Abstract
A challenge to ultra high field functional magnetic resonance imaging (fMRI) is the predominance of noise associated with physiological processes unrelated to tasks of interest. This degradation in data quality may be partially reversed using a series of preprocessing algorithms designed to retrospectively estimate and remove the effects of these noise sources. However, such algorithms are routinely validated only in isolation, and thus consideration of their efficacies within realistic preprocessing pipelines and on different data sets is often overlooked. We investigate the application of eight possible combinations of three pseudo-complementary preprocessing algorithms – phase regression, Stockwell transform filtering, and retrospective image correction (RETROICOR) – to suppress physiological noise in 2D and 3D functional data at 7 Tesla. The performance of each preprocessing pipeline was evaluated using data-driven metrics of reproducibility and prediction. The optimal preprocessing pipeline for both 2D and 3D functional data included phase regression, Stockwell transform filtering, and RETROICOR. This result supports the hypothesis that a complex preprocessing pipeline is preferable to a magnitude-only pipeline, and suggests that fMRI studies should retain complex images and externally monitor subjects’ respiratory and cardiac cycles so that these supplementary data may be used to retrospectively reduce noise and enhance overall data quality.
Keywords: functional magnetic resonance imaging (fMRI), blood oxygenation level dependent (BOLD) contrast, 7 Tesla, phase regression, Stockwell transform filtering, retrospective image correction (RETROICOR)
INTRODUCTION
Numerous preprocessing pipelines have been developed to improve the quality of data from blood oxygenation level dependent (BOLD) functional magnetic resonance imaging (fMRI) studies. Algorithms within these pipelines share the same broad goal: to identify and remove one or more sources of extraneous temporal variance (i.e., “noise”) while preserving genuine BOLD contrast related to the functional paradigm (i.e., “signal”) so that subsequent statistical analyses can reliably detect BOLD signal changes (1). Preprocessing steps commonly include k-space corrections and unaliased image reconstruction, slice timing correction, geometric unwarping, physiological noise correction, rigid-body motion correction, temporal filtering, spatial smoothing, and group alignment (1,2), and may also include de-noising based upon independent or principal component analysis (3–5). The ultimate task of optimizing the entire fMRI pipeline (data acquisition, processing, and analysis) is daunting and currently impossible due to the increase in required resources (scanner availability, storage space and/or computation time) for each variable permutation. Thus, current endeavors focus on one or a few steps with an implicit understanding that optimizing a subset of the pipeline is unlikely to result in a globally optimal fMRI pipeline. Furthermore, considering multiple steps simultaneously (e.g., 6–8,5,9–15) is methodologically preferable to evaluating algorithms individually and in isolation because the former approach permits investigations of interactions between sequential steps, thereby better characterizing the complexities of real pipelines.
It is well known that MRI data are inherently complex: each datum has a real and an imaginary part, and thus a magnitude and phase (16). Although many applications of anatomical imaging use only the magnitude component of reconstructed images, phase images contain unique and useful information that has been exploited for applications such as very low signal-to-noise ratio image detection (17), water/fat imaging (18), susceptometry (19), susceptibility weighted imaging (20), and tissue segmentation (21). Furthermore, phase image time courses have been characterized (22,23) and used to measure changes in venous blood oxygenation (24), detect activation from phase-only images (25), suppress BOLD signal contributions from large vessels (26,27), and improve the detection of genuine BOLD signal changes (28–30,12,31). It is therefore surprising that the use of magnitude-only images is still virtually ubiquitous in BOLD fMRI, and we hypothesize that a complex preprocessing pipeline that exploits magnitude and phase information is preferable to a conventional pipeline that uses only magnitude information.
As physiological noise has been shown to be the dominant noise source in high field fMRI (32–36), we elected to focus this study on three pseudo-complementary algorithms designed to mitigate the unwanted effects of physiological noise: retrospective image correction (“RETROICOR”) (37), Stockwell transform filtering (38), and phase regression (26,12). A brief description of each algorithm is provided below, and complete details may be found in the original references. Firstly, Glover et al. (37) proposed RETROICOR to reduce components of physiological noise attributed to respiration and cardiac pulsatility in data acquired with single-shot echo-planar imaging (EPI) (39,40). External monitors are used to detect the cardiac and respiratory cycles, and low order Fourier series are fit to the monitored waveforms and regressed from time series of magnitude data. Secondly, the Stockwell (S) transform of a one-dimensional time series provides an informative time-frequency representation that permits identification of higher frequency signal components (41). Goodyear et al. (38) proposed selective filtering in the S transform domain to suppress sporadic high frequency noise. Thirdly, since MR data are complex, Menon (26) proposed phase regression on a per-voxel basis to suppress BOLD signal changes from larger vessels and draining veins that can be several millimeters or more away from the site of neural activation (42). Barry et al. (12) investigated an alternative use of this algorithm to mitigate temporal variance from noise sources exhibiting correlated changes in magnitude and phase.
It would be prudent to also consider the limitations of the methods by which preprocessing algorithms are validated, and investigate plausible circumstances that may challenge their efficacies. For example, since the majority of fMRI data are acquired using 2D EPI, it follows that most algorithms have been validated using only 2D single-shot EPI data. Recent renewed interest in 3D multi-shot acquisition strategies (e.g., 43–46) to obviate challenges with EPI at higher fields suggests we reconsider whether algorithms validated using 2D EPI data at 1.5 or 3 Tesla will work just as well on 3D data and/or at ultra high (7+ Tesla) fields. Therefore, the goals of this paper are to use established data-driven metrics to (1) validate the efficacy of RETROICOR on highly multi-shot 3D functional data; (2) confirm the efficacies of S transform filtering and phase regression on ultra high field (7 Tesla) data; (3) investigate interactions between phase regression, S transform filtering, RETROICOR, and isotropic spatial smoothing; and (4) compare complex and magnitude-only preprocessing pipelines to determine if standard practices for acquiring 2D and/or 3D BOLD functional data should be updated to retain phase information whenever possible.
METHODS
Data Acquisition
Experiments were performed on a Philips Achieva 7T scanner with a quadrature transmit coil and 16-channel receive-only head coil. A complete description of the experimental setup and data acquisition may be found in Barry et al. (46), and a brief description is as follows. Twelve volunteers were studied under a protocol approved by the Vanderbilt University Institutional Review Board. The visual paradigm was a block design with four segments of 24 sec baseline (central fixation) and 24 sec activation (stationary 8 Hz flashing checkerboard wedge). Twelve slices (2 mm thick) were planned parallel to the calcarine sulcus with the shim volume situated to cover only the occipital lobe. Four functional runs were acquired alternating between 2D EPI and 3D PRESTO (Principles of Echo-Shifting with a Train of Observations) (47,48) with the following acquisition parameters: 2D EPI: voxel size = 2.19 × 2.19 × 2 mm3, TE = 28 ms, TR = volume acquisition time (VAT) = 2000 ms, θ = 87°, SENSE factor = 3.2, 96 volumes; 3D PRESTO: voxel size = 2.19 × 2.19 × 2 mm3, TE = 28 ms, TR = 22.22 ms, VAT = 1000 ms, θ = 12°, SENSE factor = 3.2, 192 volumes. Respiratory and cardiac cycles were monitored at a sampling frequency of 500 Hz using respiratory bellows (secured to the torso at the position of maximal deflection between inhalation and exhalation) and a pulse oximeter (placed on an index finger).
Preprocessing Steps
Matlab (MathWorks, Natick, MA) and AFNI (Analysis of Functional NeuroImages) (49) were used for all preprocessing steps. Stockwell transform filtering (ST) and phase regression (PR) were implemented in Matlab as described in the original papers, and RETROICOR (RI) was implemented using AFNI as described in the original paper. PR requires complex data as an input (and outputs magnitude-only data) whereas ST and RI are applied to magnitude-only data, so the two orders in which these three steps may be applied are: PR+ST+RI and PR+RI+ST. We hypothesized that ST and RI are approximately commutable operations (since ST removes aperiodic high-frequency noise and RI removes quasi-periodic low-frequency noise), and therefore excluded the latter permutation from further consideration to help control the overall computation time of the analyses. These three steps were either applied or not applied, resulting in 23 = 8 preprocessing configurations before spatial smoothing: ‘none’, PR, ST, RI, PR+ST, PR+RI, ST+RI, and PR+ST+RI.
Data Processing
The workflow for data processing and analysis was automated using software written in Matlab. Binary masks were created for each functional run, and voxels included only in both masks (for each pair of runs) that were also within the shim volume were retained for subsequent analyses. In preparation for group analyses, data from each preprocessing configuration were spatially smoothed to compensate for anatomical and functional heterogeneities (50) and group alignment issues (51). Since maximal between-subject spatial variation along the posterior calcarine sulcus is ~2 cm in group space (52,53), we considered three full-width-at-half-maximum Gaussian spatial smoothing (SS) kernel widths for this study: (1) low=8 mm, as ~6–8 mm is typically used for group analyses; (2) high=16 mm, as this is close to the upper limit of expected between-subject spatial variation; and (3) medium=12 mm, as an intermediate value between 8 and 16 mm, which resulted in 8×3=24 preprocessing pipelines (12 magnitude-only and 12 using phase information).
NPAIRS
The quality of fMRI data was evaluated via metrics of prediction and reproducibility using NPAIRS† (Non-parametric Prediction, Activation, Influence and Reproducibility re-Sampling) (54,55). Reproducibility (r ∈ [0,1]) measures the similarity (Pearson correlation coefficient) of activation maps generated from two independent data sets, and prediction (p ∈ [0,1]) evaluates the degree (e.g., posterior probability) to which a trained model can assign correct class labels to an independent test set. The current study implemented NPAIRS as described in Barry et al. (46) except that the split-half resampling procedure considered all possible splits (12C6/2 = 462) for each PC range to generate the most accurate results possible for each analysis. Reported values for prediction and reproducibility are the medians across split-half samples for the range of inclusive PCs selected to jointly maximize prediction and reproducibility for each pipeline.
RESULTS
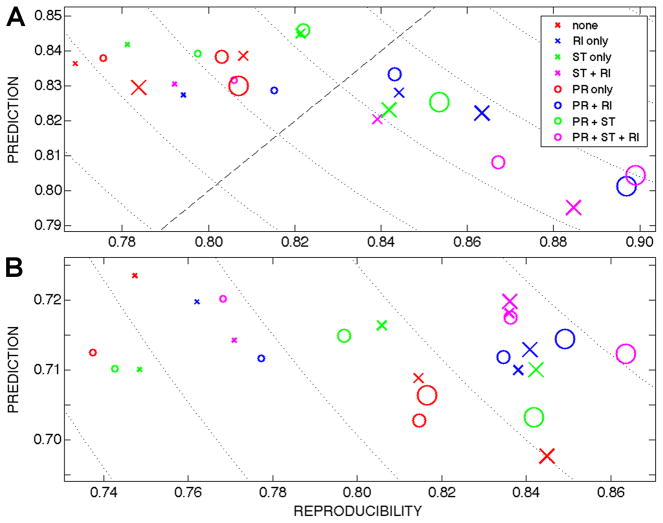
Figure 1 plots prediction vs. reproducibility for NPAIRS analyses of data acquired using (A) 2D EPI and (B) 3D PRESTO. Equal weight is given to prediction and reproducibility, so the Euclidean distance d from (p,r) to the ideal point at (p=1, r=1) is used as a scalar metric to quantify the overall performance of a given pipeline. Increasingly preferable pipelines are identified by decreasing d, and minimizing d is synonymous with identifying the optimal preprocessing steps for a given acquisition strategy. For 2D EPI data (Fig. 1A), comparisons of magnitude-only pipelines show that ST and RI in isolation (‘ST only’ or ‘RI only’) are preferable to (i.e., decreasing d) no physiological noise correction (‘none’) for all SS kernels, which confirms the original works (37,38) and is attributed primarily to increasing reproducibility. Low smoothing: ST, RI, and ST+RI demonstrate tradeoffs between small changes in p and r and all exhibit approximately the same d. Medium/high smoothing: RI is preferable to ST and ST+RI. The implementation of PR before other steps transforms pipelines from magnitude-only to complex, and decreases d in 11 of these 12 pipelines. The only pipeline exhibiting a slight increase in d after the inclusion of PR (caused by a slight decrease in r) is ‘none’ with SS=12 mm, which is presumably due to the predominance of suppression of BOLD activation from larger vessels. It should also be noted that pipelines without any physiological noise correction are included here for the sake of comparison, but are arguably less realistic because the use of at least one algorithm to reduce physiological noise is commonplace in fMRI analyses. Low smoothing: The preferable pipeline is PR+RI (d=0.252). Medium smoothing: The preferable pipeline is also PR+RI (d=0.229), although PR+ST is noteworthy in that it produces the highest overall prediction (p=0.846). High smoothing: The preferable pipeline is PR+ST+RI, which also has the highest reproducibility (r=0.899) and is the optimal 2D pipeline (d=0.220).
FIG. 1.
Plots of prediction vs. reproducibility (p,r) for NPAIRS analyses of images acquired using (A) 2D EPI and (B) 3D PRESTO. In theory, an analysis of noiseless fMRI data with a perfect model would map to the point (1,1). Concentric dotted curves mark points that are equidistant to (1,1), and the dashed line marks equal prediction and reproducibility (p = r). As defined in the legend [none = no preprocessing, PR = phase regression, ST = Stockwell transform filtering, RI = RETROICOR], each ‘x’ represents a magnitude-only pipeline, and the ‘o’ of the same color and size represents that pipeline with PR. The size of each symbol represents the degree of spatial smoothing (SS) that was applied in preparation for group analyses (small → SS = 8 mm FWHM; medium → SS = 12 mm FWHM; and large → SS = 16 mm FWHM).
Analyses of magnitude-only 3D PRESTO data (‘x’s in Fig. 1B) demonstrate similar benefits of physiological noise correction across SS kernels, with 8 of 9 magnitude-only pipelines demonstrating a decrease in d compared to no physiological noise correction (the sole exception being ST with SS=8mm). Low/medium/high smoothing: ST+RI is identified as the most preferable magnitude-only pipeline across SS kernels. As before, the implementation of PR before other steps transforms pipelines from magnitude-only to complex (‘o’s in Fig. 1B). The three pipelines without physiological noise correction exhibit increasing d after the inclusion of PR, which is, as previously stated, most likely due to the predominance of suppression of BOLD activation from larger vessels. Of the remaining 9 pipelines (ST, RI, and ST+RI for each SS kernel), the inclusion of PR decreases d in 4 pipelines, is unchanged in 2 pipelines, and increases d in 3 pipelines (PR+ST across SS kernels). Low smoothing: The preferable pipeline is PR+ST+RI (d=0.363). Medium smoothing: The ST+RI and PR+ST+RI pipelines are equivalently preferable (d=0.326). High smoothing: The preferable pipeline is PR+ST+RI, which also has the highest reproducibility (r=0.864) and is the optimal 3D pipeline (d=0.318).
These results may be summarized in three succinct points: (1) if only one of these three algorithms could be implemented to reduce physiological noise, then RETROICOR would be the best choice; (2) enhanced suppression of physiological noise is consistently achieved when phase regression precedes RETROICOR; and (3) further reduction in physiological noise may also be possible by implementing S transform filtering between phase regression and RETROICOR.
DISCUSSION
This brief report explores the dependence of physiological noise suppression on a priori decisions that pertain to data acquisition and monitoring. If MR data are magnitude-only and physiological monitoring equipment is not used (or not available), then only 2 (‘none’, ST) of the 8 configurations may be considered; if MR data are magnitude-only but physiological processes are monitored, then 4 configurations are possible (‘none’, ST, RI, ST+RI); if complex data are retained but physiological processes are not monitored, then 4 configurations are also possible (‘none’, PR, ST, PR+ST); and only if both complex data are retained and physiological processes are monitored are all 8 configurations possible. Our finding that every preferable configuration (for each spatial scale) included phase regression and RETROICOR highlights the importance of retaining complex data and externally monitoring subjects’ respiratory and cardiac cycles to improve overall data quality. Furthermore, the fact that the optimal configuration for both 2D and 3D functional data was PR+ST+RI also reinforces the importance of investigating possible synergistic interactions between algorithms when designing a preprocessing pipeline.
Each preprocessing pipeline was evaluated using data-driven metrics of reproducibility and prediction, and the Euclidean distance d from coordinates (p,r) (generated from an NPAIRS analysis) to the ideal point at (1,1) succinctly quantified the overall pipeline performance. This framework was used to both validate the application of RETROICOR on highly multi-shot 3D PRESTO data and confirm the efficacies of S transform filtering and phase regression for use on ultra high field data. However, systematic differences in the relative shifts of (p,r) points are observed between Figs. 1A (2D EPI) and 1B (3D PRESTO), suggesting a divergence in the efficacies of PR and ST that is logically attributed to the chosen acquisition sequence. For example, EPI data processed with only PR and/or ST (i.e., ST, PR, and PR+ST for each SS kernel) decreased d in 8 of these 9 pipelines relative to ‘none’ (with the exception being PR with SS=12 mm). In comparison, PRESTO data processed with only PR and/or ST decreased d in 3 (ST with SS=12 mm and SS=16 mm, and PR+ST with SS=16 mm) of these 9 pipelines relative to ‘none’; however, the addition of RI to the remaining 6 pipelines decreased d in all cases relative to the respective pipelines without RI (e.g., ST+RI vs. ST with SS=8mm), and also to beyond what was possible with ‘RI only’ in 5 of 6 instances (whilst the sixth comparison, PR+RI vs. RI only with SS=12 mm, had equivalent d=0.332). As previously mentioned, PR and ST were applied to 2D EPI and 3D PRESTO data in identical fashions exactly as described in the original papers. However, these algorithms were developed and validated using EPI data, and 2D EPI data is known to have markedly different signal and noise characteristics compared to 3D PRESTO data (43), so it is plausible that such divergent efficacies may be attributed to suboptimal implementations on 3D functional data. Thus, avenues for future work include further investigations of how the optimal application of such algorithms may differ between 2D and 3D functional data sets.
In conclusion, we have investigated the implementation of eight preprocessing configurations using three pseudo-complementary algorithms – phase regression, S transform filtering, and RETROICOR – to retrospectively suppress physiological noise in 2D and 3D functional data at 7T. Whereas S transform filtering may be applied to any fMRI data set, phase regression may only be applied if complex images are retained and RETROICOR may only be applied if subjects’ cardiac and respiratory cycles are externally monitored. The performance of each preprocessing pipeline was evaluated using data-driven NPAIRS metrics of prediction and reproducibility, which facilitated an unbiased comparison between competing preprocessing strategies. The preferable configuration for each spatial scale always included phase regression and RETROICOR, and the optimal preprocessing pipeline for both 2D EPI and 3D PRESTO data included phase regression, S transform filtering, and RETROICOR. These results support the hypothesis that a complex preprocessing pipeline is preferable to a magnitude-only pipeline, and suggest that fMRI studies should routinely retain complex images and externally monitor subjects’ physiological cycles so that these supplementary data are available to retrospectively reduce noise and enhance overall data quality. Future work will extend the application of various preprocessing algorithms to other 2D and 3D functional data sets, as well as investigate the dual role of phase regression in suppressing macrovascular BOLD signals and extraneous noise sources in single-subject fMRI analyses.
Acknowledgments
This research was supported by NIH grant 5R01EB000461 to John C. Gore and CIHR/MOP84483 to Stephen C. Strother, who also gratefully acknowledges support of the Heart & Stroke Foundation of Ontario through the Centre for Stroke Recovery.
Footnotes
NPAIRS is freely available at http://code.google.com/p/plsnpairs
References
- 1.Petersson KM, Nichols TE, Poline J-B, Holmes AP. Statistical limitations in functional neuroimaging II. Signal detection and statistical inference. Philos Trans R Soc Lond B Biol Sci. 1999;354:1261–1281. doi: 10.1098/rstb.1999.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strother SC. Evaluating fMRI preprocessing pipelines. IEEE Eng Med Biol Mag. 2006;25:27–41. doi: 10.1109/memb.2006.1607667. [DOI] [PubMed] [Google Scholar]
- 3.Thomas CG, Harshman RA, Menon RS. Noise reduction in BOLD-based fMRI using component analysis. Neuroimage. 2002;17:1521–1537. doi: 10.1006/nimg.2002.1200. [DOI] [PubMed] [Google Scholar]
- 4.Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21170. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strother S, La Conte S, Hansen LK, Anderson J, Zhang J, Pulapura S, Rottenberg D. Optimizing the fMRI data-processing pipeline using prediction and reproducibility performance metrics: I. A preliminary group analysis. Neuroimage. 2004;23:S196–S203. doi: 10.1016/j.neuroimage.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Hopfinger JB, Büchel C, Holmes AP, Friston KJ. A study of analysis parameters that influence the sensitivity of event-related fMRI analyses. Neuroimage. 2000;11:326–333. doi: 10.1006/nimg.2000.0549. [DOI] [PubMed] [Google Scholar]
- 7.LaConte S, Anderson J, Muley S, Ashe J, Frutiger S, Rehm K, Hansen LK, Yacoub E, Hu X, Rottenberg D, Strother S. The evaluation of preprocessing choices in single-subject BOLD fMRI using NPAIRS performance metrics. Neuroimage. 2003;18:10–27. doi: 10.1006/nimg.2002.1300. [DOI] [PubMed] [Google Scholar]
- 8.Shaw ME, Strother SC, Gavrilescu M, Podzebenko K, Waites A, Watson J, Anderson J, Jackson G, Egan G. Evaluating subject specific preprocessing choices in multisubject fMRI data sets using data-driven performance metrics. Neuroimage. 2003;19:988–1001. doi: 10.1016/s1053-8119(03)00116-2. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Pereira F, Lee W, Strother S, Mitchell T. Exploring predictive and reproducible modeling with the single-subject FIAC dataset. Hum Brain Mapp. 2006;27:452–461. doi: 10.1002/hbm.20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones TB, Bandettini PA, Birn RM. Integration of motion correction and physiological noise regression in fMRI. Neuroimage. 2008;42:582–590. doi: 10.1016/j.neuroimage.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Anderson JR, Liang L, Pulapura SK, Gatewood L, Rottenberg DA, Strother SC. Evaluation and optimization of fMRI single-subject processing pipelines with NPAIRS and second-level CVA. Magn Reson Imag. 2009;27:264–278. doi: 10.1016/j.mri.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Barry RL, Williams JM, Klassen LM, Gallivan JP, Culham JC, Menon RS. Evaluation of preprocessing steps to compensate for magnetic field distortions due to body movements in BOLD fMRI. Magn Reson Imag. 2010;28:235–244. doi: 10.1016/j.mri.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans JW, Todd RM, Taylor MJ, Strother SC. Group specific optimisation of fMRI processing steps for child and adult data. Neuroimage. 2010;50:479–490. doi: 10.1016/j.neuroimage.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 14.Etzel JA, Valchev N, Keysers C. The impact of certain methodological choices on multivariate analysis of fMRI data with support vector machines. Neuroimage. 2011;54:1159–1167. doi: 10.1016/j.neuroimage.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 15.Churchill NW, Oder A, Abdi H, Tam F, Lee W, Thomas C, Ween JE, Graham SJ, Strother SC. Optimizing preprocessing and analysis pipelines for single-subject fMRI. I. Standard temporal motion and physiological noise correction methods. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21238. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar A, Welti D, Ernst RR. NMR Fourier zeugmatography. J Magn Reson. 1975;18:69–83. doi: 10.1016/j.jmr.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein MA, Thomasson DM, Perman WH. Improved detectability in low signal-to-noise ratio magnetic resonance images by means of a phase-corrected real reconstruction. Med Phys. 1989;16:813–817. doi: 10.1118/1.596304. [DOI] [PubMed] [Google Scholar]
- 18.Glover GH, Schneider E. Three-point Dixon technique for true water/fat decomposition with B0 inhomogeneity correction. Magn Reson Med. 1991;18:371–383. doi: 10.1002/mrm.1910180211. [DOI] [PubMed] [Google Scholar]
- 19.Weisskoff RM, Kiihne S. MRI susceptometry: image-based measurement of absolute susceptibility of MR contrast agents and human blood. Magn Reson Med. 1992;24:375–383. doi: 10.1002/mrm.1910240219. [DOI] [PubMed] [Google Scholar]
- 20.Haacke EM, Xu Y, Cheng YN, Reichenbach JR. Susceptibility weighted imaging (SWI) Magn Reson Med. 2004;52:612–618. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- 21.Rowe DB, Haacke EM. MAgnitude and PHase Thresholding (MAPHT) of noisy complex-valued magnetic resonance images. Magn Reson Imag. 2009;27:1271–1280. doi: 10.1016/j.mri.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowe DB. Modeling both the magnitude and phase of complex-valued fMRI data. Neuroimage. 2005;25:1310–1324. doi: 10.1016/j.neuroimage.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 23.Rowe DB, Meller CP, Hoffmann RG. Characterizing phase-only fMRI data with an angular regression model. J Neurosci Methods. 2007;161:331–341. doi: 10.1016/j.jneumeth.2006.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoogenraad FGC, Reichenbach JR, Haacke EM, Lai S, Kuppusamy K, Sprenger M. In vivo measurement of changes in venous blood-oxygenation with high resolution functional MRI at 0. 95 Tesla by measuring changes in susceptibility and velocity. Magn Reson Med. 1998;39:97–107. doi: 10.1002/mrm.1910390116. [DOI] [PubMed] [Google Scholar]
- 25.Rowe DB, Logan BR. A complex way to compute fMRI activation. Neuroimage. 2004;23:1078–1092. doi: 10.1016/j.neuroimage.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 26.Menon RS. Postacquisition suppression of large-vessel BOLD signals in high-resolution fMRI. Magn Reson Med. 2002;47:1–9. doi: 10.1002/mrm.10041. [DOI] [PubMed] [Google Scholar]
- 27.Nencka AS, Rowe DB. Reducing the unwanted draining vein BOLD contribution in fMRI with statistical post-processing methods. Neuroimage. 2007;37:177–188. doi: 10.1016/j.neuroimage.2007.03.075. [DOI] [PubMed] [Google Scholar]
- 28.Lai S, Glover GH. Detection of BOLD fMRI signals using complex data. Proc ISMRM; Vancouver, BC. 1997. p. 1671. [Google Scholar]
- 29.Nan FY, Nowak RD. Generalized likelihood ratio detection for fMRI using complex data. IEEE Trans Med Imag. 1999;18:320–329. doi: 10.1109/42.768841. [DOI] [PubMed] [Google Scholar]
- 30.Hahn AD, Nencka AS, Rowe DB. Improving robustness and reliability of phase-sensitive fMRI analysis using temporal off-resonance alignment of single-echo time series (TOAST) Neuroimage. 2009;44:742–752. doi: 10.1016/j.neuroimage.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahn AD, Nencka AS, Rowe DB. Enhancing the utility of complex-valued functional magnetic resonance imaging detection of neurobiological processes through postacquisition estimation and correction of dynamic B0 errors and motion. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21217. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weisskoff RM, Baker J, Belliveau J, Davis TL, Kwong KK, Cohen MS, Rosen BR. Power spectrum analysis of functionally-weighted MR data: what’s in the noise? Proc SMRM (New York, NY) 1993:7. [Google Scholar]
- 33.Jezzard P, LeBihan D, Cuenod C, Pannier L, Prinster A, Turner R. An investigation of the contribution of physiological noise in human functional MRI studies at 1.5 Tesla and 4 Tesla. Proc SMRM (New York, NY) 1993:1392. [Google Scholar]
- 34.Noll DC, Schneider W. Respiration artifacts in functional brain imaging: sources of signal variation and compensation strategies. Proc SMR (San Francisco, CA) 1994:647. [Google Scholar]
- 35.Krüger G, Glover GH. Physiological noise in oxygen-sensitive magnetic resonance imaging. Magn Reson Med. 2001;46:631–637. doi: 10.1002/mrm.1240. [DOI] [PubMed] [Google Scholar]
- 36.Triantafyllou C, Hoge RD, Krueger G, Wiggins CJ, Potthast A, Wiggins GC, Wald LL. Comparison of physiological noise at 1. 5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. Neuroimage. 2005;26:243–250. doi: 10.1016/j.neuroimage.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Glover GH, Li T-Q, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 38.Goodyear BG, Zhu H, Brown RA, Mitchell JR. Removal of phase artifacts from fMRI data using a Stockwell transform filter improves brain activity detection. Magn Reson Med. 2004;51:16–21. doi: 10.1002/mrm.10681. [DOI] [PubMed] [Google Scholar]
- 39.Mansfield P. Multi-planar image formation using NMR spin echoes. J Phys C: Solid State Phys. 1977;10:L55–L58. [Google Scholar]
- 40.Mansfield P, Coxon R, Glover P. Echo-planar imaging of the brain at 3. 0 T: first normal volunteer results. J Comput Assist Tomogr. 1994;18:339–343. doi: 10.1097/00004728-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Stockwell RG, Mansinha L, Lowe RP. Localization of the complex spectrum: the S transform. IEEE Trans Signal Process. 1996;44:998–1001. [Google Scholar]
- 42.Turner R. How much cortex can a vein drain? Downstream dilution of activation-related cerebral blood oxygenation changes. Neuroimage. 2002;16:1062–1067. doi: 10.1006/nimg.2002.1082. [DOI] [PubMed] [Google Scholar]
- 43.Neggers SFW, Hermans EJ, Ramsey NF. Enhanced sensitivity with fast three-dimensional blood-oxygen-level-dependent functional MRI: comparison of SENSE-PRESTO and 2D-EPI at 3T. NMR Biomed. 2008;21:663–676. doi: 10.1002/nbm.1235. [DOI] [PubMed] [Google Scholar]
- 44.Koopmans PJ, Barth M, Norris DG. Layer-specific BOLD activation in human V1. Hum Brain Mapp. 2010;31:1297–1304. doi: 10.1002/hbm.20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poser BA, Koopmans PJ, Witzel T, Wald LL, Barth M. Three dimensional echo-planar imaging at 7 Tesla. Neuroimage. 2010;51:261–266. doi: 10.1016/j.neuroimage.2010.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barry RL, Strother SC, Gatenby JC, Gore JC. Data-driven optimization and evaluation of 2D EPI and 3D PRESTO for BOLD fMRI at 7 Tesla: I. Focal coverage Neuroimage. 2011;55:1034–1043. doi: 10.1016/j.neuroimage.2010.12.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu G, Sobering G, Duyn J, Moonen CTW. A functional MRI technique combining principles of echo-shifting with a train of observations (PRESTO) Magn Reson Med. 1993;30:764–768. doi: 10.1002/mrm.1910300617. [DOI] [PubMed] [Google Scholar]
- 48.Golay X, Pruessmann KP, Weiger M, Crelier GR, Folkers PJM, Kollias SS, Boesiger P. PRESTO-SENSE: an ultrafast whole-brain fMRI technique. Magn Reson Med. 2000;43:779–786. doi: 10.1002/1522-2594(200006)43:6<779::aid-mrm1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 49.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 50.Galaburda AM, Rosen GD, Sherman GF. Individual variability in cortical organization: its relationship to brain laterality and implications to function. Neuropsychologia. 1990;28:529–546. doi: 10.1016/0028-3932(90)90032-j. [DOI] [PubMed] [Google Scholar]
- 51.Hellier P, Barillot C, Corouge I, Gibaud B, Le Goualher G, Collins DL, Evans A, Malandain G, Ayache N, Christensen GE, Johnson HJ. Retrospective evaluation of intersubject brain registration. IEEE Trans Med Imag. 2003;22:1120–1130. doi: 10.1109/TMI.2003.816961. [DOI] [PubMed] [Google Scholar]
- 52.Steinmetz H, Fürst G, Freund H-J. Variation of perisylvian and calcarine anatomic landmarks within stereotaxic proportional coordinates. AJNR Am J Neuroradiol. 1990;11:1123–1130. [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson PM, Schwartz C, Lin RT, Khan AA, Toga AW. Three-dimensional statistical analysis of sulcal variability in the human brain. J Neurosci. 1996;16:4261–4274. doi: 10.1523/JNEUROSCI.16-13-04261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strother SC, Anderson J, Hansen LK, Kjems U, Kustra R, Sidtis J, Frutiger S, Muley S, LaConte S, Rottenberg D. The quantitative evaluation of functional neuroimaging experiments: the NPAIRS data analysis framework. Neuroimage. 2002;15:747–771. doi: 10.1006/nimg.2001.1034. [DOI] [PubMed] [Google Scholar]
- 55.Strother S, Oder A, Spring R, Grady C. The NPAIRS computational statistics framework for data analysis in neuroimaging. In: Lechevallier Y, Saporta G, editors. Proc 19th Intl Conf on Computational Statistics: Keynote, Invited and Contributed Papers. Physica-Verlag, Springer; Paris, France: 2010. pp. 111–120. [Google Scholar]