Abstract
Aims
Agonists that evoke smooth muscle cell hyperpolarization have the potential to stimulate both local and conducted dilation. We investigated whether the endothelium-dependent vasodilators acetylcholine (ACh) and SLIGRL stimulated conducted dilation and whether this was altered by deficiency in apolipoprotein E (ApoE−/−).
Methods and results
Isolated mesenteric arteries were cannulated, pressurized, and precontracted with phenylephrine. Agonists were either added to the bath to study local dilation or were restricted to one end of arteries to study conducted dilation. An enhanced sensitivity to both ACh and SLIGRL was observed in mesenteric arteries from ApoE−/− mice compared with wild-type controls. Inhibition of nitric oxide (NO) synthase blocked ACh responses, but had no effect on maximum dilation to SLIGRL. SLIGRL increased endothelial cell Ca2+, hyperpolarized smooth muscle cells, and fully dilated arteries. The NO-independent dilation to SLIGRL was blocked with high [KCl] or Ca2+-activated K+-channel blockers. The hyperpolarization and dilation to SLIGRL passed through the artery to at least 2.5 mm upstream. The conducted dilation was not affected by a deficit in ApoE and could also be stimulated by ACh, suggesting NO itself could stimulate conducted dilation.
Conclusion
In small mesenteric arteries of ApoE−/− mice, NO-independent dilation is enhanced. Since both NO-dependent and -independent pathways can stimulate local and conducted dilation, the potential for reducing vascular resistance is improved in these vessels.
Keywords: Conducted dilation, EDHF, Atherosclerosis, KCa-channel, Endothelial cell Ca2+
1. Introduction
Endothelial cell Ca2+-activated K+ (KCa)-channels are emerging targets for reducing arterial blood pressure.1 Hyperpolarization secondary to channel opening reduces arterial pressure,1 and transgenic animals deficient in either small (SKCa, KCa2.3) or intermediate (IKCa, KCa3.1) conductance KCa-channels have an elevated blood pressure.2 Importantly, reducing the expression of both SKCa and IKCa channels has a greater effect to lower blood pressure than either channel alone,2 reflecting the pharmacological profile of inhibiting endothelium-dependent hyperpolarization (EDH) to agonists, where both SKCa and IKCa-channels play a role. These channels can be selectively inhibited by apamin and TRAM-34, respectively, and together these agents block dilation in response to EDH in a wide range of arterial beds,3 including in humans.4,5
The dilation associated with EDH can travel along the arterial wall to evoke distant dilation in arterial segments not directly stimulated by the agonist, termed ‘spreading’ or ‘conducted’ dilation. The ability to dilate a longer length of artery both coordinates and further reduces vascular resistance, enabling blood flow to increase within a vascular bed.6,7 This process is not only absolutely dependent on hyperpolarization, but also on the presence of patent gap junctions between endothelial cells and to smooth muscle cells, with the endothelium acting as the primary conduit for conduction.8–10
Despite the evidence for endothelial dysfunction at sites of atherosclerotic lesions in humans and animal models of dyslipidaemia, relatively little known about possible equivalent effects within the resistance vasculature.11 Decreased responses to acetylcholine (ACh) and bradykinin have been reported in the forearm and coronary arteries of hypercholesterolaemic patients.12–15 In both these vascular beds, the increases in blood flow in either control or hypercholesterolaemic patients were not fully blocked by inhibition of nitric oxide (NO) synthase.12,15 There are various models of atherosclerosis in animals, including the apolipoprotein E-knockout (ApoE−/−) and mice expressing human apolipoprotein B-100 (hApoB+/+). When fed a normal chow diet, responses to ACh were reduced in the cerebral arteries of ApoE−/− mice in vivo.16 In contrast, responses to ACh are increased in isolated and pressurized gracilis arteries from hApoB+/+ mice.17 In mesenteric arteries isolated from ApoE−/− mice, endothelium-dependent relaxations to ACh remain intact at 12–1618 and 18 weeks.19 These responses were slightly reduced by the combined inhibition of cyclooxygenase and NO synthase, but again a substantial NO-independent relaxation remained, which was sensitive to apamin and TRAM-3418 or raised extracellular KCl19 the characteristic profile of EDH-mediated vasodilation. Interestingly, the responses to ACh did not appear different from control arteries from wildtype animals,19 an effect also observed in the aorta from ApoE−/− mice, despite the presence of extensive intimal lesions.20 In human mesenteric small arteries, hypercholesterolaemia can significantly reduce EDHF responses, but less so within mesenteric microvessels.21
How endothelial dysfunction in atherosclerosis affects conducted dilation responses has not been established in humans, although there is one report suggesting unaltered conduction in the cremaster of ApoE−/− mice in vivo.22 Here we characterize the extent and magnitude of local and conducted dilation in mesenteric arteries from ApoE−/− mice, an artery with robust EDH-mediated dilatation and a high density of myoendothelial gap junctions.23,24
2. Methods
ApoE−/− mice on a C57BL/6 background (Jackson laboratories, USA) and C57BL/6 mice between the ages of 9–14 weeks (young) and 40–44 weeks (old) were used in this study. Animals were housed in individually ventilated cages with 12 h light/dark cycle and controlled temperature (20–22°C). Standard chow (B&K Ltd, UK) and water were available ad libitum. Genotyping of experimental mice was performed by standard PCR techniques to confirm the presence or absence of the ApoE gene. All animal procedures were carried out in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986. The investigation conforms with the Guide for Care and Use of Laboratory Animals published by the European Commission Directive 86/609/EEC, and the University of Oxford's ethical policy is compliant with that of the NIH. Male C57BL/6 (WT) and ApoE−/− (18–40 g) mice were killed by cervical dislocation according to requirements detailed under Schedule 1 of the Animals (Scientific Procedures) Act 1986. The aorta and mesentery were removed and placed in cold 3-[N-morpholino]propane-sulfonic acid (MOPS) buffer, pH 7.40 ± 0.02. Solutions and drugs available on Supplementary material online.
2.1. Oil red O and Mac-3 staining
Isolated, pinned out aortae and isolated, cannulated mesenteric arteries were fixed, washed and either stained with oil red O or immunohistochemistry performed to stain for Mac-3, details on Supplementary material online.
2.2. Artery isolation and cannulation
Isolated aortae were mounted in a wire myograph for measurements of tension, and mesenteric arteries cannulated, pressurized, and visualized for measurements of diameter using confocal or video microscopy and offline analysis as previously described (diameter 120–250 µm),8,25,26 details on Supplementary material online. The endothelium was damaged by luminal perfusion of air bubbles. Agonists and agents were either applied to the bath, or for studies of conducted dilation, applied focally as described below.
2.3. Membrane potential recordings in pressurized arteries
For intracellular recordings, sharp microelectrodes (tip resistance 50–100 MΩ, filled with 2 M KCl solution) were positioned via a micromanipulator at ∼60° to the pressurized artery. Rapid negative deflections in potential were observed upon cell impalement, and smooth muscle cell membrane potential was measured, details as previously.27 SLIGRL was either added to the bath via the superfusion solution, or applied focally as a bolus via micropipettes. Details on Supplementary material online.
2.4. Endothelial cell Ca2+ in pressurized arteries
In separate experiments, following luminal perfusion endothelial cells of pressurized mesenteric arteries were loaded with the Ca2+-sensitive fluorescent dye Oregon Green® 488 1,2-bis[o-aminophenoxy]ethane-N,N,N',N'-tetraacetic acid -1 (BAPTA-1) and were imaged using confocal microscopy, details on Supplementary material online. SLIGRL was added directly to the bath.
2.5. Conducted dilation to luminally perfused agonists
Arteries isolated with a bifurcation were cannulated as previously described.26 Agonists together with carboxyfluorescein were luminal perfused into one side-branch and were restricted to the downstream end of the vessel. Under no conditions did flow itself stimulate dilation in mouse mesenteric arteries. Diameter and fluorescence levels in the side-branch (Br1, ‘local’ site) and the feed artery (0–2.5 mm upstream, ‘conducted’ sites) were measured simultaneously using confocal microscopy, details on Supplementary material online.
2.6. Statistical analysis
Data are presented as the mean ± standard error of the mean and n refers to the number of different animals. In all cases, one-way analysis of variance followed by Tukey post test was performed, except for concentration response curves where two-way analysis of variance was used to compare entire curves. P-values <0.05 were considered to be statistically significant.
3. Results
3.1. Detection of atherosclerotic plaques in aorta and mesenteric arteries
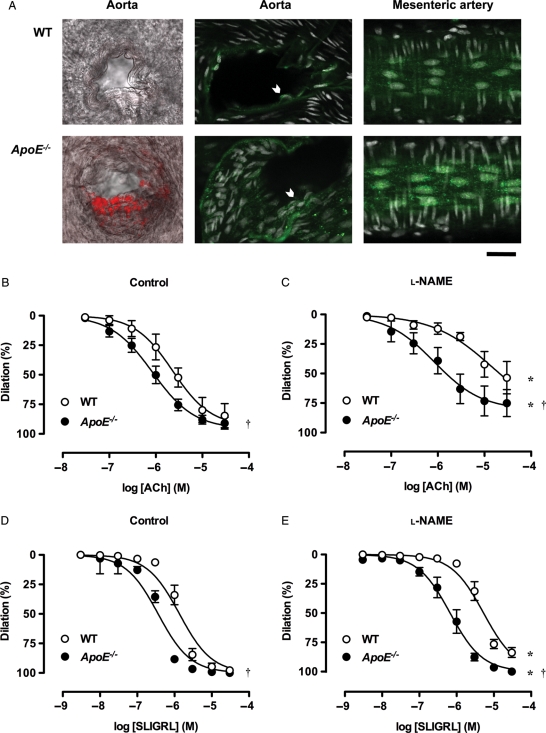
In aortae from old ApoE−/− mice, plaques were evident following staining with oil red O at the macro and micro observation level. Plaques were clearly visible at low magnification in the aortas from old ApoE−/− mice (∼25% of surface area, Supplementary material online, Figure S1). There was some evidence for plaques within branch-points from the aorta of young ApoE−/− mice when imaged using confocal microscopy (Figure 1A). As expected, there was no evidence for plaques in aortae from WT mice, nor the mesenteric bed from any mouse strain or age, including at bifurcation points of triple-cannulated mesenteric arteries.
Figure 1.
EDH-type dilation is augmented in mesenteric arteries from young ApoE−/− mice. (A) Visualization of atherosclerotic plaques (left and middle panels). Confocal micrographs of oil red O (red) and Mac-3 (green) staining in aortic branch points from WT and ApoE−/− mice. Plaque formation was only evident in the latter, although punctate intracellular staining for Mac-3 was evident in all endothelial cells (chevrons) (right panels). Images of endothelial cells in isolated, triple-cannulated and pressurized mesenteric arteries from WT and ApoE−/− mice. Mac-3 staining increased in the perinuclear region from ApoE−/− mice, but no plaques were observed using any method, either in the feed artery, or at the bifurcation. Nuclei were stained with propidium iodide (white). Bar = 30 µm. Images are representative of at least three arteries. (B–E) Dilation to endothelium-dependent agonists. Cumulative concentration response curves to ACh and SLIGRL under control conditions (B and D) and in the presence of 100 µM l-NAME (C and E) in mesenteric arteries from WT and ApoE−/− mice (n = 3–14). *P< 0.05 compared with the control response of the same strain and age; †P< 0.05 compared with WT.
3.2. Expression of Mac-3 in aortas and mesenteric arteries
The Mac-3 antibody for macrophages was used to determine macrophage infiltration into atherosclerotic plaques. Analysis of branch-points along the aorta showed some Mac-3 staining in the young ApoE−/− mice (Figure 1A), which was also dense in the aortae from older ApoE−/− mice (Supplementary material online, Figure S1). In all tissues, the endothelial cells clearly stained for Mac-3. The punctate, intracellular staining may reflect the ability of this antibody to bind lysosomal-associated membrane protein 2, thereby, indicating lysosomes (Figure 1A). For this reason, we did not quantify any variation in staining.
3.3. Effect of ApoE−/− on endothelium-dependent relaxation in aorta
As expected, in young animals the relaxation to ACh was unaffected by a deficiency in ApoE, but markedly reduced to a similar extent by the NO synthase inhibitor N(G)-nitro-l-arginine methyl ester (l-NAME) in aortas from both WT and ApoE−/− mice. Interestingly, we also observed full relaxation to the proteinase-activated receptor 2 (PAR-2) ligand SLIGRL, which was not fully sensitive to l-NAME, and was slightly, but significantly, more potent in the ApoE−/− mice (Supplementary material online, Figure S1).
3.4. Responses to bath application of agonists
Isolated, pressurized mesenteric arteries from young ApoE−/− mice were significantly more sensitive to ACh than WT controls. The pEC50 was 5.6 ± 0.2 (n = 5) in control arteries and 6.1 ± 0.1 (n = 8) in arteries from ApoE−/− mice with near maximal dilation in both cases (Figure 1B). The contribution of NO in dilation was significant in arteries from both young WT and ApoE−/− mice, but l-NAME clearly had a greater effect against ACh dilation in the arteries from WT animals (Figure 1C). Interestingly, the response to ACh in arteries from young WT animals was blocked with 45 mM KCl (10 µM: 2.8 ± 1.6% dilation, n = 4), and was fully blocked by damage to the endothelium (up to 10 µM, n = 3). Despite this apparent role for K+-channels in NO-mediated dilation, since the EDH-type (l-NAME insensitive) dilation to ACh was weak, the peptide ligand for PAR-2, SLIGRL was used to assess endothelium-dependent relaxations for the remainder of this study. SLIGRL has been shown to evoke reproducible and robust EDH-type relaxation in mouse mesenteric arteries.24,28
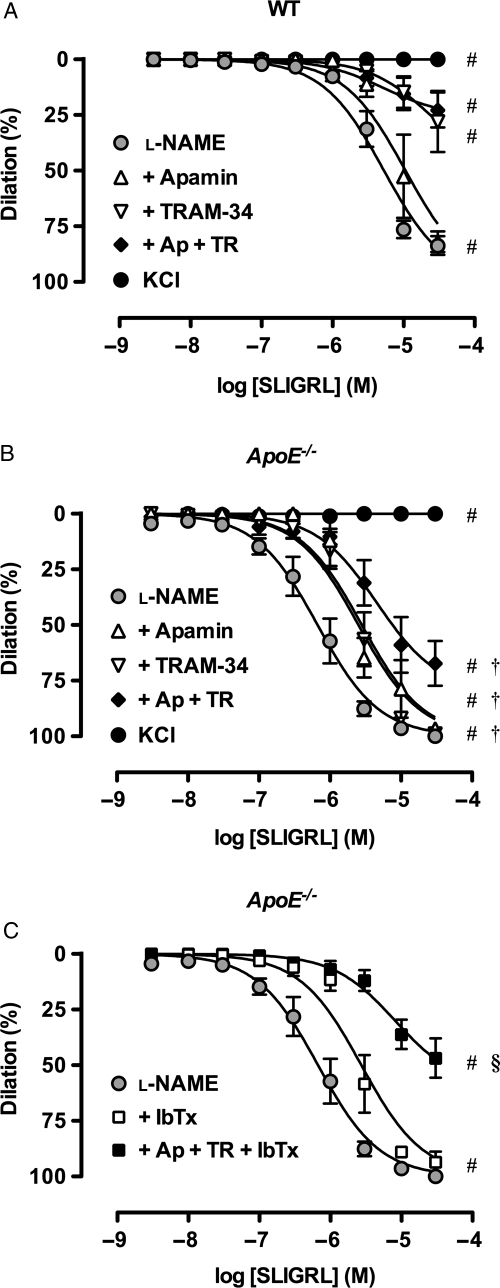
As with ACh, submaximal concentrations of SLIGRL also evoked significantly more sensitive dilation in arteries from young ApoE−/− mice (pEC50: WT 5.9 ± 0.1, n = 14; ApoE−/−: 6.4 ± 0.1, n = 13), with near maximal dilation in both cases (Figure 1D). In the presence of l-NAME, sensitivity to SLIGRL was significantly reduced in all groups as indicated by a decrease in pEC50 values (WT: 5.4 ± 0.1, n = 13; ApoE−/−: 6.2 ± 0.1, n = 12) without markedly affecting maximal dilation (Figure 1E). The additional presence of apamin (100 nM) or TRAM-34 (1 µM) significantly reduced sensitivity to SLIGRL. The most striking response was the enhanced ability of TRAM-34 to decrease SLIGRL responses in arteries from WT compared with ApoE−/− animals (Figure 2), suggesting an enhanced role for other K+ channels in the young ApoE−/− mice. The combination of apamin together with TRAM-34 reduced the dilation to SLIGRL in all groups. In the ApoE−/− mice, the large conductance (B)KCa-channel blocker iberiotoxin (IbTx, 100 nM) alone significantly right-shifted the EDH-type response to SLIGRL (pEC50: 5.6 ± 0.1, n = 3), and the addition of IbTx to apamin and TRAM-34 treated arteries further reduced the dilation to SLIGRL only in ApoE−/− animals (Figure 2C, WT data not shown). Following application of 45 mM KCl to prevent K+-channel effects, dilation to SLIGRL was almost completely inhibited in all groups (Figure 2), and was fully blocked by damage to the endothelium (up to 30 µM SLIGRL, n = 3).
Figure 2.
K+-channels activated during EDH-type dilation in mesenteric arteries. Cumulative concentration response curves to SLIGRL in mouse mesenteric arteries from young WT (A) and ApoE−/− (B and C) mice. l-NAME (100 µM) alone was compared with the additional presence of apamin (1 µM), TRAM-34 (1 µM) or IbTx (0.1 µM), and various combinations (n = 3–14). These responses were compared with arteries precontracted with 45 mM KCl (n = 3). #P< 0.05 compared with l-NAME; †P< 0.05 compared with WT; §P< 0.05 compared with l-N + Ap + TR in ApoE−/−.
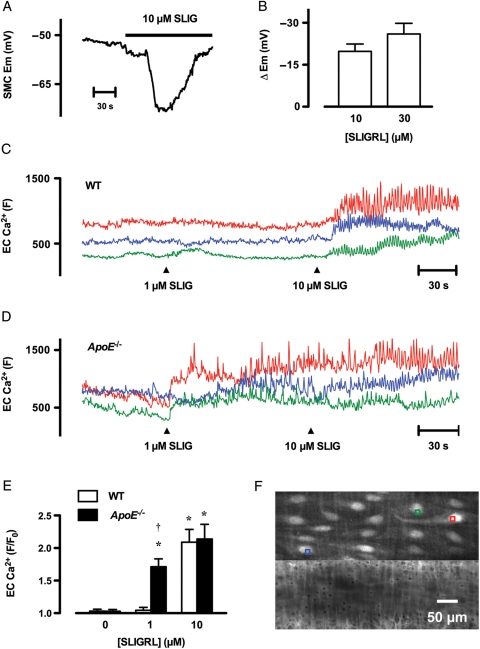
To confirm a role for K+-channels in the response to SLIGRL sharp microelectrode, measurements of membrane potential were performed. SLIGRL (10 µM and 30 µM) hyperpolarized smooth muscle cells by 19.8 ± 2.6 mV (n = 7) and 26.0 ± 3.8 mV (n = 3), respectively, from a resting membrane potential of −53.3 ± 1.8 mV (n = 10) in pressurized mesenteric arteries from young WT mice (Figures 3A and B).
Figure 3.
Changes in membrane potential and endothelial cell Ca2+ in response to SLIGRL in pressurized mesenteric arteries from young mice. (A) Time course of hyperpolarization in response to 10 µM SLIGRL in mesenteric arteries. SLIGRL was added to the superfusion solution reservoir, indicated by the bar. Note there was a short delay before SLIGRL reached the artery. (B) Summary of the peak increase in membrane potential evoked by 10 µM (n = 7) and 30 µM (n = 3) SLIGRL in arteries from WT mice. (C–D) Time course of endothelial cell Ca2+ in response to 1 and 10 µM SLIGRL in an artery from a WT (C) and ApoE−/− (D) mouse. Each colour represents the average fluorescence intensity (F) in endothelial cells. The responses were asynchronous between cells. (E) Summary of changes in endothelial cell [Ca2+]i following addition of 1 and 10 µM SLIGRL (F) relative to basal intensity (F0) in arteries from WT and ApoE−/− mice (n = 4–5). *P< 0.05 compared with the control response (0 µM SLIGRL) of the same strain; †P< 0.05 compared with WT. (F) Micrographs of the artery used to generate the data for D, showing the regions used for analysis (colours correspond to traces in D). A z-series was obtained from −10 µm (into smooth muscle cells) to +5 µm (into lumen) at 0.5 µm intervals from the plane of Ca2+-signal acquisition. The artery was loaded with Oregon Green® 488 BAPTA-1 (top panel) and exposed to 10 µM Alexa Fluor® 633 hydrazide (bottom panel) to show the internal elastic lamina (IEL) and other structures. The entire z-series was averaged to generate each image, and shows clear staining of endothelial cells and only very weak staining of smooth muscle cells. In general, the IEL could be used to monitor changes in focus during Ca2+-signal acquisition.
Next the augmented responses to SLIGRL in young ApoE−/− animals were investigated and found to be associated with altered endothelial cell Ca2+ handling. In both WT and ApoE−/− animals the increases in endothelial cell Ca2+ to a concentration of SLIGRL giving near maximum dilation (10 µM) were similar (Figure 3C–F). The responses were seen as repetitive waves across each cell, and were asynchronous between adjacent cells. In contrast, a concentration of SLIGRL that did not evoke robust dilation (1 µM) in WT animals similarly did not have a large effect on Ca2+, whereas in each ApoE−/− animal a significantly greater response was observed (Figure 3E), reflecting the augmented vasomotor responses.
Owing to the increased sensitivity to both endothelium-dependent agonists, responses were also obtained to the endothelium-independent agonist isoprenaline. These remained consistent between strains (pEC50 values 5.2 ± 0.4 and 5.2 ± 0.3 for young WT and ApoE−/− mice, respectively, n = 3) (Supplementary material online, Figure S2). Similarly, responses to the receptor-independent activator of adenylyl cyclase forskolin were unchanged (10 µM: 66.6 ± 10.2 and 66.6 ± 12.8% maximum dilation for young WT and ApoE−/− mice, respectively, n = 5). The response to forskolin was partially sensitive to 45 mM KCl (10 µM: 22.2 ± 11.2% dilation, n = 5). Dilation to either isoprenaline (-EC, 10 µM: 76.6 ± 12.4%, n = 3) or forskolin (-EC, 10 µM: 94.1 ± 5.9%, n = 3) was not reduced by damage to the endothelium.
3.5. Conducted hyperpolarization and dilation to SLIGRL
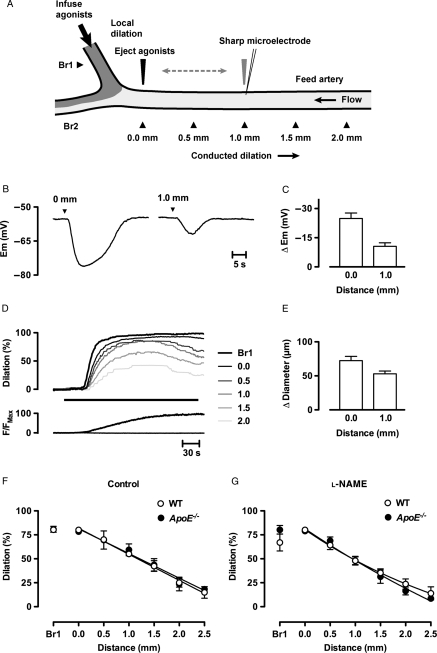
The two experimental setups for measuring conducted responses are depicted in Figure 4A. For electrophysiology in arteries from young WT mice without tone, SLIGRL was pressure-pulse ejected from a pipette moved to close proximity of the recording sharp microelectrode (shown at 1.0 mm), and stimulated smooth muscle cell hyperpolarization with an average peak increase in 24.9 ± 2.8 mV (n = 3). When the stimulating pipette was moved 1.0 mm downstream from the recording electrode (shown at 0.0 mm), a hyperpolarization of 10.6 ± 1.8 mV (n = 3) was still evident at the same impaled cell (conducted 1.0 mm upstream from ejection, Figure 4B and C). For conducted dilation responses, triple-cannulated arteries were used. The maximum outer diameter at 60 mmHg of bifurcating arteries used in triple-cannulation experiments ranged from ∼200 to 300 µm in the Feed artery and ∼120–200 µm in Branch 1. The level of phenylephrine tone was adjusted to induce 60–80% of maximal contraction. Infusion of vehicle (MOPS buffer with or without carboxyfluorescein) did not stimulation dilation in any experiment. Infusion of a high concentration of SLIGRL (30 µM) into Branch 1 evoked near maximal ‘local’ dilation in Branch 1 that spread against the direction of flow into the feed artery in all groups of animals. This conducted dilation decayed with distance, but conducted over 2.5 mm upstream from the bifurcation. The decay with distance was not significantly different between ApoE−/− and WT mice (Figure 4F). l-NAME (100 µM) had no effect against the local or conducted dilation responses to 30 µM SLIGRL (Figure 4G). These data suggested that once hyperpolarization was stimulated by SLIGRL, the ability for the signal to conduct and evoke dilation was not affected in these animals.
Figure 4.
SLIGRL evokes conducted hyperpolarization and dilation in pressurized mesenteric arteries from young mice. (A) Schematic diagram of artery for studying conducted dilation. For electrophysiology, a sharp microelectrode was used to impale a smooth muscle cell to record membrane potential, and the agonist delivered by pressure ejection either locally or 1.0 mm downstream from the recording electrode. For luminal perfusion of agonists to study conducted dilation, arteries were triple cannulated to allow a syringe pump to deliver SLIGRL with a fluorescent indicator (dark grey) through the lumen of Branch 1 (Br1). Continuous flow through the Feed artery prevented upstream diffusion of agonist, but did not in itself stimulate dilation. The entire field of view was visible to simultaneously measure diameter in Branch 1 (Br1) and the positions along the Feed artery (0.0–2.5 mm upstream). (B) Time course of local (0.0 mm) and conducted (1.0 mm) hyperpolarization to a 100 ms pulse of SLIGRL delivered to the outside of a pressurized artery with a micropipette. Summary data from mesenteric arteries cannulated from three young WT mice are shown in C. (D) Time course of local and conducted dilation to SLIGRL. SLIGRL (30 µM) together with carboxyfluorescein were infused into Branch 1 (Br1) for the period indicated by the bar. Dilation and changes in fluorescence in Br1 and at 0.5 mm intervals along the vessel (0.0–2.5 mm) were measured simultaneously. (E) Summary of the changes in diameter at the 0.0 and 1.0 mm sites under control conditions in WT animals. Maximum diameter was 253 ± 16 µm (n = 7). Summary of local (Br1) and conducted dilation (0.0–2.5 mm) in each strain of mouse under control conditions (F, n = 3–8), and in arteries incubated with 100 µM l-NAME (G, n = 3–8).
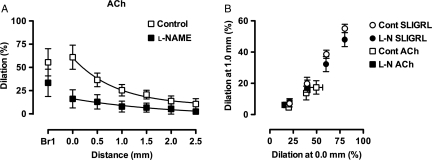
The remarkable similarity of conducted dilation to SLIGRL was investigated further by comparing responses to ACh. ACh was also able to evoke conducted dilation in mesenteric arteries from young WT mice, although less marked, at least partly due to decreased dilation at the 0.0 mm site (Figure 5). Although l-NAME had no apparent effect against responses to SLIGRL, responses to 10 µM ACh were markedly reduced (Figure 5A), confirming the greater reliance on NO release for dilation by ACh, but also suggesting that NO itself could evoke conducted dilation. However, once dilation was observed at the 0.0 mm site, the ability for signals to conduct upstream to evoke conducted dilation was very consistent between all vasodilators, both in the presence and absence of l-NAME (Figure 5B).
Figure 5.
Comparison of conducted dilation in pressurized mesenteric arteries from young mice. (A) Responses to ACh (10 µM, n = 4) in the absence (Control) and presence of 100 µM l-NAME. See Figure 4 for details. (B) The time courses of all conducted dilation experiments were analysed at points where the dilation at 0.0 mm was near 20, 40, 60, and 80% of maximum diameter, and the corresponding simultaneous dilation at the 1.0 site was plotted.
3.6. Effect of mouse age on dilation to SLIGRL
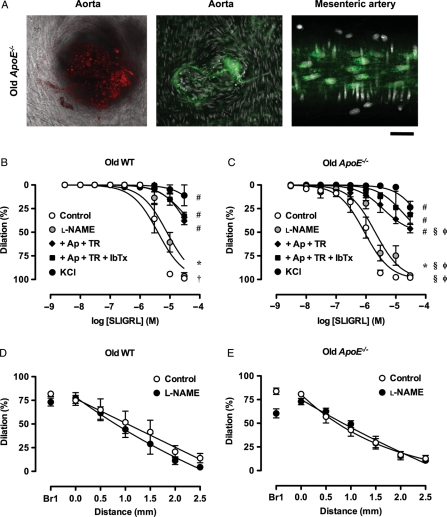
Increasing age increased the prevalence of oil red O and Mac-3 staining in the aortic branches from ApoE−/− mice, and in mesenteric arteries the density of perinuclear staining was also high in the endothelial cells from older ApoE−/− mice (Figure 6A). At the macro level, no increase in oil red O staining was observed in the old WT mouse aortae (Supplementary material online, Figure S1) and Mac-3 staining in aortic branch-points appeared similar to that in young WT mice (not shown). Increasing age significantly reduced the sensitivity to SLIGRL under control conditions in both WT and ApoE−/− mice (WT: 5.4 ± 0.1, n = 4, ApoE−/−: 6.0 ± 0.1, n = 6) but did not affect the maximum dilation to SLIGRL. In WT animals, the l-NAME insensitive EDH-type dilation was the same in young and old animals, and the various K+-channel blocker effects were the same (Figure 6). This suggests the younger WT animals had a greater contribution by NO to control dilations in response to SLIGRL. In contrast, in the ApoE−/− animals the EDH-type dilation remained significantly left-shifted in the younger animals, supporting an added contribution by BKCa-channels. Similarly, spreading dilation decay with distance was not significantly different between young and old mice or between strains (Figure 6D and E).
Figure 6.
Arterial structure and function in older mice. (A, left and middle panel). Confocal micrographs of oil red O (red) and Mac-3 (green) staining in aortic branch points from old ApoE−/− mice (right panel). Images of endothelial cells in a mesenteric artery from and old ApoE−/− mouse. Mac-3 staining was further increased in the perinuclear region compared with the young ApoE−/− mice. Bar = 30 µm. Images are representative of at least three arteries. (B–E) NO and EDH-type local and conducted dilation in mesenteric arteries from old mice. Responses to SLIGRL in mouse mesenteric arteries from old WT (A and D) and ApoE−/− (B and E) mice. (B and C) l-NAME (100 µM) alone was compared with control or the additional presence of combinations of apamin (1 µM), TRAM-34 (1 µM) and IbTx (0.1 µM) and various combinations (n = 3–7). These responses were compared with arteries precontracted with 45 mM KCl (n = 4–5). *P< 0.05 l-NAME compared with the control response of the same old strain; #P< 0.05 compared with l-NAME; †P< 0.05 compared with young WT; §P< 0.05 compared with young ApoE−/−; φP< 0.05 compared with old WT.
4. Discussion
ApoE−/− mice were used to establish the effects of hypercholesterolaemia20,29 on EDH-type dilation in mesenteric small arteries. This revealed for the first time that EDH-type dilation was paradoxically enhanced in arteries from ApoE−/− mice compared with age-matched controls. Furthermore, although the processes leading to conducted dilation were not affected, the fact that the sensitivity for initiating dilation per se was increased means that effectively the sensitivity for evoking conducted dilation is also raised and may have implications in the physiological control of blood flow.
It has previously been demonstrated that conducted (or spreading) dilation is not affected by a deficiency in ApoE,22 and here we confirm this observation. In addition, we demonstrate that the ability to stimulate dilation by endothelium-dependent agonists is improved in the young ApoE−/− mice. The augmented ability of the endothelium to produce dilation was not observed with agents acting directly on the smooth muscle cells either via receptors (isoprenaline at β-adrenoceptors) or independently of membrane receptors (adenylyl cyclase stimulated with forskolin). Thus, dyslipidaemia associated with the ApoE−/− animals appears selectively to improve the ability of endothelial cells to stimulate dilation.
Improved endothelial cell function in ApoE−/− mice has not been reported previously, but has been reported in the microcirculation of another model of dyslipidaemia.17 Studies using mesenteric arteries from ApoE−/− mice fed normal chow showed no difference in the ability of ACh to stimulate NO or EDH-type dilation.18,19 However using the same arteries from type 2 diabetic (db/db) animals with hyperlipidaemia and hypercholesterolaemia,30 the NO-mediated response was reduced, and the EDH-type relaxation maintained, albeit with a greater contribution by BKCa-channels.31 The EDH-type relaxation to ACh was fully sensitive to IbTx in the db/db mice, but not in the WT controls, and the EDH-type dilation to bradykinin in the db/db animals was insensitive to IbTx. This suggests that the expression of receptors and/or signalling pathways leading to EDH differ between these agonists. The ability of BKCa-channels to ‘rescue’ and indeed augment EDH responses was observed in the present study. We focused on responses to SLIGRL as the EDH-type dilation to ACh was poor. In both young and old ApoE−/− animals an IbTx-sensitive component was observed, which was not evident in the WT age-matched controls. This was not pursued in detail, but could be due to a cytochrome P450 metabolite, H2O2 or the actions of C-type natriuretic peptide, for example.31
An interesting aspect of the current work was the ability of raised extracellular KCl to block responses to both ACh and SLIGRL. Since both agonists had components sensitive to l-NAME, this suggests that NO relies in part on the opening K+-channels to cause dilation in this artery as has been observed to both endogenously released and exogenously applied forms of NO.32 Consistent with the actions to hyperpolarize smooth muscle cells, we observed conducted dilation to ACh in arteries from WT animals that was fully sensitive to l-NAME.
SLIGRL was able to stimulate l-NAME-insensitive ‘EDH-type’ dilation, which is associated with hyperpolarization in the pressurized arteries, as previously shown under isometric conditions.24 In WT animals, the dilation was predominantly due to activation of TRAM-34-sensitive IKCa-channels, again consistent with an earlier report.24 However this sensitivity to TRAM-34 was lost in the mesenteric arteries from ApoE−/− animals, suggesting compensation by another K+-channel, which appears to include both SKCa and BKCa-channels. Although the combination of apamin, TRAM-34 and IbTx should block all KCa-channels involved in EDH-responses, and they did markedly reduce the dilation in both WT and ApoE−/− mice, in all cases there was a residual component at the highest concentrations of SLIGRL that was sensitive to KCl. This does not appear due to KV, KATP-channels as neither 4-aminopyridine (150 µM) nor glibenclamide (5 µM) affected the response, and similarly tetraethylammonium and tetrabutylamonium (1 mM) had no effect (data not shown).
The 10-fold leftward shift in sensitivity to SLIGRL in the mesenteric arteries from ApoE−/− mice was reflected in the ability of this ligand to increase endothelial cell Ca2+. This is the first report of SLIGRL-mediated changes in endothelial cell Ca2+ in this artery, and showed that the response was not unlike that to ACh in this artery33 with global increases in Ca2+ that oscillated and were observed as waves across cells. However, block of both NOS and KCa-channels did not fully block the dilation to either 1 or 10 µM SLIGRL in the arteries from ApoE−/− mice. Thus if the residual dilation is secondary to the activation of a K+-channel activated by another Ca2+-dependent process in the endothelium, it has yet to be defined. The augmented endothelial cell Ca2+ responses to SLIGRL suggest increased expression of endothelial cell PAR-2 receptors and/or improved efficacy of signalling pathways linked to Ca2+ dependent and/or independent K+-channels.
This study is the first to assess conducted dilation responses in triple cannulated mouse mesenteric arteries and showed that SLIGRL can evoke reliable dilations capable of propagating along the vessel away from the site of application (through an arterial bifurcation). This approach is a more physiological alternative to studying the dilation responses to a bath-applied agonist and illustrates the situation in vivo whereby agonist delivery from nerves or circulating hormones spreads from the initiation site. In this respect, it is useful to know that conducted dilation along mesenteric arteries is not altered in ApoE−/−, consistent with results seen in the ApoE−/− mouse cremaster artery in vivo.22 Indeed when expressed as a function of the dilation at the 0 mm site, the conducted dilation to ACh and SLIGRL was relatively similar, either in the presence or absence of l-NAME. This work reinforces the observation that conducted dilation in mesenteric arteries is associated with hyperpolarization initiated in either the endothelium or smooth muscle8,34 and can occur separately to NO release.26 The striking relationship between the magnitude of dilation at the 0 mm site (close to but upstream from the local site) and a conducted response puts the 10-fold shift to increase sensitivity to SLIGRL into a physiological context. The augmented response to a given concentration of SLIGRL (or other PAR-2 ligand, e.g. trypsin28) would serve not only to dilate at the local site where the agonist/ligand acts but would also stimulate upstream and downstream dilation and thereby reduce vascular resistance to a greater extent than in WT animals.
Interestingly, the endothelial dysfunction reported in aortae from ApoE−/− mice is specific to the part of the aorta where a plaque has formed. Relaxation was only impaired in aortae from low-density lipoprotein receptor (LDL)/ApoE−/− double knockout mice in regions with significant lesions, and not in other regions or in aortae from the ApoE−/− single KO mice (where lesions were minimal).20 Similarly, in both ApoE−/− and diabetic ApoE−/− mice, endothelial dysfunction was only reported in plaque-prone regions of the aorta, while plaque-resistant segments maintained a normal ACh response.18 Here we show that the ACh responses are maintained in aortae from young ApoE−/− mice, and that responses to SLIGRL are actually augmented. Among other possible explanations, this may reflect an increased expression of PAR-2s in endothelial cells as has been reported in rat aortic endothelial cells under oxidative stress conditions,35 and mouse aortic smooth muscle cells in diabetic animals.36
In this study, significant lesions were only observed in the aortas from the older ApoE−/− mice, but compared with the age-matched controls, even in these older ApoE−/− mice the SLIGRL-mediated dilation of mesenteric arteries was consistently improved, at least until the full cocktail of KCa-channel blockers was present. Therefore the mechanism for the improved endothelium-dependent dilation at least partly linked to BKCa channels in the mesenteric arteries from ApoE−/− mice is still apparent in the older mice. Further, despite the reduced concentration-dependent control dilation to SLIGRL in the mesenteric arteries from WT and ApoE−/− mice, the ability to evoke conducted dilation was not affected, suggesting the function related to cell–cell coupling was not markedly reduced with age per se.
In conclusion, the current study demonstrates that hypercholesterolaemia is not detrimental to endothelium-dependent relaxations of the mesenteric arteries in the ApoE−/− mouse model of hypercholesterolaemia, despite advanced atherosclerotic plaque formation in the aorta of these mice. Surprisingly, the sensitivity for inducing this dilatation was enhanced. In addition, we demonstrate for the first time that conducted dilation in the ApoE−/− mouse mesenteric arteries is robust, qualitatively unaffected by l-NAME and similar in overall magnitude to arteries from WT controls.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by grants from the British Heart Foundation and a Programme Grant from the Wellcome Trust, UK. K.A.D. is a British Heart Foundation Senior Basic Science Research Fellow.
Supplementary Material
Acknowledgements
The authors wish to thank Professor Chris Garland for valuable discussions.
Conflict of interest: none declared.
References
- 1.Köhler R, Kaistha BP, Wulff H. Vascular KCa-channels as therapeutic targets in hypertension and restenosis disease. Expert Opin Ther Targets. 2010;14:143–155. doi: 10.1517/14728220903540257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Köhler R, Ruth P. Endothelial dysfunction and blood pressure alterations in K+-channel transgenic mice. Pflugers Arch. 2010;459:969–976. doi: 10.1007/s00424-010-0819-z. [DOI] [PubMed] [Google Scholar]
- 3.Garland CJ, Hiley CR, Dora KA. EDHF: spreading the influence of the endothelium. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2010.01148.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flammer AJ, Luscher TF. Human endothelial dysfunction: EDRFs. Pflugers Arch. 2010;459:1005–1013. doi: 10.1007/s00424-010-0822-4. [DOI] [PubMed] [Google Scholar]
- 5.Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 6.Dora KA. Coordination of vasomotor responses by the endothelium. Circ J. 2010;74:226–232. doi: 10.1253/circj.cj-09-0879. [DOI] [PubMed] [Google Scholar]
- 7.Segal SS. Communication among endothelial and smooth muscle cells coordinates blood flow control during exercise. News Physiol Sci. 1992;7:152–156. [Google Scholar]
- 8.Takano H, Dora KA, Spitaler MM, Garland CJ. Spreading dilatation in rat mesenteric arteries associated with calcium-independent endothelial cell hyperpolarization. J Physiol. 2004;556:887–903. doi: 10.1113/jphysiol.2003.060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res. 2000;86:94–100. doi: 10.1161/01.res.86.1.94. [DOI] [PubMed] [Google Scholar]
- 10.Haas TL, Duling BR. Morphology favors an endothelial cell pathway for longitudinal conduction within arterioles. Microvasc Res. 1997;53:113–120. doi: 10.1006/mvre.1996.1999. [DOI] [PubMed] [Google Scholar]
- 11.Cohen RA. The role of nitric oxide and other endothelium-derived vasoactive substances in vascular disease. Prog Cardiovasc Dis. 1995;38:105–128. doi: 10.1016/s0033-0620(05)80002-7. [DOI] [PubMed] [Google Scholar]
- 12.Casino PR, Kilcoyne CM, Quyyumi AA, Hoeg JM, Panza JA. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation. 1993;88:2541–2547. doi: 10.1161/01.cir.88.6.2541. [DOI] [PubMed] [Google Scholar]
- 13.Casino PR, Kilcoyne CM, Cannon RO, III, Quyyumi AA, Panza JA. Impaired endothelium-dependent vascular relaxation in patients with hypercholesterolemia extends beyond the muscarinic receptor. Am J Cardiol. 1995;75:40–44. doi: 10.1016/s0002-9149(99)80524-4. [DOI] [PubMed] [Google Scholar]
- 14.Egashira K, Inou T, Hirooka Y, Yamada A, Maruoka Y, Kai H, et al. Impaired coronary blood flow response to acetylcholine in patients with coronary risk factors and proximal atherosclerotic lesions. J Clin Invest. 1993;91:29–37. doi: 10.1172/JCI116183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato M, Shiode N, Teragawa H, Hirao H, Yamada T, Yamagata T, et al. The role of nitric oxide in bradykinin-induced dilation of coronary resistance vessels in patients with hypercholesterolemia. Intern Med. 1999;38:394–400. doi: 10.2169/internalmedicine.38.394. [DOI] [PubMed] [Google Scholar]
- 16.Kitayama J, Faraci FM, Lentz SR, Heistad DD. Cerebral vascular dysfunction during hypercholesterolemia. Stroke. 2007;38:2136–2141. doi: 10.1161/STROKEAHA.107.481879. [DOI] [PubMed] [Google Scholar]
- 17.Krummen S, Falck JR, Thorin E. Two distinct pathways account for EDHF-dependent dilatation in the gracilis artery of dyslipidaemic hApoB+/+ mice. Br J Pharmacol. 2005;145:264–270. doi: 10.1038/sj.bjp.0706194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding H, Hashem M, Wiehler WB, Lau W, Martin J, Reid J, et al. Endothelial dysfunction in the streptozotocin-induced diabetic apoE-deficient mouse. Br J Pharmacol. 2005;146:1110–1118. doi: 10.1038/sj.bjp.0706417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morikawa K, Matoba T, Kubota H, Hatanaka M, Fujiki T, Takahashi S, et al. Influence of diabetes mellitus, hypercholesterolemia, and their combination on EDHF-mediated responses in mice. J Cardiovasc Pharmacol. 2005;45:485–490. doi: 10.1097/01.fjc.0000159657.93922.cb. [DOI] [PubMed] [Google Scholar]
- 20.Bonthu S, Heistad DD, Chappell DA, Lamping KG, Faraci FM. Atherosclerosis, vascular remodeling, and impairment of endothelium-dependent relaxation in genetically altered hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 1997;17:2333–2340. doi: 10.1161/01.atv.17.11.2333. [DOI] [PubMed] [Google Scholar]
- 21.Urakami-Harasawa L, Shimokawa H, Nakashima M, Egashira K, Takeshita A. Importance of endothelium-derived hyperpolarizing factor in human arteries. J Clin Invest. 1997;100:2793–2799. doi: 10.1172/JCI119826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolfle SE, de Wit C. Intact endothelium-dependent dilation and conducted responses in resistance vessels of hypercholesterolemic mice in vivo. J Vasc Res. 2005;42:475–482. doi: 10.1159/000088101. [DOI] [PubMed] [Google Scholar]
- 23.Dora KA, Sandow SL, Gallagher NT, Takano H, Rummery NM, Hill CE, et al. Myoendothelial gap junctions may provide the pathway for EDHF in mouse mesenteric artery. J Vasc Res. 2003;40:480–490. doi: 10.1159/000074549. [DOI] [PubMed] [Google Scholar]
- 24.McGuire JJ, Hollenberg MD, Bennett BM, Triggle CR. Hyperpolarization of murine small caliber mesenteric arteries by activation of endothelial proteinase-activated receptor 2. Can J Physiol Pharmacol. 2004;82:1103–1112. doi: 10.1139/y04-121. [DOI] [PubMed] [Google Scholar]
- 25.Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- 26.Winter P, Dora KA. Spreading dilatation to luminal perfusion of ATP and UTP in rat isolated small mesenteric arteries. J Physiol. 2007;582:335–347. doi: 10.1113/jphysiol.2007.135202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beleznai TZ, Yarova P, Yuill KH, Dora KA. Smooth muscle Ca2+-activated and voltage-gated K+ channels modulate conducted dilation in rat isolated small mesenteric arteries. Microcirculation. 2011 doi: 10.1111/j.1549-8719.2011.00109.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGuire JJ, Hollenberg MD, Andrade-Gordon P, Triggle CR. Multiple mechanisms of vascular smooth muscle relaxation by the activation of proteinase-activated receptor 2 in mouse mesenteric arterioles. Br J Pharmacol. 2002;135:155–169. doi: 10.1038/sj.bjp.0704469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt TS, McNeill E, Douglas G, Crabtree MJ, Hale AB, Khoo J, et al. Tetrahydrobiopterin supplementation reduces atherosclerosis and vascular inflammation in apolipoprotein E-knockout mice. Clin Sci. 2010;119:131–142. doi: 10.1042/CS20090559. [DOI] [PubMed] [Google Scholar]
- 30.Tuman RW, Doisy RJ. The influence of age on the development of hypertriglyceridaemia and hypercholesterolaemia in genetically diabetic mice. Diabetologia. 1977;13:7–11. doi: 10.1007/BF00996320. [DOI] [PubMed] [Google Scholar]
- 31.Pannirselvam M, Ding H, Anderson TJ, Triggle CR. Pharmacological characteristics of endothelium-derived hyperpolarizing factor-mediated relaxation of small mesenteric arteries from db/db mice. Eur J Pharmacol. 2006;551:98–107. doi: 10.1016/j.ejphar.2006.08.086. [DOI] [PubMed] [Google Scholar]
- 32.Andrews KL, Irvine JC, Tare M, Apostolopoulos J, Favaloro JL, Triggle CR, et al. A role for nitroxyl (HNO) as an endothelium-derived relaxing and hyperpolarizing factor in resistance arteries. Br J Pharmacol. 2009;157:540–550. doi: 10.1111/j.1476-5381.2009.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, et al. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garland CJ, Yarova P, Jimenez-Altayo F, Dora KA. Vascular hyperpolarization to β-adrenoceptor agonists evokes spreading dilatation in rat isolated mesenteric arteries. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01224.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aman M, Hirano M, Kanaide H, Hirano K. Upregulation of proteinase-activated receptor-2 and increased response to trypsin in endothelial cells after exposure to oxidative stress in rat aortas. J Vasc Res. 2010;47:494–506. doi: 10.1159/000313877. [DOI] [PubMed] [Google Scholar]
- 36.Roviezzo F, Bucci M, Brancaleone V, Di Lorenzo A, Geppetti P, Farneti S, et al. Proteinase-activated receptor-2 mediates arterial vasodilation in diabetes. Arterioscler Thromb Vasc Biol. 2005;25:2349–2354. doi: 10.1161/01.ATV.0000184770.01494.2e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.