Abstract
Objective
The purpose of this study was to review the relationship between education and dementia.
Methods
A systematic literature review was conducted of all published studies examining the relationship between education and dementia listed in the PubMed and PsycINFO databases from January 1985 to July 2010. The inclusion criteria were a measure of education and a dementia diagnosis by a standardized diagnostic procedure. Alzheimer’s disease and Total Dementia were the outcomes.
Results
A total of 88 study populations from 71 articles met inclusion criteria. Overall, 51 (58%) reported significant effects of lower education on risk for dementia whereas 37 (42%) reported no significant relationship. A relationship between education and risk for dementia was more consistent in developed compared to developing regions. Age, gender, race/ethnicity, and geographical region moderated the relationship.
Conclusions
Lower education was associated with a greater risk for dementia in many but not all studies. The level of education associated with risk for dementia varied by study population and more years of education did not uniformly attenuate the risk for dementia. It appeared that a more consistent relationship with dementia occurred when years of education reflected cognitive capacity, suggesting that the effect of education on risk for dementia may be best evaluated within the context of a lifespan developmental model.
Keywords: Alzheimer’s disease, dementia, education, risk factors
The purpose of the paper is to provide an updated systematic review of the literature on the relationship between education and dementia over the past 25 years. Mortimer1 was one of the first people to propose a relationship between years of formal education and risk for dementia. He suggested that psychosocial factors such as education may be protective against diagnosable dementia by raising the level of “intellectual reserve”. The first large-scale study to investigate this relationship was a dementia prevalence survey in Shanghai, China.2 The authors found that having no formal education was strongly associated with a higher prevalence of dementia. Since this early work, many researchers have studied the relationship between level of education and risk for dementia.
Several reviews of the relationship between education and dementia have been previously published. Katzman3 and Mortimer4 both concluded that low education was likely a risk factor for dementia. In contrast, in a review of international studies, Gilleard5 argued that methodological and ascertainment biases were driving the relationship between low education and an increased risk for dementia. There have been two selective recent reviews. Caamaño-Isorna and colleagues6 conducted a meta-analysis of 19 studies and concluded that low education met epidemiologic criteria as a risk factor for dementia. In a meta-analysis of 15 incidence studies, Valenzuela and colleagues reported that high education was protective against risk for dementia.7
Broad observations from the literature and previous reviews have suggested that the relationship between education and dementia is strongest and most consistent for individuals with no or very low education, in Alzheimer’s disease cases, and in dementia prevalence studies. Criticisms have focused on ascertainment and diagnostic bias, confounding elements such as age and gender, and that education may be a surrogate for other unmeasured variables. Researchers have proposed a number of mechanisms to explain the relationship between education and risk for dementia including: brain reserve, cognitive reserve, “use it or lose it”, the brain-battering hypothesis, ascertainment/diagnostic bias, and education as a proxy for a third variable(s). However, it remains unclear as to what education is and how it can be discussed as a uniform concept across diverse populations. How can a relatively small number of years of formal education occurring early in life affect risk for dementia in old age? This review advances the literature by providing a broad systematic review of both dementia prevalence and incidence studies. We were particularly interested in examining the pattern of results across studies that evaluated multiple levels of education and potential moderators of the relationship including geographical region and gender.
METHODS
We selected a systematic review over a meta-analysis for the current paper. Meta-analyses require a standard of uniformity across studies in terms of design and statistical methods. Across the reviewed studies, level of education was categorized quite differently and the relationship with dementia was analyzed in a variety of ways. In a meta-analysis, it is also standard practice to include one result from one publication from a given study population. These requirements may have limited the number of study results available to review and obscured patterns within the literature. Finally, a primary goal of this review was to be as inclusive as possible, with the particular aim to include studies from both developed and developing regions of the world.
The systematic review of the literature was conducted using PubMed and PsycINFO databases of all published articles from January 1985 to July 2010. Search terms included combinations and variations of education, years of education, educational attainment, risk factors, Alzheimer’s disease, dementia, prevalence, and incidence. This search strategy was supplemented by reviewing the reference sections of all identified studies and previous reviews. All articles were published in English. We included studies that evaluated the relationship between years of education and Alzheimer’s disease as well as studies that combined all types of dementias into a Total Dementia outcome. We did not include studies of other dementia subtypes (e.g. vascular dementia) nor did we evaluate cognitive decline.
Both dementia prevalence and incidence studies were included and were identified in the traditional manner. Prevalence studies collected education and dementia diagnostic data at baseline. Incidence studies collected education and diagnostic data during a first wave of study. Initially disease-free individuals were then followed up at a later time point with a dementia diagnostic evaluation to identify incident dementia cases within that population.
The criteria for this review were less strict than a meta-analysis. We did not restrict the type of study (e.g. by excluding prevalence studies or case-control studies) or set a minimum sample size. The two selection criteria were a measure of actual years of education and that dementia cases were diagnosed using a standardized assessment procedure such as the Diagnostic and Statistical Manual of Mental Disorders,8–10 International Classification of Diseases,11,12 Cambridge Mental Disorders of the Elderly Examination,13 the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association.14 Two studies15,16 used the Clinical Dementia Rating (CDR)17 scale score to differentiate the dementia sample; here we selected the results based on analysis of dementia cases with CDR=1 (mild dementia) or greater.
The literature search returned over 250 articles. This number was reduced to 93 articles after the abstracts were reviewed. After the selection criteria were applied, 71 articles remained. Two outcomes were defined: Alzheimer’s disease (AD) and Total Dementia (all dementia subtypes combined). Some studies reported a result for AD or Total Dementia and some studies reported results for both outcomes. Educational attainment was categorized differently across the studies.
There was great heterogeneity across the 71 articles. Specifically, studies could have analyzed the relationship between education and dementia using years of education, 2-categories of education (e.g. illiterate vs. literate) or 3 or more categories of education as the independent variable. Any study that included three or more levels of education, analyzed populations independently, or examined both AD and Total Dementia as outcomes could have reported both significant and nonsignificant results in the same study. We were concerned that one significant result might be interpreted as evidence for an overall education-dementia relationship while any nonsignificant effects (in the same study) might be overlooked. Thus, to compare results across studies and to more exactly identify any patterns across results, we implemented a count system that identified study results as significant or nonsignificant based on reported confidence intervals or p-values.
We selected the statistical model that adjusted for age and sex when available. All adjustment variables are listed in the tables. An estimated odds ratio (OR), relative risk (RR), or hazard ratio (HR), and the respective 95% confidence intervals and p-values (when given) were extracted from the univariate or multivariate model described in each paper. ORs were reported in prevalence studies whereas an ORs, RRs or HRs may have been reported in incidence studies. In the few cases where an odds ratio was not provided and enough data were presented in the original paper, we calculated an unadjusted odds ratio. It is important to note that ORs may overestimate the effect size compared to RRs or HRs. Results were considered to be significant if the reported confidence intervals did not include zero or the p-value was less than .05.
A total of 34 studies reported results based on the relationship between high education and risk for dementia. For comparison purposes, we took the reciprocal of the risk estimate and confidence intervals so that the direction of results would be the same across studies. A total of 31 studies reported results for three or more levels of education. To facilitate comparisons, in the tables we present the results from the lowest educational category compared to the highest (reference) category. A total of 10 studies examined education as a continuous variable, and the risk estimate corresponds to change in risk per year of education. Study data are presented separately for prevalence and incidence studies (Tables 1 and 2). Within each table, study results are grouped in chronological order within geographical regions.
TABLE 1.
Study Results for Low Education and Dementia Prevalence (N=46)
| First Author (Year) | Country, Study Name | Sample Size | Age | Number & Type of Dementia Cases | Adjustment variables | Education Variable | Odds Ratios (95% CI) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| AD | p | Total Dementia | p | |||||||
| EUROPE (N=12) | ||||||||||
| Amaducci18 (1986) | Italy | 116:97 C/C | >40 | 116 AD | Unadjusted | Illiterate Literate | 4.00 (not given)^ | 0.37 | ||
| O’Connor19 (1991) | United Kingdom | 1768 | ≥ 75 | 163 TD | Unadjusted | <15 years ≥ 15 years |
1.31 (0.90–1.90)^ 1 |
|||
| Bonaiuto20 (1995) | Italy | 48:96 C/C | ≥ 60 | 48 TD | Age, sex | Illiterate Literate* | 1.40 (0.60–3.10)^ 1 |
0.44 | ||
| Ott21 (1995) | Netherlands, Rotterdam Study | 7528 | ≥ 55 | 339 AD 474 TD |
Age, sex | Primary Education >Secondary Education* | 4.00 (2.50–6.20) 1 |
3.20 (2.20–4.60) 1 |
||
| Prencipe22 (1996) | Italy, Aquila Study | 1147 | ≥ 65 | 50 AD 78 TD |
Age, sex | < 3 years ≥ 3 years |
1.80 (1.00–3.40)^ 1 |
2.00 (1.20–3.30) 1 |
||
| Azzimondi23 (1998) | Rural Sicily: Troina, Italy | 365 | >74 | 80 TD | Unadjusted | ≤ 2 years >2 years |
7.4 (3.3–18.3) 1 |
|||
| Azzimondi23 (1998) | Urban Sicily: S. Agata Militello, Italy | 408 | >74 | 116 TD | Unadjusted | ≤ 2 years > 2 years |
3.2 (1.9–5.2) 1 |
|||
| De Ronchi24 (1998) | Italy | 495 | ≥ 61 | 29 AD 56 TD |
Age, sex | No Education Any Education* | 4.7 (2.30–9.40) 1 |
5.0 (2.10–12.1) 1 |
||
| Gatz25 (2001) | Sweden | 77:154 AD C/C 131:262 TD C/C |
≥ 65 | 77 AD 131 TD |
Age, sex | ≤ 6 years > 6 years |
2.26 (1.02–5.02) 1 |
1.47 (0.82–2.62)^ 1 |
||
| Harmanci26 (2003) | Turkey, TAPS | 57:127 C/C | ≥ 70 | 57 AD | Unadjusted | No schooling Higher Education Ŧ* | 8.38 (1.78–39.56) 1 |
|||
| Tognoni27 (2005) | Italy | 1600 | ≥ 65 | 68 AD 100 TD |
Age, sex | Years of Education | 0.88 (0.77–1.00)^ | 0.91 (0.82–1.01)^ | ||
| Gatz28 (2007) | Sweden, HARMONY | 394:7786 C/C |
≥ 65 | 394 TD | Age, sex | ≥ 6 years >6 years |
1.89 (1.35–2.65) 1 |
|||
| NORTH AMERICA (N=9) | ||||||||||
| Ngandu29 (2007) | Finland, CAIDE | 1,388 | ≥ 65 | 48 AD 61 TD |
Age, sex, follow-up, community residence | 0–5 years ≥ 9 yearsŦ* |
6.67 (2.44–16.67) 1 |
5.00 (2.13–12.50) 1 |
||
| Canadian Study of Health and Aging30 (1994) | Canada, CSHA | 258:535 C/C | ≥ 65 | 258 AD | Age, sex, community residence | 0–6 years ≥ 10 years* |
4.00 (2.49–6.43) 1 |
|||
| Mortel31 (1995) | USA | 150:188 C/C | ≥ 60 | 150 AD | Unadjusted | <High School ≥ High School* |
2.43 (1.38–4.31) 1 |
|||
| Callahan32 (1996) | USA | 2212 African Americans | ≥ 65 | 48 AD 65 TD |
Unadjusted | 0–5 years >9 years Ŧ* |
3.23 (0.97–11.11)^ 1 |
0.06 | 3.85 (1.52–11.11) 1 |
|
| Hall33 (1998) | USA | 2212 African Americans | ≥ 65 | 49 AD | Age, sex | 0–6 years ≤ 10 years* |
3.49 (1.06–11.48) 1 |
|||
| Harwood34 (1999) | USA | White 393:202 C/C | ≥ 65 | White 392 AD Hispanic 188 AD |
Age, sex | White ≤ 10 years >10 years |
White 3.10 (1.80–5.9) 1 |
|||
| Hispanic 188:84 C/C | Hispanic ≤ 10 years >10 years |
Hispanic 1.10 (0.60–1.80)^ 1 |
||||||||
| Munoz35 (2000) | Canada, WODS | 115:142 C/C | ≥ 65 | 115 AD | Unadjusted | None UniversityŦ* | 2.11 (0.49–9.01)^ 1 |
|||
| Mortimer36 (2003) | USA, Nun Study | 294 | ≥ 75 | 60 TD | Age, head circumference, APOE4 | Years of EducationŦ | 1.19 (1.09–1.30) | <0.001 | ||
| Roe37 (2007) | USA ADRC data | 1835 (CERAD) | ≥ 65 | 265 AD | Age at death, sex, stroke, depression, time to death, neuropathology diagnosis stage | Years of educationŦ | 1.09 (1.01–1.16) | 0.03 | ||
| ASIA (N=13) | ||||||||||
| Zhang2 (1990) | China | 5055 | ≥ 55 | 159 TD | Unadjusted | No education > 6 years* | 5.78 (3.62–9.24) 1 |
|||
| Kondo38 (1994) | Japan | 60:120 C/C | ≥ 43 | 60 TD | Unadjusted | Elementary (6yrs) > Elementary | 3.14 (not given) 1 |
<0.001 | ||
| Chandra15 (1998) | India Indo-US study | 5126 | ≥ 55 | 32 AD 36 TD (CDR≥1.0) |
Age | Illiterate LiterateŦ* | 2.50 (0.67–10.00)^ 1 |
0.10 | 1.11 (0.50–2.50)^ 1 |
|
| Lin39 (1998) | Taiwán | 2915 | ≥ 65 | 58 AD 108 TD |
Age, sex | Years of educationŦ | 1.16 (1.02–1.33) | 0.05 | 1.04 (0.98–1.11)^ | |
| Liu40 (1998) | China, KINDS | 1736 | ≥ 65 | 35 AD 44 TD |
Age | 0 years > 0 yearsŦ |
1.43 (0.44–4.76)^ 1 |
0.14 | 2.33 (0.76–7.14)^ 1 |
0.56 |
| Yamada41 (1999) | Japan, AHS/RERF | 2222 | ≥ 60 | 74 AD | Age | Years of Education Ŧ (In 3 yr intervals) | 1.67 (1.11–2.50) | |||
| Zhang42 (2006) | China | 34,807 | ≥ 55 | 732 AD | Age | < 1 year > 12 years Ŧ* |
2.50 (1.25–5.00) 1 |
|||
| Zhou43 (2006) | China | 16,448 | ≥ 50 | 301 AD | Unadjusted | < 1 (Illiterate) Any Education* | 3.05 (2.30–4.03) 1 |
|||
| Galasko44 (2007) | Guam | 2029 | ≥ 65 | 243 TD | Age, sex | Years of education Ŧ | 1.15 (1.11–1.19) | |||
| Llibre Rodriguez45 (2008) | China (urban) 10/66 DRSG | 1160 | ≥ 65 | 35 TD | Unadjusted | Linear trend across five categories of education Ŧ | 1.04 (0.77–1.41)^ | |||
| Llibre Rodriguez45 (2008) | China (rural) 10/66 DRSG | 1002 | ≥ 65 | 24 TD | Unadjusted | Linear trend across five categories of education Ŧ | 1.22 (0.72–2.08)^ | |||
| Llibre Rodriguez45 (2008) | India (urban) 10/66 DRSG | 1005 | ≥ 65 | 90 TD | Unadjusted | Linear trend across five categories of education Ŧ | 0.79 (0.41–1.52)^ | |||
| Llibre Rodriguez45 (2008) | India (rural) 10/66 DRSG | 999 | ≥ 65 | 80 TD | Unadjusted | Linear trend across five categories of education Ŧ | 3.85 (0.71–20.00)^ | |||
| LATIN AMERICA (N=8) | ||||||||||
| Herrera46 (2002) | Brazil | 1656 | ≥ 65 | 118 TD | Unadjusted | Illiterate ≥ 8 years* | 3.82 (1.51–9.66) 1 |
|||
| Llibre Rodriguez45 (2008) | Cuba 10/66/DRSG | 2944 | 188 TD | Unadjusted | Linear trend across five categories of education Ŧ | 1.16 (1.01–1.33) | ||||
| Llibre Rodriguez45 (2008) | Dominican Republic 10/66 DRSG | 2011 | ≥ 65 | 109 TD | Unadjusted | Linear trend across five categories of education Ŧ | 1.12 (0.91–1.49)^ | |||
| Llibre Rodriguez45 (2008) | Peru (urban) 10/66 DRSG | 1381 | ≥ 65 | 43 TD | Unadjusted | Linear trend across five categories of education Ŧ | 1.37 (1.00–1.85)^ | |||
| Llibre Rodriguez45 (2008) | Venezuela 10/66 DRSG | 1904 | ≥ 65 | 36 TD | Unadjusted | Linear trend across five categories of education Ŧ | 1.47 (1.03–2.13) | |||
| Llibre Rodriguez45 (2008) | Mexico (urban) 10/66 DRSG | 1002 | ≥ 65 | 41 TD | Unadjusted | Linear trend across five categories of education Ŧ | 2.17 (1.56–3.03) | |||
| Llibre Rodriguez45 (2008) | Mexico (rural) 10/66 DRSG | 1000 | ≥ 65 | 22 TD | Unadjusted | Linear trend across five categories of education Ŧ | 0.68 (0.42–1.10)^ | |||
| Scazufca47 (2008) | Brazil, SPAH | 2005 | ≥ 65 | 100 TD | Age, sex | Illiterate Literate | 1.83 (1.18–2.86) 1 |
0.01 | ||
| MIDDLE EAST (N=2) | ||||||||||
| Bowirrat48 (2001) | Israel | 821 | ≥ 60 | 168 AD | Unadjusted | Illiterate ≥ 1 years | 9.0 (4.4–19.00) 1 |
0.01 | ||
| Kahana49 (2003) | Israel | 1501 | ≥ 75 | 165 TD | Age, sex, ethnic origin | Illiterate ≥ 12 years* | 3.99 (1.84–8.68) 1 |
0.000 | ||
| AFRICA (N=1) | ||||||||||
| Hall33 (1998) | Africa, Nigeria | 2494 | ≥ 65 | 18 AD | Age, sex | No Education Any Education | 2.15 (0.37–12.36)^ 1 |
|||
Note.
The reciprocal of original reported risk estimate is presented so that all studies are low education compared to high education.
Study reported results for 3 or more categories of education, but we present the lowest level compared to the highest level of education
Result not significant: 95% Confidence Interval includes 1.00 or p > 0.05.
AD = Alzheimer’s disease; TD= Total Dementia; CI = Confidence Interval; C/C= case control.
Study Acronyms: Turkish Alzheimer Prevalence Study (TAPS), Cardiovascular Risk Factors, Aging, and Dementia (CAIDE), Canadian Study of Health and Aging (CSHA), Western Ontario Dementia Study (WODS), Alzheimer’s Disease Research Center (ADRC), Kinmen Neurological Disorders Survey (KINDS), Adult Health Study (AHS)/Radiation Effects Research Foundation (RERF), 10/66 Dementia Research Study Group (DRSG), San Paulo Ageing & Health Study (SPAH).
TABLE 2.
Study Results for Low Education and Dementia Incidence (N=42)
| First Author (Year) | Country, Study Name | Sample Size | Age | Number & Type of Dementia Cases | Adjustment variables | Education Variable | OR/RR/HR (95% CI) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| AD | p | Total Dementia | p | |||||||
| EUROPE (N=19) | ||||||||||
| Bickel50 (1994) | Germany | 422 | ≥ 65 | 60 TD | Age | Elementary only > Elementary | 1.48 (0.60–3.40)^ 1 |
|||
| Paykel51 (1994) | United Kingdom | 1195 | ≥ 75 | 49 TD | Age | <14 years ≥ 14 years |
Not given 1 |
n.s. | ||
| Persson52 (1996) | Sweden | 374 | ≥ 70 | 38 TD | Unadjusted | > Elementary Any vocational | Not given 1 |
n.s. | ||
| Schmand53 (1997) | Amsterdam, AMSTEL | 2063 | ≥ 65 | 152 TD | Age, sex, DART-IQ, occupation, management, # of diseases, family history | ≥ 8 years >8 years |
0.86 (0.57–1.31)^ 1 |
|||
| Geerlings54 (1999) | Netherlands, AMSTEL | 3778 | ≥ 55 | 77 AD | Age, sex | ≥ 6 years > 6 years |
2.09 (1.29–3.38) 1 |
|||
| Launer55 (1999) | Europe, EURODEM | 12,943 | ≥ 65 | 352 AD | Age, age-squared, sex, study |
Women < 8 years > 11 years |
4.55 (1.64–12.57) 1 |
|||
|
Men < 8 years > 11 years |
1.00 (0.48–2.04)^ 1 |
|||||||||
| Letenneur56 (1999) | France, PAQUID study | 2881 | ≥ 65 | 140 AD 190 TD |
Age, sex | < Primary school ≥ Primary school |
1.81 (1.30–2.53) 1 |
<0.001 | 1.83 (1.37–2.44) 1 |
<0.001 |
| Nielsen57 (1999) | Denmark, Odense Study | 2452 | ≥ 65 | 102 AD | Unadjusted | < 7 years ≥ 7 years |
Not given | 0.003 | ||
| Ott58 (1999) | Netherlands, Rotterdam Study | 6827 | ≥ 55 | 97 AD 137 TD |
Age, Age-squared | Women < 7 years ≥ 11 years* | 2.00 (1.00–3.90)^ 1 |
2.10 (1.10–3.90) 1 |
||
| Men < 7 years ≥ 11 years* | 0.60 (0.20–1.70)^ 1 |
0.70 (0.30–1.50)^ 1 |
||||||||
| Gatz25 (2001) | Sweden | 54:108 AD C/C 90:180 TD C/C |
≥ 65 | 54 AD 90 TD |
Age, sex | ≤ 6 years >6 years |
2.17 (0.69–6.86)^ 1 |
1.25 (0.60–2.60)^ 1 |
||
| Qiu59 (2001) | Sweden, Kungsholmen Project | 1296 | ≥ 75 | 109 AD 147 TD |
Age, sex | < 8 years ≥ 11 years* |
2.70 (1.40–5.00) 1 |
2.10 (1.30–3.50) 1 |
||
| Anttila60 (2002) | Finland | 1449 | ≥ 65 | 70 TD | Unadjusted | Years of Education Ŧ | 1.22 (1.11–1.35) | |||
| Di Carlo61 (2002) | Italy, ILSA | 3208 | ≥ 65 | 67 AD 127 TD | Age, sex | 0–5 years 11 years Ŧ* |
2.94 (1.04–8.33) 1 |
2.27 (1.18–4.35) 1 |
||
| Karp62 (2004) | Sweden, Kungsholmen Project | 931 | ≥ 75 | 76 AD 101 TD |
Age, sex | 2–7 years >10 years* |
2.70 (1.30–5.40) 1 |
2.40 (1.30–4.40) 1 |
||
| Ravaglia63 (2005) | Italy, CSBA | 937 | ≥ 65 | 72 AD 115 TD |
Age, sex | ≤ 3 years ≥ 6 years* |
2.66 (1.02–6.89) 1 |
0.04 | 2.20 (1.07–4.53) 1 |
0.03 |
| Brayne64 (2010) | Europe, EClipSE | 872 | >65 | 486 TD | Unadjusted | Years of Education Ŧ | 1.12 (1.06–1.20) | |||
| NORTH AMERICA (N=18) | ||||||||||
| Beard65 (1992) | USA, Rochester Epidemiology Project | 241:241 C/C | Not given | 241 AD | Unadjusted | < 9 years ≥ 9 years Ŧ |
1.13 (0.69–1.85)^ 1 |
|||
| Hebert66 (1992) | USA East Boston Study | 513 | ≥ 65 | 76 AD | Unadjusted | 0–7 years ≥ 8 years Ŧ |
3.79 (2.29–6.26) 1 |
|||
| Stern67 (1994) | USA | 593 | ≥ 60 | 106 TD | Age, sex | < 8 years ≥ 8 years |
2.02 (1.33–3.06) 1 |
|||
| Cobb68 (1995) | USA, Framingham Study | 3330 | ≥ 55 | 149 AD 258 TD |
Age | < Grade School ≥ High School* |
1.04 (0.62–1.74)^ 1 |
1.31 (0.90–1.90)^ 1 |
||
| Evans69 (1997) | USA, Boston (EPESE) | 642 | ≥ 65 | 95 AD | Age, sex, follow-up interval | Years of Education Ŧ | 1.20 (1.09–1.33) | |||
| Elias70 (2000) | USA Framingham Study | 1076 | ≥ 65 | 109 AD | Unadjusted | ≤ 8th grade >High School |
0.75 (0.33–1.69)^ 1 |
|||
| Ganguli16 (2000) | USA MoVIES Project | 1298 | ≥ 65 | 94 AD 110 TD (CDR ≥ 1.0) |
Age, sex | < High School ≥ High School |
Not given^ 1 |
n.s. | Not given^ 1 |
n.s. |
| Kawas71 (2000) | USA, BLSA | 1236 | ≥ 55 | 114 AD | Age, sex | 4–12 years 17–25 years Ŧ* |
1.56 (0.84–2.94)^ 1 |
0.16 | ||
| Lindsay72 (2002) | Canada, CSHA | 194:3894 C/C | 65 | 194 AD | Age, sex | 0–8 years ≥ 13 years* |
1.90 (1.25–2.90) 1 |
|||
| Kukull73 (2002) | USA, ACT – Seattle | 2356 | ≥ 65 | 151 AD 215 TD |
Age, sex, APOE | <12 years >15 years Ŧ* |
2.08 (1.19–3.70) 1 |
1.56 (1.00–2.50)^ 1 |
||
| Wilson74 (2002) | USA, Religious Orders Study | 801 | ≥ 65 | 111 AD | Age, sex | Years of Education Ŧ | 0.99 (0.93–1.05)^ | |||
| Kuller75 (2003) | USA CHS-CS | 3608 | ≥ 65 | 480 TD | Age | < 8th grade ≥ 17 years* |
1.70 (1.16–2.41) 1 |
|||
| Tuokko76 (2003) | Canada, CSHA | 838 | ≥ 65 | 230 TD | Age, sex | ≤ 6 years ≥ 11 years |
1.10 (1.06–1.15) 1 |
<0.01 | ||
| Fitzpatrick77 (2004) | USA, CHS | White 2867 | ≥ 65 | White 401 TD | Age |
White < High School ≥ Some College* |
Not given 1 |
<0.0001 | ||
| Black 492 | Black 76 TD | Age |
Black < High School ≥ Some College* |
Not given^ 1 |
0.79 | |||||
| Shadlen78 (2006) | USA, CHS | White 2,102 | Not given | White 401 TD | Age, sex, ascertainment method |
White ≤ 10 years > 10 years |
1.8 (1.39–2.23) 1 |
|||
| Black 207 | Black 76 TD |
Black ≤ 10 years > 10 years (white) |
4.6 (3.13–6.82) 1 |
|||||||
| McDowell79 (2007) | Canada, CSHA | 5177 | ≥ 65 | 532 AD 783 TD | Age, sex, marital status | < 6 years ≥ 13 years* |
2.60 (1.91–3.53) 1 |
2.60 (2.02–3.38) 1 |
||
| ASIA (N=5) | ||||||||||
| Li80 (1991) | China | 1090 | ≥ 60 | 13 TD | Age | Illiterate Literate | 2.47 (not given)^ 1 |
0.10 | ||
| Yoshitake81 (1995) | Japan, Hisayama Study | 828 | ≥ 65 | 42 AD | Age | ≤ 6 years > 6 years |
1.18 (0.61–2.27)^ 1 |
|||
| Liu82 (1998) | Taiwan | 1507 | ≥ 65 | 25 AD 60 TD |
Age, sex | Illiterate Literate Ŧ | 1.39 (0.52–3.70)^ 1 |
1.59 (0.85–2.94)^ 1 |
||
| He83 (2000) | China | 3024 | ≥ 55 | 92 AD | Age, sex | Illiterate Any education* | 3.22 (2.15–4.82) 1 |
|||
| Yamada84 (2008) | Japan, AHS/RERF | 2286 | ≥ 65 | 80 AD 206 TD |
Age, age2, sex, sex* age | ≤ 6 years ≥7 years Ŧ |
2.50 (not given) 1 |
0.001 | 1.67 (not given) 1 |
0.003 |
| LATIN AMERICA (N=1) | ||||||||||
| Nitrini85 (2004) | Brazil | 1538 | ≥ 65 | 50 TD | Unadjusted | Illiterate (0) ≥ 8 years* |
2.07 (0.60–7.09)^ 1 |
|||
Note.
Reciprocal of original study risk estimate is given so that all studies are low education compared to high education.
Original study reported results for 3 or more categories of education, here we report the lowest level compared to the highest level of education.
Result not significant: 95% Confidence Interval includes 1.00 or p > 0.05.
AD = Alzheimer’s disease; TD= Total Dementia; CI = Confidence Interval; C/C= case control; OR= Odds Ratio; RR=Relative Risk; HR=Hazard Ratio.
Study Acronyms: Amsterdam Study of the Elderly (AMSTEL), European Studies of Dementia (EURODEM), Italian Longitudinal Study of Aging (ILSA), Conselice Study of Brain Aging (CSBA), Epidemiological Clinicopathological Studies in Europe (EClipSE), Established Populations for Epidemiologic Studies of the Elderly (EPESE), Monongahela Valley Independent Elders Survey (MoVIES), Baltimore Longitudinal Study of Aging (BLSA), Canadian Study of Health and Aging (CSHA), Adult Changes in Thought (ACT), Cardiovascular Health Study Cognition Study (CHS-CS), Cardiovascular Heatlh Study (CHS), Adult Health Study (AHS)/Radiation Effects Research Foundation (RERF).
RESULTS
Of the 71 articles that met inclusion criteria, 3 prevalence studies reported results from multiple study sites,23,33,45 two studies analyzed gender separately,55,58 and three studies analyzed multiple racial/ethnic groups separately.34,77,78 Thus, while there are 71 articles, the results reported in this paper are based on 88 total study populations. Nearly the same number of prevalence and incidence studies met our selection criteria. Study results for the relationship between low education and prevalent dementia are reported in Table 1 (N=46). Study results for the relationship between low education and incident dementia are reported in Table 2 (N=42).
Overall, 51 studies (58%) reported a significant effect of lower education on risk for dementia whereas 37 studies (42%) reported no significant relationship between lower education and a dementia outcome. Of the 88 total studies, 27 examined the relationship between education and AD, 37 studies examined the relationship between education and Total Dementia, and 24 studies analyzed the relationship for both outcomes.
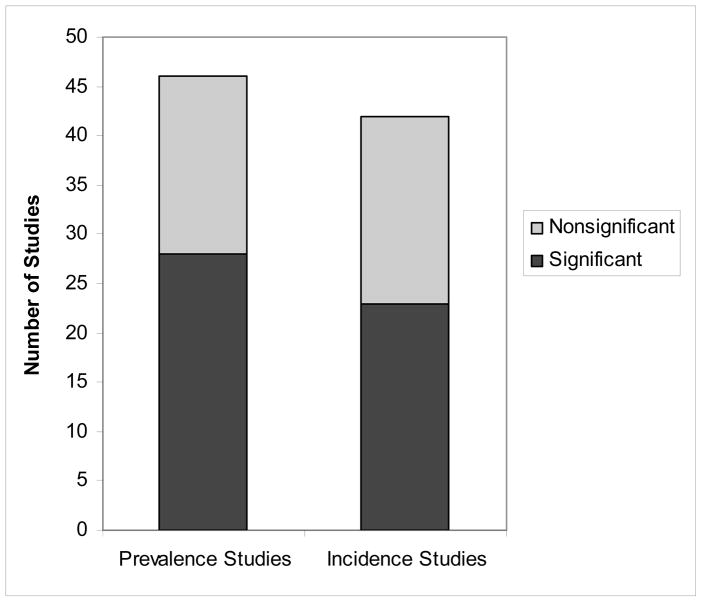
To compare across prevalence and incidence studies, we selected one result from the each of the 88 study populations. If a study examined both dementia outcomes, we selected the Total Dementia outcome. Of the 46 prevalence studies, 28 studies (61%) reported a significant effect of low education on risk for dementia. Of the 42 incidence studies, 23 studies (55%) reported a significant effect of low education on dementia risk (See Figure 1).
FIGURE 1.
Pattern of Study Results by Study Type (Prevalence or Incidence). Note. Each study is counted once. If a study analyzed both outcomes, the result for Total Dementia is shown.
In the following sections, we report results within each study type (prevalence or incidence) and study outcome (AD and/or Total Dementia). Later, we specifically discuss studies with three or more levels of education, studies by geographical region, and studies evaluating age, gender and race/ethnicity.
Education and Dementia Prevalence Studies
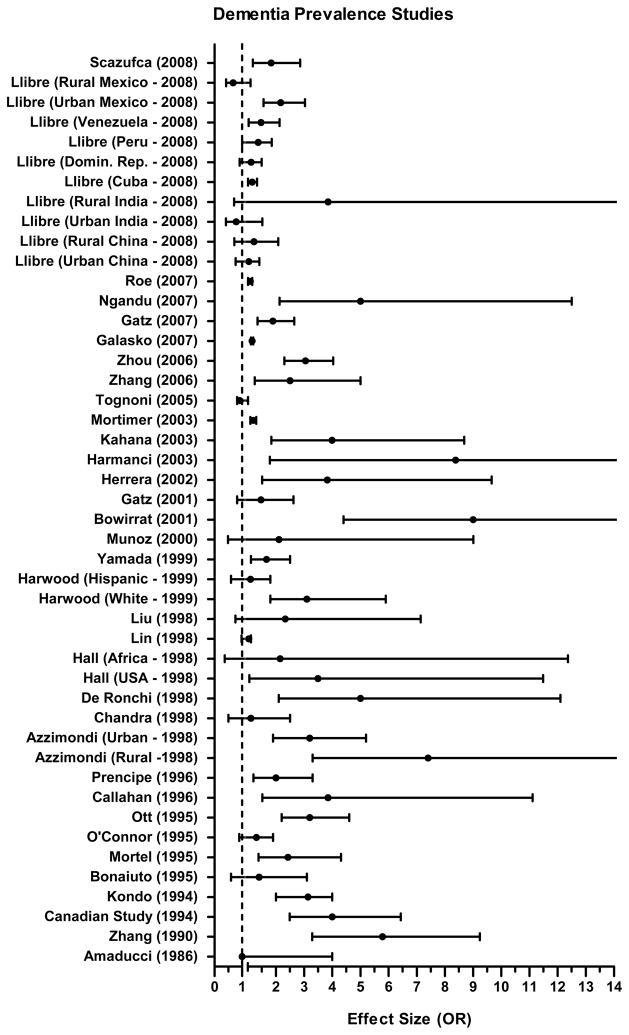
A total of 46 studies examined the relationship between low education and dementia prevalence (See Table 1). Effect sizes reported by prevalence studies are presented in Figure 2. A total of 22 studies did not adjust for any covariates (such as age or sex) in the statistical models.
FIGURE 2.
Reported Effect Size (OR) and 95% Confidence Interval from Analysis of Lower Education and Risk for Dementia Prevalence. Note. OR indicates Odds Ratio. All studies are presented without differentiation. Studies vary in how education was defined, sample size, and model covariates.
Alzheimer’s disease
Of the 14 studies that analyzed the relationship between low education and AD, 10 studies found a significant effect such that low education was associated with a significant increased risk for AD.26,30,31,33,34,37,41–43,48 In contrast, 4 studies reported no association between education and risk for AD.18,33–35 Total Dementia. Of the 22 studies that analyzed the effect of education on Total Dementia prevalence, 13 studies reported a significant effect such that lower education was associated with an increased risk for Total Dementia.2,23,28,36,38,44–47,49 In contrast, 9 studies found no significant relationship.19,20,45 Both outcomes. Of the remaining 10 prevalence studies that examined both AD and Total Dementia as outcomes, 3 studies reported a significant effect of lower education on risk for both dementia outcomes,21,24,29 3 studies reported no significant association for either outcome,15,27,40 and 2 studies found only AD to be significant;25,39 whereas, 2 other studies found only Total Dementia to be significant.22,32
Education and Dementia Incidence Studies
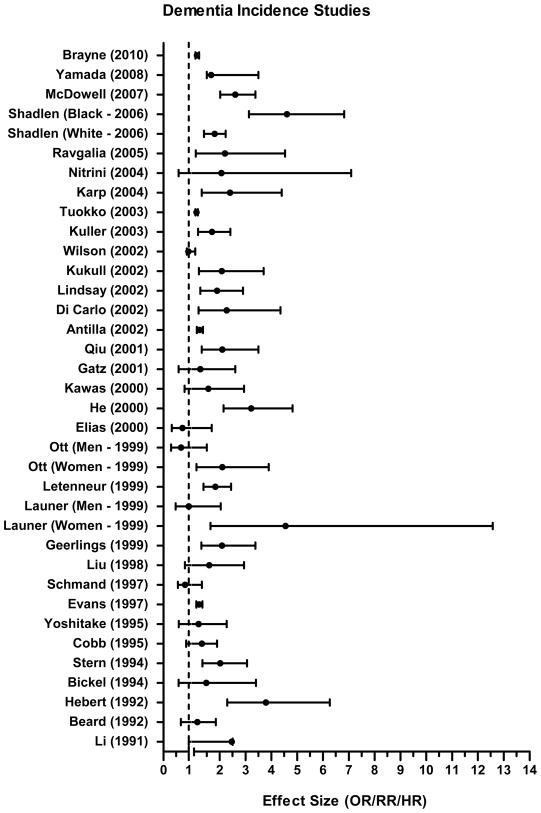
A total of 42 studies examined the relationship between low education and incident dementia (see Table 2). Effect sizes reported by incidence studies are presented in Figure 3. A total of 8 studies did not adjust for any covariates in the statistical models.
FIGURE 3.
Reported Effect Size (OR/RR/HR) and 95% Confidence Interval from Analysis of Lower Education and Risk for Dementia Incidence. Note. OR indicates Odds Ratio, RR, Relative Risk, and HR, Hazard Ratio. All studies are presented without differentiation. Studies vary in how education was defined, sample size, and model covariates.
Alzheimer’s disease
Of the 13 studies that analyzed the relationship between low education and risk for AD, 7 studies reported significant effects such that lower education was associated with an increased risk for AD.54,55,57,66,69,72,83 In contrast, 6 other studies reported no significant relationship.58,65,70,71,74,81 Total Dementia.. Of the 15 studies that examined whether low education was associated with an increased risk for Total Dementia, 8 studies reported significant effects for low education on risk for Total Dementia.60,64,67,75–78 In contrast, 7 studies reported no significant association.50–53,77,80,85 Both Outcomes. Of the remaining 14 incidence studies that examined both dementia outcomes, 7 studies found a significant relationship for both outcomes,56,59,61–63,79,84 a total of 5 reported no significant relationship for either outcome,16,25,58,68,82 and 2 reported only one of the outcomes to be significant (one AD and one Total Dementia).58,73
Multiple Categories of Education
For comparison purposes, we chose to present the lowest level of education compared to the highest level (the reference group). However, 31 studies reported results by dividing education into three or more categories and identified each level as significant or not significant. Thus, these studies could have reported a significant result for one category of education, but, a nonsignificant result for another category. Of these 31 studies, 4 reported significant results across all levels compared to the reference level and 6 studies reported no significant result for any level compared to the reference level. The remaining 21 studies reported both significant and nonsignificant results, with the lowest level of education always representing significant risk and at least one middle level not associated with risk for dementia compared to the reference level. Generally, when education is divided into more than two categories, there is likely to be reduced statistical power for finding a significant effect of a category of education closer to the reference group. Yet overall, whether low education was related to risk for dementia was unrelated to how education was defined across studies. For example, the definition of low education ranged from illiterate to less than 15 years, while the highest education level (reference category) ranged from literate to 17+ years.
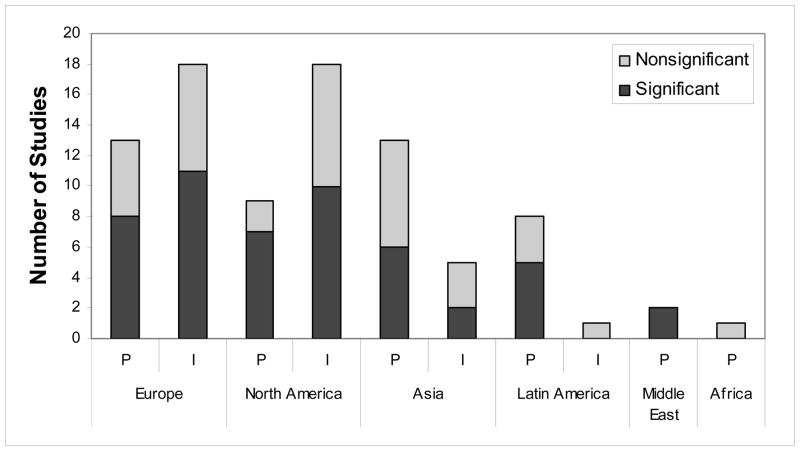
Geographical Region
Studies examining the relationship between education and dementia were carried out in both developed and developing regions. Across all identified studies, 31 studies were conducted in Europe, 27 in North America, 18 in Asia (including one study in Guam), 9 in Latin America, 2 in the Middle-East, and 1 in Africa. Again for the following, if a study examined both dementia outcomes, we selected the Total Dementia outcome in our count. Across the 31 European studies, 19 studies reported significant associations between education and a dementia outcome;21–24,26,28,29,54–64 whereas, 12 studies reported no significant association.18–20,25,27,50–53,55,58 Of the 27 North American studies, 17 studies reported significant effects of education on dementia risk;30–34,36,37,66,67,69,72,75–79 whereas, 10 studies reported no significant associations.16,34,35,65,68,70,71,73,74,77 Of the 18 studies conducted in Asia, 8 studies reported significant associations between education and a dementia outcome; 2,38,41–44,83,84 whereas, 10 studies reported no significant associations.15,39,40,45,80–82 Of the 9 Latin American studies, 5 reported significant associations between education and a Total Dementia; 45–47 whereas, 4 studies reported no significant associations.45, 85 No studies in Latin America reported on risk for AD. Two studies included in this review were conducted in Israel and reported an effect of low education on risk for AD48 and Total Dementia.49 One study conducted in Nigeria, Africa, did not find a significant association between no education and risk for AD.33 Figure 4 illustrates the results across prevalence and incidence studies within each geographical region.
FIGURE 4.
Pattern of Study Results by Prevalence and Incidence within Geographical Regions. Note. P = Prevalence; I= Incidence. Each study is counted once. If a study analyzed both dementia outcomes, the result for Total Dementia is shown.
To further evaluate the distribution by region, we divided the studies into two categories: developed and developing regions. A total of 64 studies were from primarily developed regions (North America, Europe, Japan, and Israel) and 24 studies were from primarily developing regions (Asia, Latin America, and Africa). We note that while we present the 10/66 Dementia Research Study Group reportas 10 different studies (three of which were significant), that paper reported a significant pooled meta-analyzed estimate (OR=0.83; CI=0.75–0.90), such that more years of education was associated with a decreased risk for Total Dementia.44 Of the 64 studies from developed regions, 41 studies (64%) reported significant associations between education and a dementia outcome, whereas 23 studies (36%) reported no significant findings between level of education and dementia risk. Across the 24 studies from developing regions, 10 studies (42%) reported a significant association between education and a dementia outcome. In contrast, 14 studies (58%) studies reported no significant findings between level of education and dementia risk (See Figure 5). Overall, studies from developing regions were more likely to be dementia prevalence studies and less likely to single out AD as the dementia outcome.
FIGURE 5.
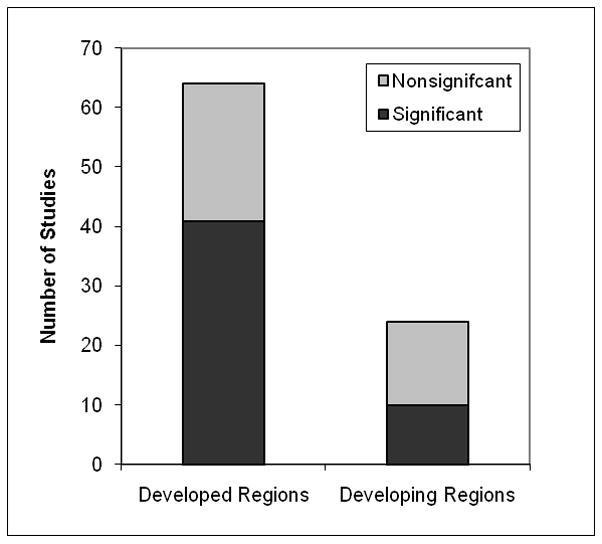
Pattern of Study Results for Developed and Developing Regions. Note. Each study is counted once. If a study analyzed both dementia outcomes, the result for Total Dementia is shown.
Age, Gender, and Race/Ethnicity
Of the 88 study populations, 30 studies did not adjust for age or any other covariates in the statistical analysis. A total of 2 studies specifically evaluated the impact of gender. In each study, men and women were analyzed separately and the results indicated a significantly increased risk for dementia in women with the lowest level of education, but no significant effect of low education on risk for dementia in men.55,58 Furthermore, Ott and colleagues58 describe a reanalysis of the cross-sectional data from the previously published dementia prevalence survey21 and confirmed a notably larger effect of low education on risk for dementia in women (OR=4.07) compared to men (OR=1.80). The results for race/ethnicity were varied. Two studies found no significant association between low education and risk for dementia within a minority group,34,77 whereas one study reported a greater risk for the minority group.78 Two other studies analyzed the same population of African Americans and found a significant effect of low education on risk for AD and Total Dementia.32,33
DISCUSSION
Collapsing across all study types and dementia outcomes, we found that 58% of studies reported significant effects of education on risk for dementia; whereas, 42% of studies reported no significant effects. Previous reviews have suggested that the effect of education was stronger in studies evaluating individuals with no or low education, in studies evaluating risk for AD, and in dementia prevalence studies. This review did not find more significant effects for studies where the lowest category was no or extremely low levels of education as compared to studies where the lowest category included more years of education. How researchers categorized education seemed to reflect cultural context, with developing countries having lower cutoffs. This review found that whether low education was associated with dementia did not depend on what cutoffs defined low education. This finding suggests that the relationship between education and dementia is perhaps more nuanced than previously suggested. Mainly, it does not appear to be the case that adding years of education adds uniform protection against risk for dementia.
Whether the relationship between education and AD is stronger than for other dementia subtypes remains unclear. In this review, AD cases often made up a large percentage of the Total Dementia outcome and we were unable to include other separate dementia outcomes such as vascular dementia. However, when examining studies that evaluated both outcomes, it was equally as common for the AD outcome to be significant as the Total Dementia outcome (when the other was not). This suggests that the relationship between education and dementia may not be unique to AD.
The results of this review suggest that prevalence studies reported a significant effect of low education on dementia risk more often than incidence studies. However, a much greater number of prevalence studies did not adjust analyses for age or sex making it difficult to compare across study type. Similarly, we found no clear evidence that the dementia prevalence studies reported larger risk estimates compared to dementia incidence studies.
Differences across study populations
Overall, there was more consistent support for an education-dementia relationship in developed compared to developing regions of the world. This finding may seem a bit paradoxical because developing regions have more older adults who are uneducated or poorly educated, yet a lower prevalence of dementia.5,86 This pattern may be explainable if we consider that individuals in developing countries generally have a shorter life expectancy and may die before either developing or evidencing symptoms of dementia. Thus, education may be unrelated to dementia due to the simultaneous lack of education and lack of dementia cases.40,43
In developing regions, even more than in developed regions, low education may be especially reflective of lifelong disadvantages such as poorer maternal health, poor nutrition, or exposure to infection.43,80 In addition, access to education may more often be based on social class than on intellectual promise or on principles of equal access regardless of ability to pay for an education 2,16,25,33,63 Further, the proportion of individuals who are illiterate or of very low education is often notably greater in rural than in urban samples, yet this more poorly educated sample is not necessarily at greater risk for dementia 20,30 In Mexico, for example, low education was associated with an increased risk for dementia in urban participants but not in rural participants.45
In terms of differences related to the age of the study population, many studies reported that the oldest participants had significantly fewer years of education and were also significantly more likely to be diagnosed with dementia – suggesting a cohort effect.44 Despite model adjustments for age, the strong tendency for older cohorts concurrently to have fewer years of education and higher rates of dementia may potentially inflate the association between education and dementia.
In terms of gender differences, although most studies adjusted for sex in the statistical models, Letenneur and colleagues56 pointed out that most studies do not consider the potential for moderation of the education-dementia association based on age or sex. This is important because women live longer, or have greater survival, and thus have more time to develop and express a dementing disease.16,87 When modeling sex separately, the Rotterdam Study58 and two separate studies using EURODEM data 55,88 found a significant effect of education on risk for dementia only in women. Women in this cohort had both fewer and qualitatively different educational opportunities compared to men (despite presumably equivalent intellectual capacity). For example, Zhang and colleagues42 noted that prior to 1950, only women belonging to the highest social class were given access to education in China. In developed regions as well, women born at the end of the nineteenth and beginning of the twentieth century may have experienced educational disadvantages reflecting lifelong socioeconomic disparities.20,42,43,55,83
In terms of race/ethnicity differences, the available studies suggest that for ethnic minority groups low education may not correspond to lesser cognitive capacity. Harwood and colleagues34 found that low education was a risk factor for AD in White participants but not in Hispanic participants (mainly Cuban immigrants), despite the fact that the Hispanic participants had an equivalent number of years of education and often worked in jobs below their educational level. Two studies evaluated African American participants compared to White participants. After adjusting for age, Fitzpatrick et al.77 reported an increased risk for dementia in White participants with low education (less than high school), but not African American participants with the same level of education. In contrast, Shadlen and colleagues78 found a considerably higher dementia risk for African American participants with low education (less than 10 years) compared to a reference group of white participants with the same level of education. However, the higher dementia risk for African American participants was notably lessened in an analysis that controlled for baseline cognitive ability – suggesting that years of education may not have been a reliable indicator of cognitive capacity. In keeping with the observation that differences in level and quality of educational opportunities available to minorities in the older cohorts may change the meaning of education, some researchers have suggested that literacy or reading level may be a better indicator of what education is thought to represent than years of education in minority groups.89 In an entirely African American sample in Indianapolis, Indiana, Hall and colleagues90 found that the combination of low education and rural residence until age 19 greatly increased risk for AD. However, when rural residence was included as a covariate, low education alone was not a significant risk factor.
Overall, across developing and developed countries, women and men, racial and ethnic minorities and individuals from the dominant majority group, education appears to have a more consistent relationship with dementia in populations where educational attainment is reflective of intellectual ability rather than privilege.
Methodological factors
The concerns illustrated above lead us into a discussion about methodological limitations in assessing the relationship between education and dementia. The potential for ascertainment and diagnostic biases was a major methodological concern raised in almost every article reviewed here as well as in previous reviews. This bias refers to the potential for individuals with low educational attainment to inherently perform more poorly on cognitive testing used to assess for dementia compared to individuals with more education, despite no differences in functional impairment.76,91 Cognitive tests have been suggested to be especially biased when evaluating illiterate or uneducated individuals.5,15,80
Studies have adopted different approaches to reduce the potential for such bias, including creating different screening cutoffs based on education,2,85 clinically evaluating a proportion of participants who initially screen negative for dementia,30,42 and developing more sensitive measures of dementia for populations in developing countries.92 In this review, two studies utilized autopsy-verified dementia outcomes. Munoz 35 reported no relationship between education and risk for dementia. In contrast, a mega-study of autopsy-confirmed dementia cases in Europe found that education was predictive of dementia diagnoses but was not predictive of differences in brain pathology.64 Overall, given the various measures taken to correct for such bias, as well as research specifically evaluating the effectiveness of these methodologies,54,76 we are reasonably confident that the results are not solely due to biased or invalid dementia diagnosis.
What is Education?
In this review, we concluded that years of education can mean different things when evaluated within the context of culture and cohort. The quantity of education that was important to lessening dementia risk varied quite dramatically by population and region and seems to be tied to the unique demands of a given society. For example, the dementia risk associated with illiteracy in a developing region83 may be similar to the dementia risk associated with completing up to 10 years in a developed region.34 We still do not know why the difference of just a few years of early education can have an association with late life risk for dementia.
A number of studies have evaluated potential mediators of the relationship between education and dementia and found that the association disappeared after accounting for factors such as familial risk and leg length.93 In contrast, other studies found a persistent association even after accounting for health, lifestyle, or familiality.28, 29,61 Studies that evaluated brain pathology at autopsy found disease pathology to be unrelated to years of education.35,64 In the papers reviewed here, the cognitive reserve theory94 was the most cited mechanism to explain the relationship between education and dementia. Based on the results of this review, where we have highlighted the broad definition of low education across study populations as well as the important mediators of this relationship, we suggest that it is important to think of education as a proxy or surrogate indicator.
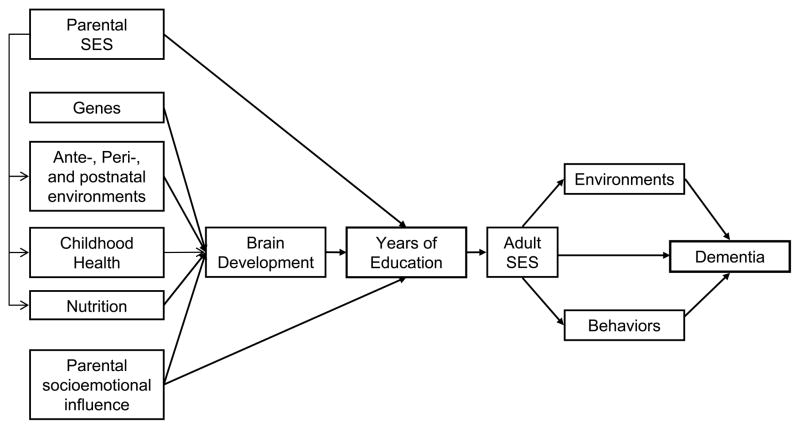
To illustrate this point, we present a developmental framework (See Figure 6) that expands on a model identifying relationship between childhood mental ability and survival.95 In our model, educational attainment is influenced by pre-education factors that contribute to cognitive development such as genetics, parental socioeconomic status (SES) and socioemotional influences. Brain development is an unmeasured baseline factor that influences access to early education and the ability to profit from education. Adult SES is a post-education factor, strongly related to educational attainment (particularly in developed regions). Adult SES, in turn, is associated with environments, such as occupation or exposure to toxins, and to behaviors such as diet, exercise, and lifestyle. In this model, level of education does not affect risk for dementia directly, but it affects (and is affected by) a multitude of factors across the lifespan.
FIGURE 6.
Lifespan Developmental Model for a Relationship between Education and Dementia. (Note. Adapted from Deary IJ, Whiteman MC, Starr JM, et al. The impact of childhood intelligence on later life: following up the Scottish mental surveys of 1932 and 1947. J Pers Soc Psychol. 2004;86:130–147 Copyright 2004 by the American Psychological Association)
In support of the cognitive reserve theory, it seems that low education has a stronger association with dementia when education is reflective of cognitive capacity rather than privilege and when low education is associated with other risk factors across the lifespan. However, the amount of education that is subsequently associated with a statistical risk for dementia is contingent on an individual’s environment and can vary greatly across populations, cohorts, gender and race/ethnicity. Finally, educational attainment is not a static event. It seems that for some individuals, years of education may correspond to a greater interest in learning or a tendency to seek out cognitively stimulating activities across the lifespan. For other individuals, years of formal education tells us little about their subsequent cognitive endeavors.
STRENGTHS AND LIMITATIONS
We attempted to be as inclusive as possible in this review covering the last 25 years of the literature. We included both prevalence and incidence studies and did not exclude case-control studies or studies with small sample sizes. However, it is possible that relevant studies were excluded because of how dementia was diagnosed or how the data were reported. As with any review, negative publication bias is an important consideration. It may be that some studies did not find an association between education and dementia and these nonsignificant findings were not reported in a published paper. Any exclusion of nonsignificant findings would warrant an even more cautious discussion of the association between education and dementia. Methodological challenges to this current review included the multiple and potentially conflicting results due to how education was defined and analyzed, multiple dementia outcomes, and varied study populations. Because it was difficult to summarize across such diverse studies, we employed a count system to examine study results. We acknowledge that this approach does not give a statistical account of the association between education and dementia. However, it seemed important to clearly present the pattern of study results across and within this diverse group of study populations.
CONCLUSION
Overall, the results of this review suggest that the education-dementia relationship may be more complex than previously suggested in the aging literature. The results suggest that lower education is associated with an increased risk for dementia for some but not all studies. Further, the level of education that was most associated with dementia risk varied considerably by study region as well as by age, gender, and race/ethnicity. We did not find clear evidence that prevalence studies reported stronger or more consistent significant effects of education on risk for dementia compared to incidence studies. The existence of an education-dementia relationship seems strongly tied to the unique demands of an individual’s environment. We suggest that education is best described as a proxy for a trajectory of life events, beginning prior to and extending beyond the years of formal education, that either increase or decrease an individual’s risk for dementia.
Acknowledgments
The authors gratefully thank Bob Knight, John J. McArdle, Nancy L. Pedersen, and Merril Silverstein for their insightful comments.
This research was supported in part by a grant from the National Institute on Aging: Multidisciplinary Research Training in Gerontology (5T32AG00037, Principal Investigator: Eileen Crimmins, PhD).
Contributor Information
Emily Schoenhofen Sharp, Email: schoenho@usc.edu, University of Southern California, Department of Psychology, 3620 McClintock Ave, Los Angeles, CA, 90089, Phone: 310-662-3494, Fax: 213-746-5994
Margaret Gatz, Email: gatz@usc.edu, University of Southern California, Department of Psychology, 3620 McClintock Ave, Los Angeles, CA, 90089, Phone: 213-740-2212, Fax: 213-746-5994
References
- 1.Mortimer JA. Do psychosocial risk factors contribute to Alzheimer’s disease? In: Henderson AS, Henderson JH, editors. Etiology of Dementia of the Alzheimer’s Type. New York: John Wiley & Sons; 1988. pp. 39–52. [Google Scholar]
- 2.Zhang M, Katzman R, Salmon DP, et al. The prevalence of dementia and Alzheimer’s disease in Shanghai China: impact of age, gender, and education. Ann Neurol. 1990;27:428–437. doi: 10.1002/ana.410270412. [DOI] [PubMed] [Google Scholar]
- 3.Katzman R. Education and the prevalence of dementia and Alzheimer’s disease. Neurology. 1993;43:13–20. doi: 10.1212/wnl.43.1_part_1.13. [DOI] [PubMed] [Google Scholar]
- 4.Mortimer JA, Graves AB. Education and other socioeconomic determinants of dementia and Alzheimer’s disease. Neurology. 1993;43:S39–S44. [Google Scholar]
- 5.Gilleard CJ. Education and Alzheimer’s disease: a review of recent international epidemiology studies. Aging Ment Health. 1997;1:33–46. [Google Scholar]
- 6.Caamaño-Isorna F, Corral M, Montes-Martínez A, et al. Education and dementia: a meta-analytic study. Neuroepidemiology. 2006;26:226–232. doi: 10.1159/000093378. [DOI] [PubMed] [Google Scholar]
- 7.Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychological Medicine. 2006;36:441–454. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, D.C: American Psychiatric Association; 1981. (DSM-III) [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, D.C: American Psychiatric Association; 1987. rev. (DSM-III-R) [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D.C: American Psychiatric Association; 1994. (DSM-IV) [Google Scholar]
- 11.World Health Organization. Mental Disorders: Glossary and Guide to their Classification in Accordance with the 9th Revision of the International Classification of Diseases. WHO: Geneva; 1977. [Google Scholar]
- 12.World Health Organization. Mental Disorders: Glossary and Guide to their Classification in Accordance with the 10th Revision of the International Classification of Diseases. WHO; Geneva: 1992. [Google Scholar]
- 13.Roth M, Huppert FA, Tym E, et al. CAMDEX: The Cambridge Examination of Mental Disorders of the Elderly. Cambridge, England: Cambridge University Press; 1988. [Google Scholar]
- 14.McKann G, Dracham D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Chandra V, Ganguli M, Pandav R, et al. Prevalence of Alzheimer’s disease and other dementias in rural India. the Indo-US study. Neurology. 1998;51:1000–1008. doi: 10.1212/wnl.51.4.1000. [DOI] [PubMed] [Google Scholar]
- 16.Ganguli M, Dodge HH, Chen P, et al. Ten-year incidence of dementia in a rural elderly US community population. The MoVIES project. Neurology. 2000;54:1109–1116. doi: 10.1212/wnl.54.5.1109. [DOI] [PubMed] [Google Scholar]
- 17.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 18.Amaducci LA, Fratiglioni L, Rocca WA, et al. Risk factors for clinically diagnosed Alzheimer’s disease: a case-control study of an Italian population. Neurology. 1986;36:922–931. doi: 10.1212/wnl.36.7.922. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor DW, Pollitt PA, Treasure FP. The influence of education and social class on the diagnosis of dementia in a community population. Psychol Med. 1991;21:219–224. doi: 10.1017/s003329170001480x. [DOI] [PubMed] [Google Scholar]
- 20.Bonaiuto S, Rocca WA, Lippi A, et al. Education and occupation as risk factors for dementia: a population-based case-control study. Neuroepidemiology. 1995;14:101–109. doi: 10.1159/000109785. [DOI] [PubMed] [Google Scholar]
- 21.Ott A, Breteler MMB, van Harskamp F, et al. Prevalence of Alzheimer’s disease and vascular dementia: association with education. The Rotterdam Study. BMJ. 1995;310:970–973. doi: 10.1136/bmj.310.6985.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prencipe M, Casini AR, Ferretti C, et al. Prevalence of dementia in an elderly rural population: effects of age, sex, and education. J Neurol Neurosurg Psychiatry. 1996;60:628–633. doi: 10.1136/jnnp.60.6.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azzimondi G, D’Alessandro R, Pandolfo G, et al. Comparative study of the prevalence of dementia in two Sicilian communities with different psychosoical backgrounds. Neuroepidemiology. 1998;17:199–209. doi: 10.1159/000026173. [DOI] [PubMed] [Google Scholar]
- 24.De Ronchi D, Fratiglioni L, Rucci P, et al. The effect of education on dementia occurrence in an Italian population with middle to high socioeconomic status. Neurology. 1998;50:1231–1238. doi: 10.1212/wnl.50.5.1231. [DOI] [PubMed] [Google Scholar]
- 25.Gatz M, Svedberg P, Pedersen NL, et al. Education and the risk of Alzheimer’s disease: findings from the study of dementia in Swedish twins. J Gerontol B Psychol Sci Soc Sci. 2001;56:292–300. doi: 10.1093/geronb/56.5.p292. [DOI] [PubMed] [Google Scholar]
- 26.Harmanci H, Emre M, Gurvit H, et al. Risk factors for Alzheimer disease: a population-based case-control study in Istanbul, Turkey. Alzheimer Dis Assoc Disord. 2003;17:139–145. doi: 10.1097/00002093-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Tognoni G, Ceravolo R, Nucciarone B, et al. From mild cognitive impairment to dementia: a prevalence study in a district of Tuscany, Italy. Acta Neurol Scand. 2005;112:65–71. doi: 10.1111/j.1600-0404.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- 28.Gatz M, Mortimer JA, Fratiglioni L, et al. Accounting for the relationship between low education and dementia: a twin study. Physiol Behav. 2007;92:232–237. doi: 10.1016/j.physbeh.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ngandu T, von Strauss E, Helkala EL, et al. Education and dementia: what lies behind the association? Neurology. 2007;69:1442–1450. doi: 10.1212/01.wnl.0000277456.29440.16. [DOI] [PubMed] [Google Scholar]
- 30.Canadian Health and Aging Study. The Canadian study of health and aging: risk factors for Alzheimer’s disease in Canada. Neurology. 1994;44:2073–2080. doi: 10.1212/wnl.44.11.2073. [DOI] [PubMed] [Google Scholar]
- 31.Mortel KF, Meyer JS, Herod B, et al. Education and occupation as risk factors for dementias of the Alzheimer and ischemic vascular types. Dementia. 1995;6:55–62. doi: 10.1159/000106922. [DOI] [PubMed] [Google Scholar]
- 32.Callahan CM, Hall KS, Hui SL, et al. Relationship of age, education, and occupation with dementia among a community-based sample of African Americans. Arch Neurol. 1996;53:134–140. doi: 10.1001/archneur.1996.00550020038013. [DOI] [PubMed] [Google Scholar]
- 33.Hall K, Gureje O, Gao S, et al. Risk factors and Alzheimer’s disease: A comparative study of two communities. Aust N Z J Psychiatry. 1998;32:698–706. doi: 10.3109/00048679809113126. [DOI] [PubMed] [Google Scholar]
- 34.Harwood DG, Barker WW, Loewenstein DA, et al. A cross-ethnic analysis of risk factors for AD in white Hispanics and white non-Hispanics. Neurology. 1999;52:551–556. doi: 10.1212/wnl.52.3.551. [DOI] [PubMed] [Google Scholar]
- 35.Munoz DG, Ganapathy GR, Eliasziw M, et al. Educational attainment and socioeconomic status of patients with autopsy-confirmed Alzheimer disease. Arch Neurol. 2000;57:85–89. doi: 10.1001/archneur.57.1.85. [DOI] [PubMed] [Google Scholar]
- 36.Mortimer JA, Snowdon DA, Markesbery WR. Head circumference, education and risk of dementia: findings from the nun study. J Clin Exp Neuropsychol. 2003;25:671–679. doi: 10.1076/jcen.25.5.671.14584. [DOI] [PubMed] [Google Scholar]
- 37.Roe CM, Xiong C, Miller JP, et al. Education and Alzheimer disease without dementia: support for the cognitive reserve hypothesis. Neurology. 2007;68:223–228. doi: 10.1212/01.wnl.0000251303.50459.8a. [DOI] [PubMed] [Google Scholar]
- 38.Kondo K, Niino M, Shido K. A case-control study of Alzheimer’s disease in Japan-Significance of life-styles. Dementia. 1994;5:314–326. doi: 10.1159/000106741. [DOI] [PubMed] [Google Scholar]
- 39.Lin R, Lai C, Tai C, et al. Prevalence and subtypes of dementia in southern Taiwan: impact of age, sex, education, and urbanization. J Neurol Sci. 1998;160:67–75. doi: 10.1016/s0022-510x(98)00225-1. [DOI] [PubMed] [Google Scholar]
- 40.Liu CH, Fuh JL, Wang SJ, et al. Prevalence and subtypes of dementia in a rural Chinese population. Alzheimer Dis Assoc Disord. 1998;12:127–134. doi: 10.1097/00002093-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Yamada M, Sasaki H, Mimori Y, et al. Prevalence and risks of dementia in the Japanese Population: RERF’s adult health study Hiroshima subjects. J Am Geriatr Soc. 1999;47:189–195. doi: 10.1111/j.1532-5415.1999.tb04577.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang ZX, Zahner GEP, Roman G, et al. Socio-demographic variation of dementia subtypes in China: methodology and results of a prevalence study in Beijing, Chengdu, Shanghai, and Xian. Neuroepidemiology. 2006;27:177–187. doi: 10.1159/000096131. [DOI] [PubMed] [Google Scholar]
- 43.Zhou DF, Wu CS, Qi H, et al. Prevalence of dementia in rural China: impact of age, gender and education. Acta Neurol Scand. 2006;114:273–280. doi: 10.1111/j.1600-0404.2006.00641.x. [DOI] [PubMed] [Google Scholar]
- 44.Galasko D, Salomon D, Gamst A, et al. Prevalence of dementia in Chamorros in Guam. Relationship to age, gender, education, and APOE. Neurology. 2007;68:1772–1781. doi: 10.1212/01.wnl.0000262028.16738.64. [DOI] [PubMed] [Google Scholar]
- 45.Llibre Rodríguez JJ, Ferri CP, Acosta D, et al. Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. Lancet. 2008;372:464–474. doi: 10.1016/S0140-6736(08)61002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrera E, Caramelli P, Silveira ASB, et al. Epidemiologic survey of dementia in a community-dwelling Brazilian population. Alzheimer Dis Assoc Disord. 2002;16:103–108. doi: 10.1097/00002093-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Scazufca M, Menezes PR, Araya R, et al. Risk factors across the life course and dementia in a Brazilian population: results from the Sao Paulo Ageing & Health Study (SPAH) Int J Epidemiol. 2008;37:879–890. doi: 10.1093/ije/dyn125. [DOI] [PubMed] [Google Scholar]
- 48.Bowirrat A, Treves TA, Friedland RP, et al. Prevalence of Alzheimer’s type of dementia in an elderly Arab population. Eur J Neurol. 2001;8:119–123. doi: 10.1046/j.1468-1331.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- 49.Kahana E, Galper Y, Zilber N, et al. Epidemiology of dementia in Ashkelon. The influence of education. J Neurol. 2003;250:424–428. doi: 10.1007/s00415-003-0999-y. [DOI] [PubMed] [Google Scholar]
- 50.Bickel H, Cooper B. Incidence and relative risk of dementia in an urban elderly population: findings of a prospective field study. Psychol Med. 1994;24:179–192. doi: 10.1017/s0033291700026945. [DOI] [PubMed] [Google Scholar]
- 51.Paykel ES, Brayne C, Huppert FA, et al. Incidence of dementia in a population older than 75 years in the United Kingdom. Arch Gen Psychiatry. 1994;51:325–332. doi: 10.1001/archpsyc.1994.03950040069009. [DOI] [PubMed] [Google Scholar]
- 52.Persson G, Skoog I. A prospective population study of psychosocial risk factors for late onset dementia. Int J Geriatr Psychiatry. 1996;11:15–22. [Google Scholar]
- 53.Schmand B, Smit JH, Geerlings MI, et al. The effects of intelligence and education on the development of dementia: a test of the brain reserve hypothesis. Psychol Med. 1997;27:1337–1344. doi: 10.1017/s0033291797005461. [DOI] [PubMed] [Google Scholar]
- 54.Geerlings MI, Schmand B, Jonker C, et al. Education and incident Alzheimer’s disease: a biased association due to selective attrition and use of a two-step diagnositic procedure? Inter J Epidemiol. 1999;28:492–497. doi: 10.1093/ije/28.3.492. [DOI] [PubMed] [Google Scholar]
- 55.Launer LJ, Andersen K, Dewey ME, et al. Rates and risk factors for dementia and Alzheimer’s disease: results from EURODEM pooled analyses. Neurology. 1999;52:78–84. doi: 10.1212/wnl.52.1.78. [DOI] [PubMed] [Google Scholar]
- 56.Letenneur L, Gilleron V, Commenges D, et al. Are sex and educational level independent predictors of dementia and Alzheimer’s disease? Incidence data from the PAQUID project. J Neurol Neurosurg Psychiatry. 1999;66:177–183. doi: 10.1136/jnnp.66.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nielsen H, Lolk A, Andersen K, et al. Characteristics of elderly who develop Alzheimer’s disease during the next two years A neuropsychological study using CAMCOG. The ODENSE Study. Int J Geriatr Psychiatry. 1999;14:957–963. doi: 10.1002/(sici)1099-1166(199911)14:11<957::aid-gps43>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 58.Ott A, van Rossum CTM, van Harskamp F, et al. Education and the incidence of dementia in a large population-based study: the Rotterdam study. Neurology. 1999;52:663–666. doi: 10.1212/wnl.52.3.663. [DOI] [PubMed] [Google Scholar]
- 59.Qiu C, Backman L, Winblad B, et al. The influence of education on clinically diagnosed dementia incidence and mortality data from the Kungsholmen project. Arch Neurol. 2001;58:2034–2039. doi: 10.1001/archneur.58.12.2034. [DOI] [PubMed] [Google Scholar]
- 60.Anttila T, Helkala E, Kivipelto M, et al. Midlife income, occupation, APOE status, and dementia: a population-based study. Neurology. 2002;59:887–893. doi: 10.1212/wnl.59.6.887. [DOI] [PubMed] [Google Scholar]
- 61.Di Carlo A, Baldereschi M, Amaducci L, et al. Incidence of dementia, Alzheimer’s disease, and vascular dementia in Italy. The ILSA study. J Am Geriatr Soc. 2002;50:41–48. doi: 10.1046/j.1532-5415.2002.50006.x. [DOI] [PubMed] [Google Scholar]
- 62.Karp A, Kareholt I, Qiu C, et al. Relation of education and occupation-based socioeconomic status to incident Alzheimer’s disease. Am J Epidemiol. 2004;159:175–183. doi: 10.1093/aje/kwh018. [DOI] [PubMed] [Google Scholar]
- 63.Ravaglia G, Forti P, Maioli F, et al. Incidence and etiology of dementia in a large elderly Italian population. Neurology. 2005;64:1525–1530. doi: 10.1212/01.WNL.0000160107.02316.BF. [DOI] [PubMed] [Google Scholar]
- 64.Brayne C, Ince PG, Keage HAD, et al. Education, the brain and dementia: neuroprotection or compensation? Brain. 2010;133:2210–2216. doi: 10.1093/brain/awq185. [DOI] [PubMed] [Google Scholar]
- 65.Beard MC, Kokmen E, Offord KP, et al. Lack of association between Alzheimer’s disease and education, occupation, marital status, or living arrangement. Neurology. 1992;42:2063–2068. doi: 10.1212/wnl.42.11.2063. [DOI] [PubMed] [Google Scholar]
- 66.Hebert LE, Scherr PA, Beckett LA, et al. Relation of smoking and alcohol consumption to incident Alzheimer’s disease. Am J Epidemiol. 1992;135:347–355. doi: 10.1093/oxfordjournals.aje.a116296. [DOI] [PubMed] [Google Scholar]
- 67.Stern Y, Gurland B, Tatemichi TK, et al. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 68.Cobb JL, Wolf PA, Au R, et al. The effect of education on the incidence of dementia and Alzheimer’s disease in the Framingham study. Neurology. 1995;45:1707–1712. doi: 10.1212/wnl.45.9.1707. [DOI] [PubMed] [Google Scholar]
- 69.Evans DA, Hebert LE, Beckett LA, et al. Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch Neurol. 1997;54:1399–1405. doi: 10.1001/archneur.1997.00550230066019. [DOI] [PubMed] [Google Scholar]
- 70.Elias MF, Beiser A, Wolf PA, et al. The preclinical phase of Alzheimer disease: A 22-year prospective study of the Framingham cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 71.Kawas C, Gray S, Brookmeyer R, et al. Age-specific incidence rates of Alzheimer’s disease. The Baltimore longitudinal study of aging. Neurology. 2000;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 72.Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 73.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence. Arch Neurol. 2002;59:1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 74.Wilson RS, Mendes de Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 75.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 76.Tuokko H, Garrett DD, McDowell I, et al. Cognitive decline in high functioning older adults: reserve or ascertainment bias? Aging Ment Health. 2003;7:259–270. doi: 10.1080/1360786031000120750. [DOI] [PubMed] [Google Scholar]
- 77.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the cardiovascular health study. J Am Geriatr Soc. 2004;52:195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 78.Shadlen M, Siscovick D, Fitzpatrick AL, et al. Education, cognitive test scores, and black-white differences in dementia risk. J Am Geriatr Soc. 2006;54:898–905. doi: 10.1111/j.1532-5415.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- 79.McDowell I, Xi G, Lindsay J, et al. Mapping the connections between education and dementia. J Clin Exp Neuropsychol. 2007;29:127–141. doi: 10.1080/13803390600582420. [DOI] [PubMed] [Google Scholar]
- 80.Li G, Shen YC, Chen CH, et al. A three-year follow-up study of age-related dementia in an urban area of Beijing. Acta Psychiatr Scand. 1991;83:99–104. doi: 10.1111/j.1600-0447.1991.tb07373.x. [DOI] [PubMed] [Google Scholar]
- 81.Yoshitake T, Kiyohara Y, Kato I, et al. Incidence and the risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: the Hisayama Study. Neurology. 1995;45:1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- 82.Liu CK, Lai CL, Tai CT, et al. Incidence and subtypes of dementia in southern Taiwan: impact of sociodemographic factors. Neurology. 1998;50:1572–1579. doi: 10.1212/wnl.50.6.1572. [DOI] [PubMed] [Google Scholar]
- 83.He YL, Zhang XK, Zhang MY. Psychosocial risk factors for Alzheimer’s disease. Hong Kong Journal of Psychiatry. 2000;10:2–7. [Google Scholar]
- 84.Yamada M, Mimori Y, Kasagi F, et al. Incidence of dementia, Alzheimer disease, and vascular dementia in a Japanese population: radiation effects research foundation adult health study. Neuroepidemiology. 2008;30:152–160. doi: 10.1159/000122332. [DOI] [PubMed] [Google Scholar]
- 85.Nitrini R, Caramelli P, Herrera E, et al. Incidence of dementia in a community-dwelling Brazilian population. Alzheimer Dis Assoc Disord. 2004;18:241–246. [PubMed] [Google Scholar]
- 86.Kalaria RN, Maestre GE, Arizaga R, et al. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gerstorf D, Herlitz A, Smith J. Stability of sex differences in cognition in advanced old age: the role of education and attrition. J Gerontol B Psychol Sci Soc Sci. 2006;61:245–249. doi: 10.1093/geronb/61.4.p245. [DOI] [PubMed] [Google Scholar]
- 88.Letenneur L, Launer LJ, Andersen K, et al. Education and the risk for Alzheimer’s disease: sex makes a difference. EURODEM pooled analyses. Am J Epidemiol. 2000;151:1064–1071. doi: 10.1093/oxfordjournals.aje.a010149. [DOI] [PubMed] [Google Scholar]
- 89.Manly JJ, Schupf N, Tang M, et al. Literacy and cognitive decline among ethnically diverse elders. In: Stern Y, editor. Cognitive Reserve: Theory and Applications. Philadelphia, PA: Taylor & Francis; 2007. pp. 219–235. [Google Scholar]
- 90.Hall KS, Gao S, Unverzagt FW, et al. Low education and childhood rural residence: risk for Alzheimer’s disease in African Americans. Neurology. 2000;54:95–99. doi: 10.1212/wnl.54.1.95. [DOI] [PubMed] [Google Scholar]
- 91.Schmand B, Lindeboom J, Hooijer C, et al. Relation between education and dementia: the role of test bias revisited. J Neurol Neurosurg Psychiatry. 1995;59:170–174. doi: 10.1136/jnnp.59.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prince MJ, Llibre Rodriguez J, Noriega L, et al. The 10/66 Dementia Research Group’s fully operationalized DSM-IV dementia computerized diagnostic algorithm, compared with the 10/66 dementia algorithm and a clinician diagnosis: a population validation study. [Accessed January 10, 2009];BMC Public Health. 2008 8:219–231. doi: 10.1186/1471-2458-8-219. [serial online]. June. Available from BioMed Central. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Llibre Rodriguez JJ, Valhuerdi A, Sanchez II, et al. The prevalence, correlates and impact of dementia in Cuba. Neuroepidemiology. 2008;31:243–251. doi: 10.1159/000165362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stern Y. Cognitive Reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Deary IJ, Whiteman MC, Starr JM, et al. The impact of childhood intelligence on later life: following up the Scottish mental surveys of 1932 and 1947. J Pers Soc Psychol. 2004;86:130–147. doi: 10.1037/0022-3514.86.1.130. [DOI] [PubMed] [Google Scholar]