160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumawinata IW, Bernadeta, Leksana B, et al. Very high risk of therapeutic failure with chloroquine for uncomplicated Plasmodium falciparum and P. vivax malaria in Indonesian Papua. Am J Trop Med Hyg. 2003;68:416–20. [PubMed] [Google Scholar]
- 7.Guthmann JP, Pittet A, Lesage A, et al. Plasmodium vivax resistance to chloroquine in Dawei, southern Myanmar. Trop Med Int Health. 2008;13:91–8. doi: 10.1111/j.1365-3156.2007.01978.x. [DOI] [PubMed] [Google Scholar]
- 8.Marlar T, Myat Phone K, Aye Yu S, Khaing Khaing G, Ma S, Myint O. Development of resistance to chloroquine by Plasmodium vivax in Myanmar. Trans R Soc Trop Med Hyg. 1995;89:307–8. doi: 10.1016/0035-9203(95)90556-1. [DOI] [PubMed] [Google Scholar]
- 9.Lee KS, Kim TH, Kim ES, et al. Short report: chloroquine-resistant Plasmodium vivax in the Republic of Korea. Am J Trop Med Hyg. 2009;80:215–7. [PubMed] [Google Scholar]
- 10.Kurcer MA, Simsek Z, Zeyrek FY, et al. Efficacy of chloroquine in the treatment of Plasmodium vivax malaria in Turkey. Ann Trop Med Parasitol. 2004;98:447–51. doi: 10.1179/000349804225021343. [DOI] [PubMed] [Google Scholar]
- 11.Teka H, Petros B, Yamuah L, et al. Chloroquine-resistant Plasmodium vivax malaria in Debre Zeit, Ethiopia. Malar J. 2008;7:220. doi: 10.1186/1475-2875-7-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Santana Filho FS, Arcanjo AR, Chehuan YM, et al. Chloroquine-resistant Plasmodium vivax, Brazilian Amazon. Emerg Infect Dis. 2007;13:1125–6. doi: 10.3201/eid1307.061386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luxemburger C, van Vugt M, Jonathan S, et al. Treatment of vivax malaria on the western border of Thailand. Trans R Soc Trop Med Hyg. 1999;93:433–8. doi: 10.1016/s0035-9203(99)90149-9. [DOI] [PubMed] [Google Scholar]
- 14.Suwanarusk R, Russell B, Chavchich M, et al. Chloroquine resistant Plasmodium vivax: in vitro characterisation and association with molecular polymorphisms. PLoS One. 2007;2:e1089. doi: 10.1371/journal.pone.0001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karunajeewa HA, Mueller I, Senn M, et al. A trial of combination antimalarial therapies in children from Papua New Guinea. N Engl J Med. 2008;359:2545–7. doi: 10.1056/NEJMoa0804915. [DOI] [PubMed] [Google Scholar]
- 16.Luxemburger C, Thwai KL, White NJ, et al. The epidemiology of malaria in a Karen population on the western border of Thailand. Trans R Soc Trop Med Hyg. 1996;90:105–11. doi: 10.1016/s0035-9203(96)90102-9. [DOI] [PubMed] [Google Scholar]
- 17.Pukrittayakamee S, Chantra A, Simpson JA, et al. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob Agents Chemother. 2000;44:1680–5. doi: 10.1128/aac.44.6.1680-1685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blessborn D, Neamin G, Bergqvist Y, Lindegardh N. A new approach to evaluate stability of amodiaquine and its metabolite in blood and plasma. J Pharm Biomed Anal. 2006;41:207–12. doi: 10.1016/j.jpba.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Berliner RW, Earle DP, Taggart JV, et al. Studies on the chemotherapy of the human malarias. Vi. The physiological disposition, antimalarial activity, and toxicity of several derivatives of 4-aminoquinoline. J Clin Invest. 1948;27:98–107. doi: 10.1172/JCI101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerra CA, Howes RE, Patil AP, et al. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis. 2010;4:e774. doi: 10.1371/journal.pntd.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White NJ. The assessment of antimalarial drug efficacy. Trends Parasitol. 2002;18:458–64. doi: 10.1016/s1471-4922(02)02373-5. [DOI] [PubMed] [Google Scholar]
- 22.Hasugian AR, Purba HL, Kenangalem E, et al. Dihydroartemisinin-piperaquine versus artesunate-amodiaquine: superior efficacy and posttreatment prophylaxis against multidrug-resistant Plasmodium falciparum and Plasmodium vivax malaria. Clin Infect Dis. 2007;44:1067–74. doi: 10.1086/512677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karunajeewa H, Lim C, Hung TY, et al. Safety evaluation of fixed combination piperaquine plus dihydroartemisinin (Artekin) in Cambodian children and adults with malaria. Br J Clin Pharmacol. 2004;57:93–9. doi: 10.1046/j.1365-2125.2003.01962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharrock WW, Suwanarusk R, Lek-Uthai U, et al. Plasmodium vivax trophozoites insensitive to chloroquine. Malar J. 2008;7:94. doi: 10.1186/1475-2875-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muhamad P, Ruengweerayut R, Chacharoenkul W, Rungsihirunrat K, Na-Bangchang K. Monitoring of clinical efficacy and in vitro sensitivity of Plasmodium vivax to chloroquine in area along Thai Myanmar border during 2009–2010. Malar J. 2011;10:44. doi: 10.1186/1475-2875-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]