Abstract
Background
The significance of EGFR expression in advanced cutaneous squamous cell carcinoma (cSCC) of the head and neck remains poorly understood.
Methods
Retrospective review of patients with advanced stage (Stage III or IV) cSCC of the head and neck (n = 56).
Results
The majority of patients (91%) had stage III disease, with 54% having regional metastasis and 9% with distant metastasis. Two-year survival was 64% and the 5-year survival was 56%. EGFR was found to be overexpressed in 56% of primary tumors and 58% of regional metastatic disease. Overall survival did not correlate with EGFR (p = 0.47) expression in primary lesions nor was it associated with an increase in regional (p = 0.74) or distant metastasis (p = 0.56). Furthermore, there was no correlation between clinicopathologic characteristics and EGFR expression
Conclusion
This data does not suggest upregulation of EGFR is associated with poor survival or aggressive disease.
Keywords: Cutaneous squamous cell carcinoma, head and neck, survival, nonmelanoma skin cancer, epidermal growth factor receptor
Introduction
With more than one million new cases reported each year, nonmelanoma skin cancer is the most common diagnosed malignancy in the United States.(1) The majority of lesions (80–90%) arise in the sun exposed areas of the head and neck(2) and are successfully treated by complete tumor excision. However, there is small percentage of nonmelanoma skin cancers which are refractory to standard surgical excision.(1, 3) Patients presenting with recurrent or advanced stage disease are at increased risk for neck metastasis, poor local control and poor outcome. The treatment for recurrent cutaneous squamous cell carcinoma (cSCC) often includes aggressive surgery with postoperative radiation. This patient population, which is primarily elderly and therefore often plagued by co-morbidities, may not always be good surgical candidates. Multiple studies have recently proposed that the use of targeted therapies with non-overlapping toxicities be used in these patients. However, advanced stage, aggressive cSCC are rare, limiting what is known about their biological behavior and preventing more directed therapy. Although well understood in mucosal SCC, the expression of epidermal growth factor receptor (EGFR) in advanced cSCC has not been investigated.
Epidermal growth factor receptor is a transmembrane glycoprotein expressed in the majority of epithelial malignancies including head and neck mucosal squamous cell carcinoma (SCC).(4) Elevated EGFR mRNA is found in 92% of mucosal SCC tumors(5) and EGFR levels are increased in poorly differentiated tumors and advanced-stage tumors.(6) High expression of EGFR protein in mucosal SCC is associated with worse prognosis and decreased disease-free survival.(5) The success of anti-EGFR therapy in mucosal SCC has led to many clinical trials evaluating its potential benefit in cSCC. However, these trials are based on histological rather than biological similarities between the two malignancies. To address this uncertainty, we assessed EGFR expression in advanced stage cSCC in patients that would be candidates for adjuvant chemotherapy.
Materials and Methods
Patient Selection
Following Institutional Review Board approval, a retrospective review of 56 patients who presented between June 1998 and December 2007 with recurrent, advanced TMN stage (III or IV) cSCC of the head and neck was performed at the University of Alabama at Birmingham. Tumors were staged according to the American Joint Committee on Cancer (AJCC)(7) guidelines and histology was confirmed by pathology.
Each patient underwent aggressive surgical resection and the majority required a neck dissection and postoperative radiation. The indication for neck dissection was suspicion of nodal metastasis on preoperative imaging or an advanced T classification of the lesion at presentation. Recommendations for adjuvant radiation included a large primary tumor, presence of more than one positive node on neck dissection, an inability to obtain negative surgical margins or evidence of perineural or lymphovascular invasion.
Analyses of EGFR Expression by Immunohistochemistry
Immunohistochemistry (IHC) staining was performed to determine EGFR expression levels. Samples were rehydrated in xylene, 95% ethanol, and 70% ethanol. Antigen retrieval was accomplished in 1 mM EDTA, pH 9.0, for 5 minutes at 100°C. Samples were then allowed to cool at room temperature and blocked with 5% BSA in TBST for 5 minutes at room temperature. Primary antibody, EGFR antibody (Abcam, #27600), was applied at the concentrations recommended and allowed to incubate for 1 hour. Secondary antibody (Pierce goat anti-rabbit HRP, #32260) was applied for 40 minutes in a humidified chamber at room temperature. DAB substrate was then applied to slides and allowed to incubate at room temperature until appropriate color developed. Samples were then counterstained with Harris Hematoxylin diluted 1:1 with tap water for 45 seconds. Finally, samples were dehydrated and counted with Permount and allowed to dry overnight.
The EGFR staining intensity and quality was scored by two independent observers who were blinded to the clinical parameters. Scoring values were assigned as follows: 0 = none to < 10% of the tumor cells staining, 1+ = light (intensity) and incomplete (quality) staining in > 10% of the tumor cells, 2+ = moderate and complete staining of > 10% of the tumor cells and 3+ = intense and complete staining > 10% (Figure 1).(8) Specimens were available for 89% (n=50) of the primary tumors and 40% (n=12) of the nodal metastasis.
Figure 1.

Statistical Analysis
Descriptive variables were summarized by mean, median and standard deviation (SD) for continuous variables and n (%) for categorical variables. The relationship between EGFR expression levels and clinicopathologic characteristics was calculated using the Fisher’s Exact Test.
The relationship between patient, clinical and treatment factors, and cancer specific survival was calculated by using the Kaplan-Meier method. Survival time was calculated as the interval from date of surgery to date of death or date of last follow-up. Deaths due to other causes were censored for these analyses. A p-value of < 0.05 was considered statistically significant. All of the analyses were two-sided. Statistical analysis was performed using SAS® Ver. 9.2 software (SAS Institute, Inc., Cary, NC).
Results
All patients underwent surgical resection of the cSCC lesion, and the majority were male (86%, n=48) and elderly (73 ± 12 years). Common comorbidities were diabetes mellitus, hypertension and hypercholesterolemia with 16% (n=9) having had a previous non-head and neck SCC malignancy. The majority of patients (91%, n=51) were TMN stage III at initial diagnosis with 54% (n=30) having evidence of regional metastasis and 9% (n=5) with evidence of distant metastasis. Almost all underwent radiation therapy (91%, n=51) at some point during treatment. A summary of patient characteristics can be found in Table 1.
Table 1.
Patient Characteristics
| Characteristic | No. of patients (%) |
|---|---|
| Age – years | |
| Mean (Range) | 73 (42–93) |
| Gender | |
| Male | 48 (86) |
| Comorbidities | |
| Diabetes mellitus | 10 (18) |
| Hypertension | 24 (43) |
| Hypercholesterolemia | 10 (18) |
| Multiple malignancies | 9 (16) |
| Congestive heart failure | 2 (4) |
| Previous transplant | 3 (5) |
| Hypothyroidism | 2 (4) |
| Coronary artery disease | 5 (9) |
| T classification | |
| T2 | 5 (9) |
| T3 | 3 (5) |
| T4 | 47 (84) |
| Tx | 1 (2) |
| TMN Stage | |
| III | 51 (91) |
| IV | 5 (9) |
| Node Positive | |
| No positive nodes | 26 (46) |
| Positive nodes | 30 (54) |
| Radiation Therapy | |
| Yes | 51 (91) |
| No | 5 (9) |
| Follow up in months (median) | 14 |
Kaplan-Meier analysis of overall survival found a two-year survival rate of 64% and 5-year survival rate of 56%. The median follow up was 14 months. Perineural invasion did not correlate with primary tumor size or location, nodal metastasis did not correlate with primary tumor size, and distant metastasis did not correlate with perineural invasion, primary tumor size or location (unpublished data).
Analysis of the immunohistochemistry staining was performed to determine EGFR expression levels (Figure 1). A total of 36% (n=18/50) of the primary tumors were negative for EGFR immunoreactivity, while 56% (n=28/50) of primary tumors were considered positive for high expression (2+ or 3+). Of these, 16% (n=8) were graded 2+ and 40% (n=20) were graded 3+. Analysis of regional lymph nodes positive for metastatic disease demonstrated similar proportion of EGFR immunoreactivity; 33% (n=4/12) were negative for EGFR expression and 58% (n=7/12) had high EGFR expression. Of these, 33% (n=4) were graded 2+ and 25% (n=3) were graded 3+ (Table 2). There was no statistically significant relationship between baseline clinicopathologic parameters (tumor stage, metastatic disease, surgical margins, perineural invasion, radiation therapy) and EGFR expression levels (Table 3). The presence of positive margins, perineural invasion or history of immunosuppression also did not correlate with recurrence. In addition, disease free survival was not affected by primary tumor size or location, perineural invasion or positive margins (unpublished data).
Table 2.
Epidermal growth factor receptor immunoreactivity in cutaneous squamous cell carcinoma.
| Specimens (No. of patients) | Immunostaining* | %Pos† | %High‡ | |||
|---|---|---|---|---|---|---|
| Score | 0 | 1+ | 2+ | 3+ | ||
|
|
||||||
| PT (50) | 18 | 4 | 8 | 20 | 64.0 | 56.0 |
| ND (12) | 4 | 1 | 4 | 3 | 66.7 | 58.3 |
Abbreviations: PT, primary tumor; ND, nodal disease
Immunohistochemical staining score: 0 = negative; 1+ = weak; 2+ = moderate; 3+ = strong
% of immunopositive tumors (score 1+ to 3+) in each subgroup
% of tumors with ≥2+ immunostaining in each subgroup
Table 3.
Clinicopathologic characteristics associated with immunohistochemical staining intensity of epidermal growth factor receptor in primary cutaneous squamous cell carcinoma lesions.
| Variable | % of EGFR staining intensity (No. of Patients) | ||
|---|---|---|---|
| Low* (22) | High † (28) | p value‡ | |
| TMN Stage | |||
| III | 41.3 (19) | 58.7 (7) | 0.31 |
| IV | 75.0 (3) | 25.0 (1) | |
| Nodal Disease | |||
| N0 | 46.2 (12) | 53.8 (14) | 0.78 |
| N+ | 41.7 (10) | 58.3 (14) | |
| Distant Metastatic Disease | |||
| M0 | 41.3 (19) | 58.7 (27) | 0.31 |
| M+ | 75.0 (3) | 25.0 (1) | |
| Surgical Margins | |||
| Negative | 46.4 (13) | 53.6 (15) | 0.78 |
| Positive | 40.9 (9) | 59.1 (13) | |
| Perineural Invasion | |||
| Negative | 48.5 (16) | 51.5 (17) | 0.55 |
| Positive | 35.3 (6) | 64.7 (11) | |
| Radiation Therapy | |||
| Neoadjuvant | 42.9 (6) | 57.1 (8) | 1.00 |
| Adjuvant | 39.3 (11) | 60.7 (17) | |
| Survival in months (median) | (34) | (30) | |
Abbreviation: EGFR, epidermal growth factor receptor
Scoring Intensity (0, 1+)
Scoring Intensity (2+, 3+)
Calculated using Fisher’s Exact Test
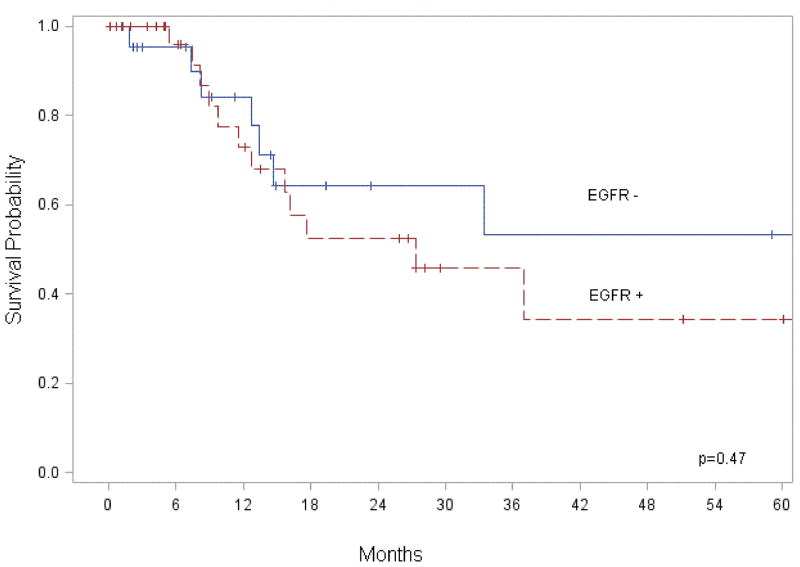
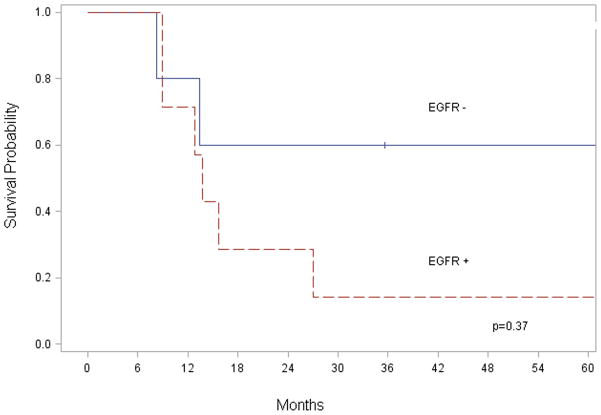
EGFR expression was not associated with an increase in regional (p = 0.78) or distant metastasis (p = 0.31). When stratified by EGFR expression there was no significant difference in survival (p = 0.47) (Figure 2). In regional lymph node specimens positive for metastatic disease, EGFR expression did not correlate to survival time (p = 0.37) (Figure 3). Although a statistically significant decrease in survival was not identified, there was a trend to suggest EGFR overexpression correlated with worse outcome. Analysis of cytoplasmic EGFR immunoreactivity was also performed. Cytoplasmic EGFR expression did not correlate with clinicopathological findings or survival.
Figure 2.
Figure 3.
Discussion
It is well known that the majority of head and neck mucosal SCC overexpress EGFR(6, 9–11) and EGFR expression is considered a predictor of prognosis.(5, 11–13) Due to the similar gross and histological appearance of cutaneous and mucosal SCC there is an underlying assumption that the biology is also similar. However, the relationship between EGFR expression in advanced cSCC remains largely unknown. This is in large part due to the low incidence and rarity of advanced cSCC. To our knowledge, this is the first report examining the relationship between EGFR expression and survival in cSCC.
When stratifying by overexpression of EGFR, we found EGFR to be overexpressed on the cell membrane in 56% of advanced cSCC. Whereas others have found EGFR overexpression in 90% of mucosal SCC.(14) Although pervious data from mucosal SCC found EGFR expression levels to correlate with survival(5, 15, 16), our data did not find a correlation with EGFR expression levels and survival in cSCC. Although the median survival was lower in patients with high EGFR expression (30 months versus 34 months), the trend was not significantly different (p=0.47). In addition, EGFR expression in the primary lesion was not associated with an increase in regional (p = 0.74) or distant metastasis (p = 0.56). Despite similarities in histology, our data suggest that EGFR expression in cSCC may have a different biological significance than in mucosal SCC.
EGFR governs cell survival and proliferation and is thought to infer radiation resistance.(11, 17) Overexpression of EGFR leads to increased activation of intracellular signaling pathways which results in neoplastic cells with a more aggressive phenotype.(6) Its overexpression in mucosal SCC and role in tumorigenesis has lead several investigators to examine the utility of targeting EGFR in cSCC. Others have found EGFR expression in 88–100% of cSCC.(18–22) The high percentage of EGFR expressing cells has been partly contributed to their cell type of origin.(19) In our study, we found 36% of tumors to demonstrate little to no EGFR immunoreactivity. This discrepancy between previous studies reporting a high rate of expression and our data is probably related to the subsection nature of quantifying EGFR expression levels. Differences in tissue sample processing and the heterogeneity of EGFR expression are other potential factors contributing to the inconsistencies.(23, 24) These previous studies did not evaluate clinical surrogates of malignant potential nor did these studies correlate EGFR expression with survival.
While it has been demonstrated that EGFR expression levels correlate with regional metastatic potential in other cancer types,(20, 22) our data did not demonstrate a higher expression of EGFR in primary tumors compared to metastatic tumors, nor did it increase the incidence of regional nodal metastasis (Table 3). In addition, there was no correlation between EGFR expression levels found in nodal specimens and survival (Figure 3). Given the small number of patients with distant metastatic disease (n=5), a comparison between the two groups (low versus high EGFR expression and metastasis) would not be productive. Since all patients had advanced, recurrent disease and were treated surgically, they were not stratified by stage, recurrence, and surgical intervention.
Similarities between advanced cSCC and mucosal head and neck cancer have led to the development of clinical trials using EGFR targeted therapies (http://www.cancer.gov/clinicaltrials), despite a limited understanding of the biological role of EGFR in advanced cSCC. Although a few case reports demonstrated isolated incidences of patients with recurrent cSCC responding to cetuximab, there has not been any significant evidence supporting the use of anti-EGFR therapies in this patient population. The absence of literature supporting a role for targeting EGFR in cSCC is likely multifactorial: 1) There are a limited number of cSCC cell lines for investigation, 2) Advanced cSCC are relatively uncommon and death rates are low, which makes it difficult to obtain a significant enough number of patients to elucidate the biology of this disease, and 3) Failure to correlate EGFR expression levels with clinical outcomes leads to negative results, which is significantly more difficult to publish and thus leads to a dearth of publications. Publication bias has been defined as the tendency for investigators or editors to fail to publish results based on the direction of the findings. One study found that data demonstrating statistical significance was submitted seven times more frequently and make up the vast majority (83%) of publications compared to those studies that did not demonstrate statistical significance.(25)
Thus far, targeted therapies against EGFR and its tyrosine kinase have demonstrated limited improvement in the mortality of patients with advanced disease when used as monotherapy.(14, 26–29) The role of EGFR as a prognostic factor in mucosal SCC is generally accepted, but the prognostic relevance of EGFR expression levels has recently been questioned.(5, 11, 23, 30, 31) Reasons for discrepancies across these studies and ours include origin of samples (biopsy versus gross tumor), power of the study, method of EGFR scoring, experience of scorer, and an inability to standardize qualitative scoring systems such as IHC.(32) These variations in grading IHC results are well known, and may explain discrepancy between our EGFR negative rate of 36% and others that range from 0–12%.(18–22) In our study examining cSCC we found no significant correlation between EGFR expression in cSCC and survival, loco-regional metastasis and time to recurrence.
There were several limitations to the current study. Because advanced stage cSCCs are relatively rare, studies such as this one are limited by small numbers of patients which of course limits statistical power. Travel time and advanced age often limited patients willingness to return for routine visits; this likely contributed to our limited follow-up time. Although median follow-up time was 14 months, patients will continue to recur from this disease over the subsequent two to four years and as a result this study may not successfully capture all recurrent disease. Further limiting the power of this study was the absence of primary tumor samples in six patients. Importantly, factors typically associated with poor outcome such as positive margins and T classification did not correlate with outcome as previously demonstrated by others.(33) Failure to reproduce these findings was most likely related the selecting patients with TMN stage III and IV disease: in this study population 44% of resections had positive margins, 54% had regional lymphadenopathy and 84% were classified as T4.
In conclusion, EGFR expression levels have been shown to correlate with aggressive biological behavior and poor prognosis in mucosal SCC, however it is not clear from this study if this is true for advanced cSCC. Elevated EGFR expression in cSCC did not correlate with metastasis or survival in this study. This study suggests the need for additional understanding of the role for EGFR in the biology of cSCC.
Acknowledgments
This work was supported by grants from the National Institute of Health (R01CA142637-01 and 2T32 CA091078-06).
Footnotes
No potential conflicts of interest were disclosed.
References
- 1.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344(13):975–83. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 2.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51(1):15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 3.Joseph AK, Mark TL, Mueller C. The period prevalence and costs of treating nonmelanoma skin cancers in patients over 65 years of age covered by medicare. Dermatol Surg. 2001;27(11):955–9. doi: 10.1046/j.1524-4725.2001.01106.x. [DOI] [PubMed] [Google Scholar]
- 4.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5(5):341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 5.Rubin Grandis J, Melhem MF, Gooding WE, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90(11):824–32. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 6.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24(17):2666–72. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 7.AJCC cancer staging manual. 7. xiv. New York: Springer; p. 648. [Google Scholar]
- 8.Atkins D, Reiffen KA, Tegtmeier CL, Winther H, Bonato MS, Storkel S. Immunohistochemical detection of EGFR in paraffin-embedded tumor tissues: variation in staining intensity due to choice of fixative and storage time of tissue sections. J Histochem Cytochem. 2004;52(7):893–901. doi: 10.1369/jhc.3A6195.2004. [DOI] [PubMed] [Google Scholar]
- 9.Herbst RS, Langer CJ. Epidermal growth factor receptors as a target for cancer treatment: the emerging role of IMC-C225 in the treatment of lung and head and neck cancers. Semin Oncol. 2002;29(1 Suppl 4):27–36. doi: 10.1053/sonc.2002.31525. [DOI] [PubMed] [Google Scholar]
- 10.Rubin Grandis J, Tweardy DJ, Melhem MF. Asynchronous modulation of transforming growth factor alpha and epidermal growth factor receptor protein expression in progression of premalignant lesions to head and neck squamous cell carcinoma. Clin Cancer Res. 1998;4(1):13–20. [PubMed] [Google Scholar]
- 11.Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62(24):7350–6. [PubMed] [Google Scholar]
- 12.Eriksen JG, Steiniche T, Askaa J, Alsner J, Overgaard J. The prognostic value of epidermal growth factor receptor is related to tumor differentiation and the overall treatment time of radiotherapy in squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys. 2004;58(2):561–6. doi: 10.1016/j.ijrobp.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 13.Temam S, Kawaguchi H, El-Naggar AK, et al. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. J Clin Oncol. 2007;25(16):2164–70. doi: 10.1200/JCO.2006.06.6605. [DOI] [PubMed] [Google Scholar]
- 14.Gold KA, Lee HY, Kim ES. Targeted therapies in squamous cell carcinoma of the head and neck. Cancer. 2009;115(5):922–35. doi: 10.1002/cncr.24123. [DOI] [PubMed] [Google Scholar]
- 15.Smith BD, Haffty BG. Molecular markers as prognostic factors for local recurrence and radioresistance in head and neck squamous cell carcinoma. Radiat Oncol Investig. 1999;7(3):125–44. doi: 10.1002/(SICI)1520-6823(1999)7:3<125::AID-ROI1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 16.Quon H, Liu FF, Cummings BJ. Potential molecular prognostic markers in head and neck squamous cell carcinomas. Head Neck. 2001;23(2):147–59. doi: 10.1002/1097-0347(200102)23:2<147::aid-hed1010>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59(2 Suppl):21–6. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 18.Bauknecht T, Gross G, Hagedorn M. Epidermal growth factor receptors in different skin tumors. Dermatologica. 1985;171(1):16–20. doi: 10.1159/000249380. [DOI] [PubMed] [Google Scholar]
- 19.Lavrijsen AP, Tieben LM, Ponec M, van der Schroeff JG, van Muijen GN. Expression of EGF receptor, involucrin, and cytokeratins in basal cell carcinomas and squamous cell carcinomas of the skin. Arch Dermatol Res. 1989;281(2):83–8. doi: 10.1007/BF00426583. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu T, Izumi H, Oga A, et al. Epidermal growth factor receptor overexpression and genetic aberrations in metastatic squamous-cell carcinoma of the skin. Dermatology. 2001;202(3):203–6. doi: 10.1159/000051637. [DOI] [PubMed] [Google Scholar]
- 21.Maubec E, Duvillard P, Velasco V, Crickx B, Avril MF. Immunohistochemical analysis of EGFR and HER-2 in patients with metastatic squamous cell carcinoma of the skin. Anticancer Res. 2005;25(2B):1205–10. [PubMed] [Google Scholar]
- 22.Ch’ng S, Low I, Ng D, et al. Epidermal growth factor receptor: a novel biomarker for aggressive head and neck cutaneous squamous cell carcinoma. Hum Pathol. 2008;39(3):344–9. doi: 10.1016/j.humpath.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Fischer C, Zlobec I, Stockli E, et al. Is immunohistochemical epidermal growth factor receptor expression overestimated as a prognostic factor in head-neck squamous cell carcinoma? A retrospective analysis based on a tissue microarray of 365 carcinomas. Hum Pathol. 2008;39(10):1527–34. doi: 10.1016/j.humpath.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 24.van Diest PJ, van Dam P, Henzen-Logmans SC, et al. A scoring system for immunohistochemical staining: consensus report of the task force for basic research of the EORTC-GCCG. European Organization for Research and Treatment of Cancer-Gynaecological Cancer Cooperative Group. J Clin Pathol. 1997;50(10):801–4. doi: 10.1136/jcp.50.10.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee KP, Boyd EA, Holroyd-Leduc JM, Bacchetti P, Bero LA. Predictors of publication: characteristics of submitted manuscripts associated with acceptance at major biomedical journals. Med J Aust. 2006;184(12):621–6. doi: 10.5694/j.1326-5377.2006.tb00418.x. [DOI] [PubMed] [Google Scholar]
- 26.Cohen EE, Davis DW, Karrison TG, et al. Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: a phase I/II study. Lancet Oncol. 2009;10(3):247–57. doi: 10.1016/S1470-2045(09)70002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart JS, Cohen EE, Licitra L, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected] J Clin Oncol. 2009;27(11):1864–71. doi: 10.1200/JCO.2008.17.0530. [DOI] [PubMed] [Google Scholar]
- 28.Cohen EE, Rosen F, Stadler WM, et al. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2003;21(10):1980–7. doi: 10.1200/JCO.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 29.Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22(1):77–85. doi: 10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 30.Numico G, Russi EG, Colantonio I, et al. EGFR status and prognosis of patients with locally advanced head and neck cancer treated with chemoradiotherapy. Anticancer Res. 30(2):671–6. [PubMed] [Google Scholar]
- 31.Gupta AK, McKenna WG, Weber CN, et al. Local recurrence in head and neck cancer: relationship to radiation resistance and signal transduction. Clin Cancer Res. 2002;8(3):885–92. [PubMed] [Google Scholar]
- 32.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97(16):1180–4. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 33.Givi B, Andersen PE, Diggs BS, Wax MK, Gross ND. Outcome of patients treated surgically for lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Head Neck. doi: 10.1002/hed.21574. [DOI] [PubMed] [Google Scholar]