Abstract
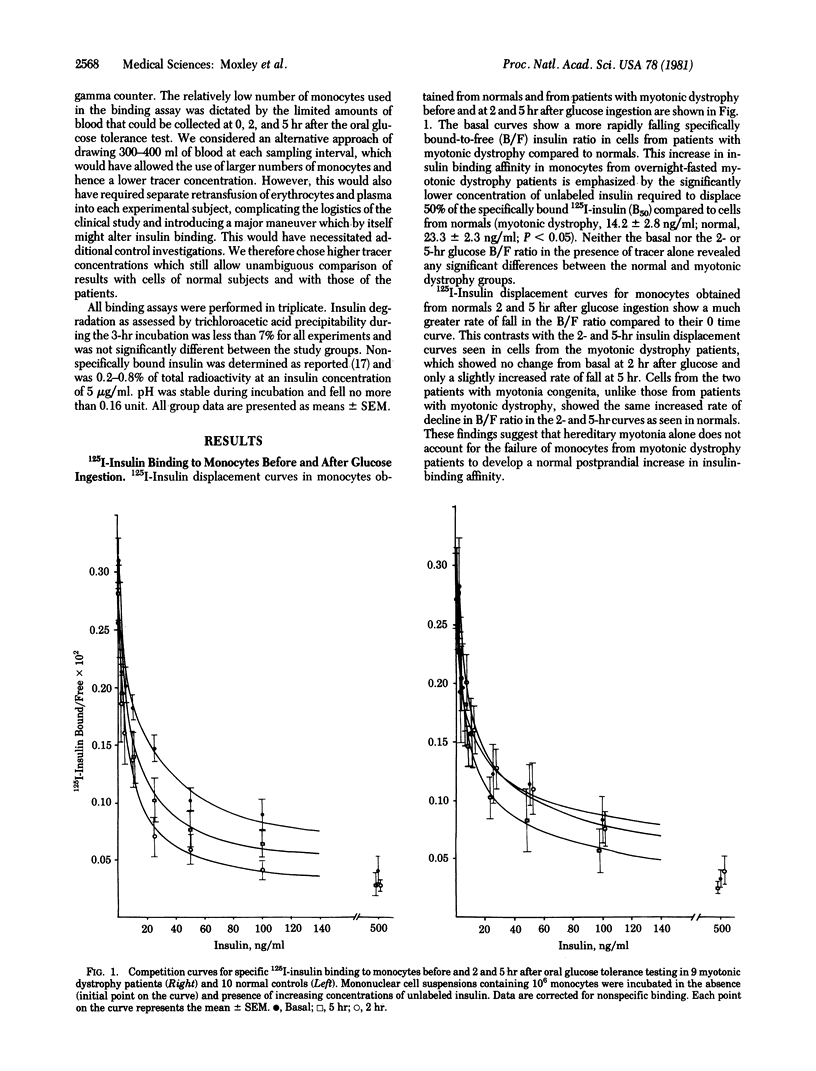
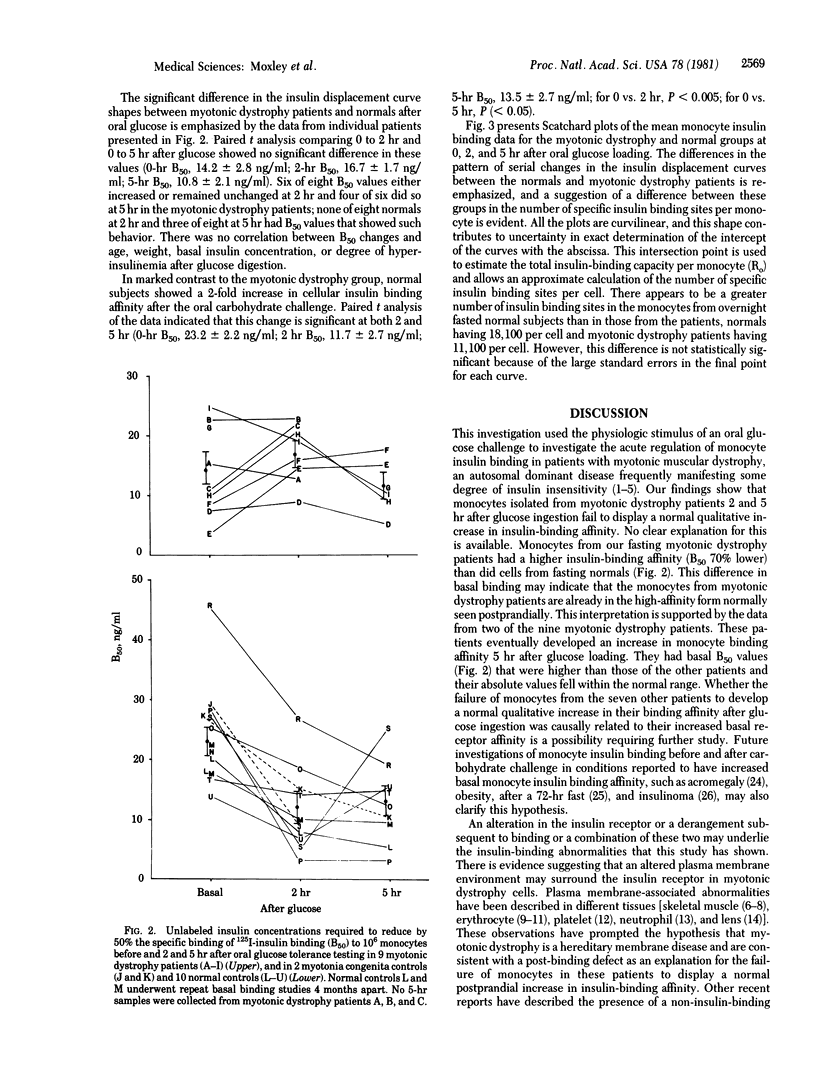
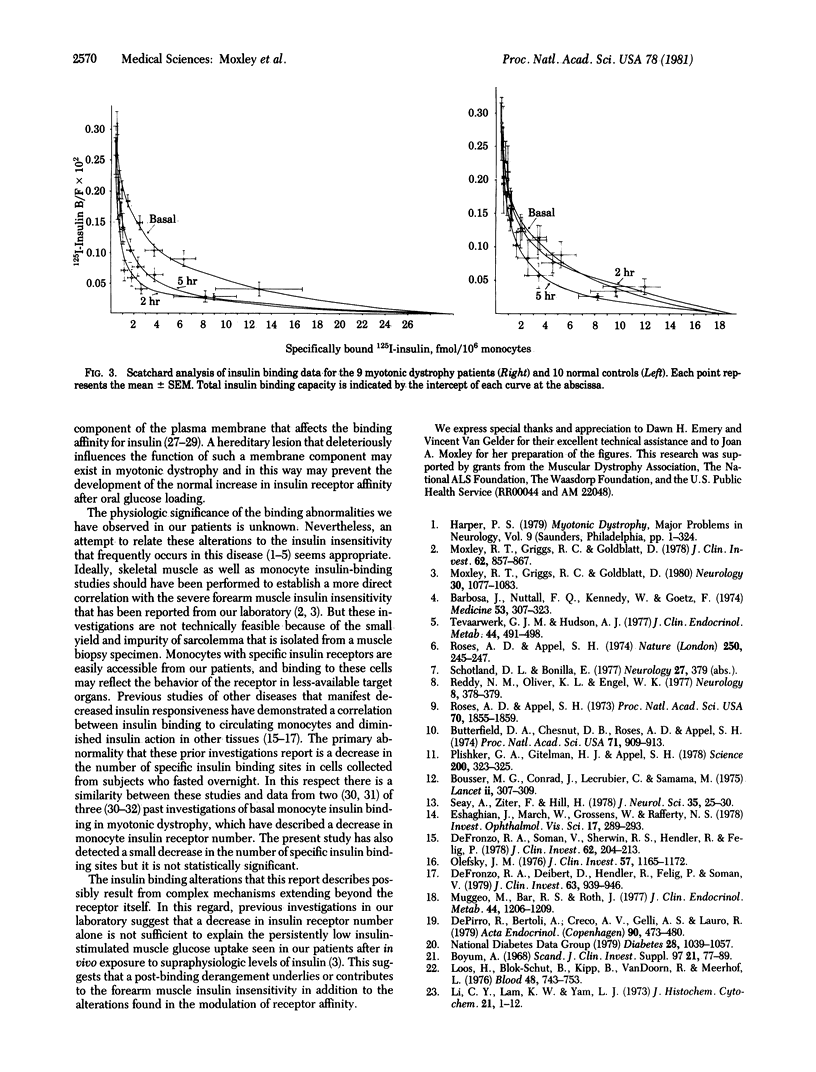
Insulin insensitivity of uncertain etiology often exists in myotonic muscular dystrophy, a multitissue, autosomal dominant disorder hypothesized to be a hereditary membrane disease. The present studies show that monocytes from patients with myotonic dystrophy fail to demonstrate the normally observed qualitative increase in insulin-binding affinity after oral glucose loading. Monocytes from 10 normal volunteers developed a significantly increased insulin-binding affinity by 2 hr after glucose ingestion (mean +/- SEM, 11.7 +/- 2.7 ng/ml compared to basal 50% insulin displacement value of 23.3 +/- 2.2 ng/ml, P less than 0.005). This increase was maintained at 5 hr (13.5 +/- 2.7 ng/ml, P less than 0.05). In contrast, no significant increase in monocyte insulin-binding affinity occurred in cells from nine myotonic dystrophy patients at 2 and 5 hr after glucose loading (50% insulin displacement values: basal, 14.2 +/- 2.8 ng/ml; 2 hr, 16.7 +/- 1.7 ng/ml; 5 hr, 10.8 +/- 2.1 ng/ml). These alterations document the presence of abnormalities in the insulin receptor or receptor-associated processes that modulate insulin binding. A hereditary plasma membrane defect may underlie these findings. This abnormality may have an etiologic role in the decreased insulin sensitivity that frequently afflicts patients with myotonic dystrophy.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar R. S., Gorden P., Roth J., Kahn C. R., De Meyts P. Fluctuations in the affinity and concentration of insulin receptors on circulating monocytes of obese patients: effects of starvation, refeeding, and dieting. J Clin Invest. 1976 Nov;58(5):1123–1135. doi: 10.1172/JCI108565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar R. S., Gorden P., Roth J., Siebert C. W. Insulin receptors in patients with insulinomas: changes in receptor affinity and concentration. J Clin Endocrinol Metab. 1977 Jun;44(6):1210–1213. doi: 10.1210/jcem-44-6-1210. [DOI] [PubMed] [Google Scholar]
- Barbosa J., Nuttall F. Q., Kennedy W., Goetz F. Plasma insulin in patients with myotonic dystrophy and their relatives. Medicine (Baltimore) 1974 Jul;53(4):307–323. doi: 10.1097/00005792-197407000-00004. [DOI] [PubMed] [Google Scholar]
- Bousser M. G., Conard J., Lecrubier C., Samama M. Increased sensitivity of platelets to adrenaline in human myotonic dystrophy. Lancet. 1975 Aug 16;2(7929):307–309. doi: 10.1016/s0140-6736(75)92735-x. [DOI] [PubMed] [Google Scholar]
- Butterfield D. A., Chesnut D. B., Roses A. D., Appel S. H. Electron spin resonance studies of erythrocytes from patients with myotonic muscular dystrophy. Proc Natl Acad Sci U S A. 1974 Mar;71(3):909–913. doi: 10.1073/pnas.71.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- De Pirro R., Bertoli A., Greco A. V., Gelli A. S., Lauro R. The effect of food intake on insulin receptor in man. Acta Endocrinol (Copenh) 1979 Mar;90(3):473–480. doi: 10.1530/acta.0.0900473. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Soman V., Sherwin R. S., Hendler R., Felig P. Insulin binding to monocytes and insulin action in human obesity, starvation, and refeeding. J Clin Invest. 1978 Jul;62(1):204–213. doi: 10.1172/JCI109108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- DeFronzo R., Deibert D., Hendler R., Felig P., Soman V. Insulin sensitivity and insulin binding to monocytes in maturity-onset diabetes. J Clin Invest. 1979 May;63(5):939–946. doi: 10.1172/JCI109394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Eshaghian J., March W. F., Goossens W., Rafferty N. S. Ultrastructure of cataract in myotonic dystrophy. Invest Ophthalmol Vis Sci. 1978 Mar;17(3):289–293. [PubMed] [Google Scholar]
- Festoff B. W., Moore W. V. Evaluation of insulin receptor in myotonic dystrophy. Ann Neurol. 1979 Jul;6(1):60–65. doi: 10.1002/ana.410060114. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Meek J. C., Streib E. The insulin receptor in myotonic dystrophy. J Clin Endocrinol Metab. 1977 Oct;45(4):821–824. doi: 10.1210/jcem-45-4-821. [DOI] [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- Loos H., Blok-Schut B., Kipp B., van Doorn R., Meerhof L. Size distribution, electronic recognition, and counting of human blood monocytes. Blood. 1976 Nov;48(5):743–753. [PubMed] [Google Scholar]
- Maturo J. M., 3rd, Hollenberg M. D. Insulin receptor: interaction with nonreceptor glycoprotein from liver cell membranes. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3070–3074. doi: 10.1073/pnas.75.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturo J. M., 3rd, Shackelford W. H., Hollenberg M. D. Characteristics of the solubilized insulin receptor of human placenta. Life Sci. 1978 Nov 13;23(20):2063–2071. doi: 10.1016/0024-3205(78)90240-0. [DOI] [PubMed] [Google Scholar]
- Moxley R. T., 3rd, Griggs R. C., Goldblatt D., VanGelder V., Herr B. E., Thiel R. Decreased insulin sensitivity of forearm muscle in myotonic dystrophy. J Clin Invest. 1978 Oct;62(4):857–867. doi: 10.1172/JCI109198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muggeo M., Bar R. S., Roth J. Change in affinity of insulin receptors following oral glucose in normal adults. J Clin Endocrinol Metab. 1977 Jun;44(6):1206–1209. doi: 10.1210/jcem-44-6-1206. [DOI] [PubMed] [Google Scholar]
- Muggeo M., Bar R. S., Roth J., Kahn C. R., Gorden P. The insulin resistance of acromegaly: evidence for two alterations in the insulin receptor on circulating monocytes. J Clin Endocrinol Metab. 1979 Jan;48(1):17–25. doi: 10.1210/jcem-48-1-17. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. Decreased insulin binding to adipocytes and circulating monocytes from obese subjects. J Clin Invest. 1976 May;57(5):1165–1172. doi: 10.1172/JCI108384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plishker G. A., Gitelman H. J., Appel S. H. Myotonic muscular dystrophy: altered calcium transport in erythrocytes. Science. 1978 Apr 21;200(4339):323–325. doi: 10.1126/science.635589. [DOI] [PubMed] [Google Scholar]
- Roses A. D., Appel S. H. Muscle membrane protein kinase in myotonic muscular dystrophy. Nature. 1974 Jul 19;250(463):245–247. doi: 10.1038/250245a0. [DOI] [PubMed] [Google Scholar]
- Roses A. D., Appel S. H. Protein kinase activity in erythrocyte ghosts of patients with myotonic muscular dystrophy. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1855–1859. doi: 10.1073/pnas.70.6.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seay A. R., Ziter F. A., Hill H. R. Defective neutrophil function in myotonic dystrophy. J Neurol Sci. 1978 Jan;35(1):25–30. doi: 10.1016/0022-510x(78)90099-0. [DOI] [PubMed] [Google Scholar]
- Tevaarwerk G. J., Hudson A. J. Carbohydrate metabolism and insulin resistance in myotonia dystrophica. J Clin Endocrinol Metab. 1977 Mar;44(3):491–498. doi: 10.1210/jcem-44-3-491. [DOI] [PubMed] [Google Scholar]
- Tevaarwerk G. J., Strickland K. P., Lin C. H., Hudson A. J. Studies on insulin resistance and insulin receptor binding in myotonia dystrophica. J Clin Endocrinol Metab. 1979 Aug;49(2):216–222. doi: 10.1210/jcem-49-2-216. [DOI] [PubMed] [Google Scholar]


