Abstract
Objective
To determine the relationship between hearing loss and cognitive function as assessed with a standardized neurocognitive battery. We hypothesized a priori that greater hearing loss is associated with lower cognitive test scores on tests of memory and executive function.
Methods
A cross-sectional cohort of 347 participants ≥ 55 years in the BLSA without mild cognitive impairment or dementia had audiometric and cognitive testing performed in 1990–1994. Hearing loss was defined by an average of hearing thresholds at 0.5, 1, 2, and 4 kHz in the better-hearing ear. Cognitive testing consisted of a standardized neurocognitive battery incorporating tests of mental status, memory, executive function, processing speed, and verbal function. Regression models were used to examine the association between hearing loss and cognition while adjusting for confounders.
Results
Greater hearing loss was significantly associated with lower scores on measures of mental status (Mini-Mental State Exam), memory (Free Recall), and executive function (Stroop Mixed, Trail Making B). These results were robust to analyses accounting for potential confounders, non-linear effects of age, and exclusion of individuals with severe hearing loss. The reduction in cognitive performance associated with a 25 dB hearing loss was equivalent to the reduction associated with an age difference of 6.8 years.
Conclusion
Hearing loss is independently associated with lower scores on tests of memory and executive function. Further research examining the longitudinal association of hearing loss with cognitive functioning is needed to confirm these cross-sectional findings.
Keywords: Hearing loss, cognition, aging, dementia
Introduction
We have previously demonstrated that audiometric hearing loss is independently associated with incident all-cause dementia in the Baltimore Longitudinal Study of Aging (BLSA) and that these results were robust to sensitivity analyses adjusting for known confounders, non-linear effects, and other potential biases (Lin, Metter et al., 2011). Mechanistic pathways hypothesized to explain this observed association include a shared pathologic etiology, the effects of hearing loss on cognitive load and cognitive reserve, and/or mediation through social isolation and loneliness (Lin, Metter et al., 2011). Most likely, a number of these hypothesized pathways co-exist and contribute to the development of cognitive impairment.
Regardless of the mechanism, a first step in exploring the pathway from hearing loss to dementia is to demonstrate that hearing loss is selectively associated with those cognitive measures and domains known to decline prior to dementia onset. Results from longitudinal studies have generally demonstrated that decrements in both measures of memory (Elias et al., 2000; Grober, Hall, Lipton et al., 2008; Linn et al., 1995; Rubin et al., 1998) and executive function(Chen et al., 2001; Fabrigoule et al., 1998; Grober, Hall, Lipton et al., 2008; Rapp & Reischies, 2005; Royall, Chiodo, & Polk, 2004) precede subsequent dementia with accelerated declines in episodic memory and executive function observed 7 years and 3 years, respectively, before diagnosis (Grober, Hall, Lipton et al., 2008; Hall, Lipton, Sliwinski, & Stewart, 2000; Hall et al., 2001). In contrast, measures of verbal intelligence do not decline until shortly before dementia diagnosis (Grober, Hall, Lipton et al., 2008).
In the present study, we investigated the association of hearing loss with cognitive function using a standardized neurocognitive battery in a cross-sectional cohort of BLSA participants without mild cognitive impairment or dementia. This neurocognitive battery included tests of mental status (Mini-Mental State Exam [MMSE]), memory (Free and Cued Selective Reminding test [FCSRT]), executive function/attention (Trail Making B, Stroop Mixed), processing and psychomotor speed (Trail Making A, Stroop Colors & Words), and verbal ability and language (Category & Letter Fluency, American Version of the Nelson Adult Reading Test [AMNART]). We hypothesized a priori that greater hearing loss is associated with lower cognitive test scores on tests of memory and executive function.
Method
Study Participants
Subjects were participants in the BLSA, an ongoing prospective study of the effects of aging that was initiated in 1958 by the National Institute on Aging (Shock et al., 1984). The BLSA cohort is comprised of community-dwelling volunteers who travel to the National Institute on Aging (NIA) in Baltimore biennially for 2.5 days of intensive testing. From 1990–1994, audiometric testing was done in conjunction with neurocognitive evaluation, and the present investigation is based on a cross-sectional cohort of participants (n = 347) who were > 55 years when examined in 1990–1994, had concurrent audiometric and neurocognitive testing, and did not have prevalent dementia or mild cognitive impairment at the time of evaluation. The NIA and the Johns Hopkins School of Medicine Institutional Review Boards approved this study, and all participants gave written informed consent.
Diagnosis of Dementia
The protocol for adjudication of dementia in the BLSA has been used continuously since 1986 and has been described previously(Kawas, Gray, Brookmeyer, Fozard, & Zonderman, 2000). If subjects were determined to have clinically-significant cognitive decline (typically memory) but did not meet criteria for dementia, they were classified as suspected dementia which corresponds to the current diagnosis of mild cognitive impairment(Petersen et al., 1999).
Cognitive Testing
Neurocognitive testing was performed by an experienced examiner accustomed to working with older adults and using a standardized protocol and neurocognitive battery. Cognitive test data available for the cohort under investigation include: MMSE (Folstein, Folstein, & McHugh, 1975) (n = 340), FCSRT (n = 343), Trail Making A and B (n = 338), Stroop (n = 314), Letter and Category Fluency (n = 345), and AMNART (n = 235). Reduced numbers of participants assessed with the Stroop and AMNART reflect changes in the test battery in 1993.
FCSRT
The FCSRT measures memory under conditions that control attention and cognitive processing(Grober, Hall, McGinn et al., 2008). It has been used in five major longitudinal aging studies (Grober, Buschke, Crystal, Bang, & Dresner, 1988a; Lindenberger U, 1999; Petersen et al., 1995; Sarazin et al., 2007; Tuokko, Vernon-Wilkinson, Weir, & Beattie, 1991) and also in the Alzheimer’s Disease Cooperative Study Instrumentation Protocol (Ferris et al., 2006). The FCSRT has been shown to be sensitive to early dementia and preclinical dementia in several cohorts (Grober, Buschke, Crystal, Bang, & Dresner, 1988b; Grober, Hall, McGinn et al., 2008; Grober & Kawas, 1997; Grober, Lipton, Hall, & Crystal, 2000; Lindenberger U, 1999; Petersen, Smith, Ivnik, Kokmen, & Tangalos, 1994; Tounsi et al., 1999; Tuokko et al., 1991) and is not associated with education (Ivnik et al., 1997) or race (Grober et al., 1988b; Grober, Hall, McGinn et al., 2008). The FCSRT begins with a study phase in which subjects are asked to search a card containing four pictures (e.g., grapes) for an item that goes with a unique category cue (e.g., fruit). After all four items are identified immediate recall of just those four items is tested. The search is performed again for items not retrieved by cued recall. The search procedure is continued until all 16 items are identified and retrieved in immediate recall. The study procedure is followed by three trials of recall each consisting of free recall followed by cued recall for items not retrieved by free recall. The sum of the three free recall trials is a measure of learning and memory and is the measure used in the present analyses.
Trail Making Test
The Trail Making Test involves drawing lines to connect consecutively numbered circles (Part A) and then connecting dots containing numbers and letters arrayed randomly on a page in alternating sequence (Part B) (Reitan, 1958). The dependent measure used in the present investigation is the reciprocal of the time (speed) taken by the subject to complete the task expressed in seconds. This procedure allows us to interpret larger scores as being associated with better cognitive performance (e.g. a score of 1/30 sec is greater than a score of 1/60 sec).
Stroop Test
The Stroop Test was administered in 3 formats. Stroop Words required the participant to read aloud the name of a color printed in black ink, and Stroop Colors required the participant to name the color of a printed series of x’s in one of 3 colors (red, blue, green). Stroop Mixed was a color-word interference task in which participants were asked to report the color in which each color word was printed when the color word was printed in ink of a different color. Scores were reported as the number of responses correctly named in 45 seconds.
Fluency
In the Letter Fluency task, subjects generated words that began with the letters F, A, and S for 1 min each (Spreen O, 1969). In the Category Fluency test, subjects had 1 minute each to generate exemplars of animals, fruits, and vegetables (Rosen, 1980). The dependent measures were the mean numbers of words generated across the three letters and the three categories, respectively.
AMNART
The AMNART was used to estimate verbal IQ. It consists of 50 words that cannot be pronounced phonetically (e.g., depot, naïve) (Nelson & O’Connell, 1978). Estimated verbal IQ was computed using number of errors on the AMNART and years of education according to the following formula: 118.56 −[.88 * (number of errors)] + (.56 * years of education).
Audiometry
From 1990–1994, audiometry was performed in the BLSA study using a semi- automated testing device (Virtual Equipment Co., Audiometer Model 320) in a sound-attenuating chamber under unaided conditions (Industrial Acoustics Company, Model 400-A) which met prevailing standards for maximal permissible ambient noise levels during air conduction audiometry (ANSI, 1977). A speech-frequency pure tone average (PTA) of air-conduction thresholds at 0.5, 1, 2, and 4 kHz was calculated for each ear, and the PTA in the better-hearing ear was used for subsequent analyses because this ear would be the principal determinant of hearing and speech perception ability on an everyday basis. All thresholds are expressed in dB HL (ANSI, 1989).
Other covariates
A diagnosis of diabetes was established based on a fasting glucose >125 mg/dL, a pathologic oral glucose tolerance test, or a positive history of a physician diagnosis plus treatment with oral anti-diabetic drugs or insulin. The diagnosis of hypertension was established based on a systolic blood pressure >140 and/or diastolic blood pressure ≥90 mmHg or treatment with antihypertensive medications. Race (white/black/other), education (in years), hearing aid use (yes/no) and smoking status (current/former/never) were based on self-report. Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977). The CES-D is a 20-item inventory of depressive symptoms, and each response is scored from zero to 3 based on the frequency of occurrence of the symptom. The range of scores is zero to 60, where higher CES-D scores indicate increased frequency and severity of depressive symptoms
Statistical Analyses
Locally weighted scatterplot smoothing (lowess) was used to graphically explore the association of hearing loss and age with cognitive scores and to identify non-linear data trends. Linear regression was then used to model the association between cognitive scores and hearing loss while adjusting for age and other covariates. A robust variance estimator was used to account for heteroscedasticity seen in the model residuals (Harrell, 2001). Age was modeled using a cubic spline when appropriate to account for possible non-linear effects of age on cognitive scores. To account for ceiling effects observed in the distribution of scores from the MMSE and AMNART, scores from these cognitive tests were converted into a 5-level ordinal categorical variable using cutpoints that approximately divided scores into 5 equal sized bins. Ordinal logistic regression was then performed, and β-coefficients from these analyses can be interpreted as the log odds of the next higher category of cognitive function associated with a 10db increase in hearing loss (i.e. negative β’s indicate poorer cognitive function with increasing hearing loss). Subjects with missing data for non-cognitive variables were excluded from analyses, and this represented ≤ 2% of the study sample in all analyses except for the one analysis that incorporated hearing aid use. Significance testing for all analyses was 2-sided with a type I error of 0.05. The statistical software used was Stata 11.1 (StataCorp, College Station, TX).
Results
Demographics for the study population considered in this report are presented in Table 1. From 1990–1994, 347 participants ≥ 55 years had concurrent audiometric and neurocognitive testing and were assessed as being free from prevalent dementia or cognitive impairment. This cohort was predominantly white (93.1%) and well-educated (mean years of education, 16.6).
Table 1.
Demographic characteristics, Baltimore Longitudinal Study of Aging 1990–1994
| Characteristic | Cohort (n = 347) |
|---|---|
| Age, mean years (S.D.) | 71.0 (7.2) |
| Hearing loss, mean dB (S.D) | 25.5 (13.7) |
| Hearing loss category, n(%) | |
| Normal (<25 dB) | 205 (59.1) |
| Mild (25–40 dB) | 99 (28.5) |
| Moderate (41–70dB) | 40 (11.5) |
| Severe (≥71dB) | 3 (0.9) |
| Sex, n(%) | |
| Female | 122 (35.2) |
| Race, n(%) | |
| White | 323 (93.1) |
| Black | 19 (5.5) |
| Other | 5 (1.4) |
| Education, mean years (S.D) | 16.6 (2.9) |
| Smoking, n(%) | |
| Never | 121 (34.9) |
| Former | 217 (62.5) |
| Current | 9 (2.6) |
| Hypertension, n(%) | 214 (61.7) |
| Diabetes, n(%) | 65 (18.7) |
| Hearing aid use n(%)a | 46 (13.3) |
Data on hearing aid use were missing for 32 participants.
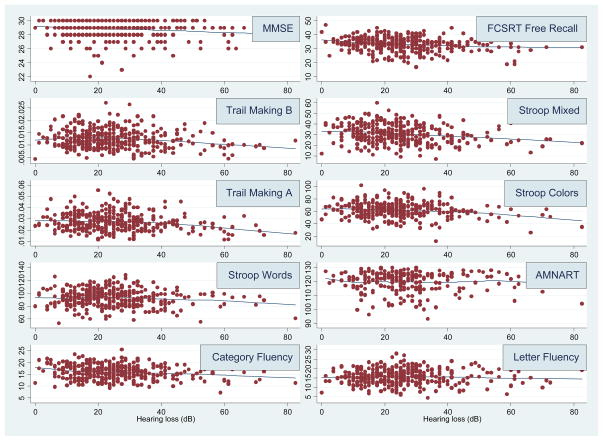
Exploratory analysis of the cross-sectional association of cognitive scores with hearing loss demonstrated that scores from all cognitive tests except AMNART and Letter Fluency generally declined linearly with increasing levels of hearing loss (Figure 1). After adjusting for age, associations between greater hearing loss and lower scores on all cognitive tests were significant or approached significance except for Trail Making A and Letter Fluency (Table 2). Stronger associations were observed between hearing loss and measures of memory (FCSRT Free Recall, p < .001) and executive function (Stroop Mixed, p = .006).
Figure 1.
Cross-sectional association of hearing loss and cognitive scores, Baltimore Longitudinal Study of Aging 1990–1994
Table 2.
Regression models of cognitive scores per 10dB of hearing loss, Baltimore Longitudinal Study of Aging 1990–1994
| Adjusted for Age | Adjusted for all covariatesc | |||
|---|---|---|---|---|
| βa (95% CI) | pd | βa (95% CI) | pd | |
| Mental Status | ||||
| MMSE | −0.16bb (−0.29 – −0.03) | * | −0.17b (−0.30 – −0.04) | * |
| Memory | ||||
| Free Recall from FCSRT | −0.76 (−1.19 – −0.33) | *** | −0.58 (−1.0 – −0.15) | ** |
| Executive Function | ||||
| Trail Making Test Part B, Speed | −2.6 × 10−4 (−5.6 × 10−4 – −4.8 × 10−5) | + | −3.0 × 10−4 (−5.9 × 10−4 – 1.1 × 10−6) | + |
| Stroop Mixed | −1.0 (−1.7 – −0.30) | ** | −0.91 (−1.7 – −0.16) | * |
| Processing & Psychomotor Speed | ||||
| Trail Making Test Part A, speed | −5.0 × 10−4 (−1.1 × 10−3 – 1.2 × 10−4) | −6.4 × 10−4 (−1.3 × 10−3 – 9.4 × 10−7) | + | |
| Stroop Colors | −1.0 (−2.1 – −0.06) | + | −0.78 (−1.9 – 0.29) | |
| Stroop Words | −1.0 (−2.2 – 0.19) | + | −0.91 (−2.2 – 0.36) | |
| Verbal Function & Language | ||||
| Category Fluency | −0.27 (−0.52 – −0.009) | * | −0.09 (−0.35 – 0.17) | |
| Letter Fluency | 0.15 (−0.83 – 1.1) | 0.25 (−0.79 – 1.3) | ||
| American Version of the Nelson Adult Reading Test | −0.18b (−0.37 – 0.012) | + | −0.12b (−0.32 – 0.07) | |
β-coefficient represents the expected difference in cognitive scores associated with a 10db increase in hearing loss. Negative β’s indicate poorer cognitive function with increasing hearing loss.
β-coefficient represents the log odds of the next higher category of cognitive function associated with a 10db increase in hearing loss. Negative β’s indicate poorer cognitive function with increasing hearing loss.
Covariates include age, sex, race, education, diabetes, smoking, hypertension
+ p<0.10;
p<0.05;
p<0.01;
p<0.001
We performed additional analyses incorporating additional covariates to test the robustness of our results. Results from a model adjusting for age, sex, education, diabetes, smoking, and hypertension demonstrated that greater hearing loss was significantly associated with lower scores on the MMSE, CSR Free Recall, and Stroop Mixed and that associations between hearing loss and Trail Making A and B approached significance (Table 2). Overall, in this fully adjusted model, stronger associations were observed between hearing loss and cognitive measures of memory and executive function than with tests of psychomotor/processing speed and verbal function.
A cubic spline model was used to account for possible non-linear effects of age in those cognitive measures (e.g. FCSRT Free Recall) which demonstrated possible non-linear associations with age (data not shown), and the results from these analyses were not substantially different from models adjusting for age as a linear variable (c.f. Table 2). Excluding participants with a history of a previous stroke (n = 7) also did not substantially change the main findings (c.f. Table 2). We also performed an analysis excluding those participants with the greatest hearing loss (severe hearing loss category, n = 3) to ensure that results were not determined by a few strongly influential data points. In this analysis, the results from the fully-adjusted models in Table 2 also remained substantially unchanged with the exception that the associations of hearing loss with Trail Making A and B were no longer significant (p > .1). Adjustment for CES-D scores also did not affect the significance of the results presented in Table 2 (data not shown).
In order to examine the role of hearing aids on cognition, we performed an analysis with an interaction term between hearing aid use and having hearing loss > 25 dB. In this model, hearing aid use was not associated with scores on any of the cognitive tests (data not shown).
To assess the magnitude of the reduction in cognitive performance associated with hearing loss, we estimated the difference in chronological age that would be equivalent to the cross-sectional effect of a 25 dB increase in hearing thresholds (analogous to shifting from normal hearing to a mild hearing loss) on cognitive scores. Cognitive tests that were associated with both age and hearing loss in fully adjusted models include Stroop Mixed and Trail Making A and B (Table 3). The difference in age equivalent to the cognitive reduction associated with a 25 dB increase in hearing loss is 6.8, 5.8, and 6.7 years for Stroop Mixed, Trail Making A, and Trail Making B, respectively.
Table 3.
Regression models of cognitive scores per year of age or 25 dB of hearing loss for cognitive tests associated with both age and hearing loss
| Age (per year) | Hearing loss (per 25 dB) | Δ Age (years) equivalent to 25 dB of hearing loss | |||
|---|---|---|---|---|---|
| βa (95% CI) | P | βb (95% CI) | P | ||
| Stroop Mixed | −0.33 (−0.48 – −0.18) | <.001 | −2.27 (−4.14 – −0.40) | .02 | 6.8 |
| Trail Making | |||||
| Part A, speed | −0.00027 (−0.00040 – −0.00015) | <.001 | −0.0016 (−0.0032 – 2.35×10−6) | .05 | 5.8 |
| Part B, speed | −0.00011 (−0.00018 – −0.000044) | .001 | −.00074 (−0.0015 – 2.74×10−6) | .05 | 6.7 |
β-coefficient represents the expected difference in cognitive scores associated with a 1 year increase in age. Negative β’s indicate poorer cognitive function with increasing age.
β-coefficient represents the expected difference in cognitive scores associated with a 25 db increase in hearing loss (analogous to shifting from normal hearing to mild hearing loss). Negative β’s indicate poorer cognitive function with increasing hearing loss.
All models adjusted for age, sex, race, education, diabetes, smoking, and hypertension
Discussion
In this cross-sectional study of adults who were free of prevalent dementia or mild cognitive impairment, hearing loss was independently associated with tests of memory and executive function, and these results were robust to analyses accounting for confounders, nonlinear effects of age, and excluding participants with severe hearing loss. The magnitude of the reduction in cognitive performance associated with hearing loss is clinically significant with the reduction associated with a 25 dB hearing loss being equivalent to an age difference of 6.8 years on tests of executive function.
Our results contribute to the literature examining the association between hearing loss and cognition. Our findings are consistent with some prior research demonstrating significant associations between greater hearing loss and poorer cognitive function on both verbal (Granick, Kleban, & Weiss, 1976; Gussekloo, de Craen, Oduber, van Boxtel, & Westendorp, 2005; Helzner et al., 2005; Lindenberger & Baltes, 1994; Ohta, Carlin, & Harmon, 1981; Tay et al., 2006; Thomas et al., 1983; Uhlmann, Larson, Rees, Koepsell, & Duckert, 1989; Valentijn et al., 2005) and non-verbal cognitive tests (Granick et al., 1976; Lindenberger & Baltes, 1994; Valentijn et al., 2005) and in both cross-sectional and prospective studies(Peters, Potter, & Scholer, 1988; Valentijn et al., 2005). In contrast, other studies have not found similar associations(Anstey, Luszcz, & Sanchez, 2001; Gennis, Garry, Haaland, Yeo, & Goodwin, 1991). While some heterogeneity in study results is explained by the choice of cognitive tests, one key limitation across multiple studies is the variability in how hearing loss was measured and how audiometric data were analyzed (e.g. choice of pure tone thresholds used to define hearing loss). Most studies utilized portable or screening audiometers (Anstey et al., 2001; Gussekloo et al., 2005; Lindenberger & Baltes, 1994; Valentijn et al., 2005) or tested participants under varying environmental conditions (e.g. home-based testing)(Lindenberger & Baltes, 1994), while some did not adequately describe their audiometric testing protocol (Gennis et al., 1991; Ohta et al., 1981; Thomas et al., 1983). The effect of biased or imprecise assessments of hearing thresholds would likely decrease sensitivity to detect associations due to increased variance. Strengths of our current study are the use of a standardized audiometric testing protocol performed in a soundproof chamber, a definition of hearing loss adjudicated by the World Health Organization (“World Health Organization Prevention of Blindness and Deafness (PBD) Program. Prevention of Deafness and Hearing Impaired Grades of Hearing Impairment http://www.who.int/pbd/deafness/hearing_impairment_grades/en/index.html ”), and a standardized neurocognitive battery evaluating multiple cognitive domains.
A number of mechanisms may be theoretically implicated in the observed association between hearing loss and cognition. Poor verbal communication associated with hearing loss may confound cognitive testing, or vice-versa there may be an over-diagnosis of hearing loss in individuals with sub-clinical cognitive impairment. Miscommunication is unlikely given that hearing loss (short of profound deafness) minimally impairs face-to-face communication in quiet environments (i.e. during cognitive testing) (Gordon-Salant, 2005) particularly in the setting of testing by experienced examiners who are accustomed to working with older adults. A previous study by Lindenberger and colleagues(Lindenberger, Scherer, & Baltes, 2001) also demonstrated that artificially-induced hearing loss (through the use of occlusive headphones) did not acutely affect the results of neurocognitive testing using both verbal and non-verbal cognitive tests. Confounding by poor verbal communication is also unlikely since the cognitive tests of memory (Free Recall) and executive function (Stroop and Trail Making) associated with hearing loss in the present study do not rely heavily on presentation of verbal information. We also conducted a sensitivity analysis excluding individuals with severe hearing loss, and the significance of the association of hearing loss with MMSE, Free Recall, and Stroop Mixed remained unchanged.
An over-diagnosis of hearing loss is also unlikely since there is no evidence that subclinical cognitive impairment would affect the reliability of audiometric testing. Behaviorally, pure-tone audiometry has been performed even in children as young as 5 years. There is also no evidence to suggest that older compared to younger adults adopt a more conservative response bias in reporting detection of the auditory signal during pure tone audiometry (Marshall, 1991).
A shared neuropathologic etiology underlying both hearing loss and cognitive decline is a possibility but our study relied on a measure that primarily reflects peripheral hearing loss. Pure tone audiometry is typically considered a measure of the auditory periphery because detection of pure tones relies on cochlear transduction and neuronal afferents to brainstem nuclei without requiring significant higher auditory cortical processing(Pickles, 2008). Neuropathology associated with Alzheimer’s disease (AD) has not been found in the peripheral auditory pathways(Baloyannis, Mauroudis, Manolides, & Manolides, 2009; Sinha, Hollen, Rodriguez, & Miller, 1993). The likelihood of another neurobiological process such as microvascular disease causing both hearing loss and dementia also cannot be fully excluded. However, risk factors for vascular disease such as diabetes, smoking, and hypertension were adjusted for in our models, and our results were robust to excluding individuals with a prior stroke.
Finally, hearing loss may be causally associated with cognitive decline, possibly through social isolation, cognitive load, or a combination of these pathways. Communication impairments caused by hearing loss can lead to social isolation and loneliness in older adults (Strawbridge, Wallhagen, Shema, & Kaplan, 2000; Weinstein & Ventry, 1982), and epidemiologic (Barnes, Mendes de Leon, Wilson, Bienias, & Evans, 2004; Fratiglioni, Wang, Ericsson, Maytan, & Winblad, 2000) and neuroanatomic studies (Bennett, Schneider, Tang, Arnold, & Wilson, 2006) have demonstrated associations between loneliness and poor social networks with cognitive decline and dementia. Mechanisms that have been implicated in the association between loneliness and cognition included direct pathophysiologic effects of altered gene expression profiles and increased inflammation in lonely individuals (Cole, Hawkley, Arevalo, & Cacioppo; Cole et al., 2007) or through psychosocial pathways of social support and influence (Berkman, Glass, Brissette, & Seeman, 2000; Uchino, 2006).
The effect of hearing loss on cognitive load is suggested by studies demonstrating that under conditions where auditory perception is difficult (i.e. hearing loss), greater cognitive resources are dedicated to auditory perceptual processing to the detriment of other cognitive processes such as working memory (Pichora-Fuller, Schneider, & Daneman, 1995;P. Rabbitt, 1990;P. M. Rabbitt, 1968; Tun, McCoy, & Wingfield, 2009). Neuroimaging studies have demonstrated a compensatory recruitment of regions in the frontal and temporoparietal cortex to maintain auditory speech processing in older adults (Wingfield & Grossman, 2006), and this pattern of neural compensation may explain the general preservation of language comprehension that is seen even in older individuals with dementia (Rousseaux, Seve, Vallet, Pasquier, & Mackowiak-Cordoliani). The cognitive load induced by hearing loss could, therefore, result in a smaller pool of resources being available for other cognitive tasks under a resource capacity model proposed by Kahneman (Kahneman, 1973). Such a hypothesis is generally consistent with our observed results demonstrating that hearing loss was primarily associated with the more challenging cognitive tests that would be expected to overwhelm available resources (e.g. Free Recall, Stroop Mixed; Trails B) rather than cognitive tests focused on less complex speeded tasks or language.
In the current study, self-reported hearing aid use was not associated with higher scores on cognitive tests among participants with hearing loss, but data on other key variables (e.g. years of hearing aid use, type of hearing aid, hours worn per day, characteristics of subjects choosing to use hearing aids, use of other communicative strategies, adequacy of rehabilitation, etc) that would affect the success of aural rehabilitation and affect any observed association were not available. Analogous to other putative risk factors for cognitive decline (e.g. microvascular disease), strategies aimed at prevention may need to take place many years before disease onset. Consequently, whether hearing devices and aural rehabilitative strategies could have an effect on cognitive decline remains unknown and will require further study.
A key limitation of our study is that our results are based on cross-sectional data rather than on longitudinal trajectories of cognitive function and hearing loss over time. Therefore, our estimates of the expected change in cognitive scores associated with hearing loss and age may be subject to bias by cohort effects or obscured by inter-individual heterogeneity in participant characteristics. However, the relative homogeneity of our study cohort in both observed and likely unobservable characteristics may help limit these potential biases. Our results also demonstrated robust associations of hearing loss with cognitive domains of memory and executive function that were consistent with our a priori hypothesis, and this differential association of hearing loss with select cognitive domains may not be readily attributable to a particular bias.
Another limitation of our study is that our results may not be broadly generalizable because our cohort consisted of primarily white, well-educated adults. This potential limitation, however, could strengthen the internal validity of our findings given the relative homogeneity of the study cohort. Residual confounding by other medical or environmental factors is also possible but speculative based on our current knowledge of risk factors for hearing loss and cognitive decline that were adjusted for in our models.
If our results are confirmed longitudinally and in other independent studies, our findings potentially have significant implications for public health. Hearing loss is highly prevalent, and the effects of hearing loss are potentially treatable with rehabilitative devices and strategies that remain grossly underutilized(Lin, Thorpe, Gordon-Salant, & Ferrucci, 2011). Further research into whether such interventions could impact cognition and dementia are needed given the lack of past research in this area.
Acknowledgments
This work was supported by National Institute on Deafness and Other Communication Disorders Grant # 1K23DC001279 to Dr. Lin and the intramural research program of the National Institute on Aging
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu
Contributor Information
Frank R. Lin, Department of Otolaryngology-Head & Neck Surgery, Johns Hopkins School of Medicine, Baltimore, Maryland and Center on Aging and Health, Johns Hopkins Medical Institutions, Baltimore, Maryland
Luigi Ferrucci, Longitudinal Studies Section, Clinical Research Branch, National Institute on Aging, Baltimore, Maryland.
E. Jeffrey Metter, Longitudinal Studies Section, Clinical Research Branch, National Institute on Aging, Baltimore, Maryland.
Yang An, Laboratory of Behavioral Neuroscience, Intramural Research Program, National Institute on Aging, Baltimore, Maryland.
Alan B. Zonderman, Laboratory of Behavioral Neuroscience, Intramural Research Program, National Institute on Aging, Baltimore, Maryland
Susan M. Resnick, Laboratory of Behavioral Neuroscience, Intramural Research Program, National Institute on Aging, Baltimore, Maryland
References
- Anstey KJ, Luszcz MA, Sanchez L. Two-year decline in vision but not hearing is associated with memory decline in very old adults in a population-based sample. Gerontology. 2001;47(5):289–293. doi: 10.1159/000052814. [DOI] [PubMed] [Google Scholar]
- Baloyannis SJ, Mauroudis I, Manolides SL, Manolides LS. Synaptic alterations in the medial geniculate bodies and the inferior colliculi in Alzheimer’s disease: a Golgi and electron microscope study. Acta Otolaryngol. 2009;129(4):416–418. doi: 10.1080/00016480802579074. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12):2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5(5):406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Glass T, Brissette I, Seeman TE. From social integration to health: Durkheim in the new millennium. Soc Sci Med. 2000;51(6):843–857. doi: 10.1016/s0277-9536(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Patterns of cognitive decline in presymptomatic Alzheimer disease: a prospective community study. Arch Gen Psychiatry. 2001;58(9):853–858. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci U S A. 108(7):3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8(9):R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MF, Beiser A, Wolf PA, Au R, White RF, D’Agostino RB. The preclinical phase of alzheimer disease: A 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57(6):808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- Fabrigoule C, Rouch I, Taberly A, Letenneur L, Commenges D, Mazaux JM, et al. Cognitive process in preclinical phase of dementia. Brain. 1998;121(Pt 1):135–141. doi: 10.1093/brain/121.1.135. [DOI] [PubMed] [Google Scholar]
- Ferris SH, Aisen PS, Cummings J, Galasko D, Salmon DP, Schneider L, et al. ADCS Prevention Instrument Project: overview and initial results. Alzheimer Dis Assoc Disord. 2006;20(4 Suppl 3):S109–123. doi: 10.1097/01.wad.0000213870.40300.21. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Wang HX, Ericsson K, Maytan M, Winblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. 2000;355(9212):1315–1319. doi: 10.1016/S0140-6736(00)02113-9. [DOI] [PubMed] [Google Scholar]
- Gennis V, Garry PJ, Haaland KY, Yeo RA, Goodwin JS. Hearing and cognition in the elderly. New findings and a review of the literature. Arch Intern Med. 1991;151(11):2259–2264. [PubMed] [Google Scholar]
- Gordon-Salant S. Hearing loss and aging: new research findings and clinical implications. J Rehabil Res Dev. 2005;42(4 Suppl 2):9–24. doi: 10.1682/jrrd.2005.01.0006. [DOI] [PubMed] [Google Scholar]
- Granick S, Kleban MH, Weiss AD. Relationships between hearing loss and cognition in normally hearing aged persons. J Gerontol. 1976;31(4):434–440. doi: 10.1093/geronj/31.4.434. [DOI] [PubMed] [Google Scholar]
- Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988a;38(6):900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988b;38(6):900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- Grober E, Hall C, McGinn M, Nicholls T, Stanford S, Ehrlich A, et al. Neuropsychological strategies for detecting early dementia. J Int Neuropsychol Soc. 2008;14(1):130–142. doi: 10.1017/S1355617708080156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J Int Neuropsychol Soc. 2008;14(2):266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Kawas C. Learning and retention in preclinical and early Alzheimer’s disease. Psychol Aging. 1997;12(1):183–188. doi: 10.1037//0882-7974.12.1.183. [DOI] [PubMed] [Google Scholar]
- Grober E, Lipton RB, Hall C, Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54(4):827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- Gussekloo J, de Craen AJ, Oduber C, van Boxtel MP, Westendorp RG. Sensory impairment and cognitive functioning in oldest-old subjects: the Leiden 85+ Study. Am J Geriatr Psychiatry. 2005;13(9):781–786. doi: 10.1176/appi.ajgp.13.9.781. [DOI] [PubMed] [Google Scholar]
- Hall CB, Lipton RB, Sliwinski M, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer’s disease. Stat Med. 2000;19(11–12):1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Hall CB, Ying J, Kuo L, Sliwinski M, Buschke H, Katz M, et al. Estimation of bivariate measurements having different change points, with application to cognitive ageing. Stat Med. 2001;20(24):3695–3714. doi: 10.1002/sim.1113. [DOI] [PubMed] [Google Scholar]
- Harrell FE. Regression Modeling Strategies. New York: Spring-Verlag; 2001. [Google Scholar]
- Helzner EP, Cauley JA, Pratt SR, Wisniewski SR, Zmuda JM, Talbott EO, et al. Race and sex differences in age-related hearing loss: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53(12):2119–2127. doi: 10.1111/j.1532-5415.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Smith GE, Lucas JA, Tangalos EG, Kokmen E, Petersen RC. Free and cued selective reminding test: MOANS norms. J Clin Exp Neuropsychol. 1997;19(5):676–691. doi: 10.1080/01688639708403753. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Attention and effort. Englewood Cliffs, NJ: Prentice-Hall; 1973. [Google Scholar]
- Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54(11):2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman A, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011 doi: 10.1001/archneurol.2010.362. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United States. [Accepted 1/2011];Journal of Gerontology:Medical Sciences. 2011 doi: 10.1093/gerona/glr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9(3):339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Lindenberger URF. Limits and potentials of intellectual functioning in old age. In: Baltes MKPB, editor. The Berlin Aging Study: Aging from 70 to 100. New York: Cambridge University Press; 1999. pp. 329–359. [Google Scholar]
- Lindenberger U, Scherer H, Baltes PB. The strong connection between sensory and cognitive performance in old age: not due to sensory acuity reductions operating during cognitive assessment. Psychol Aging. 2001;16(2):196–205. doi: 10.1037//0882-7974.16.2.196. [DOI] [PubMed] [Google Scholar]
- Linn RT, Wolf PA, Bachman DL, Knoefel JE, Cobb JL, Belanger AJ, et al. The ‘preclinical phase’ of probable Alzheimer’s disease. A 13-year prospective study of the Framingham cohort. Arch Neurol. 1995;52(5):485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- Marshall L. Decision criteria for pure-tone detection used by two age groups of normal-hearing and hearing-impaired listeners. J Gerontol. 1991;46(2):P67–70. doi: 10.1093/geronj/46.2.p67. [DOI] [PubMed] [Google Scholar]
- Nelson HE, O’Connell A. Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex. 1978;14(2):234–244. doi: 10.1016/s0010-9452(78)80049-5. [DOI] [PubMed] [Google Scholar]
- Ohta RJ, Carlin MF, Harmon BM. Auditory acuity and performance on the mental status questionnaire in the elderly. J Am Geriatr Soc. 1981;29(10):476–478. doi: 10.1111/j.1532-5415.1981.tb01753.x. [DOI] [PubMed] [Google Scholar]
- Peters CA, Potter JF, Scholer SG. Hearing impairment as a predictor of cognitive decline in dementia. J Am Geriatr Soc. 1988;36(11):981–986. doi: 10.1111/j.1532-5415.1988.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Ivnik RJ, Kokmen E, Tangalos EG. Memory function in very early Alzheimer’s disease. Neurology. 1994;44(5):867–872. doi: 10.1212/wnl.44.5.867. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Ivnik RJ, Tangalos EG, Schaid DJ, Thibodeau SN, et al. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. JAMA. 1995;273(16):1274–1278. [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA, Daneman M. How young and old adults listen to and remember speech in noise. J Acoust Soc Am. 1995;97(1):593–608. doi: 10.1121/1.412282. [DOI] [PubMed] [Google Scholar]
- Pickles JO. An introduction to the physiology of hearing. Bingley, UK: Emerald Group Publishing; 2008. [Google Scholar]
- Rabbitt P. Mild hearing loss can cause apparent memory failures which increase with age and reduce with IQ. Acta Otolaryngol Suppl. 1990;476:167–75. doi: 10.3109/00016489109127274. discussion 176., 167–175. [DOI] [PubMed] [Google Scholar]
- Rabbitt PM. Channel-capacity, intelligibility and immediate memory. Q J Exp Psychol. 1968;20(3):241–248. doi: 10.1080/14640746808400158. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rapp MA, Reischies FM. Attention and executive control predict Alzheimer disease in late life: results from the Berlin Aging Study (BASE) Am J Geriatr Psychiatry. 2005;13(2):134–141. doi: 10.1176/appi.ajgp.13.2.134. [DOI] [PubMed] [Google Scholar]
- Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Rosen W. Verbal fluency in aging and dementia. Journal of Clinical Neuropsychology. 1980;2:135–146. [Google Scholar]
- Rousseaux M, Seve A, Vallet M, Pasquier F, Mackowiak-Cordoliani MA. An analysis of communication in conversation in patients with dementia. Neuropsychologia. 48(13):3884–3890. doi: 10.1016/j.neuropsychologia.2010.09.026. [DOI] [PubMed] [Google Scholar]
- Royall DR, Chiodo LK, Polk MJ. Misclassification is likely in the assessment of mild cognitive impairment. Neuroepidemiology. 2004;23(4):185–191. doi: 10.1159/000078504. [DOI] [PubMed] [Google Scholar]
- Rubin EH, Storandt M, Miller JP, Kinscherf DA, Grant EA, Morris JC, et al. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Arch Neurol. 1998;55(3):395–401. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- Sarazin M, Berr C, De Rotrou J, Fabrigoule C, Pasquier F, Legrain S, et al. Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology. 2007;69(19):1859–1867. doi: 10.1212/01.wnl.0000279336.36610.f7. [DOI] [PubMed] [Google Scholar]
- Shock NW, Greulich RC, Aremberg D, Costa PT, Lakatta EG, Tobin JD. Normal Human Aging: the Baltimore Longitudinal Study of Aging. Wasington, D.C: National Institutes of Health; 1984. [Google Scholar]
- Sinha UK, Hollen KM, Rodriguez R, Miller CA. Auditory system degeneration in Alzheimer’s disease. Neurology. 1993;43(4):779–785. doi: 10.1212/wnl.43.4.779. [DOI] [PubMed] [Google Scholar]
- Spreen OBA. Neurosensory Center Comprehensive Examination for Aphasia: Manual of direction. Victoria, BC: Neuropsychology Laboratory, University of Victoria; 1969. [Google Scholar]
- Strawbridge WJ, Wallhagen MI, Shema SJ, Kaplan GA. Negative consequences of hearing impairment in old age: a longitudinal analysis. Gerontologist. 2000;40(3):320–326. doi: 10.1093/geront/40.3.320. [DOI] [PubMed] [Google Scholar]
- Tay T, Wang JJ, Kifley A, Lindley R, Newall P, Mitchell P. Sensory and cognitive association in older persons: findings from an older Australian population. Gerontology. 2006;52(6):386–394. doi: 10.1159/000095129. [DOI] [PubMed] [Google Scholar]
- Thomas PD, Hunt WC, Garry PJ, Hood RB, Goodwin JM, Goodwin JS. Hearing acuity in a healthy elderly population: effects on emotional, cognitive, and social status. J Gerontol. 1983;38(3):321–325. doi: 10.1093/geronj/38.3.321. [DOI] [PubMed] [Google Scholar]
- Tounsi H, Deweer B, Ergis AM, Van der Linden M, Pillon B, Michon A, et al. Sensitivity to semantic cuing: an index of episodic memory dysfunction in early Alzheimer disease. Alzheimer Dis Assoc Disord. 1999;13(1):38–46. doi: 10.1097/00002093-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol Aging. 2009;24(3):761–766. doi: 10.1037/a0014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuokko H, Vernon-Wilkinson R, Weir J, Beattie BL. Cued recall and early identification of dementia. J Clin Exp Neuropsychol. 1991;13(6):871–879. doi: 10.1080/01688639108405104. [DOI] [PubMed] [Google Scholar]
- Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behav Med. 2006;29(4):377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Uhlmann RF, Larson EB, Rees TS, Koepsell TD, Duckert LG. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 1989;261(13):1916–1919. [PubMed] [Google Scholar]
- Valentijn SA, van Boxtel MP, van Hooren SA, Bosma H, Beckers HJ, Ponds RW, et al. Change in sensory functioning predicts change in cognitive functioning: results from a 6-year follow-up in the maastricht aging study. J Am Geriatr Soc. 2005;53(3):374–380. doi: 10.1111/j.1532-5415.2005.53152.x. [DOI] [PubMed] [Google Scholar]
- Weinstein BE, Ventry IM. Hearing impairment and social isolation in the elderly. J Speech Hear Res. 1982;25(4):593–599. doi: 10.1044/jshr.2504.593. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Grossman M. Language and the aging brain: patterns of neural compensation revealed by functional brain imaging. J Neurophysiol. 2006;96(6):2830–2839. doi: 10.1152/jn.00628.2006. [DOI] [PubMed] [Google Scholar]
- World Health Organization Prevention of Blindness and Deafness (PBD) Program. Prevention of Deafness and Hearing Impaired Grades of Hearing Impairment. http://www.who.int/pbd/deafness/hearing_impairment_grades/en/index.html.