Abstract
Objectives
Examine one year outcomes of patients with small coronary arteries in the National Heart, Lung and Blood Institute Dynamic Registry (NHLBI) undergoing drug-eluting stent (DES) vs. bare-metal stent (BMS) placement.
Background
While randomized trials of DES vs. BMS demonstrate reduced target vessel revascularization, it is unclear if similar outcomes are seen in unselected patients after percutaneous coronary intervention (PCI) for small coronary arteries.
Methods
Utilizing patients from the NHLBI Registry Waves 1–3 for BMS (1997–2002) and Waves 4–5 for DES (2004 and 2006), demographic, angiographic, in-hospital and one-year outcome data of patients with small coronary arteries treated with BMS (n= 686) vs. DES (n= 669) were evaluated. Small coronary artery was defined as 2.50 – 3.00 mm in diameter.
Results
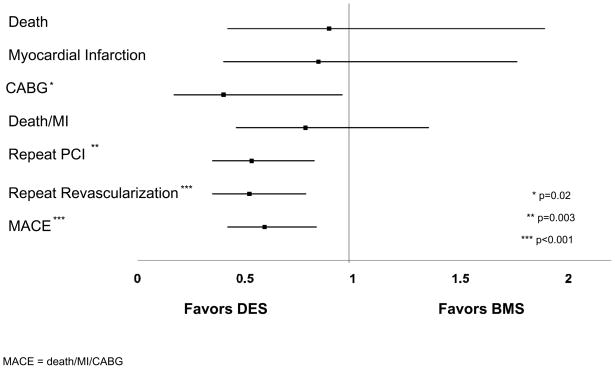
Compared to BMS-treated patients, the mean lesion length of treated lesions was longer in the DES treated group (16.7 vs. 13.1 mm, p<0.001) and the mean reference vessel size of attempted lesions was smaller (2.6 vs. 2.7 mm, p<0.001). Adjusted analyses of one year outcomes revealed that DES patients were at lower risk to undergo coronary artery bypass graft surgery (Hazard Ratio [HR] 0.40, 95% Confidence Interval [CI] 0.17–0.95, p=0.04), repeat PCI (HR 0.53, 95% CI 0.35–0.82, p=0.004), and experience the combined major adverse cardiovascular event rate (HR 0.59, 95% CI 0.42–0.83, p=0.002). There was no difference in the risk of death and myocardial infarction (MI) (HR 0.78, 95% CI 0.46–1.35, p=0.38).
Conclusions
In this real-world registry, patients with small coronary arteries treated with DES had significantly lower rates of repeat revascularization and major adverse cardiovascular events at one year compared to patients treated with BMS, with no increase in the risk of death and MI. These data confirm the efficacy and safety of DES over BMS in the treatment of small coronary arteries in routine clinical practice.
Index Words: Coronary Disease, Stents, Restenosis
INTRODUCTION
In large diameter coronary arteries, the advantages of drug-eluting stents (DES) in comparison to bare-metal stents (BMS) in reducing restenosis and decreasing rates of repeat revascularization are well known [1–2]. However, it is estimated that up to 50% of all coronary interventions are performed in coronary arteries with a reference vessel diameter less than 3.0 mm. [3]. Although randomized controlled trials have found lower rates of target lesion revascularization with DES versus BMS in patients with small coronary arteries [4–5], it is unclear if similar outcomes are seen in unselected patients after percutaneous coronary intervention (PCI) for small coronary arteries. Thus, utilizing the National Heart, Lung, and Blood Institute (NHLBI) Dynamic Registry, our primary endpoint was one year major adverse cardiac events (MACE, a combination of death, myocardial infarction (MI), and repeat revascularization) in patients with small coronary arteries treated with DES compared to BMS. The secondary endpoints of the study were the individual components of MACE.
MATERIALS AND METHODS
Design and study population
The specific methodologies and characteristics of the NHLBI Dynamic Registry have been reported previously [6]. In brief, data were collected on approximately 2,000 consecutive patients undergoing PCI during five recruitment ‘waves’ across 27 clinical centers (Wave 1: July 1997-February 1998, n=2524; Wave 2: February-June 1999, n=2105; Wave 3: October 2001-March 2002, n=2047; Wave 4: February-May 2004, n=2112; Wave 5: February-August 2006, n=2178). Patients from the BMS era were evaluated using waves 1–3 and the DES era using waves 4–5 (Table I). One year outcomes were available for patients in both the BMS and DES eras and two year outcomes were available for patients treated with DES. Patients were contacted via telephone interview at one and two years by trained nurse coordinators to assess vital status, symptoms, coronary events or cardiac-related hospitalizations. Informed consent was obtained for all patients and the study protocol was approved by Institutional Review Boards at the respective clinical sites and at the University of Pittsburgh data coordinating center.
Table I.
Enrollment Waves
| Wave 1 | Wave 2 | Wave 3 | Wave 4 | Wave 5 | |
|---|---|---|---|---|---|
| Dates enrolled | 7/97–2/98 | 2/99–6/99 | 10/01–3/02 | 2/04–5/04 | 2/06–8/06 |
| N enrolled | 2524 | 2105 | 2047 | 2112 | 2177 |
| N stented | 1696 | 1664 | 1761 | 1952 | 2062 |
| Small artery | 264 | 332 | 414 | 481 (98 BMS and 383 DES) | 474 (36 BMS and 438 DES) |
| Large artery | 1242 | 1069 | 1012 | 1164 | 1236 |
| Both small and large | 66 | 83 | 117 | 150 | 196 |
| Missing* | 124 | 180 | 218 | 157 | 156 |
Reasons why some patients are not categorized as having a small or large lesion treated include: missing artery size, diameter reference size smaller than 2.5 and not having all treated lesions stented
Definitions
Coronary artery diameter was determined by visual estimation by the operator. Small coronary arteries were defined as arteries of 2.50 –3.00 mm in diameter given restrictions in DES size availability (i.e. drug eluting stents were not available in sizes smaller than 2.50 mm diameter at the time of study enrollment). Patients receiving both DES and BMS stents were excluded. All treated lesions in the included patients had to have received at least one stent (i.e. patients where one lesion was stented and one was not were excluded). Patients presenting in cardiogenic shock (n= 9) were excluded, as well as patients undergoing PCI for restenosis (n=116). Death was defined as all cause mortality. Myocardial infarction for waves 1 and 2 was defined as evidence of two or more of the following: (1) typical chest pain > 20 minutes duration not relieved by nitroglycerin, (2) serial electrocardiogram recordings showing changes from baseline or serially in ST-T and/or Q-waves in ≥ 2 contiguous leads, (3) serum enzyme elevation of creatinine kinase-myocardial band (CK-MB) > 5% (total creatinine kinase (CK) >2X normal, lactate dehydrogenase (LDH) subtype 1 > LDH subtype 2, or troponin > 0.2 μg/ml), or (4) new wall motion abnormalities. For waves 3–5, an MI had to satisfy at least one of the 2 following criteria: (1) evolutionary ST-segment elevation, development of new Q-waves in 2 or more contiguous electrocardiogram leads, or new or presumably new left bundle branch pattern on the electrocardiogram, (2) biochemical evidence of myocardial necrosis manifested as a) CK-MB ≥ 3 times the upper limit of normal, b) total CK ≥ 3 times the upper limit of normal (if CK-MB not available), or troponin level above the upper limit of normal. Angiographic success was defined as an absolute 20% reduction in lesion severity and a final diameter stenosis of < 50%. Procedural success was defined as angiographic success without death, Q-wave MI, or emergency coronary artery bypass graft surgery (CABG). Major adverse event rate was defined as the combined endpoint of death, MI, and repeat revascularization. Stent thrombosis was defined as definite as per the Academic Research Consortium [7].
Statistical Analysis
Patients were stratified by stent type and descriptive statistics were summarized as means for continuous variables and percentages for categorical variables. Statistical comparisons by stent type for categorical data were made using either the chi-square test or Fisher’s exact test and via the Wilcoxon rank-sum for continuous data. One year event rates were calculated using the Kaplan-Meier method and survival curves were performed using the log-rank test. Patients who did not experience the outcome of interest were censored at the last known date of contact at one year if contact extended beyond the specific analysis end-point. The independent association between stent type and one-year adverse outcomes was examined using Cox proportional hazards methods. A propensity score approach was used to balance factors associated with the nonrandom assignment of treatment time (earlier recruitment wave versus later recruitment wave). The estimated propensity score for treatment with a DES once these devices were available was obtained from the fit of a logistic regression model for which the following demographic, angiographic, and procedural characteristics were considered: age, sex, race, body mass index, prior PCI, prior CABG, prior MI, diabetes mellitus, congestive heart failure, hypertension, dyslipidemia, presence of luminal irregularities, reason for revascularization, thrombolytic therapy during procedure, evidence of thrombus, total occlusion, lesions supplying collaterals, torturous lesions, class-C lesions, number of significant lesions, number of treated lesions, indication for procedure (elective versus urgent/emergent procedures), chronic kidney disease, pulmonary disease, lesion location (left circumflex versus right coronary artery/left anterior descending artery vessel), and procedural clopidogrel use. Given the change in prescribing patterns from the BMS to the DES era, all outcomes were also adjusted for pre-specified medications considered to be standard medical therapy (cholesterol modifying agents, beta blockers, angiotensin converting enzyme inhibitors or angiotensin receptor blockers, aspirin, and thienopyridines). Baseline demographic, angiographic, and procedural variables were screened for inclusion with a cut-off of <0.40. The identified variables were then placed in a model with stent type and those that were <0.30 were maintained in the final propensity score. Assumptions of proportionality were assessed and met for all models. All statistical analyses were performed with the use of SAS software, version 9.2, and a two-sided p-value of 0.05 or less was considered to indicate statistical significance.
RESULTS
Seven hundred and sixty five BMS patients and 775 DES patients undergoing small coronary artery stenting in the Dynamic Registry were analyzed. Of those receiving DES, 65.8% (n=509) were sirolimus-eluting, 34.7% (n=269) were paclitaxel-eluting, and 1.9% (n=14) were zotarolimus-eluting stents. Patients undergoing small coronary artery PCI with DES were more likely to be male, hypertensive, and diabetic, and were more likely to have a history of prior PCI than patients undergoing small coronary artery PCI with BMS (Table II). Patients undergoing small coronary artery stenting with DES presented less frequently with unstable angina; however, similar number of patients in both groups underwent PCI for stable angina and acute MI (Table III). Patients treated with DES had a greater number of significant lesions than BMS patients. In the DES patients, the mean reference vessel size of treated lesions was significantly smaller, though not clinically meaningful, and the mean lesion length and mean stent length of attempted lesions was longer. DES subjects were less likely to have evidence of thrombus at the lesion site. There was no difference in the American College of Cardiology/American Heart Association lesion class, however DES patients were more likely to have their lesion located in the circumflex artery whereas BMS patients the right coronary artery.
Table II.
Patient Demographics for Small Diameter Coronary Stenting with BMS vs. DES
| Variable | BMS (N=765) | DES (N=775) | P Value |
|---|---|---|---|
| Mean age (years) | 63.9 | 63.3 | 0.32 |
| Female | 46.6% | 35.6% | <0.001 |
| Mean Body Mass Index (kg/m2) | 28.7 | 29.3 | 0.06 |
| Prior percutaneous coronary intervention | 22.7% | 39.0 % | 0.001 |
| Prior coronary artery bypass graft | 13.6% | 18.2% | 0.04 |
| Prior myocardial infarction | 31.1% | 22.7% | 0.0005 |
| Diabetes mellitus | 30.2% | 37.8% | 0.003 |
| Insulin treated diabetes mellitus | 9.2% | 13.0% | 0.03 |
| Hypertension* | 69.3% | 78.0% | 0.003 |
| Heart failure | 10.2% | 7.1% | 0.04 |
| Hypercholesterolemia** | 68.7% | 78.1% | 0.0002 |
| Severe non-cardiac concomitant disease | 34.6% | 38.2% | 0.18 |
| Cerebrovascular disease | 7.0% | 9.0% | 0.18 |
| Pulmonary disease | 9.1% | 7.3% | 0.25 |
| Renal disease | 5.3% | 8.4% | 0.02 |
| Peripheral vascular disease | 9.2% | 7.9% | 0.40 |
| Current Smoker | 27.6% | 22.0% | 0.04 |
Hypercholesterolemia = repeated values for serum cholesterol greater than 240mg/100ml or if a physician has medically treated the participant for high cholesterol
Hypertension = blood pressure ≥ 140 systolic or ≥ 90 diastolic on two occasions or if the patient is currently on antihypertensive medications
Table III.
Angiographic and Procedural Characteristics for Small Diameter Coronary Artery Stenting with BMS vs. DES
| BMS (N=765) | DES (N=775) | P Value | |
|---|---|---|---|
|
Patient Level
| |||
| Revascularization Reason | |||
| Stable Angina Pectoris | 21.3% | 21.5% | 0.92 |
| Unstable Angina Pectoris | 45.0% | 35.0% | 0.0002 |
| Myocardial Infarction | 22.3% | 25.1% | 0.23 |
| Thrombolytic therapy | 4.2% | 1.8% | 0.009 |
| Circumstances of Procedure | 0.10 | ||
| Elective | 53.4% | 59..0% | |
| Urgent | 37.5% | 32.9% | |
| Emergent | 9.2% | 8.1% | |
| Mean left ventricular ejection fraction | 53.8% | 53.5% | 0.58 |
| Mean Significant lesions | 2.7 | 3.0 | 0.002 |
| Any total occlusion | 30.9% | 36.9% | 0.02 |
| Medications used <24hrs, prior to, or during procedure | |||
| Thienopyridine | 54.8% | 87.4% | <0.001 |
| IIb/IIIa Receptor Antagonists | 41.4% | 30.0% | < 0.001 |
| Mean number of lesions attempted | 1.1 | 1.2 | 0.04 |
| Vessel Disease | 0.02 | ||
| 1 | 42.8% | 37.8% | |
| 2 | 32.9% | 30.5% | |
| 3 | 24.1% | 31.4% | |
| Angiographic Success | 99.6% | 99.6% | 0.97 |
|
| |||
| BMS (N=765) | DES (N=775) | P Value | |
|
| |||
| Lesion Level | |||
| Mean reference vessel size (mm) | 2.7 | 2.6 | <0.0001 |
| Mean lesion length (mm) | 13.1 | 16.7 | <0.0001 |
| Mean stent length (mm) | 19.6 | 23.5 | <0.001 |
| Lesion location | 0.046 | ||
| Left main | 0.3% | 0.3% | |
| Left anterior descending | 41.5% | 40.1% | |
| Left circumflex | 24.5% | 30.8% | |
| Right coronary | 29.2% | 25.8% | |
| Graft | 4.5% | 3.0% | |
| Total occlusion | 10.7% | 10.7% | 0.99 |
| Mean diameter % stenosis | 83.4 | 84.5 | 0.83 |
| Evidence of thrombus | 16.5% | 10.5% | 0.001 |
| Calcified | 24.5% | 28.3% | 0.10 |
| Bifurcation | 12.5% | 10.2% | 0.17 |
| Ostial lesion | 4.7% | 65.9% | 0.29 |
| Lesion tortuosity | 0.08 | ||
| None/Mild | 77.0% | 73.1% | |
| Moderate/Severe | 23.0% | 26.9% | |
| American College of Cardiology/Association Heart Association Lesion Class | 0.054 | ||
| B1 | 33.7% | 34.2% | |
| B2 | 35.5% | 31.5% | |
| C | 16.6% | 21.6% | |
| Overall Stent Use | 100% | 100% | |
| Rotational Atherectomy | 2.9% | 0.9% | 0.004 |
Patients treated with DES were more likely to be discharged home on angiotensin converting enzyme inhibitors (49.9% vs. 37.6%, p<0.001), aspirin (98.1% vs. 93.9%, p<0.001), beta blockers (72.9% vs. 81.0%, p=0.004), statins (82.9% vs. 58.9%, p<0.001), and thienopyridines (94.2% vs. 98.7%, p<0.001) than patients treated with BMS. While the peri-procedural complications of major dissection (1.9% vs. 6.0%, p<0.001), embolization (0.0% vs. 0.8%, p=0.01), and abrupt vessel closure in lab (0.1% vs. 1.0%, p=0.02) were less common in treated lesions of DES patients compared to those of patients receiving BMS, there was no difference in the rate of persistent flow reduction (0.4% vs. 0.4%, p=0.99). Additionally, there was no difference in angiographic success rates (99.0% vs. 98.4%, p=0.39) between the groups. In-hospital complications were higher in BMS treated patients, including MI (1.6% vs. 0.3%, p=0.01), CABG (0.7% vs. 0.0%, p=0.03), and the combination of death/MI (1.9% vs. 0.4%, p=0.01). There were no differences in the rates of stroke (0.1% vs. 0.6%, p=0.17), entry site bleeding requiring transfusion (1.3% vs. 0.4%, p=0.09), or death (0.3% vs. 0.1%, p=0.58).
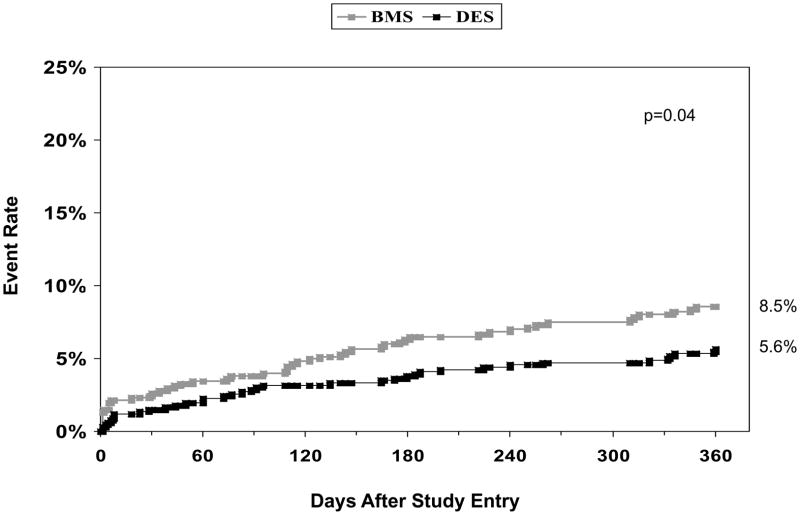
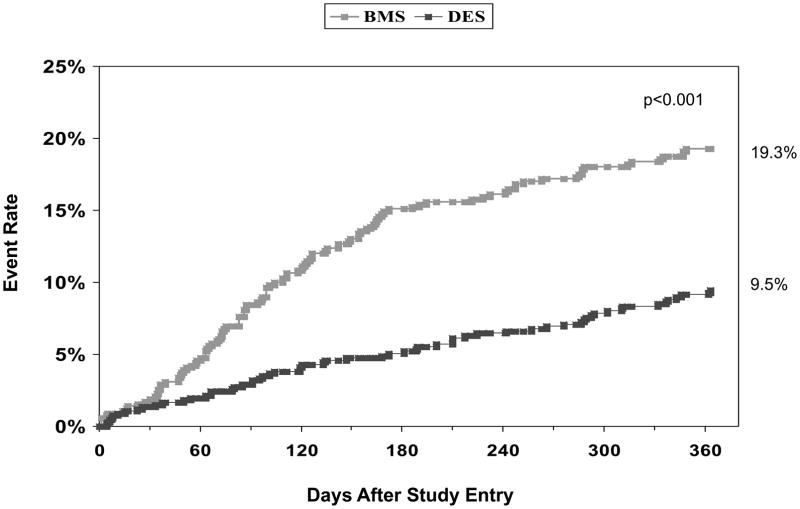
Unadjusted cumulative one year event rates are shown in Table IV including target and non-target vessel revascularization for BMS and DES treated patients (10.7% vs. 4.4%, p<0.001 and 4.5% vs. 2.7%, p=0.08) respectively. The clinical indication for target vessel revascularization in BMS patients was unstable angina (64.5%), stable angina (16.1%) and MI (9.7%). In patients who received DES and required target vessel revascularization, the clinical indications were unstable angina (53.6%), MI (21.4%), and stable angina (14.3%). The Kaplan Meier curves for the combined endpoint of death and MI and repeat revascularization are shown in Figures 1 and 2. Adjusted propensity analyses of one year outcomes did not reveal any difference in the risks of death, MI, or the combined endpoint of death/MI in patients receiving DES versus BMS. Additionally, adjusted propensity analyses including only patients that were discharged alive demonstrated no significant difference in the risk of death post discharge (Hazard Ratio [HR] 0.76, 95% Confidence Interval [CI] 0.36–1.64, p=0.49) or in the combined endpoint of death/MI post discharge (HR 0.73, 95% CI 0.41–1.31, p=0.29). Patients treated with DES were significantly less likely to undergo CABG, repeat PCI in both the target and non-target vessel, and repeat revascularization (Figure 3). This resulted in a significant reduction in the combined MACE rate in patients undergoing PCI with DES versus BMS. Cumulative one and two year outcomes for patients treated either with PES (n=269) versus SES (n=509) were evaluated and showed no significant differences in any one or two year safety or efficacy outcomes including definite stent thrombosis for patients treated with either SES only or PES only (data not shown).
Table IV.
Unadjusted Cumulative One Year Adverse Event Rates
| Adverse Event | BMS (N=686) | DES (N=669) | P Value |
|---|---|---|---|
| Death | 4.4% | 3.1% | 0.22 |
| MI | 4.4% | 3.2% | 0.24 |
| CABG | 5.7% | 1.5% | <0.001 |
| Death/MI | 8.5% | 5.6% | 0.04 |
| Repeat PCI | 15.1% | 8.1% | <0.001 |
| Target vessel revascularization | 10.7% | 4.4% | <0.001 |
| Non-target vessel revascularization | 4.5% | 2.7% | 0.08 |
| Repeat Revascularization | 19.3% | 9.5% | <0.001 |
| MACE | 24.5% | 13.0% | <0.001 |
Figure 1.
One-year incidence of death or MI by stent type in patients treated with small coronary arteries
MI = myocardial infarction, BMS = bare-metal stents, DES = drug-eluting stents
Figure 2.
One-year incidence of repeat revascularization by stent type in patients treated with small coronary arteries
BMS = bare-metal stents, DES = drug-eluting stents
Figure 3.
Adjusted One Year Hazard Ratios and Confidence Intervals for Patients with Small Coronary Arteries Undergoing PCI with DES vs. BMS
PCI = percutaneous coronary intervention, BMS = bare-metal stents, DES = drug-eluting stents, CABG = coronary artery bypass graft surgery, MI = myocardial infarction, PCI = percutaneous coronary intervention, MACE = major adverse cardiovascular events
DISCUSSION
Utilizing the NHLBI Dynamic Registry, we examined the outcomes of patients with small coronary arteries undergoing stenting with either BMS or DES. In this real world population, we observed that at one year, patients receiving DES were less likely to undergo CABG, repeat PCI, or to experience major adverse cardiac events. Additionally, patients undergoing small coronary artery stenting with DES had lower peri-procedural and in-hospital complications. These findings are consistent with prior randomized trials evaluating DES vs. BMS [4–5, 8–9].
Early trials that demonstrated the advantage of coronary artery stenting compared to balloon angioplasty did not include patients with small coronary arteries; instead, these trials required that the minimal reference vessel diameter was at least 3.0 mm [10–13]. Controversy regarding the benefit of stent implantation versus conventional angioplasty in small coronary arteries arose as studies revealed higher restenosis rates with stenting in small coronary arteries in comparison to large coronary arteries [14]. Moreno et al performed a meta-analysis of 11 randomized trials including 3,541 patients comparing bare metal stenting to balloon angioplasty in small coronary arteries (defined as 2.0–3.0 mm in diameter based on individual study design) [15]. Patients receiving bare metal stents had significantly lower rates of restenosis (25.8% vs. 35.2%, p=0.003), new target vessel revascularization (12.5% vs. 17.0%, p=0.004), and major adverse cardiac events (15.0% vs. 21.8%, p=0.002) compared to patients undergoing balloon angioplasty. With the introduction of drug-eluting stents, several randomized studies examined the use of DES compared to the standard BMS for treatment of small diameter coronary arteries, defined as 2.25 mm to 3.0 mm in diameter. These studies revealed that at 8 to 9 months, patients treated with either sirolimus-eluting or paclitaxel-eluting had lower rates of MI, restenosis, target lesion revascularization, and major adverse cardiac events [4–5, 8, 16] as compared to BMS. Data extending to 24 months as reported by investigators from the Sirolimus-Eluting vs. Uncoated Stents for the Prevention of Restenosis in Small Coronary Arteries (SES-SMART) revealed a reduction in incidence of the composite endpoint of death, MI, clinically driven TLR, and cerebrovascular accident for SES in comparison with BMS [17].
Small coronary artery interventions provide a unique challenge for operators as an inverse relationship between vessel size and severity of angiographic restenosis has been identified [18–20]. Additionally, patients with small coronary arteries have higher adverse events following PCI, including increased rates of in-hospital combined major events (death, q-wave MI, and emergency CABG) [19], lower rates of procedural success [21], and more frequent stent thrombosis [22]. In our study, treated lesions in patients receiving BMS experienced more peri-procedural complications including higher rates of dissection, distal embolization, and in-lab abrupt vessel closure. Potential explanations of this finding include patient demographics, angiographic findings, and procedure related differences in the later era of DES use. Patients receiving BMS were more likely to have unstable angina and to have lesions with thrombus at angiography Additionally, BMS patients were less likely to have received a thienopyridine within twenty-four hours prior to or during the procedure. Additionally, changes in procedural aspects in the DES era may have lead to reduced procedural complications including routine stenting instead of provisional stenting, lower predilation pressure inflation of lesions, and limited use of debulking devices, which have been associated with lower rates of post-stent dissections as compared to the BMS era [23–25]. Lastly, the strategy of stenting longer segments with DES rather than the BMS strategy of spot stenting may have contributed to these differences.
However, implications for successful small coronary artery interventions are multifold. Small coronary artery lesions comprise a significant amount of coronary vessel interventions in daily practice, with an estimated 35–67% of interventional procedures performed depending on the definition of small coronary artery that is utilized [25]. Several specific patient populations have a preponderance of small coronary arteries, including women [26] and diabetics [27]. In our study, women comprised 41% of patients receiving small coronary artery stents and diabetics 35%. Additionally, patients with small coronary arteries undergoing CABG are at higher risk for complications including poor graft patency [28] and mortality [29] than patients with larger reference vessel diameter.
Potential limitations of this study include those inherent to all observational registries, such as the existence of potentially confounding variables. The definition of small coronary arteries has not always been consistent in previous studies. The findings of this study are generalizable only to arteries greater than or equal to 2.5 mm in size since drug-eluting stents were not available in 2.25 mm at the time of study enrollment. We chose the upper limit of 3.00 mm in diameter to focus on the smallest diameter range of patients and to be consistent with recent DES trial enrollment criteria. Additionally, coronary artery diameter in this study was operator determined and not quantitatively assessed, thus limiting the ability to comment on numerically small differences in arterial diameter. Lastly, such a classification does not differentiate between a coronary artery which has a small diameter and a diffusely diseased coronary artery which appears to have a small diameter by angiographic assessment. Moreover, the definition of MI varied between waves 1–2 and waves 3–5. However, given the use of high sensitivity cardiac-specific troponin assays in waves 3–5 of the registry, a higher reported MI rate in these later waves would be expected.
In summary, in a real-world registry, patients with small diameter coronary artery lesions treated with DES were at significantly lower risk for both repeat revascularization and major adverse cardiovascular events as compared to patients treated with BMS, with no increase in the risk of death and MI. These data confirm the efficacy and safety of DES over BMS in routine clinical practice. With the availability of DES at 2.25 mm in diameter, further study of smaller coronary artery treatment outcomes will be important.
Acknowledgments
ACKNOWLEDGEMENTS
None
FUNDING
This study was supported by grant number HL-33292 from the National Heart, Lung, and Blood Institute of the National Institutes of Health
Footnotes
DISCLOSURES
None
References
- 1.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE, The SIRIUS Investigators Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 2.Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, Popma JJ, Russell ME, TAXUS-IV Investigators A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 3.Lau KW, Hung JS, Sigwart U. The current status of stent placement in small coronary arteries < 3.0 mm in diameter. J Invasive Cardiol. 2004;16(8):411–416. [PubMed] [Google Scholar]
- 4.Schampaert E, Cohen EA, Schlüter M, Reeves F, Traboulsi M, Title LM, Kuntz RE, Popma JJ C-SIRIUS Investigators. The Canadian study of the sirolimus-eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C-SIRIUS) J Am Coll Cardiol. 2004;43(6):1110–1115. doi: 10.1016/j.jacc.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Ardissino D, Cavallini C, Bramucci E, Indolfi C, Marzocchi A, Manari A, Angeloni G, Carosio G, Bonizzoni E, Colusso S, Repetto M, Merlini PA SES-SMART Investigators. Sirolimus-eluting vs uncoated stents for prevention of restenosis in small coronary arteries: a randomized trial. JAMA. 2004;292(22):2727–2734. doi: 10.1001/jama.292.22.2727. [DOI] [PubMed] [Google Scholar]
- 6.Bourassa MG, Al-Bassam M, Block PC, Coady P, Cohen H, Cowley M, Dorros G, Faxon D, Holmes DR, Jacobs A, Kelsey SF, King SB, 3rd, Myler R, Slater J, Stanek V, Vlachos HA, Detre KM. Percutaneous coronary intervention in the current era compared with 1985–1986. The National Heart, Lung, and Blood Institute Registries. Circulation. 2000;102:2945–2951. doi: 10.1161/01.cir.102.24.2945. [DOI] [PubMed] [Google Scholar]
- 7.Mauri L, Hsieh WH, Massaro JM, Ho KKL, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–1029. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 8.Schofer J, Schlüter M, Gershlick AH, Wijns W, Garcia E, Schampaert E, Breithardt G E-SIRIUS Investigators. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS) Lancet. 2003;362(9390):1093–1099. doi: 10.1016/S0140-6736(03)14462-5. [DOI] [PubMed] [Google Scholar]
- 9.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE SIRIUS Investigators. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349(14):1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor NJ, Morton JR, Birkmeyer JD, Olmstead EM, O’Connor GT. Effect of coronary artery diameter in patients undergoing coronary bypass surgery. Northern New England Cardiovascular Disease Study Group. Circulation. 1996;93(4):652–655. doi: 10.1161/01.cir.93.4.652. [DOI] [PubMed] [Google Scholar]
- 11.Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med. 1994;331:489–495. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- 12.Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, Detre K, Veltri L, Ricci D, Nobuyoshi M, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331(8):496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- 13.Betriu A, Masotti M, Serra A, Alonso J, Fernández-Avilés F, Gimeno F, Colman T, Zueco J, Delcan JL, García E, Calabuig J. Randomized comparison of coronary stent implantation and balloon angioplasty in the treatment of de novo coronary artery lesions (START): a four-year follow-up. J Am Coll Cardiol. 1999;34(5):1498–1506. doi: 10.1016/s0735-1097(99)00366-6. [DOI] [PubMed] [Google Scholar]
- 14.Akiyama T, Moussa I, Reimers B, Ferraro M, Kobayashi Y, Blengino S, Di Francesco L, Finci L, Di Mario C, Colombo A. Angiographic and clinical outcome following coronary stenting of small vessels: a comparison with coronary stenting of large vessels. J Am Coll Cardiol. 1998;32(6):1610–1618. doi: 10.1016/s0735-1097(98)00444-6. [DOI] [PubMed] [Google Scholar]
- 15.Moreno R, Fernández C, Alfonso F, Hernández R, Pérez-Vizcayno MJ, Escaned J, Sabaté M, Bañuelos C, Angiolillo DJ, Azcona L, Macaya C. Coronary stenting versus balloon angioplasty in small vessels: a meta-analysis from 11 randomized studies. J Am Coll Cardiol. 2004;43(11):1964–1972. doi: 10.1016/j.jacc.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 16.Stone GW, Ellis SG, Cannon L, Mann JT, Greenberg JD, Spriggs D, O’Shaughnessy CD, DeMaio S, Hall P, Popma JJ, Koglin J, Russell ME TAXUS V Investigators. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. 2005;294(10):1215–1223. doi: 10.1001/jama.294.10.1215. [DOI] [PubMed] [Google Scholar]
- 17.Menozzi A, Solinas E, Ortolani P, Repetto A, Saia F, Piovaccari G, Manari A, Magagnini E, Vignali L, Bonizzoni E, Merlini PA, Cavallini C, Ardissino D SES-SMART Investigators. Twenty-four months clinical outcomes of sirolimus-eluting stents for the treatment of small coronary arteries: the long-term SES-SMART clinical study. Eur Heart J. 2009;30(17):2095–2101. doi: 10.1093/eurheartj/ehp224. [DOI] [PubMed] [Google Scholar]
- 18.Foley DP, Melkert R, Serruys PW. Influence of coronary vessel size on renarrowing process and late angiographic outcome after successful balloon angioplasty. Circulation. 1994;90(3):1239–1251. doi: 10.1161/01.cir.90.3.1239. [DOI] [PubMed] [Google Scholar]
- 19.Elezi S, Kastrati A, Neumann FJ, Hadamitzky M, Dirschinger J, Schömig A. Vessel size and long-term outcome after coronary stent placement. Circulation. 1998;98(18):1875–1880. doi: 10.1161/01.cir.98.18.1875. [DOI] [PubMed] [Google Scholar]
- 20.Hirshfeld JW, Jr, Schwartz JS, Jugo R, MacDonald RG, Goldberg S, Savage MP, Bass TA, Vetrovec G, Cowley M, Taussig AS, et al. Restenosis after coronary angioplasty: a multivariate statistical model to relate lesion and procedure variables to restenosis. The M-HEART Investigators. J Am Coll Cardiol. 1991;18(3):647–656. doi: 10.1016/0735-1097(91)90783-6. [DOI] [PubMed] [Google Scholar]
- 21.Schunkert H, Harrell L, Palacios IF. Implications of small reference vessel diameter in patients undergoing percutaneous coronary revascularization. J Am Coll Cardiol. 1999;34(1):40–48. doi: 10.1016/s0735-1097(99)00181-3. [DOI] [PubMed] [Google Scholar]
- 22.Cutlip DE, Baim DS, Ho KK, Popma JJ, Lansky AJ, Cohen DJ, Carrozza JP, Jr, Chauhan MS, Rodriguez O, Kuntz RE. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001;103(15):1967–1971. doi: 10.1161/01.cir.103.15.1967. [DOI] [PubMed] [Google Scholar]
- 23.Stankovic G, Chieffo A, Iakovou I, Orlic D, Corvaja N, Sangiorgi G, Airoldi F, Colombo A. Creatine kinase-myocardial band isoenzyme elevation after percutaneous coronary interventions using sirolimus-eluting stents. Am J Cardiol. 2004;93(11):1397–1401. doi: 10.1016/j.amjcard.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 24.Biondi-Zoccai GG, Agostoni P, Sangiorgi GM, Airoldi F, Cosgrave J, Chieffo A, Barbagallo R, Tamburino C, Vittori G, Falchetti E, Margheri M, Briguori C, Remigi E, Iakovou I, Colombo A Real-world Eluting-stent Comparative Italian retrosPective Evaluation Study Investigators. Incidence, predictors, and outcomes of coronary dissections left untreated after drug-eluting stent implantation. Eur Heart J. 2006;27(5):540–546. doi: 10.1093/eurheartj/ehi618. [DOI] [PubMed] [Google Scholar]
- 25.Brown DL, Buchbinder M. Incidence, predictors, and consequences of coronary dissection following high-speed rotational atherectomy. Am J Cardiol. 1996;78(12):1416–1419. doi: 10.1016/s0002-9149(96)00639-x. [DOI] [PubMed] [Google Scholar]
- 26.Morice MC. Stenting for small coronary vessels. J Invasive Cardiol. 2003;15(7):377–379. [PubMed] [Google Scholar]
- 27.Ashby DT, Mehran R, Aymong EA, Lansky AJ, Iakovou I, Weisz G, New G, Moussa I, Dangas G, Moses JW, Stone GW, Leon MB. Comparison of outcomes in men versus women having percutaneous coronary interventions in small coronary arteries. Am J Cardiol. 2003;91(8):979–981. doi: 10.1016/s0002-9149(03)00118-8. [DOI] [PubMed] [Google Scholar]
- 28.Nicholls SJ, Tuzcu EM, Kalidindi S, Wolski K, Moon KW, Sipahi I, Schoenhagen P, Nissen SE. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol. 2008;52(4):255–262. doi: 10.1016/j.jacc.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 29.Crosby IK, Wellons HA, Jr, Taylor GJ, Maffeo CJ, Beller GA, Muller WH., Jr Critical analysis of the preoperative and operative predictors of aortocoronary bypass patency. Ann Surg. 1981;193(6):743–751. doi: 10.1097/00000658-198106000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]