Abstract
Ginsenosides are the main active ingredients in ginseng and have recently been reported to have beneficial effects on the central nervous system. Ocotillol is a derivate of pseudoginsenoside-F11, which is an ocotillol-type ginsenoside found in American ginseng. We examined the effects of ocotillol (a) on neuronal activity of projection neurons, mitral cells (MC), in a mouse olfactory bulb brain slice preparation using whole-cell patch-clamp recording, and (b) on animal behavior by measuring locomotor activity of mice in vivo. Ocotillol displayed an excitatory effect on spontaneous action potential firing and depolarized the membrane potential of MCs. The effect was concentration-dependent with an EC50 of 4 μM. In the presence of blockers of ionotropic glutamatergic and gabaergic synaptic transmission (CNQX, 10 μM; D-AP5, 50 μM; gabazine, 5 μM), the excitatory effect of ocotillol on firing was abolished. Further experiments showed that the ocotillol-induced neuronal excitation persisted in the presence of GABAA receptor antagonist gabazine but was eliminated by applying AMPA/kainate receptor antagonist CNQX and NMDA receptor antagonist D-AP5, suggesting that ionotropic glutamate transmission was involved in mediating the effects of ocotillol. Bath application of ocotillol evoked an inward current as well as an increased frequency of spontaneous glutamatergic EPSCs. Both the inward current and sEPSCs could be blocked by ionotropic glutamate receptor antagonists CNQX and D-AP5. These results indicate that the excitatory action of ocotillol on MCs was mediated by enhanced glutamate release. Behavioral experiments demonstrated that ocotillol increased locomotor activities of mice. Our results suggest that ocotillol-evoked neuronal excitability was mediated by increased release of glutamate, which may be responsible for the increased spontaneous locomotor activities in vivo.
Keywords: ocotillol, ginsenosides, glutamate receptors, excitatory postsynaptic currents, locomotor activity, brain slice
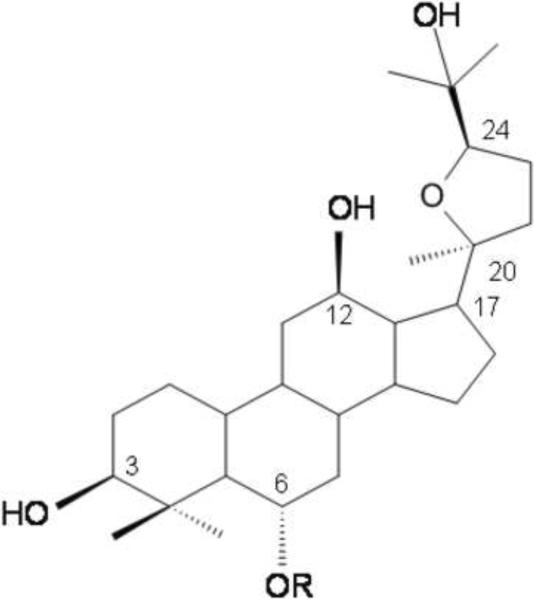
Ginseng has been used as a traditional medicine in Asia for thousands of years and is a popular natural medicine throughout the world. Ingredients of ginseng that have biological activity include more than 30 different compounds known as ginsenosides. Recent studies have reported that ginsenosides display beneficial effects on central nervous system (CNS) processes and disorders such as aging, deficit of memory and learning capabilities, and neurodegenerative diseases (Zhao et al., 2009; Yamaguchi et al., 1996; Mook-Jung et al., 2001; Li et al., 1999; Van et al., 2003; Yuan et al., 2001). Ginsenosides can be classified into four types of aglycones: protopanaxadiol, protopanaxatriol, ocotillol and oleanolic acid types (Zhu et al., 2004). Pseudoginsenoside-F11 is an ocotillol-type ginsenoside found in Panax quinquefolium (Zhang et al., 2010). As a major member of ocotillol-type ginsenosides, pseudoginsenoside-F11 increased the excitability of rat cardiac cells (Yang et al., 1994), had beneficial effects on memory impairment (Li et al., 1999), neurotoxicity (Wu et al., 2003) and morphine-induced dependence (Hao et al., 2007). Ocotillol is an aglycon of pseudoginsenoside-F11 in which the attached sugar moiety was removed (see Fig. 1). Recently, it has been prepared using methods of chemical degeneration (Ma et al., 2005). Even though a wide range of pharmacological activities of ginsenosides has been studied, the bioactivity of ocotillol and the mechanisms underlying its activity have not been reported.
Figure 1.
Chemical structure of ocotillol and pseudoginsenoside-F11. Ocotillol: R = −H; pseudoginsenoside-F11: R = −Glc2-Rha
Research into the structure-activity relationship of the antitumor effect of ginsenosides has shown that the activity of sapogenins is more potent than that of ginseng saponins (Wang et al., 2007). As an aglycon of pseudoginsenoside-F11, ocotillol may display similar bioactivities but differences in potency in vivo and in vitro. We hypothesized that ocotillol may be even more effective in modulating neuronal activity in the CNS since ocotillol is more lipophilic compared to pseudoginsenoside-F11 and thus has a more favorable blood-brain barrier permeability.
The excitability of neurons in the brain is an integral of intrinsic membrane conductances and synaptic inputs. Neurons such as mitral cells (MCs) in the mouse main olfactory bulb (MOB) display their neuronal excitability as spontaneous action potential firing, which can be modulated by membrane receptors as well as synaptic inputs (Heinbockel et al., 2004). Highly expressed proteins such as GABA receptors (GABAA, GABAB), Na+ channels, ionotropic and metabotropic glutamate receptors in MCs are involved in the modulation of neuronal excitability (Ennis et al., 2007). Thus, in this study, we used slices from the main olfactory bulb of mice to record MC activity using whole-cell patch-clamp recording. The effects of ocotillol on the activity of neurons, the underlying mechanism of its actions and its effects on locomotor behavior were determined. We report that ocotillol enhanced neuronal excitability which was mediated by increased release of glutamate. We also show that ocotillol had a positive influence on locomotor activity of mice in vivo.
EXPERIMENTAL PROCEDURES
Animals
Juvenile (16–25 days old) wild-type mice (C57BL/6J, Jackson Laboratory, Bar Harbor, ME) were used for slice preparation, and Male Swiss-Kunming mice weighing 18–23 g (supplied by the Experimental Animal Centre of Luye Pharmaceutical Company, Yantai, Shandong) were used for behavioral experiments. Both mice strains were used in agreement with Institutional Animal Care and Use Committee and NIH guidelines. The experimental procedures were approved by the local Committee on Animal Care and Use.
Behavioral experiments
Spontaneous locomotor activity of mice was evaluated in open field tests in an automated mode using an ambulometer (ZIL-2, Institute of Materia Medica, Chinese Academy of Medical Sciences, China). Animals were placed individually into a chamber, which consisted of opaque perspex walls and floors and transparent perspex lids. The chamber was equipped with infrared photobeams connected to a computer to collect spontaneous locomotor activity. The spontaneous locomotor activity (total accumulated counts of a horizontal single photobeam interruption) was collected for a 15-min period.
Slice preparation
Wild type juvenile mice were anesthetized with 2-bromo-2-chloro-1,1,1-trifluoroethane (Sigma, St. Louis, MO), and decapitated, the MOBs were dissected out and immersed in artificial cerebrospinal fluid (ACSF, see below) at 4°C, as previously described (Heinbockel et al., 2004). Horizontal slices (400 μm-thick) were cut parallel to the long axis using a vibratome (Vibratome Series 1000, Ted Pella Inc., Redding, CA). Slices were incubated in a holding bath in normal ACSF equilibrated with carbogen (95% O2 – 5% CO2) at room temperature (22°C). After recovery for 30 min, the slices were used. For recording, a brain slice was placed in a recording chamber mounted on a microscope stage and maintained at 30 ± 0.5°C by superfusion with oxygenated ACSF flowing at 2.5–3 ml/min.
Electrophysiological Recording and Data Acquisition
Visually-guided recordings were obtained from MCs in the mitral cell layer of the MOB with near-infra red differential interference contrast optics and a BX51WI microscope (Olympus Optical, Tokyo, Japan) equipped with a camera (C2400-07, HamamatsuPhotonics, Japan). Images were displayed on a Sony Trinitron Color Video monitor (PVM-1353MD, Sony Corp. Japan). Recording pipettes (5–8 MΩ) were pulled on a Flaming-Brown P-97 puller (Sutter Instrument Co., Novato, CA) from 1.5 mm O.D. borosilicate glass with filament (Sutter Instrument Co., Novato, CA). Liquid junction potential was 9–10 mV and reported measurements were not corrected for this potential. Data were obtained using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA) and a Digidata 1440A Interface (Molecular Devices) and digitized at 10 kHz. Data acquisition was controlled by Clampex 10.1 (Molecular Devices). Signals were low-pass Bessel filtered at 2 kHz and saved on computer disc. Membrane resistance was calculated from the difference of voltage value obtained by injecting −100 pA current to recorded cells to hyperpolarize the cell. The detection of events [intracellularly recorded excitatory postsynaptic currents (EPSCs)] was performed off-line using Mini Analysis program (Synaptosoft, Decatur, GA). Membrane potentials were calculated from the steady-state membrane potential that occurred after an action potential (resting potential) during control or drug treatment, respectively.
The ACSF consisted of (in mM): NaCl 124, KCl 3, CaCl2 2, MgSO4 1.3, glucose 10, sucrose NaHCO3 26, NaH2PO4 1.25 (pH 7.4, 300 mOsm), saturated with 95 O2/5% CO2 (modified from Heyward et al., 2001). For intracellular recording of spiking activity, pipette-filling solution consisted of (mM) K gluconate 125, MgCl2 2, HEPES 10, Mg2ATP 2, Na3GTP 0.2, NaCl 1, EGTA 0.2. For intracellular recordings of EPSCs, electrodes were filled with a solution of the following composition (in mM): 125 cesium methanesulfonate CsMeSO3, 1 NaCl, 10 phosphocreatine ditris salt, 2 MgATP, 0.3 GTP, 0.5 EGTA, 10 HEPES, and 10 QX-314 [2(triethylamino)-N-(2,6-dimethylphenyl) bromide], pH 7.3 with 1N CsOH (osmolarity, 290 Osm).
Drugs
The following drugs were applied during the experiments: ocotillol (purity > 92%, provided by Luye Pharmaceutical Company, China), L-2-amino-5-phosphonopentanoic acid (D-AP5), 6-cyano-7-nitroquinoxaline-2-3-dione (CNQX), (2-(3-carboxypropyl)-3-amino-6-(4 methoxyphenyl)-pyridazinium bromide (gabazine, SR-95531). Ocotillol was dissolved in dimethyl sulfoxide (DMSO) to make 10 mM stock solution (final concentration of DMSO in bath < 0.1%). For all experiments, the drugs were applied by bath perfusion that was made by dissolving aliquots of stock in the ACSF. Control recordings showed that 0.1% DMSO had no detectable effects on the firing rate and membrane potential of MCs. Chemicals and drugs were supplied by Sigma-Aldrich (St. Louis, MO) and Tocris (Ellisville, MO).
Numerical data are expressed as the mean ± SEM. Tests for statistical significance (p <0.05) were performed using paired Student's t-tests, and one-way ANOVA followed by the Bonferroni test for multiple comparisons.
RESULTS
Ocotillol increased spiking activity of mitral cells
The MOB contains principal neurons (mitral cells, MCs) that transmit olfactory information to higher order olfactory structures. MCs play a crucial role in processing sensory information in MOB. They receive direct synaptic inputs from the axons of olfactory receptor neurons, send excitatory projections to piriform cortex, and receive strong feedback inhibition primarily through reciprocal dendrodendritic synapses (Shepherd et al., 2004; Ennis et al., 2007). In slices, MCs generate spontaneous action potentials in the range of one to six Hz. Here, we took advantage of the intrinsic properties of MCs such as spontaneous firing, membrane potential, membrane currents, and synaptic inputs such as spontaneous EPSCs to investigate the actions of ocotillol on neuronal activity and the possible cellular mechanism underlying the behavioral action of ocotillol.
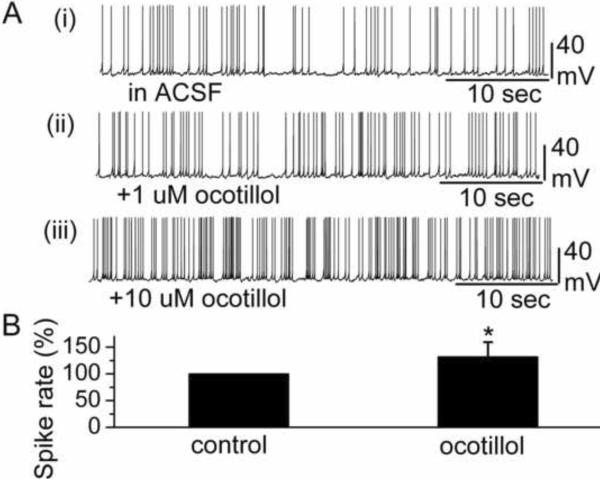
MCs were identified visually by their soma location and relatively large soma size, and by their input resistance (283.3 ± 31.6 MΩ, n = 19). The membrane potential of MCs in this study was −51.5 ± 1.9 mV (n=19). Bath application of ocotillol (5 μM) reversibly increased the MC firing rate (in control: 3.2 ± 0.5 Hz; in ocotillol: 4.0 ± 0.6 Hz) (n = 12; p < 0.001, paired t test) (Fig. 2B). The increased spiking was accompanied by depolarization of the membrane potential. Compared to control conditions, 5 μM ocotillol depolarized MCs by 1.1 ± 0.1 mV (n = 12; p < 0.001, paired t test). Fig. 2A shows an individual recording trace from one MC cell in the absence and presence of ocotillol.
Figure 2.
Ocotillol enhanced the spike rate of MCs. A Original recording from a representative MC illustrated the increased spike rate following bath application of ocotillol. The traces labeled (i), (ii), (iii) show the original recording from a MC in control condition, 1 μM ocotillol and 10 μM ocotillol, respectively. B A normalized and averaged bar graph showed the increase in spontaneous spiking of MCs. The spike rate in the presence of 5 μM ocotillol was normalized with respect to control condition (n = 12, * p < 0.001).
Concentration-response curve of ocotillol
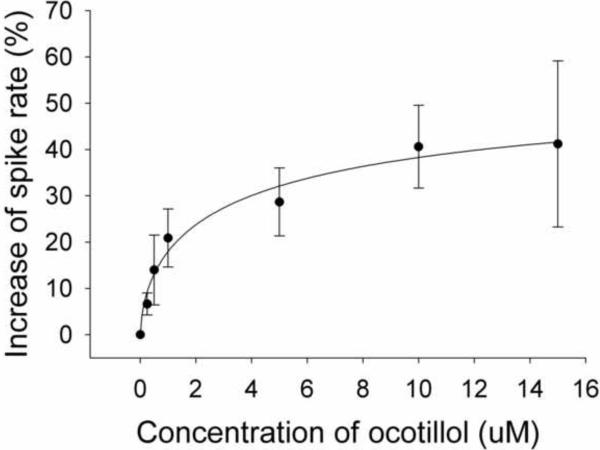
To characterize the excitatory effects of ocotillol on neurons, the concentration-response relationship for ocotillol was determined (Fig. 3). The averaged data for the effect of ocotillol on the firing rate of MCs at varying concentrations was fit to the Hill equation to estimate an EC50 and maximal excitation. Ocotillol generated a concentration-dependent enhancement of the firing rate of MCs. The estimated maximal increase of the firing rate was 60.1%, and the estimated value of EC50 was 4.0 μM.
Figure 3.
Concentration-response curves for the enhancement of spiking of MCs by ocotillol. The spike rates in the presence of ocotillol were normalized with respect to the control condition, and averaged (each point was the mean ± SEM of 4 to 12 cells). The lines are fits for the data to the equation y = Axn/(Kdn + xn), where y is the increase of spiking rate, A is maximal inhibition, Kd is the apparent dissociation constant for ocotillol, and n is the Hill coefficient. Kd and A were estimated using a Marquadt nonlinear least-squares routine.
The excitatory effect of ocotillol was abolished in the presence of blockers of fast synaptic transmission
Excitatory ionotropic glutamate receptors and inhibitory GABAA receptors are highly expressed in MCs. They play a critical role in the regulation of neuronal excitability (Ennis et al., 2007). Either activation of ionotropic glutamate receptors or blockade of GABA receptors may enhance neuronal excitability. To determine whether the excitatory effect of ocotillol was mediated by ionotropic glutamate receptors or GABA receptors, we examined the ocotillol-evoked increase of MC activity in the presence of blockers of ionotropic glutamate receptors and GABAA receptors.
A cocktail of synaptic blockers (gabazine, 5 μM; CNQX, 10 μM; D-AP5, 50 μM) can be used to efficiently block fast synaptic transmission to MCs (Heinbockel et al., 2004). Among these receptor inhibitors, CNQX is a potent AMPA/kainate receptor antagonist, D-AP5 is a potent NMDA receptor antagonist and gabazine is a GABAA receptor antagonist. In the presence of the cocktail of synaptic blockers, 5 μM ocotillol failed to enhance the neuronal excitability of MCs. Compared to control condition, in synaptic blockers (3.9 ± 0.4 Hz), the ocotillol-evoked excitatory effect on MCs was completely blocked (3.7 ± 0.4 Hz, n = 4, p > 0.05, paired t test). The results showed that blockade of GABAA receptor and ionotropic glutamate receptors reversed ocotillol-evoked excitatory action, suggesting that either synaptic transmission via GABA receptors or ionotropic glutamate receptors was involved in the excitatory effect of ocotillol.
Ocotillol-evoked neuronal excitation was not mediated by GABA receptors, but by glutamatergic synaptic transmission
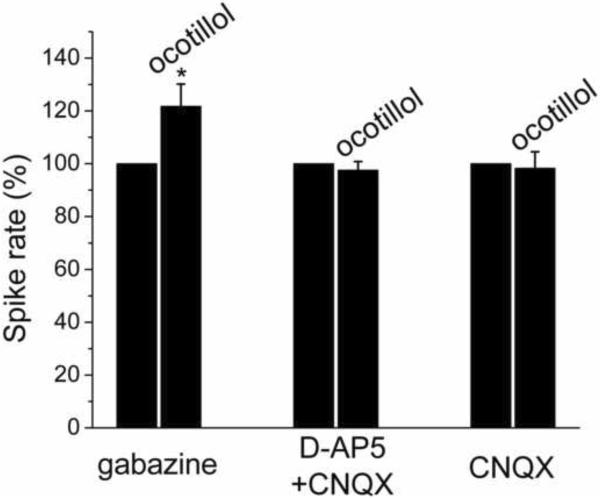
Subsequently, the role of GABAA receptors for the observed ocotillol-induced excitatory effect was examined by applying ocotillol in the presence of the GABAA receptor blocker gabazine. Fig. 4 is a normalized and averaged bar graph, showing the increased spike rate of MCs in response to ocotillol in the presence of gabazine. With gabazine present, ocotillol (5 μM) increased the spike rate from 3.6 ± 0.5 Hz to 4.4 ± 0.5 Hz (n = 4; p < 0.05, paired t test) and depolarized MCs by 1.14 ± 0.40 mV (n = 4; p < 0.05, paired t test). The persistence of the excitatory ocotillol effect in gabazine indicated that GABAA receptors were not involved in the excitatory action of ocotillol on MCs. The change in spike rate and membrane potential in the presence of gabazine in response to ocotillol was not different from ocotillol-evoked effects in control condition (in ACSF, see Fig. 2) (p > 0.05 determined by ANOVA and Bonferroni post-hoc analysis) (firing rate: p = 0.64409; Vm: p = 0.35841).
Figure 4.
The ocotillol-induced increase of MC spike rate was mediated by ionotropic glutamate receptors. The data were normalized to the control condition in the presence of gabazine (n = 4; * p < 0.05, paired t test), or D-AP5 + CNQX (n = 13; p > 0.05, paired t test), or CNQX (n = 7, p > 0.05, paired t test), respectively.
The NMDA receptor is an important type of ionotropic glutamate receptor that controls neuronal excitability. AMPA/kainate receptors are non-NMDA type ionotropic trans-membrane receptors for glutamate that mediate fast synaptic transmission in the central nervous system (CNS). Blockade of NMDA and AMPA/kainate receptors will inhibit the excitability of neurons. Therefore, we determined the role of NMDA and AMPA/kainate receptors for ocotillol-induced excitatory activity of MCs. In the presence of NMDA receptor antagonist D-AP5 (50 μM) and AMPA/kainate receptor antagonist CNQX (10 μM), the spike rate and membrane potential of MCs were not significantly modified by applying ocotillol (spike rate: 2.8 ± 0.4 Hz in D-AP5+CNQX vs. 2.9 ± 0.5 Hz in D-AP5+CNQX plus ocotillol, n = 13; p > 0.05, paired t test; membrane potential: 50.5 ± 0.7 mV vs. 50.1 ± 0.8 mV, n = 13; p > 0.05, paired t test) (Fig. 4). The results suggested that ionotropic glutamate receptors contributed to the excitatory effect of ocotillol.
Ocotillol was also bath applied in the presence of CNQX (10 μM) alone. In the presence of the CNQX, ocotillol (5 μM) failed to increase the firing rate of MCs. Compared to the control condition in CNQX (3.0 ± 0.4 Hz), no change in MC activity was observed in response to ocotillol (in CNQX plus ocotillol: 3.1 ± 0.5 Hz, n = 7, p > 0.05, paired t test; change in membrane potential: 0.3 ± 0.2 mV, n = 7, p > 0.05, paired t test). The blockade of AMPA/kainate receptors prevented the ocotillol-evoked increase of MC activity which indicated that ocotillol either enhanced glutamate levels in the slice or directly acted on AMPA/kainate receptors.
Ocotillol induced inward currents and enhanced EPSCs in a concentration-dependent manner
MC dendrites and somata express high levels of ionotropic glutamate receptors, NMDA, AMPA, and kainate receptors. MC apical dendrites respond to glutamate released from the olfactory nerve (ON) via AMPA and NMDA receptors (Ennis et al., 2001, 2007; Aroniadou-Anderjaska et al., 1999, 2000). Glutamate released from glutamatergic neurons such as olfactory receptor neurons at ON terminals or MCs/tufted cell activates MCs, evokes inward currents, and increases sEPSC frequency (Ennis et al., 2007).
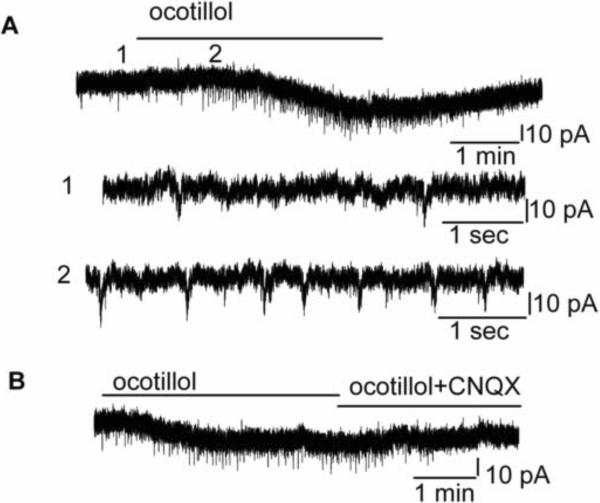
Further evidence for the excitatory effects of ocotillol was obtained by measuring MC ionic currents using voltage-clamp recording (Fig. 5A). The holding potential was set to −60 mV. Ocotillol (1 μM) evoked an inward current of 19.5 ± 3.5 pA in MCs (n = 8; the steady state currents at −60 mV in ocotillol were measured and subtracted from that in ACSF). In the presence of D-AP5 and CNQX, the ocotillol-evoked inward currents were blocked (0.6 ± 1.1 pA, n = 5, p > 0.05, paired t test).
Figure 5.
Ocotillol elicited inward currents, and increased spontaneous EPSCs in MCs. A. Original recording illustrates an ocotillol-evoked inward current of a MC and enhancement of sEPSCs. Time points 1 and 2 in the upper trace are shown at higher time resolution in the second and third trace, resp. B. The sEPSCs were abolished by CNQX. A and B are from the same cell.
In addition to the evoked inward currents, bath application of ocotillol also increased the frequency of spontaneous glutamatergic EPSCs. Fig. 5A, B illustrates the inward current and enhanced sEPSC frequency evoked by ocotillol in a representative MC. Ocotillol (5 μM) increased sEPSC frequency from 0.2 ± 0.1 Hz to 0.5 ± 0.1 Hz (n = 6, p < 0.05, paired t test), indicating that the ocotillol-evoked excitatory effects was mediated by increased glutamate. The sEPSCs could be blocked by CNQX or blockers of ionotropic glutamate receptors (CNQX plus D-AP5), indicating that sEPSCs were mediated by ionotropic glutamate receptors, mostly by AMPA receptors.
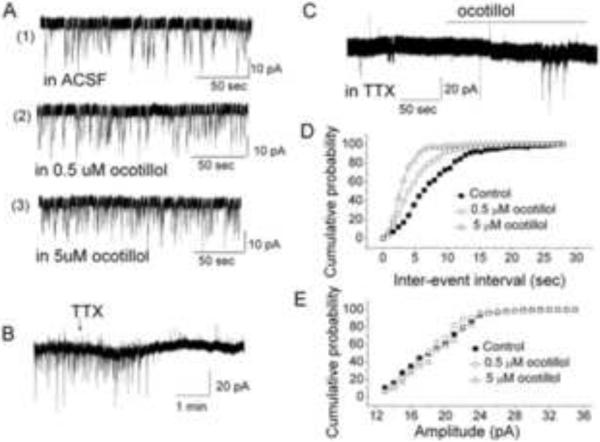
The change of sEPSC frequency evoked by ocotillol was determined at two drug concentrations (0.5 μM and 5 μM). Bath application of ocotillol resulted in a concentration-dependent increase of sEPSC frequency in MCs. Fig. 6A shows the original recording from a representative MC. With 0.5 μM and 5 μM ocotillol, the averaged cumulative inter-sEPSC interval distribution exhibited a leftward shift, or shorter inter-sEPSC intervals (Fig. 6D). The averaged cumulative sEPSC amplitude distribution did not show a change with 0.5 μM and 5 μM ocotillol (Fig. 6E). To determine a pre- or postsynaptic localization of the action of ocotillol, TTX was included in the ACSF solution to block action-potential dependent glutamate release. Under this condition, 1 μM TTX completely blocked sEPSCs in most of the cells tested (Fig. 6B). Only one of eight MCs displayed a few sEPSCs, and, in this cell, ocotillol increased the frequency of sEPSCs (Fig. 6C), indicating that most spontaneous glutamate release was action-potential dependent from MCs and not from olfactory receptor neurons at ON terminals. The result is consistent with the observation that MCs release glutamate and produce self-excitation (Ennis et al., 2007).
Figure 6.
Ocotillol affects glutamate release in MCs. A Ocotillol increased sEPSC frequency in a concentration-dependent manner. Original recording from a representative MC showing sEPSCs in control (ACSF), 0.5 μM and 5 μM ocotillol. B TTX (1 μM) blocked sEPSCs in most mitral cells. C Ocotillol (5 μM) increased sEPSCs in one mitral cell in the presence of TTX. D Cumulative inter-sEPSC interval distributions. Ocotillol evoked a left shift of the curves, n = 4. E Cumulative amplitude distributions in the absence and presence of ocotillol. n = 4.
Ocotillol increased spontaneous locomotor activity of mice
The experiments described above suggested that ocotillol displayed the characteristic of a neuronal stimulant due to its ability to increase glutamate release. An increase in glutamate receptor activity can result in increased activity of principal neurons in the brain, and might be responsible for changes in locomotor activity of animals. Therefore, we determined whether ocotillol regulates spontaneous locomotor activity of mice in vivo.
The effects of ocotillol on spontaneous locomotor activity were measured using the optical beam technique (Liu et al., 2008). Male mice were randomly divided into 1 control group (saline) and 4 treatment groups to test four different ocotillol doses. Ocotillol was injected (i.p.) once a day for 3 days. After administration of ocotillol (dose at 1, 2, 4, 8 mg/kg, i.p.) on the third day and a waiting period of 30 min, the animals were placed individually into the test chamber to collect data on spontaneous locomotor activity for a 15-min period.
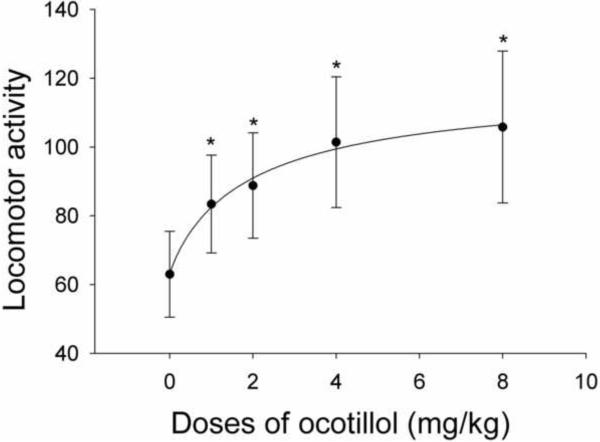
As shown in Fig. 7, ocotillol significantly increased the spontaneous locomotor activity of mice when administered at doses of 1–8 mg/kg. The behavioral effect of ocotillol displayed dose-dependency and was detectable even at the lowest dose of 1 mg/kg. The averaged locomotor activities induced by varying doses of ocotillol were well fit by the Hill equation, and gave rise to an estimated EC50 of 2.0 mM/kg. The similar shape of the concentration-response curve in vitro (Fig. 3) and the dose-response curve in vivo (Fig. 7) suggested that the ocotillol-evoked activation of principal neurons, which was mediated by glutamate, may be related to the increased spontaneous locomotor activity evoked by ocotillol in vivo.
Figure 7.
Dose-response curve of the ocotillol-evoked increase of spontaneous activity of mice. Each point was the mean value ± SEM of 30 mice. The line is the fit of the data to the Hill equation: y = Y0 + Axn/(Kdn + xn), where y is locomotor activities, Y0 is minimal locomotor activities, A is maximal enhanced activities, Kd is the apparent dissociation constant for agents, and n is the Hill coefficient. Kd was estimated using a Marquadt nonlinear least-squares routine. * indicates a statistical difference from the control group (saline) alone. p < 0.05 determined by ANOVA and Bonferroni post-hoc analysis.
DISCUSSION
In the present study, we demonstrate that ocotillol has excitatory effects on a principal neuronal type in the brain, namely MCs in the MOB. The excitatory action of ocotillol was concentration-dependent with an estimated EC50 value of 4.0 μM. In the presence of fast synaptic blockers, the excitatory action of ocotillol on spontaneous action potential firing was abolished. Further electrophysiological dissection of the increased excitability of MCs revealed that inhibitory GABAA receptors were not involved in mediating the effect of ocotillol, but the effect could be blocked by NMDA receptor antagonist D-AP5 and AMPA/kainate receptor antagonist CNQX. The increased frequency of spontaneous glutamatergic EPSCs evoked by ocotillol implies that ocotillol enhanced neuronal activity by increasing release of glutamate in the MOB. The increased locomotor activity in response to ocotillol in vivo indicates that ocotillol acts as a neuronal stimulant. The excitatory actions of ocotillol in vivo are consistent with the electrophysiological results in vitro, implying that the glutamate-mediated neuronal activity in vitro may result in a locomotor activity increase in vivo.
MCs in the MOB offer three advantages to test the molecular and cellular mechanisms underlying the action of ocotillol. Firstly, MCs are principal neurons in the MOB and functional proteins such as GABAA receptors, sodium channels, ionotropic and metabotropic glutamate receptors are highly expressed in MCs (Ennis et al., 2007). Secondly, MCs exhibit spontaneous action potential firing, and all the above-mentioned proteins participate in the regulation of neuronal excitability. Thirdly, the activity of MCs is regulated by olfactory input and synaptic transmission in the MOB. Thus, MCs in the MOB slice serve as a good model to test the effects of medicinal compounds on neuronal activity and to determine the mechanisms underlying the compound's effects.
In most of the commercially available ginseng-derived products, major and abundant components consist of ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, Rg1 and others (Harkey et al., 2001). Biological, physiological, and physico-chemical factors influence the absorption, distribution, metabolism, and excretion (ADME) of ginsenosides in the body. These factors limit systemic exposure to most ginsenosides and their deglycosylated metabolites, and result in a low oral bioavailability of ginsenosides (Xu at al., 2003). Studies about metabolism and pharmacokinetics of ginsenosides have demonstrated that most ginsenosides are poorly absorbed in the gastrointestinal tract (Tawab et al., 2003; Xu et al., 2003; Akao et al., 1998). In general, ginsenosides display poor membrane permeability. Some ginsenosides are transformed in the gastrointestinal tract by gastric acid and microflora or are deglycosylated by intestinal bacteria (Hasegawa et al., 1997; Bae et al., 2000; Lee et al., 2002; Karikura et al., 1990). Therefore, the biologically active constituents and their plasma concentration of ginsenosides in the body become very complicated which may limit their pharmaceutical efficacy to treat CNS disorders. As an aglycon of pseudoginsenoside-F11, ocotillol may display a higher bioavailability because of its more lipophilic property, absence of deglycosylation by intestinal bacteria, and better membrane permeability than pseudoginsenoside-F11. Therefore, it is reasonable to speculate that ocotillol might be more efficacious to treat CNS disorders than pseudoginsenoside-F11.
Ginsenosides exhibit different neuronal activities and various molecular mechanisms are responsible for these activities. Recent studies have found that ginsenosides display inhibitory effects on voltage-dependent and ligand-gated ion channels. For example, it was observed that Rg3 inhibits voltage dependent Ca2+ (P/Q-type), K+ (Kv1.4), and Na+ (Nav1.2 and Nav1.5) channel activities (Jeong et al. 2004). The actions of ginsenosides on ion channels imply that they may modulate neuronal activity. Indeed, application of extracts of Panax quinquefolius (mostly containing ginsenosides) to brainstem compartments was reported to modulate neuronal discharge frequency in brainstem unitary activity (Yuan et al., 2001). More recently, ginsenosides were observed to influence the glutamate system (Chang et al., 2008). The action of ginsenoside Rg1 or Rb1 on the glutamate exocytotic system was enhanced, which was mediated by activation of protein kinase C. Facilitation of glutamate exocytosis by ginsenosides may be a potentially important mechanism to strengthen the central glutamatergic system during learning and memory processes. Meanwhile, it was reported that ginsenoside Rh2, Rg1 and Rb3 display inhibitory effects on NMDA receptors (Lee et al., 2006; Peng et al., 2009; Zhang et al., 2008). Ginsenoside Rh2 and 20(S)-protopanaxadiol inhibit the release of Na+ channel-activated amino acid neurotransmitters in the mammalian brain (Duan and Nicholson, 2008). In this study, we found that ocotillol modulates neuronal activity by increasing glutamate release. Therefore, the modulation of the glutamatergic system by ginsenosides varies with the chemical structures of ginsenosides. Our results provide direct evidence that an ocotillol-type compound exhibits its modulatory effect on the gluamatergic system.
Here we show that the shape of the concentration-response curve in vitro and dose-response curve in vivo is similar, implying that the ocotillol-evoked enhancement of glutamate transmission may be responsible for the increased spontaneous locomotor activities in vivo. Strengthening the central glutamatergic system by ocotillol may be beneficial in learning and for memory deficits in vivo.
CONCLUSION
In conclusion, this study demonstrated that the ocotillol-evoked neuronal excitability was mediated by increased release of glutamate, which may be responsible for the increased spontaneous locomotor activities in vivo.
Ocotillol is a ginsenoside and a main active ingredient found in American ginseng.
Ocotillol increases firing and depolarizes mitral cells in olfactory bulb slices.
The effect of ocotillol on mitral cells results from enhanced glutamate release.
In behavioral experiments, ocotillol increases locomotor activities of mice.
The behavioral effect might result from ocotillol-evoked neuronal excitability.
Acknowledgments
This work was supported in part by the Howard University New Faculty Research Program, Howard University Health Sciences Faculty Seed Grant Program, the Whitehall Foundation, National Institutes of Health National Institute of General Medical Sciences (S06GM08016), National Institutes of Health National Institute of Neurological Disorders and Stroke (U54NS039407), National Institutes of Health Division of Research Infrastructure, National Center for Research Resources, RCMI Program (2G12 RR003048).
Abbreviations
- ACSF
artificial cerebrospinal fluid
- NMDA
N-methyl-D-aspartate
- MOB
main olfactory bulb
- MC
mitral cells
- CNS
central nervous system
- EPSCs
excitatory postsynaptic currents
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Akao T, Kida H, Kanaoka M, Hattori M, Kobashi K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J Pharm Pharmacol. 1998;50:1155–1160. doi: 10.1111/j.2042-7158.1998.tb03327.x. [DOI] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Ennis M, Shipley MT. Current-source density analysis in the rat olfactory bulb: laminar distribution of kainate/AMPA and NMDA receptor-mediated currents. J Neurophysiol. 1999;81:15–28. doi: 10.1152/jn.1999.81.1.15. [DOI] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. J Neurophysiol. 2000;84:1194–1203. doi: 10.1152/jn.2000.84.3.1194. [DOI] [PubMed] [Google Scholar]
- Bae EA, Park SY, Kim DH. Constitutive beta-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol Pharm Bull. 2000;23:1481–1485. doi: 10.1248/bpb.23.1481. [DOI] [PubMed] [Google Scholar]
- Chang Y, Wang S-J. Ginsenoside Rg1 and Rb1 enhance glutamate exocytosis from rat cortical nerve terminals by affecting vesicle mobilization through the activation of protein kinase C. Eur J Pharmacol. 2008;590:74–79. doi: 10.1016/j.ejphar.2008.05.032. [DOI] [PubMed] [Google Scholar]
- Duan Y, Nicholson RA. 20(S)-protopanaxadiol and the ginsenoside Rh2 inhibit Na+ channel-activated depolarization and Na+ channel-dependent amino acid neurotransmitter release in synaptic fractions isolated from mammalian brain. Comp Biochem Physiol C Toxicol Pharmacol. 2008;147:351–356. doi: 10.1016/j.cbpc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Ennis M, Hamilton KA, Hayar A. Neurochemistry of the main olfactory system. In: Lajtha A, editor. Handbook of Neurochemistry and Molecular Neurobiology. 3rd edition. vol. 20. Springer; Heidelberg: 2007. pp. 137–204. Sensory Neurochemistry (Johnson, D.A., Volume Editor) [Google Scholar]
- Ennis M, Zhou F-M, Ciombor K, Aroniadou-Anderjaska V, Hayar A, Borrelli E, Zimmer LA, Margolis F, Shipley MT. Dopamine D2 receptor-mediated presynaptic inhibition of olfactory nerve terminals. J Neurophysiol. 2001;86:2986–2997. doi: 10.1152/jn.2001.86.6.2986. [DOI] [PubMed] [Google Scholar]
- Harkey MR, Henderson GL, Gershwin ME, Stem JS, Hackman RS. Variability in commercial ginseng products: an analysis of 25 preparations. Am J Clin Nutr. 2001;73:1101–1106. doi: 10.1093/ajcn/73.6.1101. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Sung JH, Benno Y. Role of human intestinal Prevotella oris in hydrolyzing ginseng saponins. Planta Med. 1997;63:436–440. doi: 10.1055/s-2006-957729. [DOI] [PubMed] [Google Scholar]
- Hao Y, Yang JY, Wu CF, Wu MF. Pseudoginsenoside-F11 decreases morphine-induced behavioral sensitization and extracellular glutamate levels in the medial prefrontal cortex in mice. Pharmacol Biochem Behav. 2007;86:660–666. doi: 10.1016/j.pbb.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Heinbockel T, Heyward P, Conquet F, Ennis M. Regulation of main olfactory bulb mitral cell excitability by metabotropic glutamate receptor mGluR1. J Neurophysiol. 2004;92:3085–3096. doi: 10.1152/jn.00349.2004. [DOI] [PubMed] [Google Scholar]
- Heyward P, Ennis M, Keller A, Shipley MT. Membrane bistability in olfactory bulb mitral cells. J Neurosci. 2001;21:5311–5320. doi: 10.1523/JNEUROSCI.21-14-05311.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SM, Lee JH, Kim JH, Lee BH, Yoon IS, Lee JH, Kim DH, Rhim H, Kim Y, Nah SY. Stereospecificity of ginsenoside Rg3 action on ion channels. Mol Cells. 2004;18(3):383–389. [PubMed] [Google Scholar]
- Karikura M, Miyase T, Tanizawa H, Takino Y, Taniyama T, Hayashi T. Studies on absorption, distribution, excretion and metabolism of ginseng saponins. V. The decomposition products of ginsenoside Rb2 in the large intestine of rats. Chem Pharm Bull (Tokyo) 1990;38:2859–2861. doi: 10.1248/cpb.38.2859. [DOI] [PubMed] [Google Scholar]
- Lee DS, Kim YS, Ko CN, Cho KH, Bae HS, Lee KS, Kim JJ, Park EK, Kim DH. Fecal metabolic activities of herbal components to bioactive compounds. Arch Pharm Res. 2002;25:165–169. doi: 10.1007/BF02976558. [DOI] [PubMed] [Google Scholar]
- Lee E, Kim S, Chung KC, Choo MK, Kim DH, Nam G, Rhim H. 20(S)-ginsenoside Rh2, a newly identified active ingredient of ginseng, inhibits NMDA receptors in cultured rat hippocampal neurons. Eur J Pharmacol. 2006;536:69–77. doi: 10.1016/j.ejphar.2006.02.038. [DOI] [PubMed] [Google Scholar]
- Li Z, Guo YY, Wu CF, Li X, Wang JH. Protective effects of pseudoginsenoside-F11 on scopolamine-induced memory impairment in mice and rats. J Pharm Pharmacol. 1999;51:435–440. doi: 10.1211/0022357991772484. [DOI] [PubMed] [Google Scholar]
- Liu YL, Semjonous NM, Murphy KG, Ghatei MA, Bloom SR. The effects of pancreatic polypeptide on locomotor activity and food intake in mice. Int J Obes (Lond) 2008;32:1712–1715. doi: 10.1038/ijo.2008.160. [DOI] [PubMed] [Google Scholar]
- Ma SG, Jiang YT, Song SJ, Wang ZH, Bai J, Xu SX, Liu K. Alkaline-degradation products of ginsenosides from leaves and stems of Panax quinquefolium. Yao Xue Xue Bao. 2005;40:924–930. [PubMed] [Google Scholar]
- Mook-Jung I, Hong HS, Boo JH, Lee KH, Yun SH, Cheong MY, Joo I, Huh K, Jung MW. Ginsenoside Rb1 and Rg1 improve spatial learning and increase hippocampal synaptophysin level in mice. J Neurosci Res. 2001;63:509–515. doi: 10.1002/jnr.1045. [DOI] [PubMed] [Google Scholar]
- Peng LL, Shen HM, Jiang ZL, Li X, Wang GH, Zhang YF, Ke KF. Inhibition of NMDA receptors underlies the neuroprotective effect of ginsenoside Rb3. Am J Chin Med. 2009;37:759–770. doi: 10.1142/S0192415X09007223. [DOI] [PubMed] [Google Scholar]
- Shepherd GW, Chen WR, Greer CA. Olfactory bulb. In: Shepherd GM, editor. The Synaptic Organization of the Brain. New York, Oxford: 2004. pp. 165–216. [Google Scholar]
- Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- Van Kampen J, Robertson H, Hagg T, Drobitch R. Neuroprotective actions of the ginseng extract G115 in two rodent models of Parkinson's disease. Exp Neurol. 2003;184:21–29. doi: 10.1016/j.expneurol.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhao YQ, Rayburn ER, Hill DL, Wang H, Zhang R. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother Pharmacol. 2007;59:589–601. doi: 10.1007/s00280-006-0300-z. [DOI] [PubMed] [Google Scholar]
- Wu CF, Liu YL, Song M, Liu W, Wang JH, Li X, Yang JY. Protective effects of pseudoginsenoside-F11 on methamphetamine-induced neurotoxicity in mice. Pharmacol Biochem Behav. 2003;76:103–109. doi: 10.1016/s0091-3057(03)00215-6. [DOI] [PubMed] [Google Scholar]
- Xu QF, Fang XL, Chen DF. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmacol. 2003;84:187–192. doi: 10.1016/s0378-8741(02)00317-3. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Higashi M, Kobayashi H. Effects of ginsenosides on impaired performance caused by scopolamine in rats. Eur J Pharmacol. 1996;312:149–151. doi: 10.1016/0014-2999(96)00597-3. [DOI] [PubMed] [Google Scholar]
- Yang SJ, Chen X, Zhang WJ. Effect of PQS-pseudoginsenoside-F11 on action potential of cultivated rat cardiac cells. Acta Pharmacol Sin. 1994;10:284–287. [Google Scholar]
- Yuan CS, Wang X, Wu JA, Attele AS, Xie JT, Gu M. Effects of Panax quinquefolius L. on brainstem neuronal activities: comparison between Wisconsin-cultivated and Illinois-cultivated roots. Phytomedicine. 2001;8(3):178–183. doi: 10.1078/0944-7113-00027. [DOI] [PubMed] [Google Scholar]
- Zhao H, Li Q, Pei X, Zhang Z, Yang R, Wang J, Li Y. Long-term ginsenoside administration prevents memory impairment in aged C57BL/6J mice by up-regulating the synaptic plasticity-related proteins in hippocampus. Behav Brain Res. 2009;201:311–317. doi: 10.1016/j.bbr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Zhu S, Zou K, Cai S, Meselhy MR, Komatsu K. Simultaneous Determination of Triterpene Saponins in Ginseng Drugs by High-Performance Liquid Chromatography. Chem Pharm Bull (Tokyo) 2004;52:995–998. doi: 10.1248/cpb.52.995. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ma X, Si B, Zhao Y. Simultaneous determination of five active hydrolysis ingredients from Panax quinquefolium L. by HPLC-ELSD. Biomed Chromatogr. 2010 Aug 25; doi: 10.1002/bmc.1495. DOI: 10.1002/bmc.1495 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Zhang YF, Fan XJ, Li X, Peng LL, Wang GH, Ke KF, Jiang ZL. Ginsenoside Rg1 protects neurons from hypoxic-ischemic injury possibly by inhibiting Ca2+ influx through NMDA receptors and L-type voltage-dependent Ca2+ channels. Eur J Pharmacol. 2008;586:90–99. doi: 10.1016/j.ejphar.2007.12.037. [DOI] [PubMed] [Google Scholar]