Abstract
Aims
To determine the retinal nerve fibre layer (RNFL) thickness at which visual field (VF) damage becomes detectable and associated with structural loss.
Methods
In a prospective cross-sectional study, 72 healthy and 40 glaucoma subjects (one eye per subject) recruited from an academic institution had VF examinations and spectral domain optical coherence tomography (SD-OCT) optic disc cube scans (Humphrey field analyser and Cirrus HD-OCT, respectively). Comparison of global mean and sectoral RNFL thicknesses with VF threshold values showed a plateau of threshold values at high RNFL thicknesses and a sharp decrease at lower RNFL thicknesses. A ‘broken stick’ statistical model was fitted to global and sectoral data to estimate the RNFL thickness ‘tipping point’ where the VF threshold values become associated with the structural measurements. The slope for the association between structure and function was computed for data above and below the tipping point.
Results
The mean RNFL thickness threshold for VF loss was 75.3 μm (95% CI: 68.9 to 81.8), reflecting a 17.3% RNFL thickness loss from age-matched normative value. Above the tipping point, the slope for RNFL thickness and threshold value was 0.03 dB/μm (CI: −0.02 to 0.08) and below the tipping point, it was 0.28 dB/μm (CI: 0.18 to 0.38); the difference between the slopes was statistically significant (p<0.001). A similar pattern was observed for quadrant and clock-hour analysis.
Conclusions
Substantial structural loss (~17%) appears to be necessary for functional loss to be detectable using the current testing methods.
The structural and functional relationship in glaucoma has been extensively studied, but the point at which the clinical functional loss as measured by visual fields (VFs) becomes detectable and related to structural changes is unknown. Fundus photography demonstrated the occurrence of retinal nerve fibre layer (RNFL) defects before measurable VF defects.1 Longitudinal observations revealed that RNFL thinning was associated with future VF damage.2,3 Detection of structural changes that precede the loss of visual function could be used to improve disease management and preserve vision.
Precise measurements of RNFL thickness are possible with optical coherence tomography (OCT).4–6 Previous studies proposed models to relate RNFL thickness and functional assessment, but none of them identified the threshold where functional changes can be expected to be detected clinically.7,8 Our hypothesis is that there is an RNFL thickness threshold at which VF loss becomes clinically observable, where for values above the threshold there will be little correspondence between OCT measured structure and VF measured function, and in values below the threshold there will be a strong association between RNFL thickness and VF. The purpose of this study is to determine the RNFL thickness at which VF damage becomes detectable and associated with structural loss.
METHODS
Subjects were consecutively enrolled from the Pittsburgh Imaging Technology Trial, a prospective longitudinal study designed to assess ocular structure over time carried out at the University of Pittsburgh Eye Center. The study followed the principles of the Declaration of Helsinki and was performed in accordance with the Health Insurance Portability and Accountability Act. Institutional review board and ethics committee approval was obtained, and all participants gave their informed consent to be enrolled.
Subjects
Subjects were included if they were either healthy volunteers or open-angle glaucoma subjects of age 18 years or older. Subjects were excluded if any of the following was present: history of diabetes mellitus or any other systemic disease or medical treatment that might affect VF or retinal thickness, history of intraocular surgery except for uncomplicated cataract extraction at least a year prior to enrolment, best corrected visual acuity worse than 20/40, refractive error outside −12.00 to +8.00 dioptres and any ocular abnormality other than glaucoma. One eye of each subject was randomly selected. In unilateral glaucoma cases, the affected eye was selected.
In order to prevent selection bias in a study that evaluates the relationship between structure and function, the diagnostic definition of all subjects was based solely on VF findings without consideration of structural appearance. Healthy subjects had a normal VF examination in both eyes. Glaucoma subjects had typical and reproducible glaucomatous scotomas. Subjects who were clinically suspected of having glaucoma due to optic nerve head or RNFL appearance or because of elevated intraocular pressure but with normal VFs were included in the healthy group to ensure representation of the full spectrum of disease.
VF testing
Swedish Interactive Thresholding Algorithm standard 24-2 perimetry (Carl Zeiss Meditec, Dublin, California (CZM)) was performed in all subjects. Qualified tests had a false positive of <15% and fixation loss and false negative of <30%. Healthy subjects had a glaucoma hemifield test (GHT) within normal limits and their mean deviation (MD) and pattern SD within 95% of healthy population. Glaucomatous VFs were defined as those with at least one of the following confirmed on two consecutive visits: GHToutside normal limits or pattern standard deviation probability outside the 95% of the healthy population.
VF total deviation values were recorded in all 52 testing points. The individual values were grouped in correspondence with the distribution of the sectors as given by spectral domain optical coherence tomography (SD-OCT). The allocation of the individual threshold points to their group was determined according to Garway-Heath et al distribution map.9 Total deviation values were unlogged, and the average of all values in each group was log transformed back to the decibel scale.7 A secondary analysis was performed using the mean of the unlogged total deviation values. MD was also recorded.
SD-OCT
SD-OCT was acquired at the same visit as the clinical examination and VF. Detailed descriptions of the principles of SD-OCT have been published previously.10 All OCT scans were performed using the Cirrus HD-OCT (CZM). Optic disc 200×200 cube scans were obtained, quantifying a 6×6×2 mm volume. Scan quality scores >7 were acceptable, with no overt eye movement as detected by observing blood vessel discontinuity or distortion in the OCTenface image. Mean, quadrant and clock hour peripapillary RNFL thicknesses were used in the statistical analysis. SD-OCTsectors corresponding to a single VF threshold value (clock hours 2–4 and 10) were removed from the analysis because the reliability of the data might be limited.
Statistical analysis
VF values and OCT data were registered at the right eye orientation. Scatter plot of RNFL thicknesses with threshold values shows a plateau of VF at high RNFL thicknesses and a steep decrease at lower RNFL thicknesses. A ‘broken stick’ nonlinear statistical model was fit to the data to calculate the ‘tipping point’.11 The estimation is considered to be nonlinear because the tipping point is simultaneously estimated along with the slopes. An initial estimate of the tipping point was determined using Davies’ test,12 then a segmented regression model with unknown breakpoint was fit to the data using the estimated threshold from Davies’ test as the initial value:
where Yj denotes the threshold for subject i, X denotes the RNFL thickness for subject i, α is the intercept, β1 is the slope for the segment below the threshold Ψ, β1+β2 is the slope for the segment above the threshold Ψ and ε is a random error which is assumed to be statistically independent and normally distributed with mean 0 and SD σ. Note that if there is no breakpoint, β2 will equal zero. Similar analysis was performed in the secondary analysis using the 1/L values.
All analyses were conducted using R Language and Environment for Statistical Computing program (http://www.R-project.org)13 and the segmented R library.14 P<0.05 was considered statistically significant.
RESULTS
One hundred twelve subjects were recruited: 72 healthy and 40 glaucoma subjects; 44 men and 68 women. Demographics are summarised in table 1.
Table 1.
Demographic characteristics of the healthy and glaucomatous subjects
| Healthy n=72 | Glaucoma n=40 | p | |
|---|---|---|---|
| Age (yrs) | 50.0±16.4 | 65.5±10.9 | <0.001† |
| Female/Male | 39/33 | 29/11 | 0.06* |
| VF MD (dB) | −0.21±0.80 | −5.04±4.64 | <0.001‡ |
| VF PSD (dB) | 1.46±0.25 | 5.98±3.97 | <0.001‡ |
| OCT mean RNFL (μm) | 90.8±8.6 | 69.8±12.1 | <0.001† |
VF MD, visual field mean deviation; VF PSD, visual field pattern SD; OCT, optical coherence tomography; RNFL, retinal nerve fiber layer.
χ2.
Student t test.
Wilcoxon test.
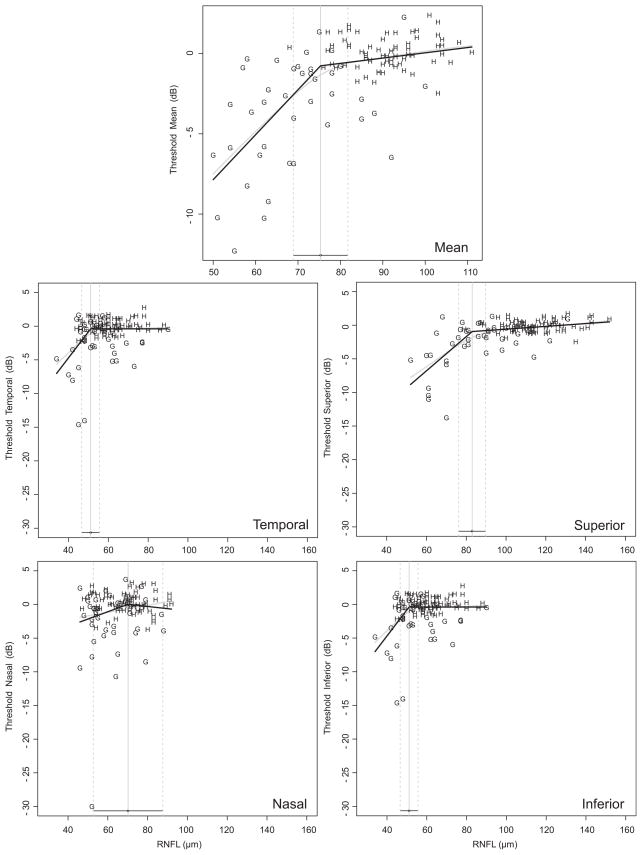
Scatter plots of RNFL thickness and VF threshold values showed a region where the OCT measured RNFL thickness was unrelated to VF measured visual function followed by a region where the relationship between these parameters was strong (figure 1). Estimates of the statistically optimal tipping points between these regions for average and quadrant RNFL thicknesses are listed in table 2. The global mean and the superior, temporal and inferior quadrants showed tight CI around the deflection point and the location of the tipping point was statistically significant (all p<0.05, Davies’ test). The nasal quadrant showed a wide CI and the tipping point location was not significant (p=0.82). Using population-based normative data of RNFL thickness values at the mean age of the participants in this study (personal communication with Michael V Patella, OD, Carl Zeiss Meditec), the percentage of RNFL loss necessary to reach the tipping point was also calculated for the mean and quadrants (table 2). The tipping point for the mean RNFL measurement occurred after a loss of 17.3% from the expected aged-matched value and approximately 25% loss in the superior and inferior quadrants. The RNFL thickness at the tipping point for the global mean and the inferior quadrant was considered as a borderline measurement based on the comparison with the normative data set criteria set by the manufacturer and outside normal limits for the superior quadrant.
Figure 1.
Healthy (H) and glaucoma (G) retinal nerve fibre layer (RNFL) thickness in the mean and in quadrants with corresponding visual field threshold values. Spline fit is the grey line; ‘broken stick’ model is the black line.
Table 2.
Retinal nerve fibre layer thickness tipping point with corresponding optical coherence tomography normative values.
| Tipping point
|
Normative values
|
% Loss for tipping point from mean normative | Davies’ test p value | ||||
|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | BDL cut-off | Outside normal limit cut-off | |||
| Mean | 75.3 | 68.9 to 81.8 | 91.1 | 77.5 | 69.9 | 17.3 | 0.003 |
| Temporal | 51.1 | 46.6 to 55.6 | 63.1 | 46.4 | 42.2 | 19.0 | 0.002 |
| Superior | 83.0 | 76.3 to 89.7 | 115.0 | 92.1 | 85.6 | 27.8 | <0.001 |
| Nasal | 70.2 | 52.7 to 87.7 | 68.2 | 51.3 | 43.0 | NA | 0.82 |
| Inferior | 87.5 | 73.6 to 101.4 | 117.9 | 93.4 | 85.8 | 25.8 | 0.002 |
| 1:00 | 62.7 | 53.1 to 72.2 | 104.4 | 76.2 | 61.9 | 39.9 | 0.003 |
| 5:00 | 73.4 | 66.7 to 80.1 | 95.5 | 65.7 | 56.8 | 23.1 | <0.001 |
| 6:00 | 103.8 | 83.3 to 124.3 | 129.8 | 90.1 | 81.2 | 20.0 | 0.010 |
| 7:00 | 97.2 | 77.2 to 117.3 | 128.5 | 88.9 | 74.0 | 24.4 | 0.004 |
| 8:00 | 46.0 | 41.2 to 50.7 | 63.4 | 43.4 | 36.2 | 27.4 | <0.001 |
| 9:00 | 41.3 | 36.7 to 45.9 | 50.7 | 37.0 | 31.9 | 18.5 | 0.012 |
| 11:00 | 94.4 | 82.3 to 106.5 | 124.2 | 89.9 | 71.1 | 24.0 | <0.001 |
| 12:00 | 82.1 | 69.2 to 95.0 | 116.5 | 74.1 | 63.2 | 29.5 | 0.002 |
BDL, borderline; NA, non-applicable.
All measurements are reported in micrometers.
Tipping points for clock hours with percent loss from the mean normative values are summarised in table 2. The percentage of RNFL loss from the mean of age-matched normative thickness was highest for 1:00 (39.9%), while for all other sectors the range was 18.5–29.5%, with the lowest loss in the 9:00 location. The tipping point was located in the borderline zone for 1:00 when compared with the normative values, and for all other locations the tipping point occurred within the normal range.
The slopes for VF threshold as a function of RNFL thickness above and below the tipping points are listed in table 3. The models for the slope estimate did not converge for clock hours 1, 5 and 8, and therefore the slope could not be determined with confidence in these particular locations. The slopes above the tipping point in all tested locations were not different than a zero slope (CI included zero), except for the inferior quadrant and 1:00. The slopes below the tipping point were statistically significantly different than a zero slope except for the nasal quadrant. The slopes below the tipping points were statistically significantly steeper than the slopes above the tipping point for all locations except for the nasal quadrant.
Table 3.
Fitted slopes of retinal nerve fibre layer thickness and visual field threshold above and below the tipping point. All slopes are in decibels per micrometre
| Slope below tipping point (95% CI) | Slope above tipping point (95% CI) | Difference between slopes (95% CI) | P value | |
|---|---|---|---|---|
| Mean | 0.28 (0.18 to 0.38) | 0.03 (−0.02 to 0.08) | 0.25 (0.13 to 0.36) | <0.001 |
| Temporal | 0.39 (0.14 to 0.63) | −0.01 (−0.06 to 0.06) | 0.39 (0.13 to 0.64) | 0.003 |
| Superior | 0.25 (0.16 to 0.34) | 0.02 (−0.01 to 0.05) | 0.23 (0.14 to 0.33) | <0.001 |
| Nasal | 0.11 (−0.02 to 0.24) | −0.03 (−0.20 to 0.14) | 0.14 (−0.07 to 0.36) | 0.20 |
| Inferior | 0.16 (0.09 to 0.23) | 0.03 (0.01 to 0.06) | 0.13 (0.05 to 0.20) | <0.001 |
| 1:00 | 0.55 (0.07 to 1.03) | 0.04 (0.13 to 0.77) | 0.51 (0.02 to 0.99) | <0.001 |
| 5:00 | 0.35 (0.20 to 0.49) | 0.01 (−0.03 to 0.05) | 0.33 (0.18 to 0.48) | <0.001 |
| 6:00 | 0.11 (0.06 to 0.15) | 0.02 (−0.01 to 0.05) | 0.09 (0.03 to 0.14) | 0.001 |
| 7:00 | 0.17 (0.09 to 0.25) | 0.01 (−0.04 to 0.06) | 0.16 (0.06 to 0.26) | <0.001 |
| 8:00 | 0.98 (0.39 to 1.57) | −0.09 (−0.19 to 0.01) | 0.95 (0.35 to 1.54) | 0.002 |
| 9:00 | 0.52 (0.02 to 1.01) | −0.09 (−0.19 to 0.01) | 0.61 (0.10 to 1.12) | 0.018 |
| 11:00 | 0.17 (0.10 to 0.24) | 0.01 (−0.02 to 0.04) | 0.16 (0.09 to 0.24) | <0.001 |
| 12:00 | 0.15 (0.06 to 0.23) | 0.01 (−0.02 to 0.03) | 0.14 (0.05 to 0.22) | 0.002 |
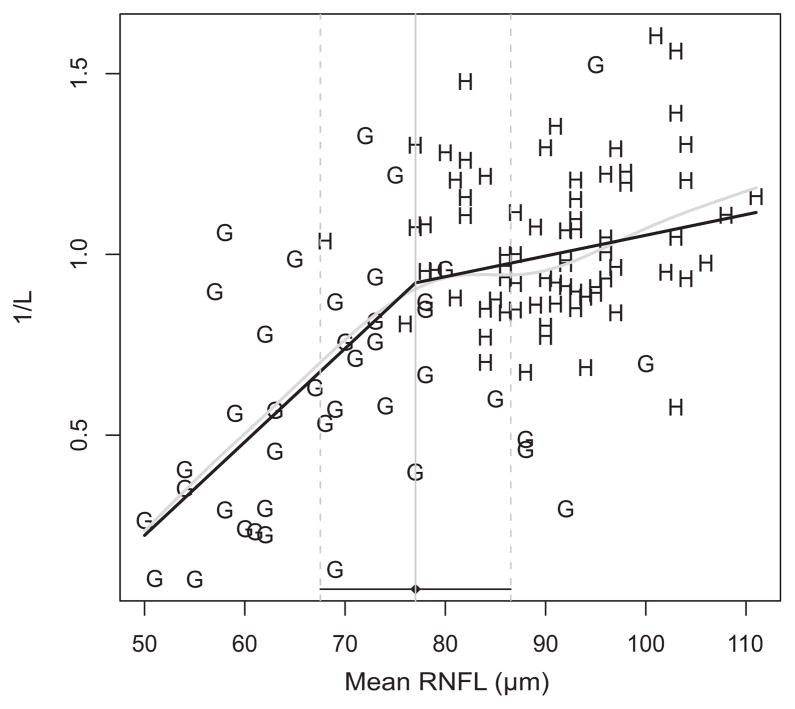
A similar segmented relationship was noted in the secondary analysis using 1/L values (figure 2). The estimated tipping point was 77.0 μm (95% CI: 67.5 to 86.5), with a slope above the tipping point of 0.01 1/(L×μm) (CI: −0.01 to 0.01) and a slope below the tipping point of 0.03 1/(L×μm) (CI: 0.02 to 0.04). The difference between the slopes was 0.02 1/(L×μm) (CI: 0.01 to 0.03) and statistically significant (p=0.003).
Figure 2.
Scatter plot of healthy (H) and glaucoma (G) values using unlogged (1/l) visual field threshold values. The grey line is a spline fit, and the black line is the two-segment ‘broken stick’ model. RNFL–retinal nerve fiber layer, L–Lambert.
Employing the same approach using VF MD instead of the threshold values identified the tipping point at 76.7 μm (CI: 71.3 to 86.5), with slopes above the tipping point of 0.03 dB/μm (CI:−0.03 to 01.0) and below the tipping point of 0.39 dB/μm (CI:0.28 to 0.51), with a statistically significant difference between the slopes.
DISCUSSION
In the present study, analysis of ocular structural and functional data by a ‘broken stick’ statistics identifies a tipping point at which the relationship between RNFL thickness and VF threshold values are clearly different. The tipping point allows us to define the RNFL threshold where strong association with VF abnormalities can be expected. This study reveals that substantial structural loss (approximately 17%) appears to be necessary for functional loss to be detectable, using the current testing methods.
Similar to numerous previous studies, we demonstrate that thicker RNFL values are not associated with abnormalities in the VF threshold.7,8,15–18 Moreover, our analysis indicates that in this region (above the tipping point) the slope of VF threshold versus RNFL is not statistically significantly different than zero for global and most sectoral analysis. This can be mostly attributed to the higher variability in VF outcomes than structural outcome in the early stage of glaucoma.7,8 From the clinical perspective, this can be interpreted as an indication that structural evaluation is a more sensitive measure of health at the early stage of glaucoma, as it was suggested previously by histology and disc photography assessment.1–3 Below the tipping point, there is a strong association between structure and function with a rate of change that varies substantially by RNFL location.
The tipping point could be detected in all tested locations except for the nasal quadrant. The estimate for the tipping point in the nasal quadrant presented with a wider CI than the other quadrants, reflecting the lack of prominent deflection point between the subpopulation of data. The wide CI in the nasal quadrant may be the result of undersampling (relative to the other quadrants) of the corresponding tissues in the VF. In addition, OCT measurements in the nasal quadrant have the lowest test-retest reproducibility.19 The combined influences of fewer nasal VF data points and lower nasal OCT reproducibility may have masked a tipping point in the nasal quadrant. Additionally, the statistical model did not converge in clock hours 1, 5 and 8; although a tipping point was detected, the accuracy of the actual value is questionable. Because the analysis is based on detection of the optimal deflection point in multidimensional plane, it is difficult to discern the reason for the non-convergence.
Eye movements have a greater impact on sectoral measurement variability than global variability, resulting in lower sectoral reproducibility19 and wider range of the sectoral CIs (table 2). This may have contributed to the tipping point being outside the normal range for mean, while inferior and superior quadrants being within the normal range in the corresponding clock hours.
Ageing has been shown to cause gradual thinning of the RNFL20 and reduction in visual sensitivity.21 In order to account for this effect, we use VF total deviation threshold values that are age corrected. For RNFL measurements, we use the mean age-adjusted normative value, allowing us to identify the percentage tissue loss while accounting for the ageing effect. The tipping point occurs with a mean RNFL thickness 17% below the level that would be expected in the age-matched healthy population. In the superior and inferior quadrants and clock hours, the tipping point occurs after ~25% loss. The highest loss (40%) occurs at the 1:00 location, but as stated above the reliability of the analysis in this location is questionable. The lowest sectoral percentage loss is at 8:00 and the temporal quadrant. This finding might be explained by the high concentration of thinner axons in the papillomacular bundle passing through this location.22 Therefore, in the same RNFL thickness as in other locations, the number of axons passing through the temporal quadrant is larger, leading to the appearance of VF abnormality after a smaller RNFL thickness loss. A ‘floor effect’, where no further structural damage can be detected while functional changes can still occur, was not identified in this study, but this could be expected in situations where very advanced stages of structural damage exist.
Numerous studies evaluate the relationship between structure and function in glaucomatous eyes. Most of the studies employ a single model throughout the data, either linear or curvilinear.7,8,15–17 Ajtony et al have speculated that a mean RNFL thickness of approximately 70 μm might represent a profound threshold value in glaucomatous structure changes.18 In the present study, we utilise a statistical method to accurately determine this location that is found to be at 75.3 μm. Identifying the actual location where VF abnormality is expected to occur is of utmost importance in clinical management especially for individuals suspected of having glaucoma.
Our secondary analysis shows that with both decibel and 1/L units, the tipping point appears approximately at the same RNFL thickness, thus confirming the true basis of this range for distinguishing between phases of structure and function relationship. We also performed an analysis using VF MD because this parameter is commonly used in clinical practice. The tipping point for MD occurs at an RNFL thickness of 77 μm.
The limitations of the study include its cross-sectional nature and the population data used. Caution should be used when applying these results to individual subjects. A person might start with a thicker or thinner RNFL and their actual tipping point RNFL thickness value might vary from the one reported herein. The study design, however, is appropriate for initial testing of the hypothesis.
In conclusion, the ‘broken-stick’ analysis allows the identification of a structural and functional tipping point. It appears that ‘preperimetric’ glaucoma (using current standard automated perimetry) can be identified by OCT RNFL thickness, with the detectable VF loss first appearing with RNFL approximately 17% below that expected for healthy eyes.
Acknowledgments
Funding NIH R01-EY013178, R01-EY011289, 1T32EY017271, P30-EY08098 Other Funders: Air Force Office of Scientific Research; Research to Prevent Blindness. Supported in part by National Institute of Health contracts R01-EY013178, R01-EY011289, 1T32EY017271, P30-EY08098 (Bethesda, MD); Air Force Office of Scientific Research FA9550-070-1-0101; The Eye and Ear Foundation (Pittsburgh, PA); and unrestricted grants from Research to Prevent Blindness (New York, NY). Financial Disclosures: Drs Wollstein and Duker received research funding from Carl Zeiss Meditec and Optovue. Drs Wollstein, Ishikawa and Schuman have intellectual property licenced by the University of Pittsburgh to Bioptigen. Dr Duker received research funding from Topcon Medical Systems, and is a consultant to Alcon, Genetech and Ophthotech and is a member of the advisory board of Paloma Pharmaceuticals. Dr Fujimoto is a scientific advisor and has stock options in Optovue. Drs Fujimoto and Schuman receive royalties for intellectual property licensed by Massachusetts Institute of Technology to Carl Zeiss Meditec.
Footnotes
Presented in part at the Association of Research in Vision and Ophthalmology (ARVO) annual meeting, Ft. Lauderdale, Florida, May 2009, American Glaucoma Society annual meeting, Naples, Florida, Mar 2010, and the American Ophthalmological Society annual meeting, White Sulphur Springs, West Virginia, May 2010.
Ethics approval This study was conducted with the approval of the University of Pittsburgh.
Provenance and peer review Not commissioned; externally peer reviewed.
Competing interests Drs Wollstein and Duker received research funding from Carl Zeiss Meditec and Optovue. Drs Wollstein, Ishikawa and Schuman have intellectual property licenced by the University of Pittsburgh to Bioptigen. Dr Duker received research funding from Topcon Medical Systems, is a consultant to Alcon, Genetech and Ophthotech and is a member of the advisory board of Paloma Pharmaceuticals. Dr Fujimoto is a scientific advisor and has stock options in Optovue. Drs Fujimoto and Schuman receive royalties for intellectual property licensed by Massachusetts Institute of Technology to Carl Zeiss Meditec.
References
- 1.Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109:77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]
- 2.Quigley HA, Katz J, Derick RJ, et al. An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. Ophthalmology. 1992;99:19–28. doi: 10.1016/s0161-6420(92)32018-4. [DOI] [PubMed] [Google Scholar]
- 3.Quigley HA, Enger C, Katz J, et al. Risk factors for the development of glaucomatous visual field loss in ocular hypertension. Arch Ophthalmol. 1994;112:644–9. doi: 10.1001/archopht.1994.01090170088028. [DOI] [PubMed] [Google Scholar]
- 4.Schuman JS, Hee MR, Puliafito CA, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol. 1995;113:586–96. doi: 10.1001/archopht.1995.01100050054031. [DOI] [PubMed] [Google Scholar]
- 5.Wollstein G, Ishikawa H, Wang J, et al. Comparison of three optical coherence tomography scanning areas for detection of glaucomatous damage. Am J Ophthalmol. 2005;139:39–43. doi: 10.1016/j.ajo.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 6.Schuman JS, Pedut-Kloizman T, Pakter H, et al. Optical coherence tomography and histologic measurements of nerve fiber layer thickness in normal and glaucomatous monkey eyes. Invest Ophthalmol Vis Sci. 2007;48:3645–54. doi: 10.1167/iovs.06-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26:688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harwerth RS, Wheat JL, Fredette MJ, et al. Linking structure and function in glaucoma. Prog Retin Eye Res. 2010;29:249–71. doi: 10.1016/j.preteyeres.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garway-Heath DF, Poinoosawmy D, Fitzke FW, et al. Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology. 2000;107:1809–15. doi: 10.1016/s0161-6420(00)00284-0. [DOI] [PubMed] [Google Scholar]
- 10.Drexler W, Fujimoto JG. State-of-the-art retinal optical coherence tomography. Prog Retin Eye Res. 2008;27:45–88. doi: 10.1016/j.preteyeres.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Muggeo VM. Estimating regression models with unknown break-points. Stat Med. 2003;22:3055–71. doi: 10.1002/sim.1545. [DOI] [PubMed] [Google Scholar]
- 12.Davis RB. Hypothesis testing when a nuisance parameter is present only under the alternative. Biometrika. 1987;74:33–43. [Google Scholar]
- 13.R Development Core Team. [accessed 1 Sep 2009];R: A Language and Environment for Statistical Computing. http://www.R-project.org.
- 14.Muggeo VM. Segmented: an R package to fit regression models with broken-line relationships. RNews. 2008;8:5. [Google Scholar]
- 15.Schlottmann PG, De Cilla S, Greenfield DS, et al. Relationship between visual field sensitivity and retinal nerve fiber layer thickness as measured by scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2004;45:1823–9. doi: 10.1167/iovs.03-0692. [DOI] [PubMed] [Google Scholar]
- 16.Leung CK, Chong KK, Chan WM, et al. Comparative study of retinal nerve fiber layer measurement by StratusOCT and GDx VCC, II: structure/function regression analysis in glaucoma. Invest Ophthalmol Vis Sci. 2005;46:3702–11. doi: 10.1167/iovs.05-0490. [DOI] [PubMed] [Google Scholar]
- 17.Badlani V, Shahidi M, Shakoor A, et al. Nerve fiber layer thickness in glaucoma patients with asymmetric hemifield visual field loss. J Glaucoma. 2006;15:275–80. doi: 10.1097/01.ijg.0000212298.23787.bf. [DOI] [PubMed] [Google Scholar]
- 18.Ajtony C, Balla Z, Somoskeoy S, et al. Relationship between visual field sensitivity and retinal nerve fiber layer thickness as measured by optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48:258–63. doi: 10.1167/iovs.06-0410. [DOI] [PubMed] [Google Scholar]
- 19.Budenz DL, Fredette MJ, Feuer WJ, et al. Reproducibility of peripapillary retinal nerve fiber thickness measurements with stratus OCT in glaucomatous eyes. Ophthalmology. 2008;115:661–6 e4. doi: 10.1016/j.ophtha.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 20.Sung KR, Wollstein G, Bilonick RA, et al. Effects of age on optical coherence tomography measurements of healthy retinal nerve fiber layer, macula, and optic nerve head. Ophthalmology. 2009;116:1119–24. doi: 10.1016/j.ophtha.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spry PG, Johnson CA. Senescent changes of the normal visual field: an age-old problem. Optom Vis Sci. 2001;78:436–41. doi: 10.1097/00006324-200106000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Jonas JB, Muller-Bergh JA, Schlotzer-Schrehardt UM, et al. Histomorphometry of the human optic nerve. Invest Ophthalmol Vis Sci. 1990;31:736–44. [PubMed] [Google Scholar]