Abstract
To explore the role of adult hippocampal neurogenesis in novelty processing, we assessed novel object recognition (NOR) in mice after neurogenesis was arrested using focal x-irradiation of the hippocampus, or a reversible, genetic method in which glial fibrillary acidic protein-positive neural progenitor cells are ablated with ganciclovir. Arresting neurogenesis did not alter general activity or object investigation during four exposures with two constant objects. However, when a novel object replaced a constant object, mice with neurogenesis arrested by either ablation method showed increased exploration of the novel object when compared with control mice. The increased novel object exploration did not manifest until 4–6 weeks after x-irradiation or 6 weeks following a genetic ablation, indicating that exploration of the novel object is increased specifically by the elimination of 4–6-week-old adult born neurons. The increased novel object exploration was also observed in older mice, which exhibited a marked reduction in neurogenesis relative to young mice. Mice with neurogenesis arrested by either ablation method were also impaired in 1-trial contextual fear conditioning (CFC) at 6 weeks but not at 4 weeks following ablation, further supporting the idea that 4–6-week old adult born neurons are necessary for specific forms of hippocampal-dependent learning, and suggesting that the NOR and CFC effects have a common underlying mechanism. These data suggest that the transient enhancement of plasticity observed in young adult-born neurons contributes to cognitive functions.
Keywords: dentate gyrus, hippocampus, neurogenesis, novel object recognition, learning
Introduction
The hippocampus (HPC) is integral to novelty processing, which includes the detection of novelty, the deployment of attention, and memory encoding, all of which are critical for adaptive behavior (Sokolov, 1963; O'Keefe and Nadel, 1978; Brown and Aggleton, 2001). Functional imaging studies indicate that the HPC is one of a small number of brain regions that exhibits novelty-related activation (Tulving et al., 1994; Stern et al., 1996; Kirchhoff et al., 2000; Yamaguchi et al., 2004). In humans and primates, hippocampal lesions have been shown to impair aspects of novelty processing (Cave and Squire, 1991; Reed and Squire, 1997; Beason-Held et al., 1999; Zola et al., 2000), and to attenuate the autonomic response to novel stimuli as well as a characteristic novelty-evoked event-related potential, the P300 (Knight, 1996). In rodents, the effects of HPC lesions on novelty detection are debated, but the balance of evidence suggests that these lesions impair novelty detection when novelty has a spatial or contextual component (Forwood et al., 2005; Goodrich-Hunsaker et al., 2005; Clelland et al., 2009; cf. McTighe et al., 2009) or when the retention interval is long (> 24 h) (Vnek and Rothblat, 1996; Clark et al., 2000; Gaskin et al., 2003; Hammond et al., 2004), but may fail to impair novelty detection that is purely object-based (Winters et al., 2004).
Within the HPC, neurons are continuously generated throughout adulthood from progenitor cells in the subgranular zone of the dentate gyrus (DG) (Gage, 2000; Ming and Song, 2005). There is growing evidence that adult-born hippocampal neurons make a functionally significant contribution to learning, memory, and mood regulation (Kempermann et al., 2004; Doetsch and Hen, 2005; Lledo et al. 2006; Drew and Hen, 2007). These neurons develop functional synapses, exhibit synaptic plasticity, and are activated in situations that evoke hippocampal-dependent learning (Wang et al., 2000; Ge et al., 2006; Kee et al., 2007). Although ablation of hippocampal neurogenesis has been shown to impair performance in some HPC-dependent tasks, the literature contains many inconsistencies, which suggest that adult-born neurons are not required for all forms of HPC-dependent learning and may instead be required for some tasks only under particular conditions (Shors et al., 2002; Meshi et al., 2006; Saxe et al., 2006).
Several lines of evidence suggest that young adult-born neurons may respond differently to novelty than their mature counterparts. Exposure to a novel environment increases the firing rate of inhibitory neurons in the DG (Nitz and McNaughton, 2004), and, perhaps as a result of this, exploration of a novel environment is associated with increased dendritic inhibition of dentate granule cells (Moser, 1996). Because young adult-born neurons are insensitive to or excited by GABA during the first weeks after their terminal division (Ge et al., 2006), exploration of a novel environment, and the concomitant increase in inhibitory tone, may magnify their contribution to DG information processing. Consistent with this idea, the rate of neurogenesis in the DG is negatively correlated with locomotor reactivity to novelty (Lemaire et al., 1999). However, from these correlational data, it is unclear whether the rate of neurogenesis has a causal role in novelty processing or whether neurogenesis rates are secondary to other processes that control the novelty response.
Here, we investigate the contribution of adult-generated neurons to novelty processing by examining how the arrest of adult hippocampal neurogenesis affects novel object recognition (NOR). We report that mice with arrested neurogenesis show a surprising increase in exploration of a novel object when compared with control mice. This increase in novel object exploration does not manifest until 4–6 weeks after the arrest of neurogenesis, suggesting that the behavioral effect depends on the depletion of highly plastic 4–6 week-old neurons. We further demonstrate that an impairment in contextual fear conditioning has a similar time-course after the arrest of neurogenesis, suggesting that this impairment and the NOR phenotype share a common underlying mechanism.
Materials and Methods
Mice
129/SvEv age-matched adult male mice were purchased from Taconic (Hudson, NY) at 7 weeks of age and x-irradiated at either 9, 13, or 15 weeks of age. Transgenic (TG) mice expressing herpes simplex virus thymidine kinase from the mouse glial fibrillary acidic protein (GFAP-TK) promoter (line 7.1) were generated as previously described (Johnson et al., 1995; Bush et al., 1998). We backcrossed the GFAP-TK transgene onto a 129/SvEv background for at least 6 generations and used approximately 6-week-old male littermates derived from heterozygote x wild-type matings. Mice were housed four or five per cage in a 12-h (06:00–18:00) light–dark colony room at 22°C. Food and water were provided ad libitum. Behavioral testing was performed during the light phase. The procedures described herein were conducted in accordance with National Institutes of Health regulations and approved by the Institutional Animal Care and Use Committees of Columbia University and the New York State Psychiatric Institute.
X-irradiation
This procedure was performed as previously described (Santarelli et al., 2003) with the exception that mice used for novel object recognition paradigm experiments were anesthetized with pentobarbital sodium (Nembutal® sodium solution) (6 mg/kg).
Drugs
Ganciclovir sodium (GCV) (Cytovene®-IV, Roche, Indianapolis, IN) was dissolved in sterile saline at a concentration of 25 mg/ml and delivered through Alzet osmotic minipumps (0.25 µl per hour, 28 days) (Palo Alto, CA) implanted subcutaneously under isoflurane anesthesia. Osmotic minipumps were rotated under the skin two to three times per week.
Novel Object Recognition Paradigm
We used a modified version of the novel object recognition task described by Ennaceur and Delacour (1988). The testing room was lit with two 60 W light bulbs and behavior sessions were recorded with a video camera affixed to a tripod above the testing arena. The testing arena was a white, plastic transport box (55 × 40 × 15 cm) and was divided into two equal halves so that two sessions could occur simultaneously. The divider was made of the same white plastic as the transport box and measured 15 cm in height. Mice could not contact or see one another during the exposures. The transport box was filled with wooden bedding. The light intensity was equal in all parts of the arena (approximately 20 lx).
We used 3 different objects; each object was available in triplicate. The objects were (1) a blue, ceramic shoe (diameter 9.5 cm, maximal height 6 cm); (2) a black, shiny box (8 × 3 × 9.5 cm); and (3) a clear, plastic funnel (diameter 8.5 cm, maximal height 8.5 cm). The objects elicited equal levels of exploration as determined in pilot experiments (data not shown). A mouse could not displace the objects. The objects and the placement of the objects were fully randomized.
Mice were transported to the testing room in their home cages. The novel object recognition (NOR) paradigm consisted of five 5-min exposures with 3-min inter-exposure intervals (Fig. 1A). Mice were placed in the center of the arena at the start of each exposure. Between exposures, mice were held individually in standard cages, the objects and arenas were cleaned using PDI Sani-Cloth® HB Germicidal Disposable Wipes (Orangeburg, NY, USA), and the bedding was replaced. Exposures 1–4 were habituation sessions with two objects placed symmetrically on either end of the arena approximately 5 cm away from the wall. In exposure 5, one of the objects was replaced with a novel object. All mice were returned to their home cages at the end of the 5th exposure.
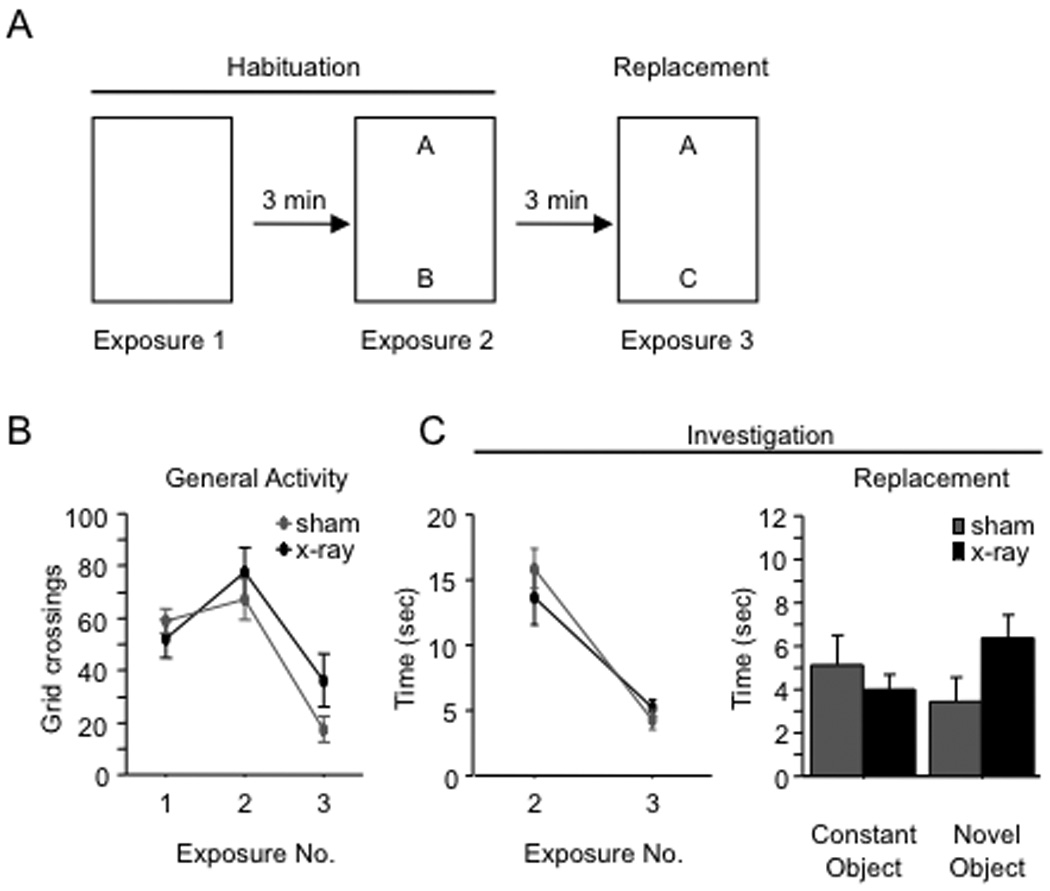
Figure 1. X-irradiation increases novel object investigation.
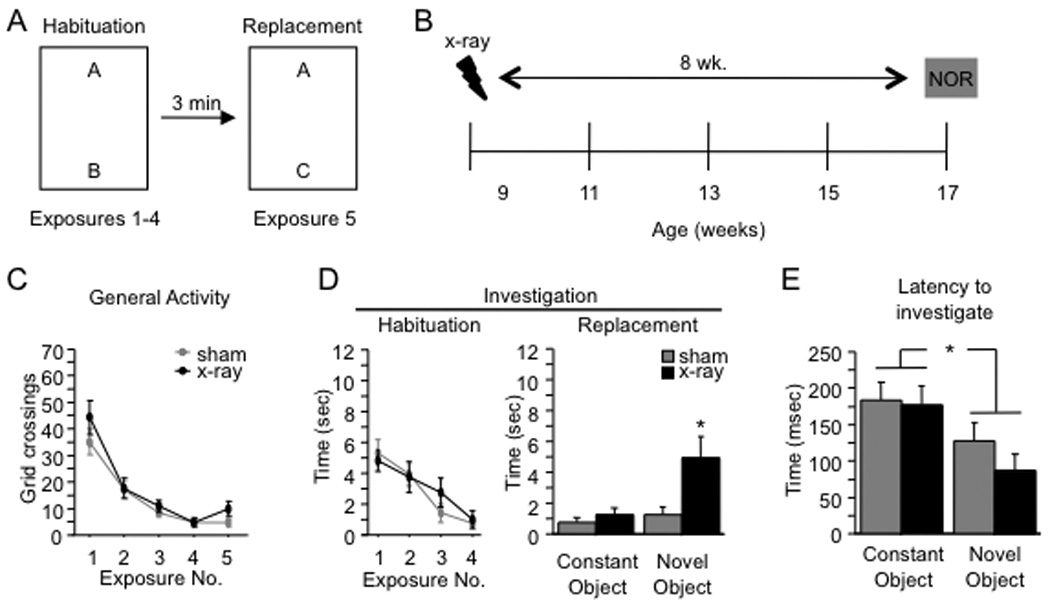
(A) Schematic diagram of the NOR paradigm. Mice received 4 exposures to two objects. For exposure 5, one of the objects was replaced with a novel object. (B) Mice were x-irradiated or sham-irradiated at 9 weeks of age and the NOR paradigm was administered 8 weeks later at 17 weeks of age. (C–D) General activity and investigation (habituation; averaged across both objects) declined across exposures 1–4 for both x-irradiated and sham mice. There was no effect of x-irradiation on either variable [F’s(1,19) < 1]. In exposure 5 (replacement), x-irradiated mice investigated the novel object more than sham mice (p = 0.02), but the groups did not differ in exploration of the constant object (p = 0.42). General activity during exposure 5 was not affected by x-irradiation (p = 0.11). (F) The latency to investigate the novel object was shorter than the latency to investigate the constant object for both groups of mice (p < 0.01), and the latencies did not differ between groups (p = 0.36). * p < 0.05. ** p < 0.01. Error bars represent ± SEM.
For the pre-exposure experiment, mice were pre-exposed to the NOR arena for a 5-min, given a 3-min inter-exposure interval, and then given a 5-min exposure of the arena plus objects. A 3-min inter-exposure interval followed and a novel object replaced a constant object in the 3rd exposure.
Behavior was scored on videos by an observer blind to treatment or to genotype using the Stopwatch+ program (Center for Behavioral Neuroscience, Atlanta, GA, USA). Each test session was scored continuously in its entirety. As a proxy for locomotor activity, a grid of 6 boxes was superimposed over the arena, and grid crossings were counted. Object investigation was defined as orientation of the head toward the object with the nose within 1 cm of the object. Investigation was not scored if the mouse was on top of the object or completely immobile.
Contextual Fear Conditioning
The procedure was based on those of Wiltgen et al. (2006) and Drew et al. (2010). The fear conditioning procedure took place over two consecutive days. On day 1, mice were placed in the conditioning chamber, received a shock 180 s later (2 s, 0.75 mA), and were removed 15 s following the shock. Mice were returned to the conditioning chamber on the following day for 240 s for a test of context-elicited freezing. (Data from the 6 wk. group only were previously published in Drew, Denny, and Hen 2010).
Variations of the 1 shock procedure were used for the GFAP-TK TG mice. GFAP-TK mice received a shock at 180 s or at 360 s (2s, 0.75 mA), and were removed 15 s following the shock. As with the previous one shock procedure, mice were returned to the conditioning chamber on the following day for 240 s for a test of context-elicited freezing.
BrdU Injections
129SvEv mice were injected with 5’-bromo-2’-deoxyuridine (BrdU) (Roche, Indianapolis, IN) (150 mg/kg) twice a day intraperitoneally (i.p.) for 2 days (300 mg/kg per d in 0.9% NaCl) at 1 day, or 1, 2, 4, or 6 weeks before the start of x-irradiation (n = 5 mice / group). Mice were sham- and x-irradiated as previously described (Santarelli et al., 2003). Mice were deeply anesthetized and brains were collected for immunohistochemistry 1 week following the last day of x-irradiation treatment.
Immunohistochemistry
Mice were deeply anesthetized with ketamine (100 mg/kg) and transcardially perfused with cold 0.1 M phosphate buffer saline (PBS), followed by cold 4% paraformaldehyde (PFA) / 0.1 M PBS. Brains were postfixed overnight in 4% PFA at 4°C, then cryoprotected in 30% sucrose / 0.1 M PBS, and stored at 4°C. Serial coronal section (35 µm) were cut through the entire hippocampus on a cryostat, and stored in 0.1 M PBS with 0.1% NaN3.
For doublecortin (DCX) immunohistochemistry, sections were washed in 0.1 M PBS and then quenched in 0.3% H2O2 in 0.1 M PBS / CH3OH (1:1) for 15 min at room temperature. Sections were then washed and blocked in 10% normal donkey serum in 0.1 M PBS with 0.5% Triton X-100 for 2 h at room temperature. Incubation with primary antibody was performed at 4°C overnight (goat anti-doublecortin, 1:500, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, #SC 8066) in 0.1 M PBS with 0.5% Triton X-100. Sections were then washed in 0.1 M PBS and incubated with a biotinylated secondary antibody (donkey anti-goat; 1:250, Jackson ImmunoResearch, West Grove, PA) for 2 h at room temperature. For all experiments, excluding the time course DCX analysis, sections were then washed in 0.1 M PBS and treated next with avidin-biotin-peroxidase complex (ABC Elite Kit, Vector Labs, Burlingame, CA) followed by a 3,3’diaminobenzidine as a substrate for staining (Vector, Burlingame, CA). For the time course analysis (Supplemental Fig. 1), sections were then washed in 0.1 M PBS and incubated with avidin-Cy3 (1:125, Jackson ImmunoResearch, West Grove, PA) and Hoechst (1:1000) for 1 h at room temperature.
For BrdU immunohistochemistry in sham and x-irradiated mice, sections were first adhered to slides. Slides were soaked in 10 mM citrate buffer (pH 6.0) for 2 h at 95°C and then rinsed in 0.1 M PBS. Incubation with primary antibody was performed at room temperature overnight (mouse anti-BrdU, 1:100, Becton Dickinson, San Jose, CA, 347580) in 0.1 M PBS with 0.1% Triton X-100. Slides were then washed in 0.1 M PBS and incubated with a biotinylated secondary antibody (goat anti-mouse; 1:200, Jackson ImmunoResearch, West Grove, PA) for 1 h at room temperature. Slides were washed in 0.1 M PBS and treated next with avidin-biotin-peroxidase complex (ABC Elite Kit, Vector Labs, Burlingame, CA) followed by a 3,3’diaminobenzidine as a substrate for staining (Vector, Burlingame, CA). Finally, slides were counterstained using Nuclear fast red.
Cell Quantification
DCX+ and BrdU+ cells were counted on Axioplan-2 upright microscope. Every sixth section throughout the entire extent of the dentate gyrus was counted. Throughout the experiment, the investigator was blind to the treatment status.
Statistical analysis
Data were analyzed using StatView 5.0 software (SAS Institute, Cary, NC). The measures of interest were number of grid crossings (general activity) and duration of object investigation. For exposures 1 through 4, we analyzed the mean duration of investigation taken across the two objects. The data for exposures 1 through 4 were subjected to an Exposure X Treatment (x-irradiated v. sham) or Exposure X Genotype (GFAP-TK transgenic (TG) v. wild-type (WT)) ANOVA, with Exposure as a repeated measure. For exposure 5, investigation times were subjected to an Object (constant v. novel) X Treatment ANOVA, with Object as a repeated measure, and grid crossing data were subjected to t-tests with Treatment as the independent variable. In the initial experiment (Figs. 1C, 1D, 1E), interactions were probed using t-tests with a Bonferroni-corrected alpha level. In subsequent experiments, planned comparisons were performed using t-tests as described below. Alpha was set to 0.05 for all analyses.
Results
Targeted hippocampal x-irradiation increases novel object investigation
Mice were tested in the novel object recognition (NOR) paradigm (Fig. 1A) 8 weeks after x-irradiation (Fig. 1B). General activity (grid crossings) (Fig. 1C) and object investigation (averaged across the two objects) (Fig. 1D) were assessed during the exposures 1–5. General activity and object investigation declined across exposures 1–4. An Exposure X Treatment (x-irradiation vs. sham) ANOVA yielded a significant effect of exposure on general activity [F(3,57) = 54.1, p < 0.01] and on investigation [F(3,57) = 18.0, p < 0.01]. There was no effect of treatment on either variable [F’s (1,19) < 1], nor was there a significant Exposure X Treatment interaction [F’s (3,57) < 1.1, p’s > 0.38], indicating that x-irradiation did not affect behavior prior to the introduction of the novel object. For exposure 5, we compared the investigation durations for the constant and the novel objects (Fig. 1D). X-irradiated mice investigated the novel object more than sham mice, but x-irradiated and sham mice investigated the constant object for similar amounts of time. The Treatment X Object ANOVA on the investigation durations yielded a significant interaction effect [F(1,19) = 4.9, p = 0.04]. Post-hoc (Bonferroni) tests confirmed that x-irradiated mice investigated the novel object more than sham mice (p = 0.02), but the groups did not differ in investigation of the constant object (p = 0.42). General activity in exposure 5 was not affected by x-irradiation [t(19) = 1.7, p = 0.11].
Sham mice investigated the novel object and constant object in approximately equal amounts, suggesting that these mice failed to detect the novel object. This result is perplexing because WT mice typically exhibit a preference for novel objects (Dodart et al., 1997; Tang et al., 1999; Şık et al., 2003). We thus asked whether another measure of performance would provide evidence that sham mice detected the novel object. We examined the latency to the first bout of investigation for both the constant and the novel object in exposure 5 (Fig. 1E). Both x-irradiated and sham mice tended to investigate the novel object earlier than the constant object, and the latency to investigate the novel object did not differ between groups. The data were subjected to a 2 (Treatment) X 2 (Object) ANOVA, which yielded a significant effect of Object [F(1,48) = 8.9, p = 0.0044] but no effect of Treatment [F(1,48) = 0.9, p = 0.36] or the interaction [F(1,48) = 0.5, p = 0.48]. We subjected the latency analysis data to planned comparisons (paired t-tests comparing the latencies to the novel and constant objects). The analysis showed that both sham and x-irradiated mice approached the novel object earlier than the constant object (p’s < 0.031, one-tailed). This result indicates that both sham and x-irradiated mice detected the novel object.
The irradiation-induced increase in novel object investigation has a delayed onset
Next, we examined the time-course with which the NOR phenotype manifests after x-irradiation. Irradiation kills dividing cells and very young neurons (Mizumatsu et al., 2003), but many immature neurons are spared after x-irradiation. These spared immature neurons are presumably depleted only gradually through maturation. Because of the dynamic nature of the ablation, we hypothesized that examination of the time-course with which the NOR phenotype manifests after x-irradiation should reveal the cell-age at which adult-generated neurons influence behavioral performance in NOR.
The NOR procedure was conducted 2, 4, and 6 weeks after x-irradiation in different groups of mice (Fig. 2A). To confirm that the irradiation-induced arrest of neurogenesis lasted for the full duration of behavioral testing, we assayed doublecortin (DCX) immunoreactivity in x-irradiated and sham mice at 2 and 8 weeks post x-irradiation (Fig. 2B, Supplemental Fig. 1). DCX immunoreactivity was absent at both time points.
Figure 2. The x-irradiation-induced increase in novel object investigation has a delayed onset.
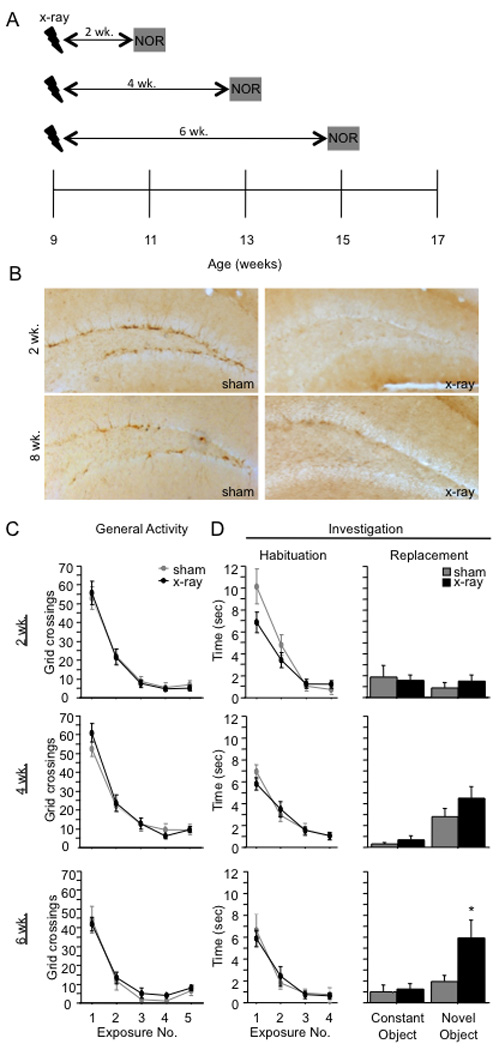
(A) Schematic diagram of the experimental time-course. (B) DCX immunoreactivity is absent 2 and 8 weeks following x-irradiation, demonstrating that neurogenesis was arrested at all points during behavioral testing. (C–D) At 2 weeks post x-irradiation, there was no effect of x-irradiation on any parameter. At 4 and 6 weeks post x-irradiation, x-irradiated and sham mice exhibited similar levels of general activity and object investigation in exposures 1–4 [F’s < 1]. At 4 weeks post x-irradiation, x-irradiated mice appeared to investigate the novel object more than sham mice during exposure 5, but the effect did not reach significance [F(1,20) = 2.95, p = 0.10]. At 6 weeks following x-irradiation, x-irradiated mice explored the novel object significantly more than sham mice during exposure 5 ([t(13) = 2.2, p = 0.047]). * p < 0.05. Error bars represent ± SEM.
At 2 weeks post x-irradiation (Fig. 2C–2D), sham and x-irradiated mice performed similarly. Although there appeared to be a modest effect of x-irradiation on investigation during exposures 1 to 4, evidenced by a significant Treatment X Exposure interaction effect [F(3,42) = 2.9, p = 0.05], post-hoc comparisons (x-irradiated v. sham) did not reach significance in any individual time bin (p’s > 0.09). In exposure 5, neither sham nor x-irradiated mice showed a preference for the novel object [F(1,14) < 1], and there was no effect of x-irradiation on investigation (p = 0.55) or on activity (p = 0.83). At 4 weeks post x-irradiation, x-irradiated and sham mice again performed similarly in exposures 1 through 4. There was no effect of x-irradiation on crossings or investigation (F’s < 1). In exposure 5, both x-irradiated and sham mice investigated the novel object more than the constant object [F(1,20) = 12.1, p < 0.01], but the preference was not significantly enhanced in x-irradiated mice; neither the effect of Treatment [F(1,20) = 2.95, p = 0.10] nor the interaction effect [F(1,20) = 1.1, p = 0.32] reached significance. At 6 weeks post x-irradiation, there was again no effect of x-irradiation during exposures 1 through 4 [F’s < 1]. However, in exposure 5, x-irradiated mice investigated the novel object more than sham mice. Planned comparisons (t-tests) confirmed that x-irradiated mice investigated the novel object more than sham mice [t(13) = 2.2, p = 0.047], but x-irradiated and sham mice did not differ in investigation of the constant object (p = 0.718).
To confirm that the onset of the NOR phenotype was related to the amount of time after x-irradiation and not to the absolute age of the mice, we x-irradiated a group of mice at 15 weeks of age and tested it in the NOR paradigm 2 weeks later (17 weeks of age) (Fig. 3A). Performance was similar to that of the group that was x-irradiated at 9 weeks of age and tested 2 weeks later. In exposures 1 through 4, there was a significant effect of Exposure on crossings [F(3,54) = 121.5, p < 0.001] and overall investigation [F(3,54) = 28.6, p < 0.001] but no effect of Treatment [F’s(1,18) < 2.4, p’s > 0.14] or of the interaction [F’s(3,54) < 1.6, p’s > 0.20] (Fig. 3B–3C). In exposure 5, both groups of mice investigated the novel object more than the constant object [F(1,22) = 24.3, p < 0.001], but there was no effect of the x-irradiation treatment [F’s(1,22) < 1] (Fig. 3C).
Figure 3. NOR performance is related to the length of time after x-irradiation rather than the absolute age of the mouse.
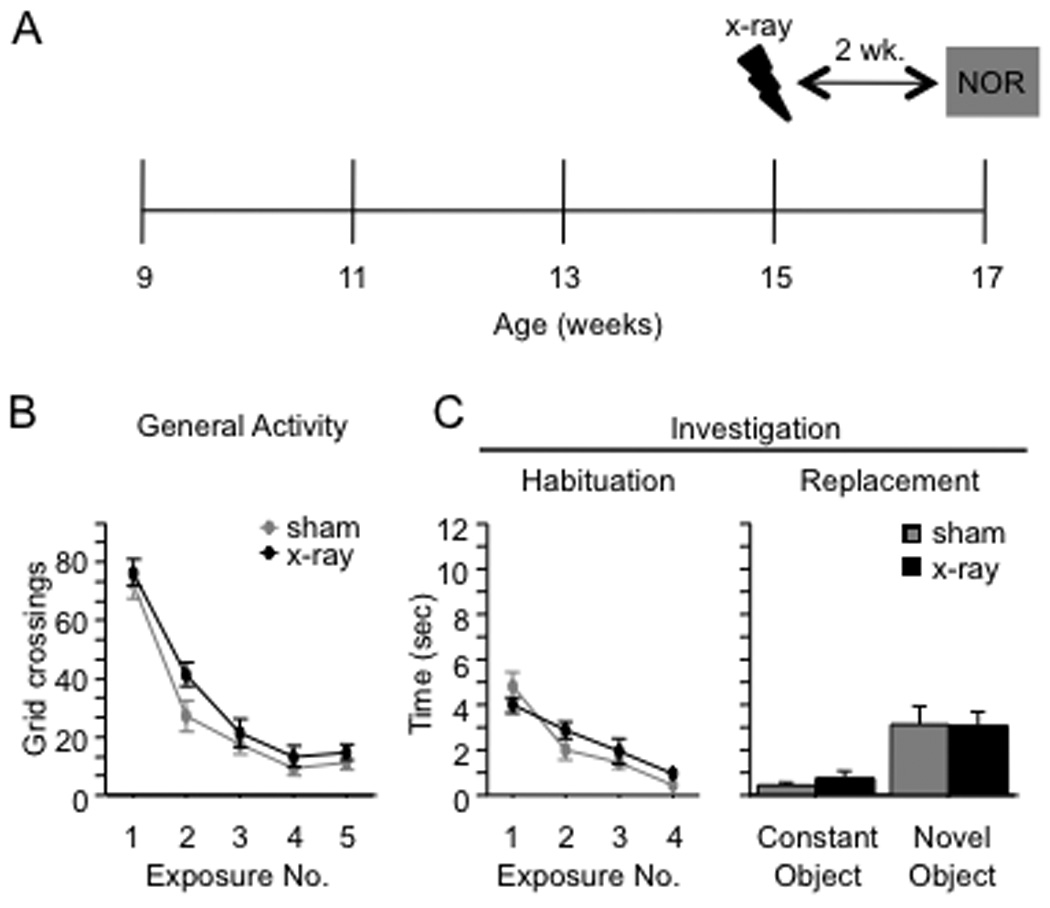
(A) Schematic diagram of the experimental time-course. (B–C) X-irradiated and sham mice did not differ in general activity or investigation (habituation) during exposures 1 through 4 [F’s(1,18) < 2.4, p’s > 0.14]. Both groups of mice investigated the novel object more than the constant object [F(1,22) = 24.3, p < 0.001], and there was no effect of the x-irradiation treatment [F’s(1,22) < 1]. Error bars represent ± SEM.
The control mice in these NOR experiments showed only a modest preference for the novel object, which may be due to the significantly weaker exploratory drive of the 129SvEv mice. Since pre-exposure appears to greatly enhance novel object preference (Stefanko et al., 2009), and, as a result, is a common feature of NOR protocols, sham and x-irradiated mice were given pre-exposure to the arena before introduction to the objects (Fig. 4A). However, when sham- and x-irradiated mice were given pre-exposure to the NOR arena, overall investigation of the objects increased, but this did not enhance novel object preference in either group of mice (Figures 4B–4C).
Figure 4. Pre-exposure to the NOR arena fails to increase novel object preference.
(A) Schematic diagram of the experimental time-course. Mice were pre-exposed to the arena before introduction of the objects. (B–C) Pre-exposure to the arena increased overall levels of object investigation in both groups of mice when a novel object was introduced; however, both groups explored the constant and novel object similarly (n = 6–8 mice/group). Error bars represent ± SEM.
In summary, although x-irradiation produces an immediate arrest of neuronal proliferation, the behavioral effect of x-irradiation in the NOR paradigm has a delayed onset. The NOR behavioral effect appears to depend on the gradual depletion of young adult-generated neurons as a function of time after x-irradiation.
Cells killed by x-irradiation are less than 4 weeks old
To more precisely identify the age of the cells that were directly ablated by hippocampal x-irradiation, mice were injected with BrdU 6, 4, 2, or 1 week(s), or 1 d before the start of x-irradiation (Fig. 5A). The mean number of BrdU+ cells was most greatly reduced in x-irradiated mice injected with BrdU 1 d prior to irradiation (75±8% reduction in x-irradiated mice when compared to sham mice; p < 0.01) (Fig. 5C). BrdU+ cell counts were reduced to a lesser extent in mice injected with BrdU 1 or 2 weeks prior to x-irradiation (47±19% and 47±7%, respectively; p’s < 0.05) (Fig. 5B), indicating that the ablation is not strictly limited to dividing cells. Finally, there was no reduction in BrdU+ cells in x-irradiated mice that were injected with BrdU at 4 weeks (p = 0.75) and 6 weeks (p = 0.81) before x-irradiation, indicating that cells 4 weeks and older are spared by x-irradiation. These results indicate that cells 1 d old and younger are the most likely to be killed by x-irradiation, but cells between 1 and 28 d old are also killed by x-irradiation, thereby limiting the precision with which the age of cells ablated by irradiation can be identified.
Figure 5. Hippocampal x-irradiation targets adult-born neurons 2-weeks old and younger.
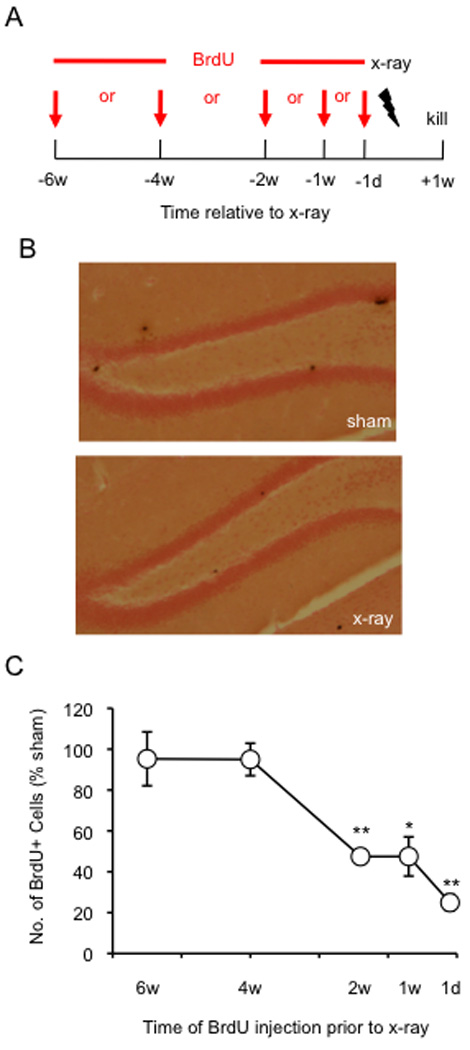
(A) Schematic diagram of the experimental BrdU injection protocol. (B) Representative images of the DG of sham and x-irradiated mice injected with BrdU at 2 weeks before x-irradiation and processed for BrdU immunoreactivity. (C) Mean number BrdU+ cells expressed as percent of sham mice. Mean number of BrdU+ cells is significantly lower in x-irradiated mice when compared with sham mice at 2 w, 1 w, and 1 d before x-irradiation. * p < 0.05, ** p < 0.01. Error bars represent ± SEM.
Aging is associated with a decline in hippocampal neurogenesis and an increase in novel object investigation
Neurogenesis rapidly declines with age in adults of numerous species, including mice and rats (Seki and Arai, 1995; Kuhn et al., 1996; Kempermann et al., 1998; Rao et al., 2005; Ben Abdallah et al., 2010). Therefore, we conducted NOR in aged mice, hypothesizing that the NOR phenotype should be present in mice in which neurogenesis is naturally reduced by aging. Mice were x-irradiated at 9 weeks of age and NOR was performed 5 months later (Fig. 6A). Interestingly, while DCX immunoreactivity is present in sham mice 6 weeks following sham-irradiation, it is absent in sham mice 5 months following sham-irradiation (Fig. 6B, Supplemental Fig. 1). DCX immunoreactivity is absent in x-irradiated mice at both time points. There was no effect of Treatment on general activity (Fig. 6C) or on object investigation (Fig. 6D) [F’s (1,18) < 1]. In exposure 5, both sham and x-irradiated mice explored the novel object more than the constant object [F(1,18) = 34.9, p < 0.0001]; neither the effect of Treatment [F(1,18) < 1, p = 0.9957] nor the interaction effect [F(1,18) < 1, p = 0.8027] reached significance. Performance in both groups was similar to that of the x-irradiated mice at 6 and 8 weeks post x-irradiation. These results are in accordance with our data suggesting that the NOR behavioral effect appears after a depletion of young adult-generated neurons. Here, the depletion occurred as a function of age rather than as a function of time after x-irradiation.
Figure 6. Aging is associated with a decline in hippocampal neurogenesis and an increase in novel object exploration.
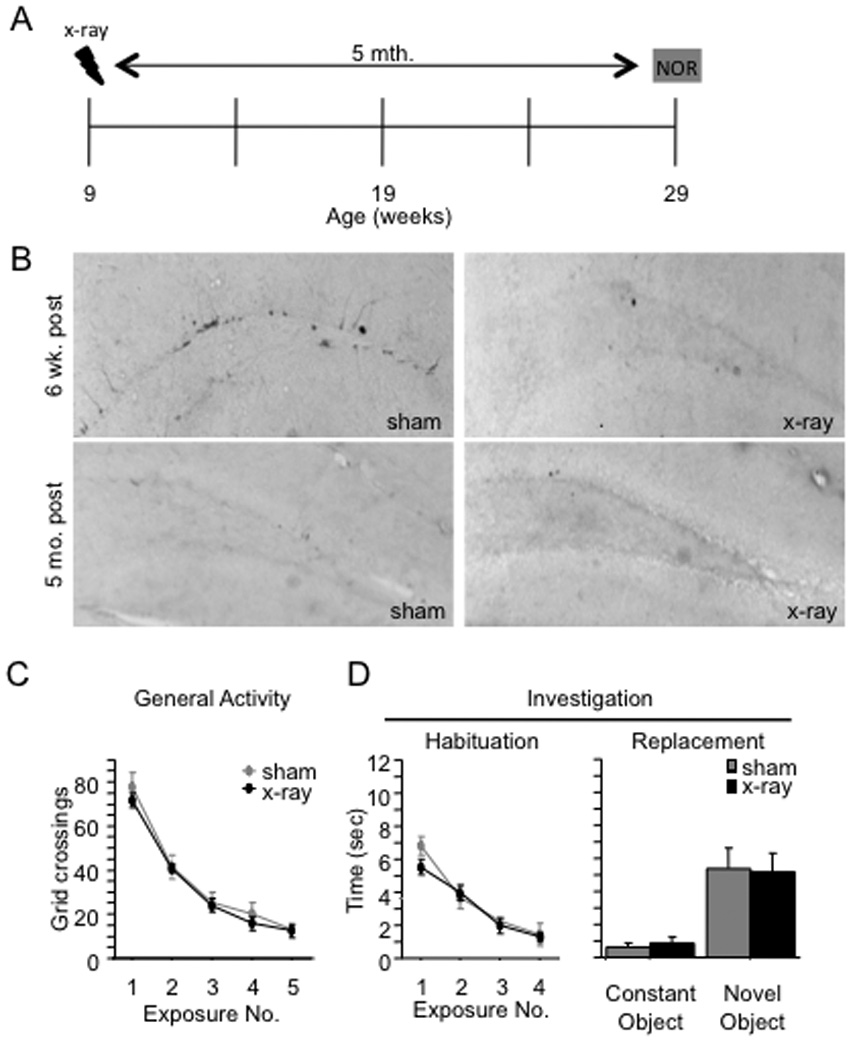
(A) Schematic diagram of the experimental time-course. (B) While DCX immunoreactivity is present in sham mice at 6 weeks post x-irradiation, DCX immunoreactivity is absent in sham mice at 5 months post x-irradiation. DCX immunoreactivity is abolished in x-irradiated mice at 6 weeks and 5 months post x-irradiation, indicating a permanent ablation of hippocampal neurogenesis. (C–D) In exposures 1 through 5, x-irradiated and sham mice exhibited similar levels of general activity [F(1,18) < 1, p = 0.596] and investigation [F(1,18) < 1, p = 0.39]. Both groups of mice investigated the novel object more than the constant object, and there was no effect of x-irradiation on investigation of either object. Error bars represent ± SEM.
Genetic arrest of adult neurogenesis increases novel object investigation
To confirm that the NOR phenotype is related to the arrest of adult neurogenesis rather than to some other effect of the x-irradiation procedure, we assessed NOR performance in GFAP-TK TG mice in which adult neurogenesis was suppressed with ganciclovir (GCV) for 4 weeks.
GCV treatment for 4 weeks (Fig. 7A) did not alter body weight in either WT or GFAP-TK TG mice (Fig. 7B). The number of DCX+ neurons was significantly reduced in GFAP-TK TG mice at both time points (p’s < 0.05) (Figs. 7C and 7D), indicating that GCV had significantly reduced neurogenesis. This observation suggests that neurogenesis was arrested in GFAP-TK TG mice.
Figure 7. GCV treatment increases novel object exploration in GFAP-TK TG mice.
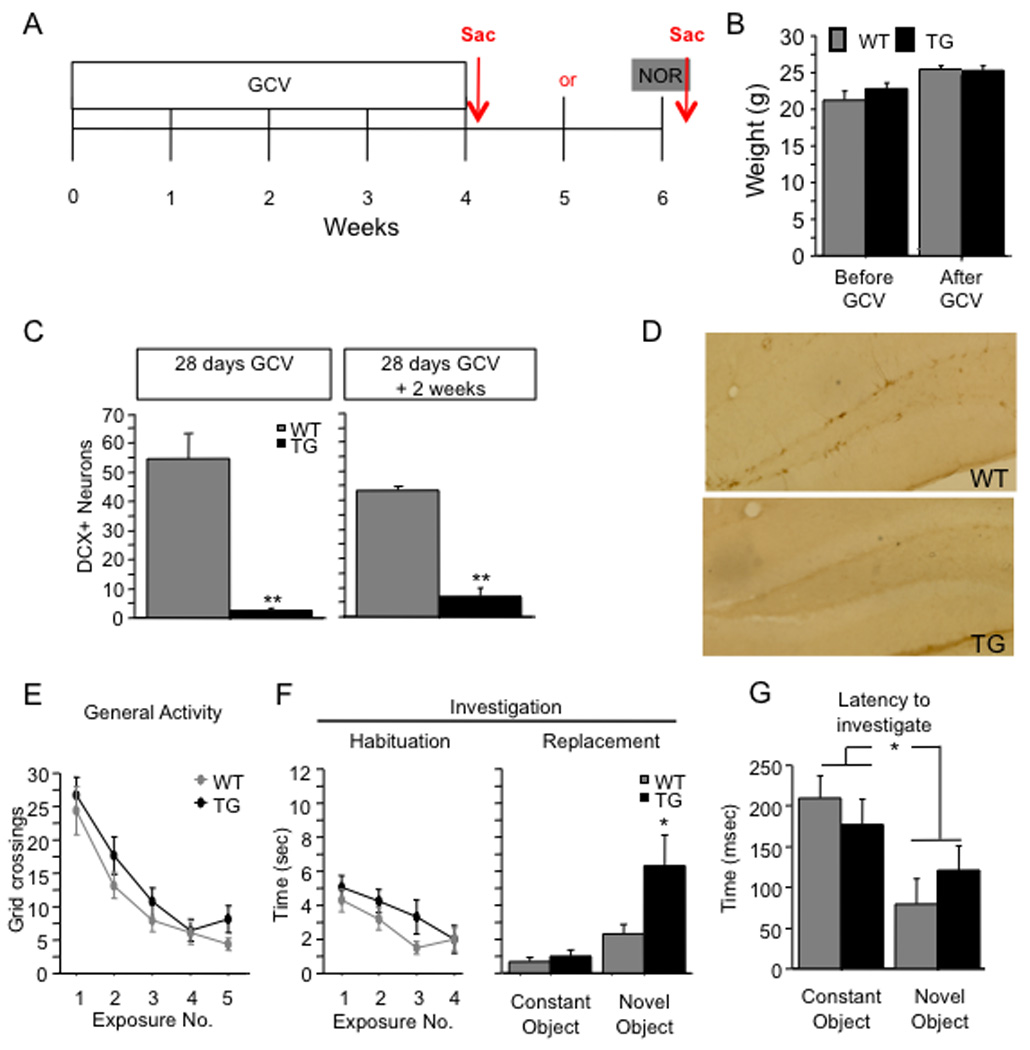
(A) Schematic diagram of the experimental time-course. Mice were treated with GCV for 28 days and then were tested in the NOR paradigm 2 weeks later. (B) GCV treatment did not induce body weight loss as indicated by comparable body weights between GFAP-TK TG and WT mice before and after GCV treatment. (C) DCX+ young neurons were significantly reduced in GFAP-TK TG mice treated with GCV when compared with WT mice, indicating that GCV had significantly reduced neurogenesis. (D) Representative images of the DG processed for DCX immunoreactivity. (E–F) During exposures 1 through 4, WT and GFAP-TK TG mice exhibited similar levels of general activity [F(1,44) = 1.4, p = 0.25] and investigation [F(1,44) = 1.3, p = 0.255]. In exposure 5, GFAP-TK TG mice explored the novel object more than WT mice [t(44) = 2.1, p = 0.041], but the groups did not differ in investigation of the constant object (p = 0.453). (G) The latency to the novel object was shorter than that to the constant object for both groups of mice (p < 0.01). There was no effect of genotype on the latency to either object (p = 0.87). * p < 0.05. ** p < 0.01. Error bars represent ± SEM.
WT and GFAP-TK TG mice were given a 4-week GCV treatment and then tested in the NOR procedure 2 weeks later (Fig. 7A). NOR performance of GFAP-TK TG mice was similar to that of x-irradiated mice. There was no effect of genotype on general activity (Fig. 7E) [F(1,44) = 1.4, p = 0.250] or on object investigation (Fig. 7F) [F(1,44) = 1.3, p = 0.255] during exposures 1–4, and the Genotype X Exposure interactions were nonsignificant [F’s(3,132) < 1.1, p’s > 0.38]. However, in exposure 5, GFAP-TK TG mice explored the novel object more than WT mice (Fig. 7F). Planned comparisons confirmed that the GFAP-TK TG mice investigated the novel object more than WT mice [t(44) = 2.1, p = 0.041], but the groups did not differ in investigation of the constant object (p = 0.453). General activity in exposure 5 did not differ significantly by genotype (p = 0.098).
Similar to the sham mice in the x-irradiation experiments, WT mice investigated the novel and constant objects in approximately equal amounts, suggesting that these mice failed to detect the novel object. Therefore, we examined the latency to the first bout of investigation for both objects in exposure 5, as we did with sham and x-irradiated mice. WT and GFAP-TK TG mice tended to investigate the novel object earlier than the constant object (Fig. 7G). The latency data were subjected to a 2 (Treatment) X 2 (Object) ANOVA, which yielded a significant effect of Object [F(1,30) = 10.2, p = 0.0033] but no effect of Treatment [F(1,30) = 0.03, p = 0.87] or of the interaction [F(1,30) = 1.6, p = 0.22]. We subjected the latency analysis data to planned comparisons (paired t-tests comparing the latencies to the novel and constant objects). The analysis showed that both WT and GFAP-TK TG mice approached the novel object earlier than the constant object (p’s < 0.045, one-tailed). These data indicate that both WT and GFAP-TK TG mice are capable of detecting the novel object.
The effect of x-irradiation on 1-trial contextual fear conditioning has a delayed onset
To assess the generality of the time-course of the NOR phenotype, we asked whether another hippocampal-dependent task would reveal a similar cell-age at which adult-generated hippocampal neurons influence learning. We used a 1-trial contextual fear conditioning (CFC) paradigm that has been previously shown by our lab to be sensitive to the arrest of adult hippocampal neurogenesis (Drew et al., 2010).
The CFC procedure was conducted 2, 4, and 6 weeks after x-irradiation in different groups of mice (Fig. 8A). At 2 and 4 weeks post x-irradiation (Fig. 8B), sham and x-irradiated mice did not differ in their levels of (post-training) context-elicited freezing [F’s(1,18) < 1]. At 6 weeks post x-irradiation, x-irradiated mice exhibited significantly less context-elicited freezing than did sham mice [F(1,32) = 7.1, p = 0.012].
Figure 8. The effect of x-irradiation on 1-trial contextual fear conditioning has a delayed onset.
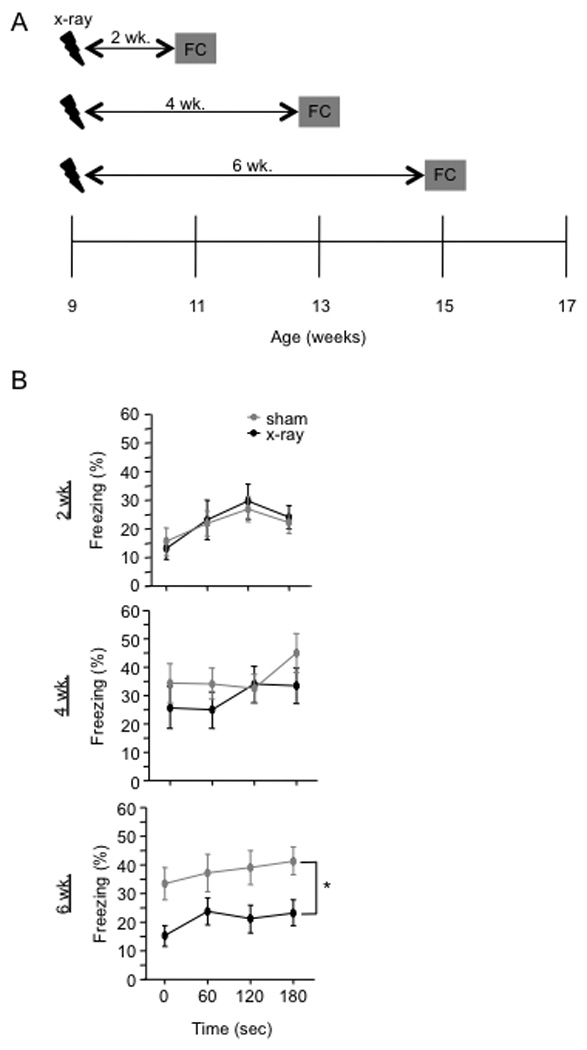
(A) Schematic diagram of the experimental time-course. Mice were fear conditioned 2, 4, or 6 weeks after x-irradiation. Contextual fear conditioning was produced by placing a mouse in the conditioning chamber and delivering one footshock 180 s later. Mice were returned to the conditioning chamber 24 h later to assess for context-elicited freezing. (B) Context-elicited freezing was significantly reduced in x-irradiated mice when compared with sham mice at 6 weeks following x-irradiation [F(1,32) = 7.1, p = 0.0119] but not at 2 or 4 weeks following x-irradiation [F’s(1,18) < 1]. * p < 0.05. Error bars represent ± SEM.
To confirm that the onset of the CFC phenotype was related to the amount of time after x-irradiation and not to the absolute age of the mice, we x-irradiated a group of mice at 13 weeks of age and tested it in the CFC paradigm 2 weeks later (15 weeks of age) (Fig. 9A). Performance was similar to that of the group that was x-irradiated at 9 weeks of age and tested 2 weeks later (Fig. 8B). Sham and x-irradiated mice did not differ in their levels of context-elicited freezing [F(1,27) < 1] (Fig. 9B).
Figure 9. 1-trial contextual fear conditioning performance is related to the length of time after x-irradiation rather than the absolute age of the mouse.
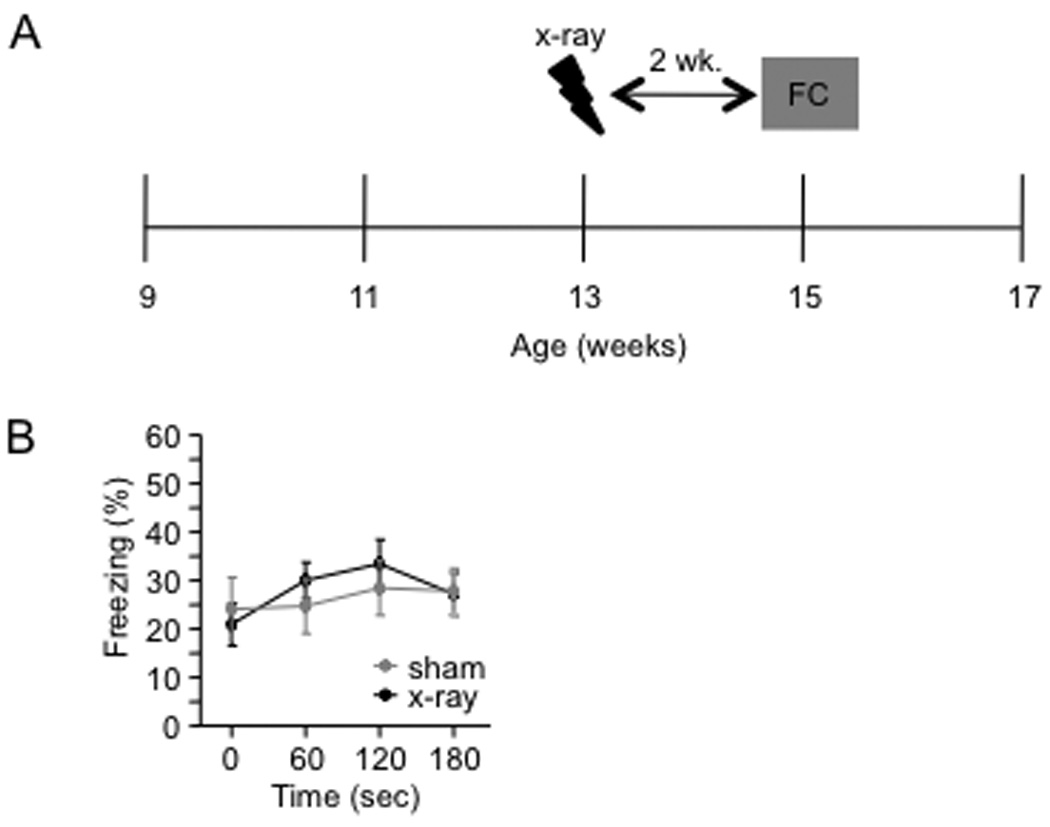
(A) Schematic diagram of the experimental time course. Mice were x-irradiated at 13 weeks of age and then fear conditioned two weeks later. (B) X-irradiated and sham mice did not differ in the test of context-elicited fear conducted 24 h following fear conditioning [F(1,27) < 1, p = 0.77]. Error bars represent ± SEM.
GCV treatment impairs 1-trial contextual fear conditioning in GFAP-TK TG mice
To confirm that the CFC phenotype is related to the arrest of adult neurogenesis rather than to some other effect of the x-irradiation procedure, we assessed CFC performance in GFAP-TK TG mice in which adult neurogenesis was suppressed with GCV for 4 weeks.
WT and GFAP-TK TG mice were given a 4-week GCV treatment and then tested in the CFC procedure 2 weeks later (Fig. 10A). Fear conditioning was produced by placing a mouse in the conditioning chamber and delivering one footshock at 180 s or 360 s later. In the test of context-elicited fear 24 h following training, GFAP-TK TG mice exhibited significantly less freezing than WT mice when the initial placement to shock interval (PSI) was 180 s [F(1,66) = 4.0, p = 0.0498] (Fig. 10B). However, GFAP-TK TG mice exhibited similar levels of freezing as WT mice when the initial PSI was 360 s [F(1,15) < 1, p = 0.8682] (Fig. 10C). These data suggest that ablation-induced deficit in single-trial CFC can be rescued by providing increased exposure during the conditioning to the context.
Figure 10. GCV treatment impairs 1-trial contextual fear conditioning in GFAP-TK TG mice.
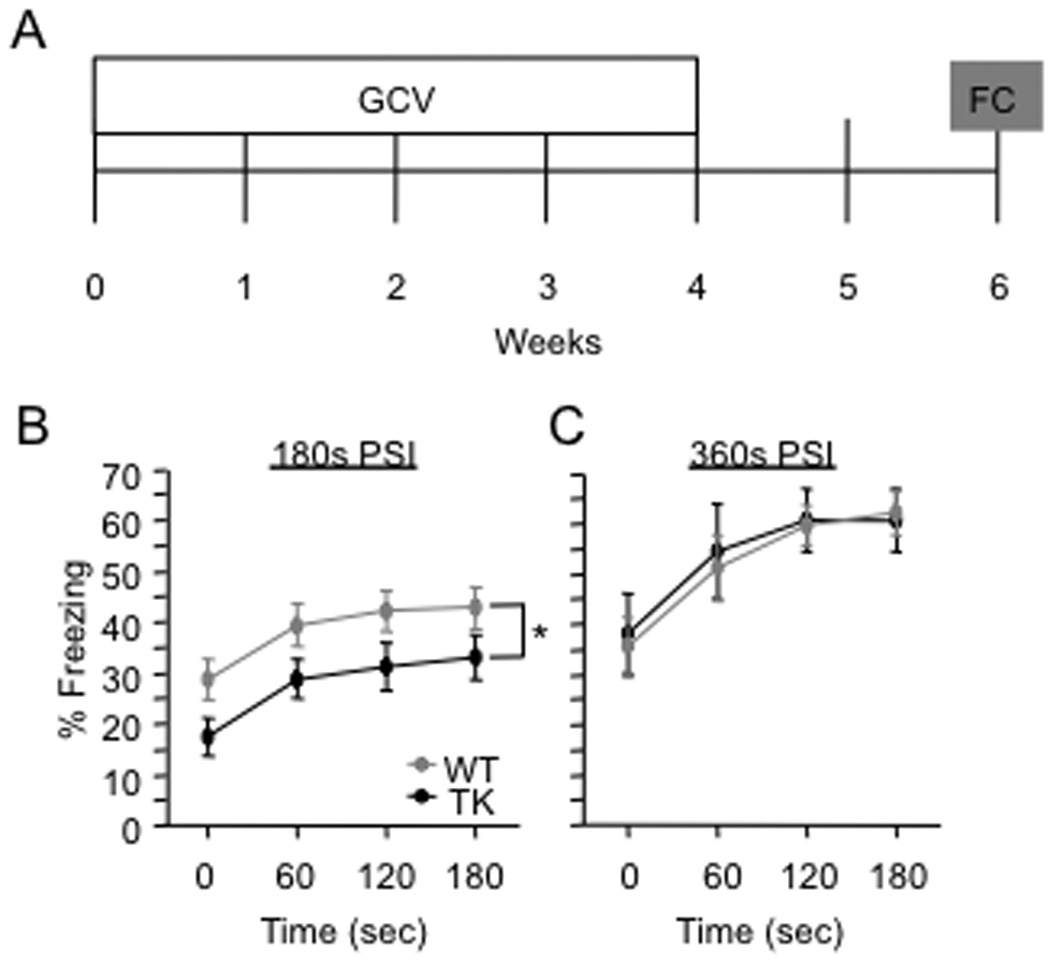
(A) Schematic diagram of the experimental time-course. GFAP-TK TG and WT mice were treated with GCV for 4 weeks and then fear conditioned 2 weeks later. Contextual fear conditioning was produced by placing a mouse in the conditioning chamber and delivering one footshock at 180 s or at 360 s later. In the test of context-elicited fear 24 h following training, GFAP-TK TG mice exhibited significantly less freezing than WT mice when the placement to shock interval (PSI) was 180 s (B) [F(1,66) = 4.0, p = 0.0498]. However, when the PSI was 360 s (C), GFAP-TK TG mice exhibited similar levels of freezing as WT mice in the test of context-elicited fear conducted 24 h following training [F(1,15) < 1, p = 0.8682]. * p < 0.05. Error bars represent ± SEM.
Discussion
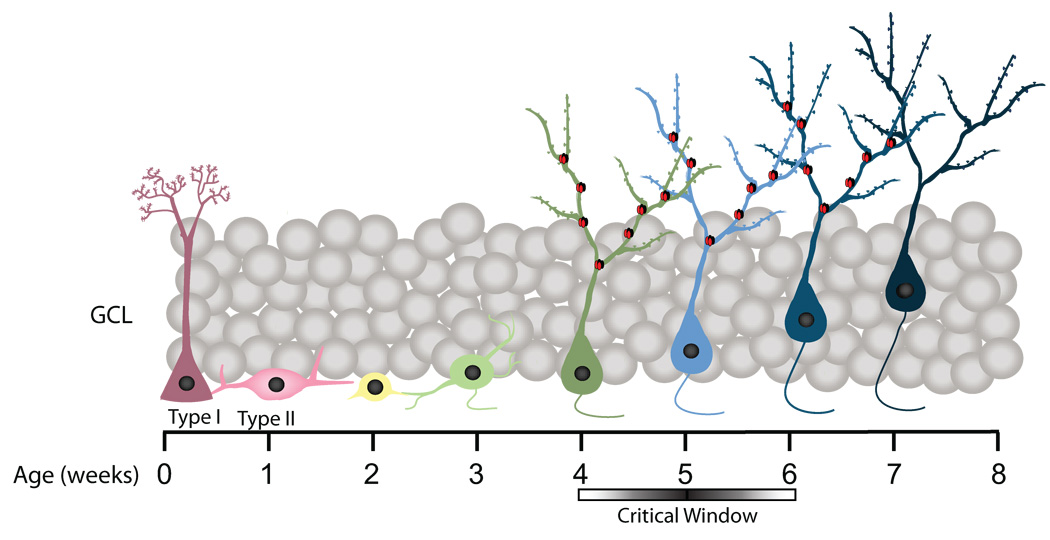
This series of experiments yielded two main results: (1) mice with arrested neurogenesis showed a surprising increase in the exploration of a novel object relative to control mice, and (2) this behavioral phenotype, as well as an impairment in contextual fear conditioning (CFC), did not manifest until 4–6 weeks after the arrest of neurogenesis. Both behavioral phenotypes were produced by two independent methods of arresting neurogenesis, suggesting that both phenotypes are due to the arrest of neurogenesis, not side effects of the manipulations. Moreover, increased novel object exploration was also observed in older mice that exhibited a marked reduction in neurogenesis relative to young mice. These data suggest the arrest of adult hippocampal neurogenesis alters novel object recognition (NOR) and CFC specifically because of the loss of 4–6 week old neurons (Fig. 11).
Figure 11. A schematic summary of neuronal differentiation in the dentate gyrus.
Cells aged 4–6 weeks old modulate NOR and CFC performance. At this time point, adult-born neurons have unique features that enhance plasticity, such as elevated expression of NR2B-containing NMDA receptors (Ge et al., 2007). NR2B-containing NMDA receptors are denoted as red and black subunits.
Immature, adult-generated granule cells have unique properties relative to mature granule cells. A recent study has found that cells between 1 and 3 weeks of age showed excitatory GABAergic responses, but that their GABA response changes from excitatory to inhibitory after about 3 weeks of age (Ge et al., 2006). However, it appears that young neurons older than 3 weeks of age are still less sensitive to GABA than their more mature counterparts (Ge et al., 2008). Several studies have identified a second developmental window between 4 and 6 weeks after mitosis during which neurons exhibit enhanced synaptic plasticity, evidenced by increased long-term potentiation (LTP) amplitude, decreased LTP induction threshold, and increased immediate-early gene expression during behavioral tasks (Ge et al., 2007; Kee et al. 2007). This is the time window during which these young neurons express NR2B-containing NMDA receptors at high levels, a characteristic thought to contribute to enhanced plasticity (Ge et al., 2007). Our results provide evidence that immature neurons in the 4–8 week old range modulate information processing and suggest, therefore, that the unique properties of immature neurons may be critical for mediating NOR and CFC performance.
Our birthdating experiment (Fig. 5) reveals some imprecision in the use of x-irradiation to estimate cell ages. Although the prepotent effect of x-irradiation is the killing of cells 1d old and younger, x-irradiation kills some cells older than 1 d. Thus, from the x-irradiation experiments alone, it is conceivable that the elimination of cells older than 6 weeks contributes to the NOR and CFC effects. However, the additional information provided by our GFAP-TK experiments argues against a role for these older cells. In GFAP-TK transgenic (TG) mice, ganciclovir (GCV) halts neuronal proliferation but does not kill quiescent cells such as neurons (Garcia et al., 2004). The presence of behavioral effects 6 weeks after the onset of GCV treatment indicates that the elimination of cells 6 weeks old or younger is sufficient to produce our behavioral effects. However, it is also possible that the behavioral delay reflects the gradual increase in the total number of missing neurons until a threshold is reached. Another possibility we cannot exclude is that compensating changes in mature granule cells contribute to the observed phenotype.
In contrast to numerous other studies, control mice in our experiments showed only a modest preference for the novel object. There are two likely explanations for this peculiar finding. Firstly, while the majority of other studies used C57Bl/6J mice, our experiments mainly used 129SvEv/Tac mice, which have a significantly weaker exploratory drive (Smith et al., 2009). However, when our NOR paradigm is administered to C57Bl/6J mice, we do not detect an impact of ablation of hippocampal neurogenesis, possibly because these mice never fully cease investigation and exploring during the habituation exposures (Supplemental Fig. 2). Therefore, in a strain exhibiting high exploration, we do not observe a further increase in NOR investigation after ablation of hippocampal neurogenesis. Secondly, in the majority of our experiments, mice were not given pre-exposure to the NOR arena. In mice, pre-exposure appears to greatly enhance novel object preference (Stefanko et al., 2009), and, as a result, this is a common feature of NOR protocols. However, when sham- and x-irradiated mice were given pre-exposure to our NOR arena, overall investigation of the objects increased, but this did not enhance novel object preference in our 129SvEv strain. Irrespective of the underlying cause, the low level of preference exhibited by our control mice was advantageous because it maximized the ability to detect an increase in the treatment groups.
The importance of the hippocampus (HPC) in object recognition processes has been debated. Many studies have focused on the importance of parahippocampal regions of the temporal lobe, namely the perirhinal cortex (PRC) (Murray et al. 2000; Gilbert and Kesner, 2003). Lesions of the PRC disrupt object recognition memory (Aggleton et al., 1997; Bussey et al., 1999; Liu and Bilkey, 2001; Warburton et al., 2003; Winters et al., 2004; Winters and Bussey, 2005), whereas hippocampal lesions sometimes impair object recognition (Murray and Mishkin, 1998; Mumby et al., 1999; Baxter and Murray, 2001; Mumby, 2001; Zola and Squire, 2001) and sometimes do not (Cave and Squire, 1991; Vnek and Rothblat, 1996; Reed and Squire, 1997; Beason-Held et al., 1999; Clark et al., 2000; Gaskin et al., 2003). It has been suggested that NOR paradigms taking place within a complex spatial environment may be more sensitive to hippocampal lesions than NOR paradigms taking place in an impoverished environment (Winters et al., 2004). In the complex environment, the HPC may generate holistic or conjunctive memory representations of the objects within their larger context, whereas in the impoverished environment, the PRC may be sufficient to represent objects independently of context. Our NOR paradigm is sensitive to hippocampal manipulations possibly because the apparatus offered visual access to the complex extra-maze environmental cues.
It is conceivable that the arrest of neurogenesis increases novel object investigation because it alters the balance between hippocampal- and PRC-mediated information processing. The impairment in CFC after x-irradiation or transgenic arrest of neurogenesis adds to the growing body of evidence that the arrest of adult hippocampal neurogenesis impairs aspects of spatial and/or contextual processing (Shors et al., 2002; Saxe et al., 2006; Drew et al., 2010). Because the HPC often competes with other memory systems for dominance (Poldrack and Packard, 2003), the impairment in hippocampal processing caused by the arrest of neurogenesis may increase reliance on extra-hippocampal processing resources, such as the PRC. Increased reliance on the PRC’s object-centered encoding may, in turn, render mice more sensitive to object novelty. Indeed, recent evidence shows that hippocampal activity can interfere with object memory in NOR. Oliveira et al. (2010) found that deactivating the HPC after NOR training improved subsequent NOR performance. Interestingly, this improvement was only present in mice that did not receive pre-exposure to the NOR environment (like our mice), perhaps because the pre-exposure reduced the amount of hippocampal context processing occurring at the time of object encoding and/or consolidation. The increased novel object investigation in our neurogenesis-arrested mice may thus stem from an impairment in hippocampal context encoding, which reduces contextual interference with object processing. The notion of decreased interference is something we already observed in a working memory paradigm where animals without neurogenesis were performing better than controls (Saxe et al., 2007).
An alternate view is that the increased novel object exploration represents an impairment in acquisition efficiency. The cessation of object investigation is presumably controlled, at least in part, by the satiation of object encoding processes. If so, then a slowing of encoding processes would increase the duration of object investigation. There is evidence that dentate gyrus (DG) manipulations can alter the rate of hippocampal encoding processes. It was recently shown that overexpression of the gene NCS-1 in the DG is sufficient to speed acquisition in the NOR paradigm (Saab et al., 2009). In rodents, the rate of acquisition of the trace eyeblink conditioning is positively correlated with the amount of hippocampal neurogenesis (Leuner et al., 2004). We have recently shown that single- but not multiple-trial CFC is impaired in neurogenesis-arrested mice, suggesting that hippocampal neurogenesis is necessary for the rapid acquisition of one-trial CFC but is not necessary for the more gradual acquisition that takes place with multiple-trial CFC (Drew et al., 2010). Furthermore, our current CFC data suggest that neurogenesis-arrested mice are impaired in single-trial CFC when the training interval is short but perform as WT mice if exposure to the training context is sufficiently long. The increased novel object investigation in neurogenesis-arrested mice may therefore reflect a slowing of memory encoding rather than an enhancement of NOR.
In summary, our studies demonstrate that the arrest of neurogenesis increases the response to novel objects and impairs CFC, and that both of these effects depend on the elimination of 4–6 week old adult-born hippocampal neurons. Adult-born hippocampal neurons of this age may participate in these behavioral processes through their engagement in context learning (thereby modulating the balance between context and object processing) or by facilitating rapid memory acquisition. We further hypothesize that the enhanced plasticity of these young neurons is critical for their contribution to these behavioral processes.
Supplementary Material
DCX immunoreactivity is absent following arrest of hippocampal neurogenesis. (A) DCX+ young neurons were significantly reduced in x-irradiated mice when compared with sham mice at 2, 6, 8 weeks, and 5 mths following x-irradiation, demonstrating that neurogenesis was arrested at all points during behavioral testing. (B) Representative images of the DG processed for DCX immunoreactivity. ** p < 0.01, *** p < 0.001. Error bars represent ± SEM.
Arrest of hippocampal neurogenesis in C57Bl/6J mice does not alter novel object investigation. (A) Schematic diagram of the experimental time-course. (B-C) X-irradiated and sham mice did not differ in general activity or investigation (habituation). Both groups of mice investigated the novel object more than the constant and there was no effect of the x-irradiation treatment (n = 5 mice / group). (D) Schematic diagram of the experimental time-course for GCV treatment. Mice were implanted with two 28 d GCV pumps and the NOR paradigm was administered following 8 wk of GCV treatment. (E) WT and GFAP-TK TG mice investigated the novel object more than the constant object, and there was no effect of the GCV treatment. Error bars represent ± SEM.
Acknowledgements
C.A.D. was supported by the Ruth L. Kirschstein Research Service Awards for Individual Predoctoral Fellows 1F31MH084529-01A1 and the National Institute of Health Training Grant 5 T32 GM008798. N.S.B. was supported by the Neurobehavioral Sciences Research Training Program 5T32MH015174-31. R.H. was supported by the National Alliance for Research on Schizophrenia and Depression (NARSAD), the New York Stem Cell Initiative, and the National Institues of Health Grant R01 MH068542. M.R.D. was supported by the National Institute of Mental Health Grant 1 K99 MH083943-01, the Charles H. Revson Foundation Senior Fellowship in Biomedical Science, and NARSAD Young Investigator Award.
We are grateful to Neena Rao, Laura Weiner, and Anil Jonathan for their assistance with the behavioral experiments. Cognitive and behavioral phenotyping experiments utilized the facilities of the Rodent Models Neurobehavioral Analysis Core of the Lieber Center for Schizophrenia Research at Columbia University and the New York State Psychiatric Institute.
R.H. receives compensation as a consultant for BrainCells, Inc. in relation to the generation of novel antidepressants.
References
- Aggleton JP, Keen S, Warburton EC, Bussey TJ. Extensive cytotoxic lesions involving both the rhinal cortices and area TE impair recognition but spare spatial alternation in the rat. Brain Res Bull. 1997;43:279–287. doi: 10.1016/s0361-9230(97)00007-5. [DOI] [PubMed] [Google Scholar]
- Gray A. The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. Oxford: Oxford University Press; 1982. p. 548. [Google Scholar]
- Baxter MG, Murray EA. Opposite relationship of hippocampal and rhinal cortex damage to delayed nonmatching-to-sample deficits in monkeys. Hippocampus. 2001:61–71. doi: 10.1002/1098-1063(2001)11:1<61::AID-HIPO1021>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9:562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Ben Abdallah NM-B, Slomianka L, Vyssotski AL, Lipp H-P. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging. 2010;31:151–161. doi: 10.1016/j.neurobiolaging.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nature Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Aggleton JP. Functionally dissociating aspects of event memory: the effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. J Neurosci. 1999;19:495–502. doi: 10.1523/JNEUROSCI.19-01-00495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave CB, Squire LR. Equivalent impairment of spatial and nonspatial memory following damage to the human hippocampus. Hippocampus. 1991;1:329–340. doi: 10.1002/hipo.450010323. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neuroscience. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A Functional Role for Adult Hippocampal Neurogenesis in Spatial Pattern Separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodart JC, Mathis C, Ungerer A. Scopolamine-induced deficits in a two-trial object recognition task in mice. Neuroreport. 1997;8:1173–1178. doi: 10.1097/00001756-199703240-00023. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Hen R. Young and excitable: the function of new neurons in the adult mammalian brain. Curr Opin Neurobiol. 2005;15:121–128. doi: 10.1016/j.conb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Drew MR, Hen R. Adult hippocampal neurogenesis as target for the treatment of depression. CNS Neurol Disord Drug Targets. 2007;6:205–218. doi: 10.2174/187152707780619353. [DOI] [PubMed] [Google Scholar]
- Drew MR, Denny CA, Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav Neurosci. 2010;124:446–454. doi: 10.1037/a0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Forwood SE, Winters BD, Bussey TJ. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus. 2005;15:347–355. doi: 10.1002/hipo.20059. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Garcia ADR, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constituteive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Gaskin S, Tremblay A, Mumby DG. Retrograde and anterograde object recognition in rats with hippocampal lesions. Hippocampus. 2003;13:962–969. doi: 10.1002/hipo.10154. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Sailor KA, Ming G, Song H. Synaptic integration and plasticity of new neurons in the adult hippocampus. J Physiol (Lond) 2008;586:3759–3765. doi: 10.1113/jphysiol.2008.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. Recognition memory for complex visual discriminations is influenced by stimulus interference in rodents with perirhinal cortex damage. Learn Mem. 2003;10:525–530. doi: 10.1101/lm.64503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker N, Hunsaker M, Kesner R. Dissociating the role of the parietal cortex and dorsal hippocampus for spatial information processing. Behavioral neuroscience. 2005;119:1307–1315. doi: 10.1037/0735-7044.119.5.1307. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Johnson WB, Ruppe MD, Rockenstein EM, Price J, Sarthy VP, Verderber LC, Mucke L. Indicator expression directed by regulatory sequences of the glial fibrillary acidic protein (GFAP) gene: in vivo comparison of distinct GFAP-lacZ transgenes. Glia. 1995;13:174–184. doi: 10.1002/glia.440130304. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nature Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Brandon EP, Gage FH. Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr Biol. 1998;27:939–942. doi: 10.1016/s0960-9822(07)00377-6. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci. 2000:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Aurousseau C, Le Moal M, Abrous DN. Behavioural trait of reactivity to novelty is related to hippocampal neurogenesis. Eur J Neurosci. 1999;11:4006–4014. doi: 10.1046/j.1460-9568.1999.00833.x. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo P, Alonso M, Grubb M. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc. Natl. Acad. Sci. USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Bilkey DK. The effect of excitotoxic lesions centered on the hippocampus or perirhinal cortex in object recognition and spatial memory tasks. Behav Neurosci. 2001;115:94–111. doi: 10.1037/0735-7044.115.1.94. [DOI] [PubMed] [Google Scholar]
- McTighe SM, Mar AC, Romberg C, Bussey TJ, Saksida LM. A new touchscreen test of pattern separation: effect of hippocampal lesions. Neuroreport. 2009;20:881–885. doi: 10.1097/WNR.0b013e32832c5eb2. [DOI] [PubMed] [Google Scholar]
- Ming G-L, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- Moser EI. Altered inhibition of dentate granule cells during spatial learning in an exploration task. J Neurosci. 1996;16:1247–1259. doi: 10.1523/JNEUROSCI.16-03-01247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG. Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behav Brain Res. 2001:159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Astur RS, Weisend MP, Sutherland RJ. Retrograde amnesia and selective damage to the hippocampal formation: memory for places and object discriminations. Behav Brain Res. 1999:97–107. doi: 10.1016/s0166-4328(99)00097-2. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J Neurosci. 1998:6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Hampton RR, Saksida LM. The parahippocampal region and object identification. Annals of the New York Academy of Sciences. 2000;911:166–174. doi: 10.1111/j.1749-6632.2000.tb06725.x. [DOI] [PubMed] [Google Scholar]
- Nitz D, McNaughton B. Differential modulation of CA1 and dentate gyrus interneurons during exploration of novel environments. J Neurophysiol. 2004;91:863–872. doi: 10.1152/jn.00614.2003. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Oxford University Press; 1978. p. 570. [Google Scholar]
- Oliveira AMM, Hawk JD, Abel T, Havekes R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learning & Memory. 2010:155–160. doi: 10.1101/lm.1625310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21:464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Impaired recognition memory in patients with lesions limited to the hippocampal formation. Behav Neurosci. 1997;111:667–675. doi: 10.1037//0735-7044.111.4.667. [DOI] [PubMed] [Google Scholar]
- Saab BJ, Georgiou J, Nath A, Lee FJS, Wang M, Michalon A, Liu F, Mansuy IM, Roder JC. NCS-1 in the Dentate Gyrus Promotes Exploration, Synaptic Plasticity, and Rapid Acquisition of Spatial Memory. Neuron. 2009;63:643–656. doi: 10.1016/j.neuron.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang J-W, Malleret G, David DJ, Monckton JE, Garcia ADR, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew M. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MW, Kandel ER, Hen R. Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci USA. 2007;104:4642–4646. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6:2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şik A, van Nieuwehuyzen P, Pickaerts J, Blokland A. Performance of different mouse strains in an object recognition task. Behav Brain Res. 2003;147:49–54. doi: 10.1016/s0166-4328(03)00117-7. [DOI] [PubMed] [Google Scholar]
- Smith DR, Burruss DR, Johnson AW. An assessment of olfaction and responses to novelty in three strains of mice. Behav Brain Res. 2009;201:22–28. doi: 10.1016/j.bbr.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Sokolov EN. Higher nervous functions; the orienting reflex. Annu Rev Physiol. 1963:545–580. doi: 10.1146/annurev.ph.25.030163.002553. [DOI] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci USA. 2009;106:9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Corkin S, González RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y-P, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Kapur S, Habib R, Houle S. Novelty encoding networks in the human brain: positron emission tomography data. Neuroreport. 1994;5:2525–2528. doi: 10.1097/00001756-199412000-00030. [DOI] [PubMed] [Google Scholar]
- Vnek N, Rothblat LA. The hippocampus and long-term object memory in the rat. J Neurosci. 1996;16:2780–2787. doi: 10.1523/JNEUROSCI.16-08-02780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J-W, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, Koder T, Cho K, Massey PV, Duguid G, Barker GRI, Aggleton JP, Bashir ZI, Brown MW. Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron. 2003;38:987–996. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- Winters B, Forwood S, Cowell R, Saksida L, Bussey T. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci. 2004;24:5901. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J Neurosci. 2005;25:52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola SM, Squire LR. Relationship between magnitude of damage to the hippocampus and impaired recognition memory in monkeys. Hippocampus. 2001;11:92–98. doi: 10.1002/hipo.1027. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DCX immunoreactivity is absent following arrest of hippocampal neurogenesis. (A) DCX+ young neurons were significantly reduced in x-irradiated mice when compared with sham mice at 2, 6, 8 weeks, and 5 mths following x-irradiation, demonstrating that neurogenesis was arrested at all points during behavioral testing. (B) Representative images of the DG processed for DCX immunoreactivity. ** p < 0.01, *** p < 0.001. Error bars represent ± SEM.
Arrest of hippocampal neurogenesis in C57Bl/6J mice does not alter novel object investigation. (A) Schematic diagram of the experimental time-course. (B-C) X-irradiated and sham mice did not differ in general activity or investigation (habituation). Both groups of mice investigated the novel object more than the constant and there was no effect of the x-irradiation treatment (n = 5 mice / group). (D) Schematic diagram of the experimental time-course for GCV treatment. Mice were implanted with two 28 d GCV pumps and the NOR paradigm was administered following 8 wk of GCV treatment. (E) WT and GFAP-TK TG mice investigated the novel object more than the constant object, and there was no effect of the GCV treatment. Error bars represent ± SEM.