Abstract
Despite the key role of γ-aminobutyric acid (GABA) neurons in the modulation of cerebral cortical output, little is known about their development in the human cortex. We analyzed several GABAergic parameters in standardized regions of the cerebral cortex and white matter in a total of 38 human fetuses and infants from 19 gestational weeks to 2.7 postnatal years utilizing immunocytochemistry, Western blotting, tissue autoradiography and computer-based cellular quantitation. At least 20% of GABAergic neurons in the white matter migrated toward the cortex over late gestation. After term, migration declined and ended within 6 postnatal months. In parallel, the GABAergic neuronal density increased in the cortex over late gestation, also with a peak at term. From midgestation to infancy, the pattern of GABAA receptor binding changed from uniformly low across all cortical layers to high levels concentrated in the middle laminae; glutamic acid decarboxylase (GAD65 and GAD67) levels differentially increased. Thus, the second half of gestation is a period of rapid development of the cortical GABAergic system that continues into early infancy. This time period corresponds to the peak window of vulnerability to perinatal hypoxia-ischemia in which GABAergic neurons are potentially developmentally susceptible, including in the preterm infant.
Keywords: Autoradiography, Doublecortin, GAD65/67, Neuronal migration, Periventricular leukomalacia
INTRODUCTION
The microcircuitry of the cerebral cortex depends upon precise interrelationships between inhibitory γ-aminobutyric acid (GABAergic) granular neurons and excitatory (glutamatergic) pyramidal neurons in columnar modules with region-specific connections (1). Cognitive processing in turn involves modulation of excitatory events by inhibitory neurons, as well as a coordinated balance between excitation and inhibition maintained over a large range for many stimuli (1–4). The axons of the GABAergic inhibitory neurons arborize within and across cortical columns; unlike pyramidal neurons, they do not typically project to distant subcortical sites. Yet, recent evidence suggests that a GABAergic subset in the cortex and white matter have projections that extend over long distances in adult rats and primates (5). While excitatory pyramidal neurons account for 70–85% of all cortical neurons and are relatively homogeneous, the small numbers of inhibitory neurons (16–30% of cortical neurons) display far more diverse morphological, physiological, molecular, and synaptic characteristics (1, 6–14). Interneurons are mainly GABAergic (7, 10, 11, 14). Interneuron diversity is postulated to provide sufficient sensitivity, complexity, and dynamic range for the inhibitory system to match excitation regardless of the intensity and complexity of the excitatory stimulus (1, 15). During mammalian, and particularly primate evolution, the number and complexity of GABAergic interneurons greatly increased relative to projection (glutamatergic) neurons (16). These increases in the upper cortical layers (the hallmark of the human neocortex) suggest that GABAergic neurons are involved in sophisticated cognitive processing in humans. Indeed, Cajal (as cited by Hill and Walsh), proposed that “the functional superiority of the human brain is intimately bound up with the prodigious abundance and unusual wealth of forms of the so-called neurons with short axons”, i.e. interneurons (16). During development, GABAergic interneurons play a role in the regulation of neuronal proliferation and migration during corticogenesis, as well as in the development of the cortical microcircuitry (17–19) and the determination of critical periods (20).
Limited human data suggest that GABAergic neurons migrate outward from the pallium of the dorsal telencephalon and ganglionic eminence across the intermediate zone of the white matter to reach their final addresses in the cerebral cortex (21, 22). Additionally, many middle- and late-born GABAergic neurons migrate radially inward from the marginal zone to the cortical plate (23, 24). GABAergic neurons have been reported in the human cerebral white matter, (presumably via outward migration) as late as 31 gestational weeks (25), but the precise timetable of the GABAergic migration to the cortex in human gestation and the developmental profile of the organization of GABAergic neurons within the overlying cortex are largely unknown.
In this study, we examined selected parameters of GABAergic development in the human cerebral cortex and underlying white matter in the second half of gestation. We focused on this time frame because it is the peak window of vulnerability of the premature infant to cerebral white matter damage, i.e. periventricular leukomalacia (PVL). This lesion could potentially destroy late migrating GABAergic neurons in the deep white matter and adversely affect the GABAergic development of the overlying cortex, thereby impairing subsequent cognitive development in preterm survivors. Indeed, a decrease in the density of GABAergic neurons has been reported in the cerebral white matter in 10 preterm infants with white matter damage compared to 5 controls (25).
We tested the overall hypothesis that the GABAergic system in the human cerebral cortex is structurally and neurochemically immature at midgestation, and that it develops relatively late, over its second half of gestation. We determined the presence and analyzed the morphology of GABAergic neurons in fetal white matter using the immunomarker for glutamic acid decarboxylase (GAD), the key biosynthetic enzyme for GABA; the percent of white matter GABAergic neurons expressing doublecortin (DCX), a marker of postmitotic migrating neurons (26–28); the density of GABAergic neurons in the overlying cortex; the cortical levels of the 2 isoforms of GAD (GAD65 and GAD67) by Western blotting; and the cortical binding patterns of the GABAA receptor by tissue receptor autoradiography.
MATERIALS AND METHODS
Clinical Database
Tissue samples from a total of 38 fetal and infant brains were obtained from the Departments of Pathology, Children’s Hospital Boston, MA, and Brigham and Women’s Hospital, Boston, MA, and the Maryland Developmental Brain and Tissue Bank. The cases had no clinical history of neurological disease and no or minimal changes in the brain upon standard neuropathologic examination. The tissues were variously processed depending upon the procedure under analysis (Table 1). Different sample sizes and tissue methods for different GABAergic parameters were used because not all procedures were possible in all cases due to lack of tissue availability.
Table 1.
Summary of Sample Sizes and Methods for GABA-related Analyses
| Cases (n) | Method | Location | Age Range | |
|---|---|---|---|---|
| GABAergic Neurons in Cortex | 16 | Immunocytochemistry and Cell Counting | Frontal Association Cortex | 27 GA weeks to 2 PN years |
| GABAergic Neurons in White Matter | 18 | GAD65/67 Immunocytochemistry | Combined Frontal, Parietal, Occipital and Temporal White Matter | 26 GA weeks to 2 PN years |
| 12 | DCX and GAD65/67 Immunocytochemistry | 24 GA weeks to 2 PN years | ||
| GAD67/65 Levels | 8 | Western Blotting | Parietal Association Cortex | 21 GA weeks to 2.7 PN years |
| GABA Receptors | 11 | Autoradiography and Immunocytochemistry | Parietal Association Cortex | 19 GA weeks to 2.7 PN years |
BA, Brodmann area; GA, gestational age; GABA, γ-aminobutyric acid; PN, postnatal.
Cortical samples from the frontal association cortex, (inclusive of Brodmann Areas 6, 8, 9, 10, 23, and 24), were fixed in 4% paraformaldehyde and processed for cell counting and immunocytochemical analyses (Table 1). Cortical samples from the parietal association cortex at the level of the atrium of the lateral ventricle (inclusive of Brodmann Areas 22 and 40) were frozen immediately at autopsy at −80°C for GABAA receptor autoradiography and Western blot analysis (Table 1). Autopsy reports were reviewed for major clinical findings and systemic diagnoses. Cases with known disorders of cortical maldevelopment and/or neuronal migration disturbances were excluded. Age was expressed as post-conceptional age (gestational age + postnatal age). Parental consent for research analysis was obtained in all cases and the study was approved by the relevant Institutional Review Boards.
Immunocytochemistry
Single-label Immunocytochemistry
The paraffin-embedded cortical tissue samples were cut on a conventional microtome at 5 μm. Serial sections were deparaffinized and rehydrated with xylene and ethanol gradient. We identified GABAergic interneurons in tissue sections with antibodies to GAD65/67 and the GABAA receptor subunits GABAAα1 and GABAAα3 (29–34) (Table 2). Cells undergoing programmed death were identified with an antibody to active caspase-3 (Table 2). Antigen retrieval was performed in sodium citrate buffer (pH 6.0) for 20 minutes in 199°F in a microwave. The sections were incubated in peroxidase blocking reagent (S2-1, DAKO, Carpinteria, CA) for 10 minutes at room temperature (RT) to reduce the endogenous peroxidase activity. Nonspecific staining was blocked by incubating sections in Tris-buffered saline (TBS) with 0.1% Tween-20 (Sigma-Aldrich, St. Louis, MO) (TBST, pH7.4) containing 10% normal goat serum for 2 hours at RT. Primary antibody incubations were performed overnight at 4°C. After 2 washes (5 minutes each) in TBST, the sections were incubated in biotinylated goat anti-rabbit IgG secondary antibody (1:100 in TBS, Vectastain ABC kit, Vector Laboratories, Burlingame, CA) for 1 hour at RT. After 2 washes in TBST, the sections were incubated for 1 hour at RT in avidin-biotin solution (Vector Laboratories) (1:100 in TBS) followed by diaminobenzidine detection. A control for GAD65/67 antibody was performed; tissue sections were incubated with primary antibody pre-absorbed with a saturating concentration of the target peptide from which the antibody was produced (Invitrogen, Carlsbad, CA). No immunostaining was observed following pre-absorption (35). Analysis of immunostaining was with a standard light microscope.
Table 2.
Antibodies
| Antibody | Host | Catalog No./Source | Dilution | Labels | References |
|---|---|---|---|---|---|
| GAD67/65 | Rabbit Polyclonal IgG | ab49832/Abcam, Cambridge, MA | 1:1000 and 1:10,000 | Brain GAD 65 and GAD 67 isoforms | (30) |
| MAP2 | Mouse Monoclonal IgG | SMI-52R/Covance, Dedham, MA | 1:800 | MAP2a and b and a doublet at 68 kDa that may represent MAP2c | (31) |
| GFAP | Mouse Monoclonal IgG | SMI-22/Covance | 1:500 | Astrocytes and Bergmann glia | (32) |
| Double-cortin (C18) | Goat polyclonal IgG | SC8066/Santa Cruz Biotechnology, Santa Cruz, CA | 1:100 | Doublecortin | (33) |
| Active caspase-3 | Rabbit Monoclonal IgG | 559565/BD Biosciences, Franklin Lakes, NJ | 1:20,000 | Active form of Caspase-3 | (34) |
| GABAA (α1) | Rabbit Polyclonal IgG | AGA001/Alomone, Jerusalem, Israel | 1:200 | GABA(A) receptor α1 | (29) |
| GABAA (α3) | Rabbit Polyclonal IgG | AGA003/Alomone | 1:200 | GABA(A) receptor α3 | (29) |
GABA, γ-aminobutyric acid; GAD, glutamic acid decarboyxlase; GFAP, glial fibrillary acidic protein; MAP2, microtubule-associated protein-2
Double-label immunocytochemistry
Tissue was processed as above and sections were incubated with antibody to GAD65/67, glial fibrillary acidic protein (GFAP), microtubule-associated protein 2 (MAP2), or DCX. Double labeling was performed with GFAP to exclude the possibility of the expression of GAD65/67 by astrocytes; the neuronal marker MAP2 was used to confirm the neuronal phenotype of the GAD65/67-immunopositive cells (31). Double-label immunocytochemistry was performed with DCX to determine if the GAD65/67 cells were in the process of migration as DCX is an immunomarker of postmitotic migrating neurons (26–28). Fluorescence was detected with Alexa Fluor donkey anti-rabbit 594 and Alexa Fluor donkey anti-goat or donkey anti-mouse 488 (1:1000; Molecular Probes, Eugene, OR). Negative controls omitted the primary antibodies. Immunofluorescence was visualized with an Olympus BX51 microscope (Olympus America Inc., Melville, NY) using FITC and TRITC filters, images were captured by a Coolsnap fx camera (Photometrics, Tucson, AZ) and then exported to computer software (MCID Elite 6.0, Imaging Research, Inc., Mississauga, ONT, Canada).
Computer-based GABAergic Cell Quantitation
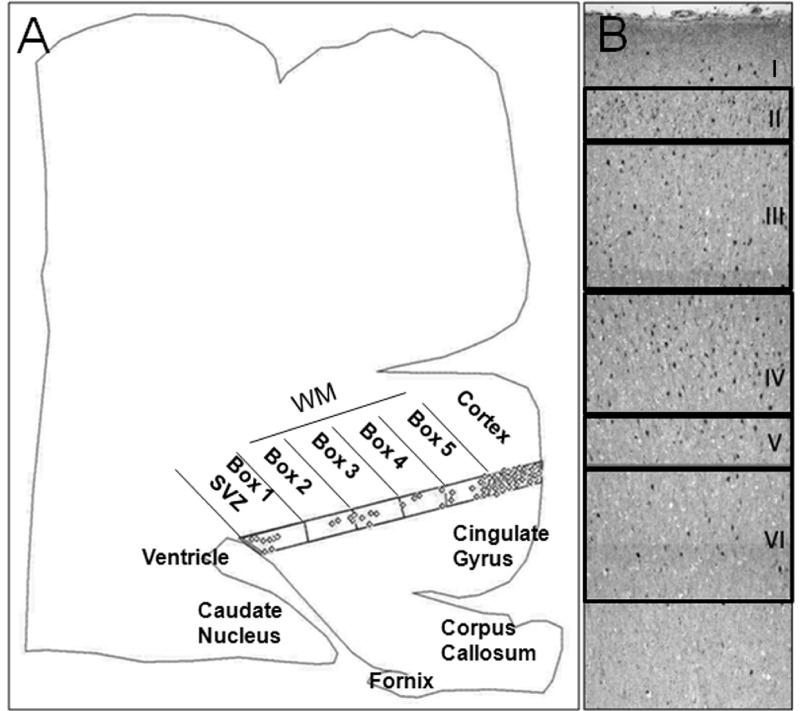
The densities of GAD65/67 immunostained cells were determined in the frontal association cortex and underlying white matter in 1) ventricular/subventricular zone, 2) white matter from the subventricular region to the subplate region, 3) the subplate proper directly underneath the cerebral cortex, and 4) Layers I–VI (Fig. 1). For all regions, computer-based methods were used with Neurolucida version 6.02.2 (Microbrightfield, Inc., Williston, VT). For white matter GABAergic analysis, only tissue sections that spanned the entire distance from the ventricular/subventricular zone to the border of the cerebral cortex were used. The boundary of the entire section and the counting area of the white matter were traced at low magnification (x4) with Neurolucida. The white matter was divided into 5 grid boxes with a width of 600 to 700 μm, and labeled progressively from 1 to 5. The 5 regions analyzed beneath the cerebral cortex were subventricular/ventricular region (Box 1), periventricular white matter (Box 2), deep white matter (Box 3), central white matter (Box 4), and subplate (subcortical) region (Box 5) (Fig. 1). We performed cell counts in 1 section/case for boxes 1 to 5 because in a pilot study cell counts in each of 4 adjacent sections showed a high correlation (correlation coefficient 0.82), with no significant differences in cell density (p = 0.30) between sections utilizing analysis of covariance (ANOVA) to test for differences between sections (data not shown). For the cerebral cortex a grid counting procedure was used based upon modifications from Benes et al (36, 37) in the human adult cortex and in the developing human cortex (31). The counting grids were placed over the same region in each of 3 serially sections sampled that were separated by 10 μm. Of note, pilot analysis of cortical cell counts in the frontal association cortex demonstrated significant differences in GABAergic neuronal density in each lamina between sections (ANOVA, p > 0.05), thereby underscoring the need for determining the mean from more than 1 section/case (data not shown). The cerebral cortex in the frontal association cortex was divided into 6 laminae based upon cytoarchitectonic features, with a rectangular box encompassing each lamina (Fig. 1) (31, 36, 37). GABAergic neurons were counted in each of the 5 white matter grid boxes and/or 6 laminae at high magnification (x20). The GABAergic neuronal densities in white matter and cortex were calculated by dividing the number of cells by the box area, (mm2) and expressed as neurons/mm2. The mean GABAergic neuronal density and standard error in each cortical laminae in the 3 sections sampled was calculated. As an additional assessment of the potential effects of rapid growth of the cerebral cortex on putatively changing GABAergic neuronal populations with development, we used the thickness of the cortex at the site of the GABAergic cell density measurement as a “correction factor” for cortical growth. We measured the section thickness in millimeters with the Neurolucida tool, “quick measure”, and divided the mean cortical GABAergic cell density by the section width in μm at each post-conceptional age analyzed. Statistical tests in all analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC) with p < 0.05 considered significant. To model simultaneously the age effect and spatial distribution (over boxes) of GABAergic neuronal density in the white matter, a repeated measures regression model was used, with multiple boxes per case. The best fit was a cubic effect of age and a quadratic effect of spatial distribution (boxes). Loess curves (38) with 95% confidence intervals were fit to model changes in GABAergic neuronal density with age in the cortex and white matter. Loess curves are non-parametric smoothed curves, for which confidence intervals are plotted to determine the variability surrounding the shape of the curve (38). They are often used when data cannot be fit by a parametric model such as linear or quadratic. The confidence interval around the curve gives the measure of variability (38). A “flat” confidence interval indicates that the variability in the data is high such that a “shape” to it is not clearly seen; when the confidence interval follows the trend of the curve there is reasonable confidence as to the shape of the curve.
Figure 1.
γ-Aminobutyric acid (GABA)ergic) cell counting methods. (A) A computer-generated plot from a tracing of a tissue section of cerebral cortex in a term infant shows the distribution of GABAergic neurons in the ventricular/subventricular zone (SVZ), periventricular, deep, and central white matter (WM) and overlying cerebral cortex. The SVZ, WM and subplate region is equally divided into 5 boxes by an overlying grid: Box 1 is located at the SVZ; Box 5 is located directly underneath Layer VI (subplate proper), with the periventricular, deep, and central WM in the intervening boxes. GABAergic neuron density in each box is obtained by dividing neuronal number by box area. (B) A low-power view demonstrates the subdivisions of the cerebral cortex for counting in a tissue section immunostained for glutamic acid decarboxylase (GAD65/67). The cortex is divided into 6 boxes according to the individual cytoarchitectonic layers.
Migrating GABAergic Neurons in the Developing White Matter
Because double-labeling observations necessitate the use of the florescent microscope in our laboratory, we were unable to apply Neurolucida (dependent upon non-florescent microscopy) for cell counting of GABAergic neurons co-expressing DCX with the grid system. Thus, a different counting approach was utilized in which the GAD65/67-positive cells with or without DCX co-expression in the periventricular and central white matter (the same areas sampled by the grid approach) were counted in 5 to 10 high-power fields (x40). Because GABAergic neurons are rare in the white matter at mid-gestation and postnatally in fields analyzed at x40 magnification, the particular field was selected by their visual presence and not randomly; however, no field was selected based upon the presence of DCX expression. Of note, the areas counted were smaller than those counted with the grid system to determine the overall density of GABAergic neurons in the white matter. The percent of migrating GABAergic neurons of the total GABAergic neurons in the 5 to 10 high-power fields was calculated by dividing the DCX and GAD65/67 double-labeled cells by the total GAD65/67-positive cells (with and without DCX expression) in each field. The mean percent of the values from 5 to 10 high-power fields was calculated for each case. The cases (n = 12) were divided into 3 age groups: fetal (<37 post-conceptional weeks) (n = 4); term (37–42 post-conceptional weeks) (n = 4); and infant (>42 post-conceptional weeks) (n = 4). Statistical analysis involved an ANOVA model by age group.
GABAergic Neuronal Size in the Developing Cerebral Cortex
To determine the size of the GABAergic neurons in the cerebral cortex, GAD65/67-immunopositive neurons with distinct nuclei were randomly selected from Layer IV in the frontal associative cortex, and the longest cytoplasmic diameter of 10 GAD65/67 immunopositive neurons was measured with the ‘Quick measure line’ tool (Neurolucida software version 6.02.2). The size of the GABAergic neurons in each age- group reflected the mean size of the individual cases with the standard deviation provided. The statistical analysis treated age as a continuous variable; thus, the model produced a single p value without comparisons between age groups, although the data are presented as bar groups binned by age.
GAD65 and GAD67 Levels in the Developing Cerebral Cortex
Western blot analysis was used to measure levels of GAD65/67 on frozen tissue samples from the parietal association cortex. The samples were homogenized to a final concentration of 10% weight per volume and a modified Lowry method was used for protein quantification (39). After separation with SDS-PAGE, proteins were transferred electrophoretically to an Immunology-P membrane (Millipore, Bedford, MA), overnight and incubated with GAD65/67 antibody (Table 2) at a concentration of 1:10,000. The 65-kDa and 67-kDa bands were detected using a goat anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody (1:10,000; Bio-RAD, Hercules, CA), followed by Chemiluminescence ECL (Perkin Elmer, Waltham, MA), and quantified from densitometry bands (MCID Elite 6; Imaging Research Inc, Ontario, CA), standardized to adult cortex tissue run on the same gel. Values were expressed as a percentage to the adult standard. Loess curves (38) were fit to model changes in GAD65 and GAD67 expression levels in the cerebral cortex with age.
GABAA Binding in the Developing Cerebral Cortex
Tissue sections at 20 μm sectioned on a Leica motorized cryostat were prepared from frozen blocks of the parietal association area. Total GABAA receptor binding was determined by incubation of 2 unfixed slide-mounted tissue sections in 50 nM 3H-GABA (Perkin Elmer) for 20 minutes at RT (35). The tissue sections were pre-incubated in 50 mM Tris-HCL (pH 7.4) containing 100 μm Baclofen (GABAB agonist) for 45 to 60 minutes at RT. Nonspecific binding in 1 tissue section was determined by addition of 100 μm isoguvacine to the solution. Following the incubation, the sections were washed in 50 mM Tris-HCL (pH 7.4) for 10 seconds. The sections were then rinsed in ice-cold water for 10 seconds before drying in a warm stream of air. Tissue sections were placed in cassettes and exposed to 3H-Hyperfilm (Amersham, Buckinghamshire, UK) for 8 weeks at 4°C with a set of tissue equivalent (μCi/g) 3H standards (Amersham) for conversion of optical density of silver grains to fmol/mg of tissue to determine GABAA binding. Quantitative densitometry of autoradiograms was performed using a MCID 5+ imaging system (Imaging Research).
Receptor binding was measured in 7 defined rectangle boxes (taken from 2 specific binding sections) laid over the entire thickness of the cortex from the pial surface to the gray-white matter junction. The binding was expressed as the mean of the 7 measurements at each percent of the distance from the pial surface to the gray-white matter junction, thereby providing the spatial distribution of binding levels across the layers of the cerebral cortex from superficial to deep, as well as absolute binding levels. Thus, 0% from the pial surface was the superficial border of Layer I; 100% was Layer VI at the junction with the underlying subplate region; and the middle Layers III and IV are around 50% from the pial surface. To model simultaneously the effect of age upon the levels and the spatial distribution of GABAA receptor binding we used a multi-stage process. First, models were fit to each individual case to determine the shape of the spatial distribution; a cubic model was generally a good fit and was therefore used in the final model. Next, the effect of age was modeled at 3 different spatial locations (i.e. at both ends and in the middle of the cortex); ultimately, a log transformation of age was found to produce a linear fit. The final evaluation involved a repeated measures regression model with an interaction term to test for differences in the spatial distribution over time (age). Postmortem interval was not included in the analysis because we found that it had no significant effect upon binding levels (data not shown)
RESULTS
GABAergic Neurons in the Developing White Matter
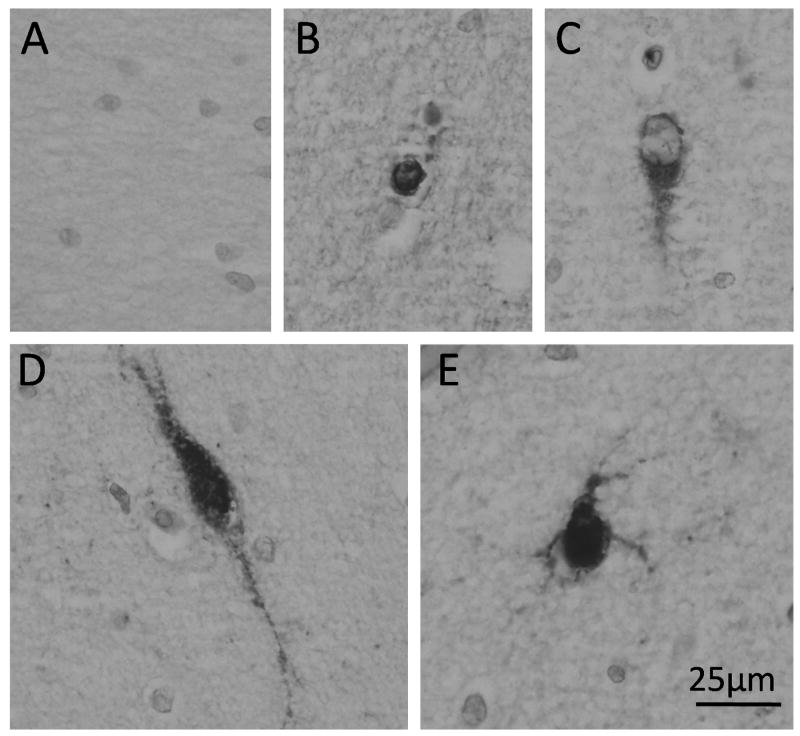
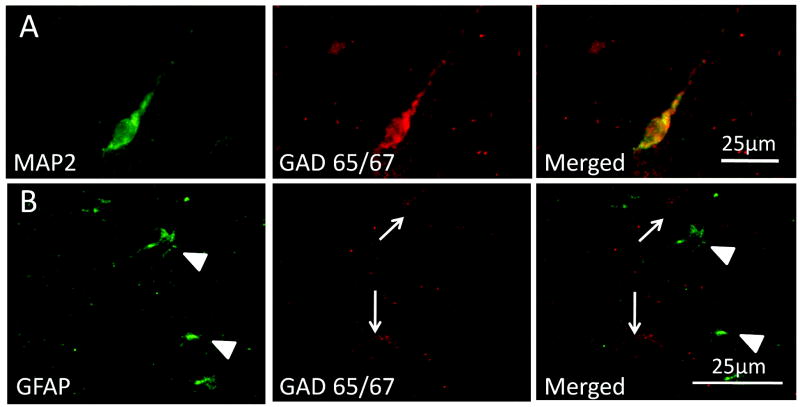
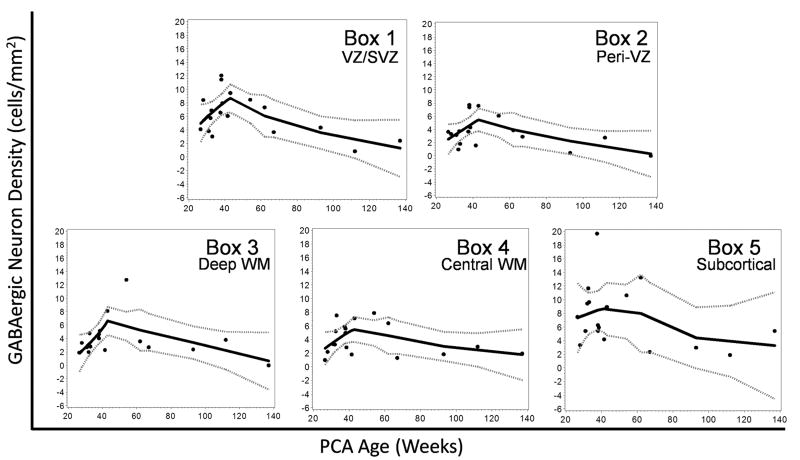
We analyzed the density and distribution of GABAergic neurons in the frontal lobe white matter in 18 cases ranging in age from 26.5 gestational weeks to 2 postnatal years. There were 4 morphological subtypes of GAD65/67-immunopositive neurons in the white matter: 1) small round granular neurons with negligible cytoplasm and no dendritic processes; 2) oval unipolar neurons with a single process; 3) oval bipolar neurons 2 oppositely aligned processes; and 4) relatively large multipolar neurons with round abundant cytoplasm and more than 3 dendritic processes (Fig. 2). The neuronal phenotype of these GABAergic cells was confirmed by their co-expression with the neuronal marker MAP2 (Fig. 3). They did not co-express the astroglial marker GFAP, thereby excluding GABAergic expression by astrocytes (Fig. 3). Granular, unipolar, and bipolar neurons were present in all areas of the white matter from the ventricular/subventricular zone to the subplate region. The multipolar neurons were observed more frequently in the subplate region than in the deeper zones and were not identified in the ventricular/subventricular zone. GABAergic granular neurons were noted in the subventricular/ventricular zone as well as all other regions, including the subplate. At the ventricular/subventricular zone (Box 1), all white matter sites (Boxes 2–4) and subplate region (Box 5), the GABAergic neuronal density significantly increased with age, peaked around term, and decreased thereafter (p < 0.001 for a cubic effect of age, while controlling for a quadratic effect of box) (Fig. 4). However, this change was less marked in the subplate region (Box 5) compared to the underlying regions (Fig. 4). There were no significant effects of postmortem interval upon cell density measurements.
Figure 2.
Morphological types of γ-aminobutyric acid (GABA)ergic neurons in cerebral white matter. (A) Negative control staining with omission of the primary antibody. (B) Small, round granular neuron with negligible cytoplasm and no processes. (C) Oval unipolar neuron with negligible cytoplasm and a single process. (D) Oval bipolar neuron with processes aligned opposite to each other. (E) Relatively large, round multipolar neuron with abundant cytoplasm and more than 3 dendritic processes. Immunocytochemistry with anti-glutamic acid decarboxylase (GAD65/67) antibody.
Figure 3.
Glutamic acid decarboxylase (GAD65/67)-immunopositive cells in the white matter are neurons and not astrocytes, as determined by double-label immunocytochemistry with the neuronal marker microtubule-associated protein 2 (MAP2) and the astrocytic marker glial fibrillary acidic protein (GFAP), respectively. (A) A unipolar MAP2-immunopositive neuron co-expresses GAD65/67. (B) Two granular GABAergic neurons (arrows) are immunopositive for GAD65/67 but negative for GFAP (arrowheads).
Figure 4.
The density of γ-aminobutyric acid (GABA)ergic neurons in the subventricular/ventricular zone (VZ/SVZ) (Box 1), white matter (WM) (Boxes 2–4), and subplate (Box 5) increase between midgestation and term, peak at term, and decline thereafter. This developmental change is statistically significant (p < 0.001). Loess curves demonstrate the changes in GABAergic density for each region (box) from midgestation through infancy. The solid black line in each graph is the best curve fit of the density of GABAergic neurons; gray dotted lines indicate the 95% confidence intervals.
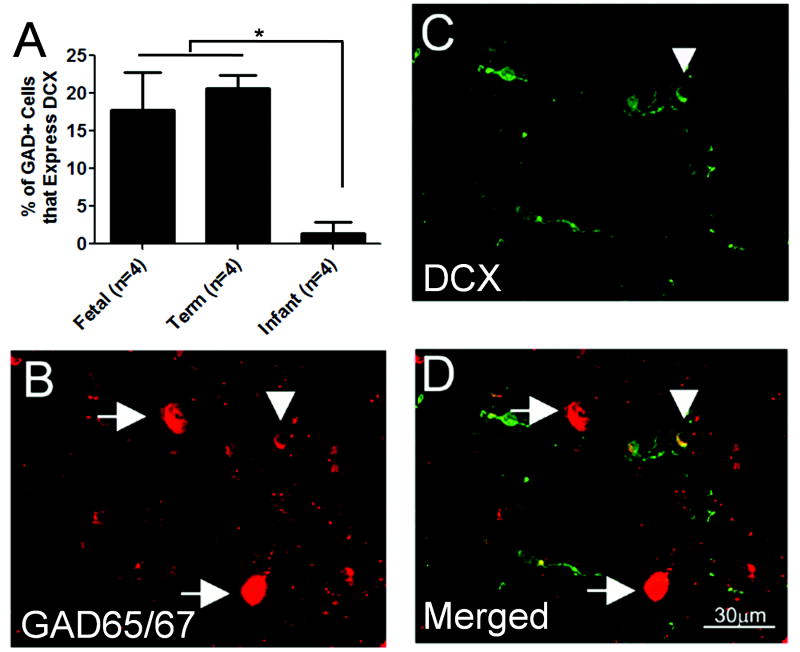
In the non-grid analysis of migrating GABAergic neuronal density in the periventricular and central white matter, co-expression with DCX demonstrated the migrating phenotype of a subpopulation of the GABAergic neurons. This subpopulation was comprised of small cells with 1 to 2 delicate trailing processes (Fig. 5); the GABAergic neurons that were DCX-immunonegative tended to be larger with intense labeling for GAD65/67 (Fig. 5). The percent of GABAergic neurons in the white matter that were migrating (i.e. co-expressed DCX) was 17.8% in the late fetal period and 20.7% at term, with a precipitous decline after birth to 1.5% (Fig. 5, 6). In the 3 cases ≥6 postnatal months of age, no migrating GABAergic neurons were detected (Fig. 6). The change in the percent of migrating GABAergic neurons with age was significant (p = 0.004, ANOVA); post-hoc analysis revealed significant differences between combined fetal/term versus infant cases but no significant difference between the fetal and term cases. There was no significant effect of postmortem interval when included in the model with age.
Figure 5.
Percentages of migrating γ-aminobutyric acid (GABA)ergic neurons in the white matter. (A) Densities of GABAergic neurons that express doublecortin (DCX) are expressed as the percent of total GABAergic neuronal density in the periventricular and central white matter in fetal (26–34 gestational weeks), term (37–43 post-conceptional weeks), and infant (3 post-natal months to 2 postnatal years) subjects. The percent of GABAergic migrating neurons decreases significantly after term (ANOVA, p = 0.003). (B–D) A relatively small GABAergic neuron in the white matter expresses both DCX and glutamic acid decarboxylase (GAD65/67) (arrowhead), suggesting that it is migrating. GABAergic neurons that do not express DCX are relatively large with oval cell bodies, intensely staining for GAD65/67, and often have 1 or more processes.
Figure 6.
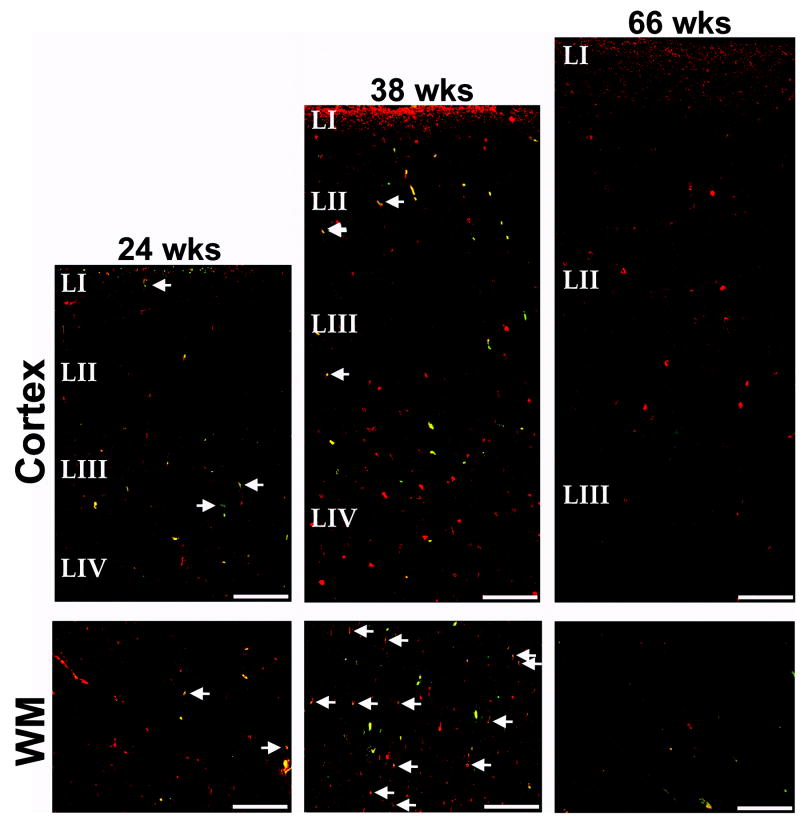
Migrating γ-aminobutyric acid (GABA)ergic (glutamic acid decarboxylase (GAD65/67-positive, doublecortin (DCX)-positive) neurons in the developing human cerebral cortex and white matter (WM) at 23 post-conceptional weeks (mid-gestation), 38 weeks (term), and 66 weeks (infancy). The density of double positive and GAD65/67-positive, DCX-negative neurons is low in the fetal white matter, increases at term, and decreases in infancy. Double-labeled, migrating GABAergic neurons are rare in the cortex at all ages. GAD65/67 neurons are red; DCX-positive neurons are green; and GAD65/67-positive neurons expressing DCX are yellow (merged, arrows). Blood vessels show as yellow due to autofluorescence, and are not marked by arrows. Scale bar = 200 μm.
GABAergic Neurons in the Developing Cerebral Cortex
Marked changes occurred in the developmental profile of GABAergic neurons in the cerebral cortex from midgestation through infancy in the frontal association cortex. At midgestation, only rare GABAergic neurons were detected in the cortex, in contrast to a heavy concentration of these neurons at term (Fig. 7). After term there was an obvious decrease in neuronal density with increasing age to 2 postnatal years (Fig.7). Migrating GABAergic (GAD65/67-positive, DCX-positive) neurons were scattered in the overlying cortex during gestation and around term without a distinct laminar pattern; they were not detected in the cortex after the neonatal period (Fig. 6). To examine the possibility that the decrease in neuronal density after term birth reflected programmed cell loss due to apoptosis, we examined the cortical expression of caspase-3, an immunomarker of apoptosis (40), but found essentially no caspase-3-immunopositive cells at any age in the cerebral cortex (data not shown).
Figure 7.
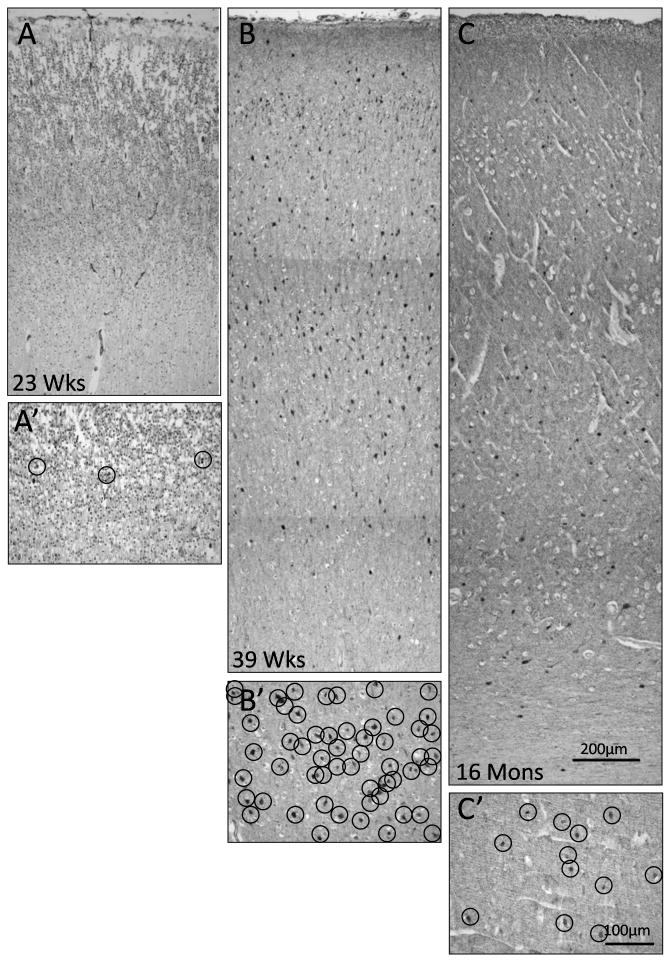
Developmental changes in γ-aminobutyric acid (GABA)ergic neuronal density in the frontal association cortex. (A–C) Twenty-three weeks (mid-gestation) (A), 37 weeks (term) (B), and 16 postnatal momths (C), are shown. The density is markedly increased at term compared to midgestation or postnatally. A′–C′ are high power of A–C at Layer IV; each circle outlines a GABAergic neuron demonstrated by glutamic acid decarboxylase (GAD65/67) immunostaining.
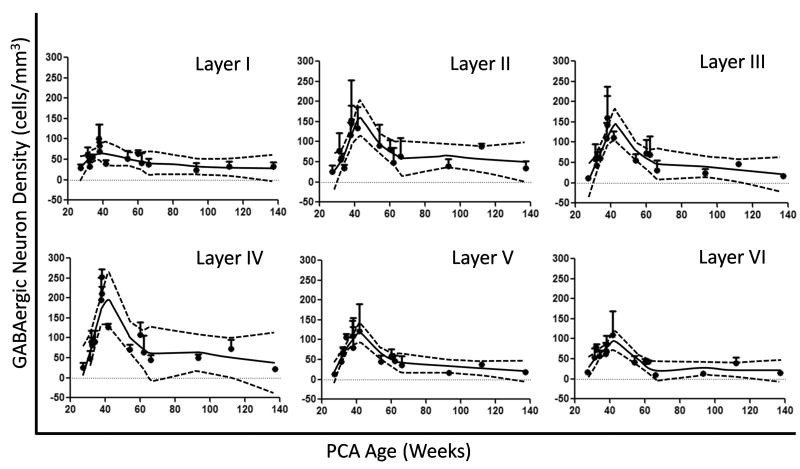
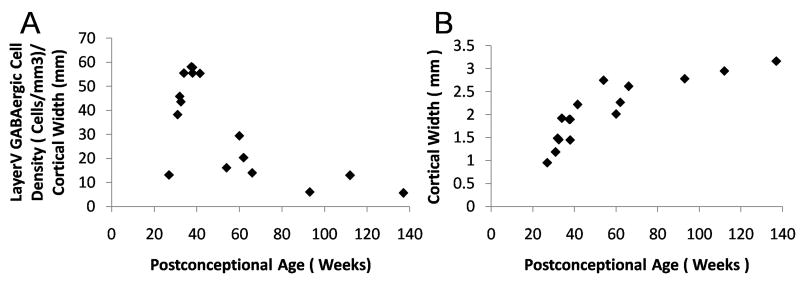
Developmental changes in GABAergic neuronal density were age- and layer-specific in the frontal association cortex in a total of 16 cases ranging in age from 27 gestational weeks to 2 postnatal years. Overall, the neuronal density in Layers I–VI increased through the second half of gestation, peaked around term, and decreased in infancy (Fig. 8). The GABAergic cell density in Layers II–V increased 88–100% from midgestation to term; after term the density decreased from the peak by 69–83% (Fig. 8). GABAergic neurons were unevenly distributed across the cortical layers at all ages (Fig. 8); their density was highest in the upper laminae such that the density was 140–200 cells/mm2 in Layers II–IV vs. 98–120 cells/mm2 in Layers V–VI. The lowest neuronal density at all ages was in Layer I (Fig. 8). Using the width of the cerebral cortex as a correction factor for the development of cerebral cortex over time, the same developmental pattern of an increase in GABAergic cell density across the last half of gestation with a peak at term and decline thereafter was present in parallel with a continuous increase in cortical width at the same site (Fig. 9). Of note, the cortical thickness increased most rapidly from midgestation until approximately 3 postnatal months, with a steady rise thereafter (Fig. 9).
Figure 8.
γ-Aminobutyric acid (GABA)ergic neuronal density in the frontal association cortex (FA) during early human development, as represented by Loess curves. The density of GABAergic neurons increases from midgestation to term, peaks at term, and decreases thereafter in infancy in Layers I–VI. The solid black line in each graph is the best curve fit of the density of GABAergic neurons; gray dotted lines indicate 95% confidence intervals.
Figure 9.
Changes in γ-aminobutyric acid (GABA)ergic cell density parallel a continuous increase in cortical width. (A, B) Using the width of the cerebral cortex in mm as a correction factor for the development of cerebral cortex over the time-span of the study, the same developmental pattern of an increase in GABAergic cell density over the last half of gestation with a peak at term and decline thereafter is present (as demonstrated for Layer V in the frontal association cortex) (A) in parallel with a continuous increase in cortical width at the same site, with the most rapid increase from midgestation until approximately 3 postnatal months (B).
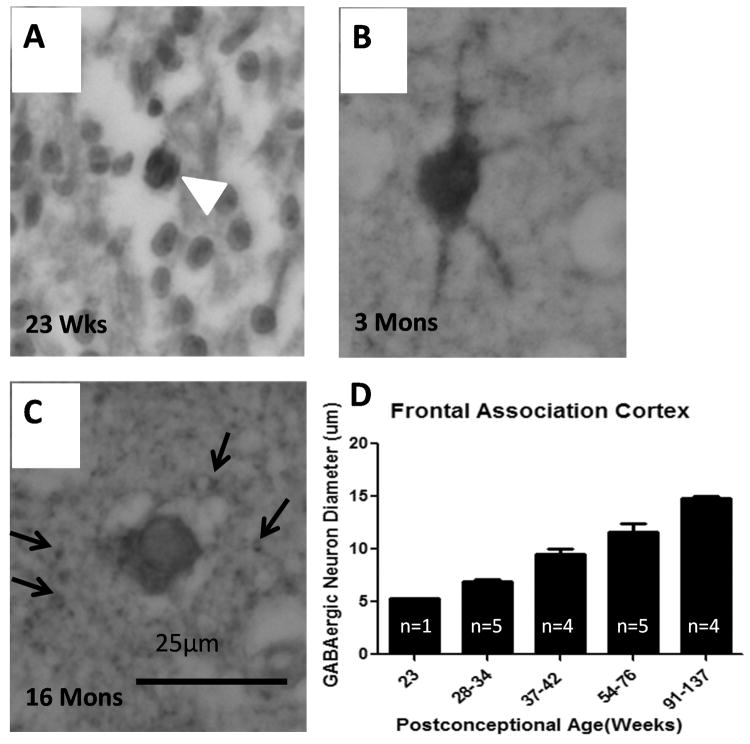
There were dramatic changes in GABAergic morphology and cell size in the fetal and infant cortex across development. At midgestation, GABAergic neurons were small and round with scant cytoplasm (Fig. 10); at term to 3 postnatal months they appeared multipolar with distinct processes (Fig. 10). From 5 months onward they had an adult morphology with large, oval shape without obvious processes (Fig. 10). At 5 postnatal months there was abundant punctuate GABAergic immunostaining in the neuropil, the site of dendritic processes, spines and axonal terminals. There was a continual increase in cell diameter with age in the frontal cortex (n = 19), with a higher rate of increase during the fetal compared to term period and a lower rate (but continued increase) in infancy (quadratic regression model, p < 0.001) (Fig. 10). The mean diameter of the GABAergic neurons was 5.3 ± 0 μm at 23 gestational weeks, 9.9 ± 0.4 μm at term, and 15.0 ± 0.2 μm at 2 postnatal years, representing a total 183% increase in size over the time period studied (Fig. 10). This increase in cell diameter was statistically significant (p < 0.001). There was no significant effect of postmortem interval upon cell diameter.
Figure 10.
Developmental changes in the morphology and size of γ-aminobutyric acid (GABA)ergic neurons. (A) At 23 gestational weeks, GABAergic neurons (demonstrated by glutamic acid decarboxylase (GAD65/67) immunocytochemistry) are small and round with scant cytoplasm (arrowhead). (B) At 3 postnatal months they are large with abundant cytoplasm and multiple processes. (C) At 16 postnatal months they have a mature morphology with a granular appearance and without cellular processes. There is punctate GAD65/67 immunostaining in the neuropil at this age. (D) GABAergic neuronal diameters in the frontal association cortex increase significantly with age (p < 0.001, analyzed with age as continuous variable).
GAD65/67 Expression Levels in the Developing Cerebral Cortex
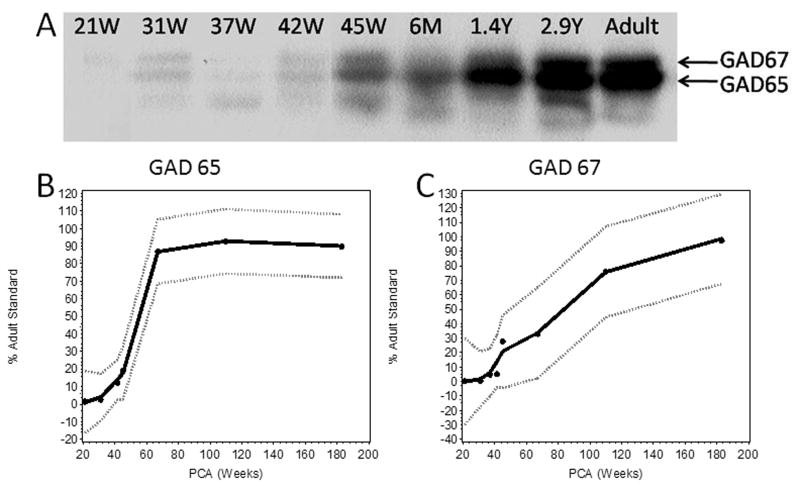
We performed Western blot analysis to determine the developmental profile of GAD65 and GAD67 expression in the parietal association cortex in 8 cases ranging in age from 21 gestational weeks to 2.9 postnatal years. There were 2 main bands detected that correlated with the known molecular weight of 65 KD and 67 KD for the GAD65 and GAD67 isoforms, respectively (Fig. 11). The Loess curves for GAD65 and GAD67 expression levels showed different developmental profiles for these isoforms (Fig. 11). The expression of GAD65 was less than 10% of the adult standard over the last half of gestation, increased 10% around 40 post-conceptional weeks, and dramatically increased to approximately 85% of the adult standard between 45 post-conceptional weeks and 6 postnatal months, with a plateau thereafter. The expression of GAD67 was less than 5% of adult standard up to term, then increased more gradually post-term, and did not reach 80% of the adult standard until 13 postnatal months.
Figure 11.
Different expression profiles of glutamic acid decarboxylase (GAD)65 and GAD67 isoforms in the parietal cortex in the human fetus and infant. (A) In Western blots, the 2 main bands (arrows) are 67 and 65 KDa, representing the GAD67 and GAD65 isoforms, respectively. Expression levels are expressed as a percent of a pooled human adult standard. (B, C) Developmental expression profiles of GAD65 and GAD67 are illustrated by Loess curves; the best curve fit in the middle line is surrounded by the 95% confidence intervals. GAD65 expression (B) dramatically increases from term to 6 postnatal months at which time it reaches a plateau at 95% of the adult standard. GAD67 expression increases with age from negligible levels at 21 gestational weeks to 90% at 2.9 postnatal years (C).
GABAergic Receptors the Developing Human Cerebral Cortex
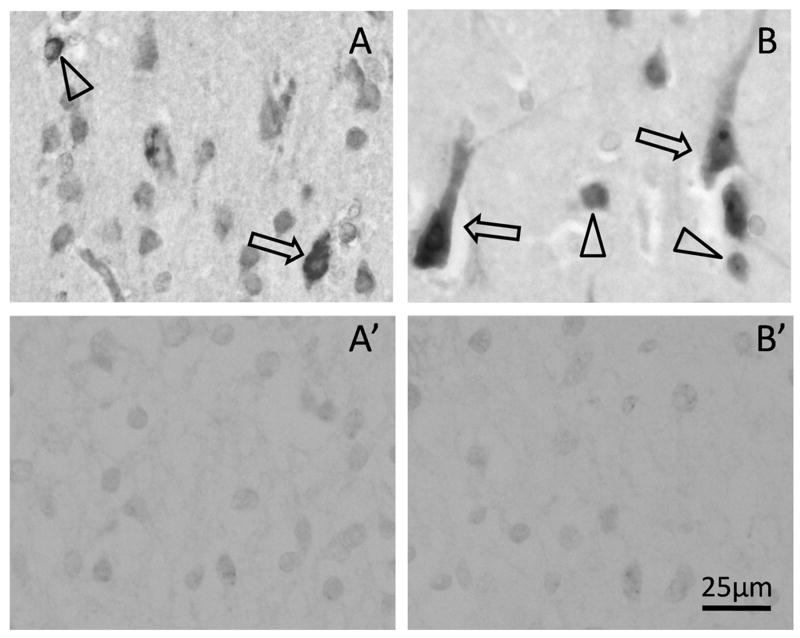
The cellular localizations of the α1 and α3 subunits of the GABAA receptor were determined by immunocytochemistry in the frontal association cortex of 8 cases ranging in age from 25 gestational weeks to 2 postnatal years. The intensity of the immunostaining for both subunits was low in the fetal and term cortex, but increased around 5 postnatal months. At this age, the immunostaining was intense and punctuate on the somata and dendrites of both pyramidal and granular neurons in all laminae (Fig. 12). A transition from soma to dendritic immunostaining appeared around 6 postnatal months.
Figure 12.
γ-Aminobutyric acid (GABA)A receptor subunit expression. (A, B) GABAA subunit is expressed by both pyramidal neurons (arrows) and granular neurons (arrows), as demonstrated by immunocytochemistry to GABAA receptors α1 (A) and α3 (B) subunits. A′ and B′ are adjacent tissue sections stained following pre-absorption of the primary antibody with the antigen peptide; there is no non-specific staining.
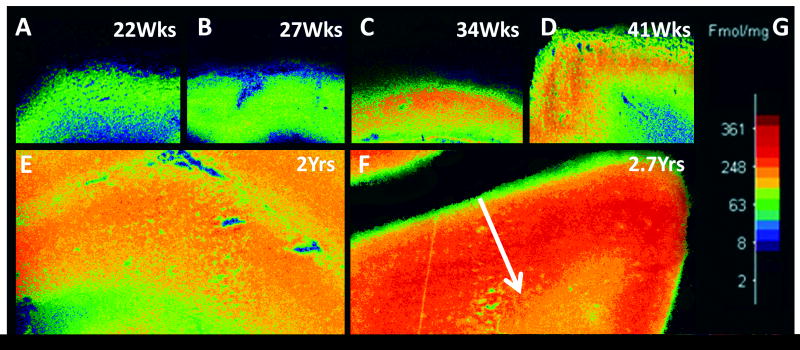
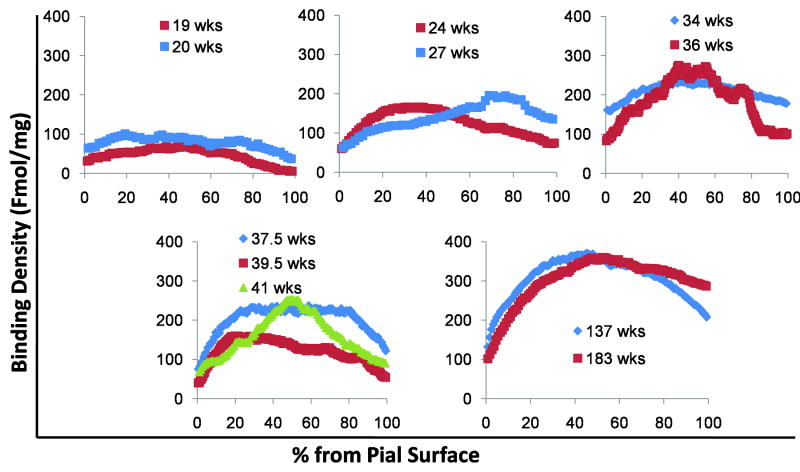
We analyzed GABAA receptor binding with tissue section autoradiography in the parietal association cortex in 11 cases ranging in age from 19 gestational weeks to 2.7 postnatal years. The binding of GABAA receptors was uniformly low (<100 fmol/mg tissue) across all laminae from 19 to 20 weeks (Fig. 13) and corresponded with the low intensity of immunostaining for the α1 and α3 subunits in this time frame (see above). Absolute binding began to increase in the middle layers by 24 weeks (100–200 fmol/mg tissue) and remained low in the superficial and deep layers (Fig. 13). At 34 to 36 weeks and into infancy, a curve with higher binding in the middle layers (200–400 fmol/mg tissue) became increasingly pronounced. Thus, in the plots of the spatial distribution of the cortical binding, the pattern changed from a relatively flat line to a definite curve with higher absolute levels in the middle layers (Fig. 14). The developmental changes in both binding levels and spatial distribution were highly significant (p < 0.001) for both the effect of age and its interaction with spatial distribution.
Figure 13.
Tissue autoradiography for γ-aminobutyric acid (GABA)A receptor binding in the parietal association cortex across early human development. The binding pattern changes from uniformly low across all laminae at 22 weeks to concentrated binding in the middle laminae at 34 weeks and thereafter. (A) 22 weeks; (B) 27 weeks; (C) 34 weeks; (D) 41 weeks (term); (E) 2 postnatal years; (F) 2.9 postnatal years. The white arrow indicates the site of measurement of cortical binding from the pial surface to the gray-white junction.
Figure 14.
Quantitative distribution of γ-aminobutyric acid (GABA)A receptor in cerebral cortex during development from 19 gestational weeks to 2.7 postnatal years. GABAA receptor binding is increased with age. Between 19 to 27 gestational weeks the binding pattern is low and homogeneous throughout the cortex. From 34 gestation weeks onward the binding pattern becomes a curve, with high binding in the middle layers. The curve becomes more pronounced with age.
DISCUSSION
The major finding of this study is that the GABAergic system in the human cerebral cortex and white matter develops relatively late, i.e. over the second half of gestation and into infancy. This late development parallels the peak window of vulnerability to perinatal brain injury, including in the preterm infant. This GABAergic development may, therefore, be at particular risk to the hypoxic-ischemic and other metabolic and inflammatory insults that complicate prematurity and neonatal intensive care (41). This possibility is supported by the previous report of decreased GABAergic neurons in the cerebral white matter in preterm infants with white matter damage (25). Given that GABAergic neurons in the subventricular region, white matter, subplate and cortex have all been implicated in developmental processes, including neuronal migration and differentiation (17–20, 42), injury to any or all of them in the preterm and full term infant may disrupt cortical formation in early life and compound the primary destructive processes. Here, we found a complex and rapidly changing profile in the development of different GABAergic parameters, as summarized in the schematic diagram in Figure 15. Aspects of this profile are highlighted in the following discussion.
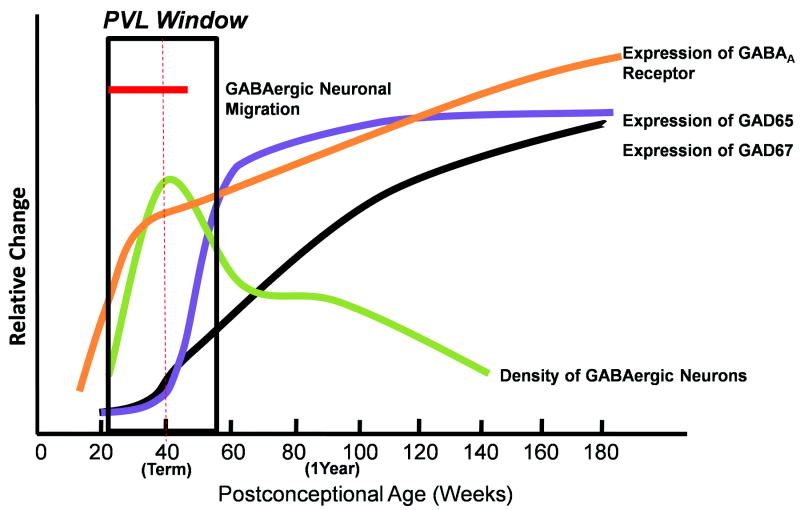
Figure 15.
Summary of developmental profiles of different γ-aminobutyric acid (GABA)ergic parameters relative to each other in the human cerebral cortex and white matter in the fetus and infant. Rapid and dramatic changes occur from midgestation into infancy with marked increases and intersecting decreases in the developmental trajectory of the structural and neurochemical components of the GABAergic system. These rapid changes define a critical period in the development of this system is therefore likely to be susceptible to injury. GAD, glutamic acid decarboxylase; PVL, periventricular leukomalacia.
The Late Migration of GABAergic Neurons
Cortical formation depends upon the proliferation and migration of glutamatergic and GABAergic neurons from different germinal zones according to different time tables (43, 44). Glutamatergic neurons form in the dorsal telencephalic pallium and migrate along radial glia fibers early in gestation (42–46). In the human brain, approximately two thirds of GABAergic neurons arise from the dorsal telencephalic zone; the remaining third originates in the ganglionic eminence and migrate tangentially to the cortex (21, 22). In mice and rats, GABAergic interneurons also migrate radially inward from the marginal zone to the cortical plate (23, 24). Our combined cortical (Layers I–VI) and white matter data suggest that human GABAergic neurons migrate into the cerebral cortex via the white matter throughout the second half of gestation and become “fixed” in number around birth; migration appears to decline after birth and completely cease by 6 postnatal months (Fig. 15). Even with correction for the increasing width of the cortex, the density of cortical GABAergic neurons increases over the last half of gestation, peaks at term, and declines thereafter, a progression of events visually striking upon microscopic examination. The demonstration of an increase in GABAergic neuronal density prior to term birth suggests that the GABAergic number increases in greater proportion relative to the known increasing cortical volume. Hence, the increased neuronal number is likely due to increased migration of GAD67/65-expressing neurons into the cortex across the second half of gestation and/or increased expression of GAD67/65 expression in neurons destined to be GABAergic that are already in the cortex. On the other hand, the decrease in GABAergic cell density in the cerebral cortex from term into infancy suggests that there is a programmed loss of neurons (which we consider unlikely due to the lack of caspase 3 immunostaining across this age in the cortex), or alternatively, an increase in neuropil (reflecting dendritic and/or axonal arborization and/or increased synaptogenesis and thereby cortical volume) relative to a constant cell number. Our finding that the upper layers of the cortex (Layers II–IV) have a higher density of GABAergic neurons than the deeper layers (Layers V–VI) is consistent with the concept that these upper layers contain the greatest density of interneurons in the highly evolved human cortex (16).
Importantly, we found that at least 20% of the GABAergic neurons migrate to the cortex into late gestation and continuing to term, as determined by co-expression with DCX. This time-frame is 9 weeks longer than that suggested in the white matter for 5 fetuses analyzed previously between 26 and 31 post-conceptional weeks (25). It is possible that 20% is an underestimate given that other markers, such as Lis (26), are expressed in migrating populations of cells that are DCX-negative. For the same reason, it is also possible that GABAergic migration may extend beyond term. Further analysis of other migration markers is needed to exclude these possibilities. The presence of GABAergic granule neurons in the human ventricular/subventricular zone suggests that the GABAergic phenotype is expressed in germinal zones prior to migration. In experimental animals, GABAergic neurons at this site influence their own differentiation into unipolar and bipolar neurons by the release of GABA in a paracrine manner (17). Their presence in the human ventricular/subventricular zone as late as one postnatal year suggests that GABAergic neurons could arise from this germinal zone well after the fetal period.
The question arises as to what the functions are of the majority of GABAergic neurons in the white matter and the subplate that appear not to be migrating, i.e. those white matter neurons that are GAD65/67-positive and DCX-negative. Our finding that the GABAergic neuronal density in the subplate region (Box 5) does not dramatically change from midgestation through infancy suggests that GABAergic neurons may become relatively “fixed” at this site by midgestation. Thus, these non-migrating GABAergic neurons may be an integral part of the transient subplate zone and play a critical role in the establishment of connections between the cortex and thalamus (47, 48). In humans, the subplate peaks around 24 to 36 gestational weeks, with a programmed loss of the majority of its neurons in the first postnatal year (47, 48). In animal models, GABAergic neurons in the subplate are thought to function in local circuits at the stage when thalamocortical axons form transient synaptic contacts prior to innervating the cortical plate (49). These non-migrating GABAergic neurons may also form part of a permanent population that persists in the subplate and white matter into adulthood to influence mature cortical function (49–51).
Differentiation of the GABAergic System in the Developing Human Cerebral Cortex
We found that the relative density of human GABAergic neurons varies among different laminae from midgestation into infancy, as demonstrated in the frontal association cortex. It is well recognized that the density of neurons varies across different functional areas of the cerebral cortex, as indicated as the defining features of neuronal density and relative proportions of pyramidal and granular neurons for Brodmann areas. Monkey studies indicate that the relative proportion of GABA neurons in vertical columns among cortical areas is also different (52, 53), but we did not address this issue here. GABAergic interneurons vary greatly in their somatic, dendritic, and axonal morphologies (54), and are further subdivided by the differential expression of calcium-binding proteins and neuropeptides (55, 56). The subclassification of GABAergic neurons based upon morphology, calcium-binding proteins, and neuropeptides was beyond the scope of this study. We found, however, that the morphology of GABAergic neurons changes from small granular to multipolar to large granular over the last half of gestation and into infancy. In parallel with changes in GABAergic neuronal density and size, there were changes in expression levels of the enzymatic isoforms GAD65 and GAD67. GAD67 is widely distributed in cell bodies where it is thought to be involved in GABA synthesis for general metabolic activity (57); GAD65, on the other hand, targets membranes and nerve endings where it is involved in synaptic transmission (57–59). We found that the increase in GAD expression levels in the human cortex begins before birth but continues well beyond term. Notably, the increase in GAD65 is most rapid in the postnatal weeks following the peak in GABAergic cell density (Fig. 15). The continued GAD expression suggests progressive GABA production by the fixed population of GABAergic neurons as the neuropil (dendrites, spines, and axonal terminals) expands and GABAergic connectivity matures.
From midgestation through infancy, GABAergic differentiation is further characterized by changes in GABAA receptors (Fig. 15). We focused upon the GABAA receptor in this study because it mediates the major fast inhibitory action of GABA (1, 60). GABAA receptors are both pre- and postsynaptic and are found on both glutamatergic pyramidal neurons and GABAergic interneurons (61, 62); they are also present on white matter neurons in the human fetus (25). We found that α1 and α3 GABAergic receptor subunits localize to the somata of both pyramidal and granular neurons in the term cortex and are concentrated upon somata and dendrites in both neuronal subtypes thereafter, with a peak in dendrites around 6 postnatal weeks. From the late fetal period onward, tissue receptor autoradiography of the parietal association cortex demonstrated an absolute increase in GABAA binding levels with a change in the profile of binding from uniform across all layers to a curved pattern with a binding peak in the middle layers. The increase in binding over the second half of gestation may reflect the increase in density in GABAergic neurons with the emergence of pre- and/or postsynaptic GABAA receptors (Fig. 15). Yet, after the number of GABAergic neurons is putatively fixed at term, there is a continued increased in binding levels with concentration in the middle layers. The rise in receptor binding density in the middle layers that begins in late gestation occurs simultaneously with an overall (prenatal) increase and (postnatal) decrease in GABAergic cell density in all layers. The increase in the absolute values of GABAA receptor binding and its concentration in the middle layers, including Layer IV, with increasing age may reflect changes in the neuropil and the ingrowth of thalamocortical fibers during this time period and a parallel upregulation of inhibitory (GABAergic) modulation to counterbalance the presumed increase of glutamatergic inputs. Direct synaptic contacts between thalamocortical axonal terminals and cortical GABAergic neurons have been demonstrated in this regard (13).
The developmental role of chloride transporters in the developing function of GABAA receptors must be considered relative to the changes in GABAA receptor binding. Early in development, GABAA receptors appear to be excitatory because of the high intracellular chloride content as determined by the balance of chloride transporters. In immature neurons, the intracellular chloride concentration is higher than the extracellular concentration, and GABAA activation leads to an efflux of chloride ions which results in depolarization, an effect attributed to the sodium-potassium-chloride cotransporter (NKCC) 1, a chloride importer (63–65). In mature neurons, however, activation of GABAA receptors hyperpolarizes neurons by causing chloride influx, an effect attributed to the increased expression of the chloride exporter potassium-chloride cotransporter (KCC) 2 (65–67). In the human cerebral cortex, the expression of NKCC1, as determined by Western blotting rapidly increases from 20 gestational weeks to term and then dramatically decreases, reaching a plateau after 4 postnatal months (68); the expression of KCC2, on the other hand, increases from 20 gestational weeks to adulthood (68). Combining our data with these reported human observations concerning chloride cotransporters, GABAA receptors are likely to be immature and excitatory during late gestation and to switch to an inhibitory phenotype during the first postnatal year, after the surge in the absolute levels of GABAA receptor binding in the second half of gestation (Fig, 15). Of note, a loss of expression of both NKCC1 and KCC2 in the subplate and white matter has been reported in preterm infants with white matter damage (69).
Potential Study Limitations
A potential limitation of our study is the nature of the control (baseline) population. This population is comprised of autopsied infants who were critically ill before death, and thus may not be truly representative of normal living infants. This limitation relates to the fact that “normal” preterm brains at autopsy are extraordinarily rare, if non-existent, because normal preterm infants do not die, and preterm infants rarely, if ever, die in pediatric health care centers without intensive care and terminal metabolic derangements, including hypoxia-ischemia. Nevertheless, within these constraints, we have selected for analysis tissue samples from fetuses and infants without neurological signs or, most importantly, neuropathologic abnormalities at autopsy.
A second potential limitation of the study is the use of a non-stereological method for assessing GABAergic neuronal density in the developing human cerebral cortex. The “optimal” methodology of cell counting in the human cerebral cortex is intensely debated, with proponents for 3D approaches (stereology with optical dissector techniques) (70–72), and those advocating 2-D approaches (36, 37). Each approach has its own strengths and weaknesses, as well as inherent biases (36). Consequently, the selection of a particular method depends upon the goals, use of immunocytochemistry (not recommended for stereological counts) (73), tissue availability, and financial and technical resources of a particular study (substantial for stereological procedures) (36). It should be noted, however, that the 2D and 3D methods can provide comparable results concerning neuron counts, including in the human cerebral cortex (37). In the present study, we modified the 2-dimensional sampling method of Benes et al (36, 74), utilizing immunocytochemical preparations with an antibody to GAD67/65 to identify the GABAergic phenotype; we applied a computer-based grid system with a large (600–700 micron) x-y sampling frame in which we determined the mean density of GABAergic neurons in each laminae based upon 3 step sections, as opposed to a single section. We did not seek to determine absolute GABAergic neuron number in the baseline cases across development in this study, but rather, relative differences in cell density (number of neurons/unit volume) with increasing age. Thus, our focus upon relative as opposed to absolute changes is a justification for the modified 2D as opposed to 3D method, the latter putatively preferable for absolute measures. In our opinion, the validity of this modified 2D sampling procedure is reinforced by appreciation that the GABAergic neuronal density measurements accurately reflect the changes we observe directly under the microscope, i.e. in multiple tissue sections across ages we observed directly that the density of GABAergic neurons obviously increases until around term and then decreases thereafter. Thus, the modified 2D sampling procedure captured our qualitative observations.
Conclusions
We report rapid and dramatic changes in several GABAergic parameters in the human cerebral cortex and white matter over the last half of gestation, suggesting a critical period in the development of the GABAergic system during this timeframe (Fig. 15). Yet, we also found equally as dramatic and rapid changes in an additional set of GABA parameters later, in the first postnatal year, suggesting that this critical period extends beyond birth (Fig. 15). The changes in the second half of gestation include major increases in the density of GABAergic neurons in the cerebral cortex and white matter, the density of migrating GABAergic neurons in the white matter, the size of GABAergic cell bodies, and the receptor binding levels. The changes in the first year of life include increases in cortical GAD65 and GAD67 levels, decreases in the density of GABAergic neurons in the cerebral cortex, white matter, and subplate, decreases in the density of GABAergic migrating neurons, and ongoing changes in the spatial patterning and levels of cortical GABAA receptor binding. Consequently, the GABAergic system is likely to be developmentally susceptible to injury not only in the last half of gestation (e.g. the peak period of PVL), but also in early infancy (e.g. during the entire period of vulnerability to PVL) (75), as well as the period of neonatal hypoxic-ischemic encephalopathy and seizures (41). Of note, PVL can originate at term and into the first few postnatal months as well, again underscoring the vulnerability of the premyelinating white matter to this lesion at and beyond term birth (75), given that active myelin sheath synthesis does not occur in the telencephalic white matter until 2 to 4 postnatal months (76, 77). Indeed, our findings are relevant to multiple pediatric neurological disorders in which the GABAergic system has been implicated, including epilepsy (78–80) and autism (81–86). The developmental events at greatest risk in PVL, which preferentially affects the preterm brain, include GABAergic migration through the cerebral white matter with the potential end-result of altered GABAergic cortical development and subsequent cognitive impairments due to an imbalance of excitation and inhibition. This baseline definition of the developmental sequences of the cortical GABAergic system in early life forms the foundation for future neuropathologic studies of developmental GABAergic disorders.
Acknowledgments
The authors thank Ms. Sarah Andiman for technical support and the Eunice Kennedy Shriver NICHD Brain and Tissue Bank for Developmental Disorders (NO1-HD-9-0011) for the provision of developmental cerebral cortical tissue.
This work was supported the National Institute of Neurological Diseases and Stroke (PO1-NS38475) (HCK, JJV), Hearst Foundation (RLH), and Eunice Shriver Kennedy National Institute of Child Health and Development Intellectual and Developmental Disabilities Research Center, Children’s Hospital Boston (P30-HD018655). The content of the manuscript is the view of the authors and not that the official view of the Eunice Shriver Kennedy National Institute of Child Health and Development.
References
- 1.Markram H, Toledo-Rodriguez M, Wang Y, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 2.Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–94. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–6. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- 4.Zheng W, Knudsen EI. Gabaergic inhibition antagonizes adaptive adjustment of the owl’s auditory space map during the initial phase of plasticity. J Neurosci. 2001;21:4356–65. doi: 10.1523/JNEUROSCI.21-12-04356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clancy B, Teague-Ross TJ, Nagarajan R. Cross-species analyses of the cortical GABAergic and subplate neural populations. Front Neuroanat. 2009;3:20. doi: 10.3389/neuro.05.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–86. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 7.Butt SJ, Fuccillo M, Nery S, et al. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 8.de Lima AD, Voigt T. Identification of two distinct populations of gamma-aminobutyric acidergic neurons in cultures of the rat cerebral cortex. J Comp Neurol. 1997;388:526–40. [PubMed] [Google Scholar]
- 9.Hevner RF. Layer-specific markers as probes for neuron type identity in human neocortex and malformations of cortical development. J Neuropathol Exp Neurol. 2007;66:101–9. doi: 10.1097/nen.0b013e3180301c06. [DOI] [PubMed] [Google Scholar]
- 10.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–96. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 11.Yuste R. Origin and classification of neocortical interneurons. Neuron. 2005;48:524–7. doi: 10.1016/j.neuron.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Ewell LA, Jones MV. Frequency-tuned distribution of inhibition in the dentate gyrus. J Neurosci. 2010;30:12597–607. doi: 10.1523/JNEUROSCI.1854-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones EG. GABAergic neurons and their role in cortical plasticity in primates. Cereb Cortex. 1993;3:361–72. doi: 10.1093/cercor/3.5.361-a. [DOI] [PubMed] [Google Scholar]
- 14.Kubota Y, Hattori R, Yui Y. Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res. 1994;649:159–73. doi: 10.1016/0006-8993(94)91060-x. [DOI] [PubMed] [Google Scholar]
- 15.Capogna M, Pearce RA. GABA A, slow: causes and consequences. Trends Neurosci. 2011;34:101–12. doi: 10.1016/j.tins.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Hill RS, Walsh CA. Molecular insights into human brain evolution. Nature. 2005;437:64–7. doi: 10.1038/nature04103. [DOI] [PubMed] [Google Scholar]
- 17.Manent JB, Represa A. Neurotransmitters and brain maturation: early paracrine actions of GABA and glutamate modulate neuronal migration. Neuroscientist. 2007;13:268–79. doi: 10.1177/1073858406298918. [DOI] [PubMed] [Google Scholar]
- 18.Wang DD, Kriegstein AR. Defining the role of GABA in cortical development. J Physiol. 2009;587:1873–9. doi: 10.1113/jphysiol.2008.167635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz ML, Meinecke DL. Early expression of GABA-containing neurons in the prefrontal and visual cortices of rhesus monkeys. Cereb Cortex. 1992;2:16–37. doi: 10.1093/cercor/2.1.16. [DOI] [PubMed] [Google Scholar]
- 20.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–88. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 21.Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–9. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- 22.Petanjek Z, Dujmovic A, Kostovic I, et al. Distinct origin of GABA-ergic neurons in forebrain of man, nonhuman primates and lower mammals. Coll Antropol. 2008;32 (Suppl 1):9–17. [PubMed] [Google Scholar]
- 23.Rymar VV, Sadikot AF. Laminar fate of cortical GABAergic interneurons is dependent on both birthdate and phenotype. J Comp Neurol. 2007;501:369–80. doi: 10.1002/cne.21250. [DOI] [PubMed] [Google Scholar]
- 24.Hevner RF, Daza RA, Englund C, et al. Postnatal shifts of interneuron position in the neocortex of normal and reeler mice: evidence for inward radial migration. Neuroscience. 2004;124:605–18. doi: 10.1016/j.neuroscience.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 25.Robinson S, Li Q, Dechant A, et al. Neonatal loss of gamma-aminobutyric acid pathway expression after human perinatal brain injury. J Neurosurg. 2006;104:396–408. doi: 10.3171/ped.2006.104.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer G, Perez-Garcia CG, Gleeson JG. Selective expression of doublecortin and LIS1 in developing human cortex suggests unique modes of neuronal movement. Cereb Cortex. 2002;12:1225–36. doi: 10.1093/cercor/12.12.1225. [DOI] [PubMed] [Google Scholar]
- 27.des Portes V, Pinard JM, Billuart P, et al. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92:51–61. doi: 10.1016/s0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- 28.Gleeson JG, Allen KM, Fox JW, et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 29.Yazulla S, Studholme KM. Neurochemical anatomy of the zebrafish retina as determined by immunocytochemistry. J Neurocytol. 2001;30:551–92. doi: 10.1023/a:1016512617484. [DOI] [PubMed] [Google Scholar]
- 30.Broadbelt KG, Paterson DS, Rivera KD, et al. Neuroanatomic relationships between the GABAergic and serotonergic systems in the developing human medulla. Auton Neurosci. 2010;154:30–41. doi: 10.1016/j.autneu.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andiman SE, Haynes RL, Trachtenberg FL, et al. The cerebral cortex overlying periventricular leukomalacia: analysis of pyramidal neurons. Brain Pathol. 2010;20:803–14. doi: 10.1111/j.1750-3639.2010.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vick WW, Wikstrand CJ, Bullard DE, et al. The use of a panel of monoclonal antibodies in the evaluation of cytologic specimens from the central nervous system. Acta Cytol. 1987;31:815–24. [PubMed] [Google Scholar]
- 33.Hodge RD, Kowalczyk TD, Wolf SA, et al. Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J Neurosci. 2008;28:3707–17. doi: 10.1523/JNEUROSCI.4280-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–6. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 35.Broadbelt KG, Paterson DS, Rivera KD, et al. Neuroanatomic relationships between the GABAergic and serotonergic systems in the developing human medulla. Auton Neurosci. 2010;154:30–41. doi: 10.1016/j.autneu.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benes FM, Lange N. Two-dimensional versus three-dimensional cell counting: a practical perspective. Trends Neurosci. 2001;24:11–7. doi: 10.1016/s0166-2236(00)01660-x. [DOI] [PubMed] [Google Scholar]
- 37.Todtenkopf MS, Vincent SL, Benes FM. A cross-study meta-analysis and three-dimensional comparison of cell counting in the anterior cingulate cortex of schizophrenic and bipolar brain. Schizophr Res. 2005;73:79–89. doi: 10.1016/j.schres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 38.Cleveland WS, Devlin SJ, Gross E. Regression by local fitting. J Econometrics. 1988;37:87–114. [Google Scholar]
- 39.Duncan JR, Paterson DS, Hoffman JM, et al. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–7. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicholson DW, Ali A, Thornberry NA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 41.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–24. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nadarajah B, Alifragis P, Wong RO, et al. Neuronal migration in the developing cerebral cortex: observations based on real-time imaging. Cereb Cortex. 2003;13:607–11. doi: 10.1093/cercor/13.6.607. [DOI] [PubMed] [Google Scholar]
- 43.Hevner RF. From radial glia to pyramidal-projection neuron: transcription factor cascades in cerebral cortex development. Mol Neurobiol. 2006;33:33–50. doi: 10.1385/MN:33:1:033. [DOI] [PubMed] [Google Scholar]
- 44.Howard B, Chen Y, Zecevic N. Cortical progenitor cells in the developing human telencephalon. Glia. 2006;53:57–66. doi: 10.1002/glia.20259. [DOI] [PubMed] [Google Scholar]
- 45.Parnavelas JG, Anderson SA, Lavdas AA, et al. The contribution of the ganglionic eminence to the neuronal cell types of the cerebral cortex. Novartis Found Symp. 2000;228:129–39. doi: 10.1002/0470846631.ch10. [DOI] [PubMed] [Google Scholar]
- 46.Anderson SA, Kaznowski CE, Horn C, et al. Distinct origins of neocortical projection neurons and interneurons in vivo. Cereb Cortex. 2002;12:702–9. doi: 10.1093/cercor/12.7.702. [DOI] [PubMed] [Google Scholar]
- 47.Kostovic I, Rakic P. Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol. 1980;9:219–42. doi: 10.1007/BF01205159. [DOI] [PubMed] [Google Scholar]
- 48.Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297:441–70. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- 49.Kostovic I, Judas M, Sedmak G. Developmental history of the subplate zone, subplate neurons and interstitial white matter neurons: relevance for schizophrenia. Int J Dev Neurosci. 2011;29:193–205. doi: 10.1016/j.ijdevneu.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Suarez-Sola ML, Gonzalez-Delgado FJ, Pueyo-Morlans M, et al. Neurons in the white matter of the adult human neocortex. Front Neuroanat. 2009;3:7. doi: 10.3389/neuro.05.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chun JJ, Shatz CJ. Interstitial cells of the adult neocortical white matter are the remnant of the early generated subplate neuron population. J Comp Neurol. 1989;282:555–69. doi: 10.1002/cne.902820407. [DOI] [PubMed] [Google Scholar]
- 52.Houser CR, Hendry SH, Jones EG, et al. Morphological diversity of immunocytochemically identified GABA neurons in the monkey sensory-motor cortex. J Neurocytol. 1983;12:617–38. doi: 10.1007/BF01181527. [DOI] [PubMed] [Google Scholar]
- 53.Jones EG, Huntley GW, Benson DL. Alpha calcium/calmodulin-dependent protein kinase II selectively expressed in a subpopulation of excitatory neurons in monkey sensory-motor cortex: comparison with GAD-67 expression. J Neurosci. 1994;14:611–29. doi: 10.1523/JNEUROSCI.14-02-00611.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeFelipe J. Cortical interneurons: from Cajal to 2001. Prog Brain Res. 2002;136:215–38. doi: 10.1016/s0079-6123(02)36019-9. [DOI] [PubMed] [Google Scholar]
- 55.Akil M, Lewis DA. Differential distribution of parvalbumin-immunoreactive pericellular clusters of terminal boutons in developing and adult monkey neocortex. Exp Neurol. 1992;115:239–49. doi: 10.1016/0014-4886(92)90058-x. [DOI] [PubMed] [Google Scholar]
- 56.Hof PR, Glezer, Conde F, et al. Cellular distribution of the calcium-binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: phylogenetic and developmental patterns. J Chem Neuroanat. 1999;16:77–116. doi: 10.1016/s0891-0618(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 57.Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci. 1998;19:500–5. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- 58.Kaufman DL, Houser CR, Tobin AJ. Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem. 1991;56:720–3. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reetz A, Solimena M, Matteoli M, et al. GABA and pancreatic beta-cells: colocalization of glutamic acid decarboxylase (GAD) and GABA with synaptic-like microvesicles suggests their role in GABA storage and secretion. EMBO J. 1991;10:1275–84. doi: 10.1002/j.1460-2075.1991.tb08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olsen RW, Tobin AJ. Molecular biology of GABAA receptors. FASEB J. 1990;4:1469–80. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- 61.Christie SB, Li RW, Miralles CP, et al. Synaptic and extrasynaptic GABAA receptor and gephyrin clusters. Prog Brain Res. 2002;136:157–80. doi: 10.1016/s0079-6123(02)36015-1. [DOI] [PubMed] [Google Scholar]
- 62.Galarreta M, Hestrin S. Electrical synapses between GABA-releasing interneurons. Nat Rev Neurosci. 2001;2:425–33. doi: 10.1038/35077566. [DOI] [PubMed] [Google Scholar]
- 63.Delpire E. Cation-Chloride Cotransporters in Neuronal Communication. News Physiol Sci. 2000;15:309–12. doi: 10.1152/physiologyonline.2000.15.6.309. [DOI] [PubMed] [Google Scholar]
- 64.Payne JA, Rivera C, Voipio J, et al. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 65.Yamada J, Okabe A, Toyoda H, et al. Cl- uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J Physiol. 2004;557:829–41. doi: 10.1113/jphysiol.2004.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang C, Shimizu-Okabe C, Watanabe K, et al. Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain. Brain Res Dev Brain Res. 2002;139:59–66. doi: 10.1016/s0165-3806(02)00536-9. [DOI] [PubMed] [Google Scholar]
- 67.Plotkin MD, Snyder EY, Hebert SC, et al. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA’s excitatory role in immature brain. J Neurobiol. 1997;33:781–95. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 68.Dzhala VI, Talos DM, Sdrulla DA, et al. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–13. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- 69.Robinson S, Mikolaenko I, Thompson I, et al. Loss of cation-chloride cotransporter expression in preterm infants with white matter lesions: implications for the pathogenesis of epilepsy. J Neuropathol Exp Neurol. 2010;69:565–72. doi: 10.1097/NEN.0b013e3181dd25bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saper CB. Any way you cut it: a new journal policy for the use of unbiased counting methods. J Comp Neurol. 1996;364:5. doi: 10.1002/(SICI)1096-9861(19960101)364:1<5::AID-CNE1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 71.West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- 72.Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–20. [PubMed] [Google Scholar]
- 73.Manaye KF, Zweig R, Wu D, et al. Quantification of cholinergic and select non-cholinergic mesopontine neuronal populations in the human brain. Neuroscience. 1999;89:759–70. doi: 10.1016/s0306-4522(98)00380-7. [DOI] [PubMed] [Google Scholar]
- 74.Benes FM, Vincent SL, Todtenkopf M. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiatry. 2001;50:395–406. doi: 10.1016/s0006-3223(01)01084-8. [DOI] [PubMed] [Google Scholar]
- 75.Kinney HC, Panigrahy A, Newburger JW, et al. Hypoxic-ischemic brain injury in infants with congenital heart disease dying after cardiac surgery. Acta Neuropathol. 2005;110:563–78. doi: 10.1007/s00401-005-1077-6. [DOI] [PubMed] [Google Scholar]
- 76.Kinney HC, Brody BA, Kloman AS, et al. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol. 1988;47:217–34. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- 77.Brody BA, Kinney HC, Kloman AS, et al. Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J Neuropathol Exp Neurol. 1987;46:283–301. doi: 10.1097/00005072-198705000-00005. [DOI] [PubMed] [Google Scholar]
- 78.Sen A, Martinian L, Nikolic M, et al. Increased NKCC1 expression in refractory human epilepsy. Epilepsy Res. 2007;74:220–7. doi: 10.1016/j.eplepsyres.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 79.Munoz A, Mendez P, DeFelipe J, et al. Cation-chloride cotransporters and GABA-ergic innervation in the human epileptic hippocampus. Epilepsia. 2007;48:663–73. doi: 10.1111/j.1528-1167.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- 80.Palma E, Amici M, Sobrero F, et al. Anomalous levels of Cl- transporters in the hippocampal subiculum from temporal lobe epilepsy patients make GABA excitatory. Proc Natl Acad Sci U S A. 2006;103:8465–8. doi: 10.1073/pnas.0602979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lawrence YA, Kemper TL, Bauman ML, et al. Parvalbumin-, calbindin-, and calretinin-immunoreactive hippocampal interneuron density in autism. Acta Neurol Scand. 2010;121:99–108. doi: 10.1111/j.1600-0404.2009.01234.x. [DOI] [PubMed] [Google Scholar]
- 82.Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathol. 2007;113:559–68. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]
- 83.Yip J, Soghomonian JJ, Blatt GJ. Increased GAD67 mRNA expression in cerebellar interneurons in autism: implications for Purkinje cell dysfunction. J Neurosci Res. 2008;86:525–30. doi: 10.1002/jnr.21520. [DOI] [PubMed] [Google Scholar]
- 84.Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD65 mRNA levels in select subpopulations of neurons in the cerebellar dentate nuclei in autism: an in situ hybridization study. Autism Res. 2009;2:50–9. doi: 10.1002/aur.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fatemi SH, Reutiman TJ, Folsom TD, et al. mRNA and protein levels for GABAAalpha4, alpha5, beta1 and GABABR1 receptors are altered in brains from subjects with autism. J Autism Dev Disord. 2010;40:743–50. doi: 10.1007/s10803-009-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oblak AL, Gibbs TT, Blatt GJ. Reduced GABA(A) receptors and benzodiazepine binding sites in the posterior cingulate cortex and fusiform gyrus in autism. Brain Res. 2011;1380:218–28. doi: 10.1016/j.brainres.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]