Abstract
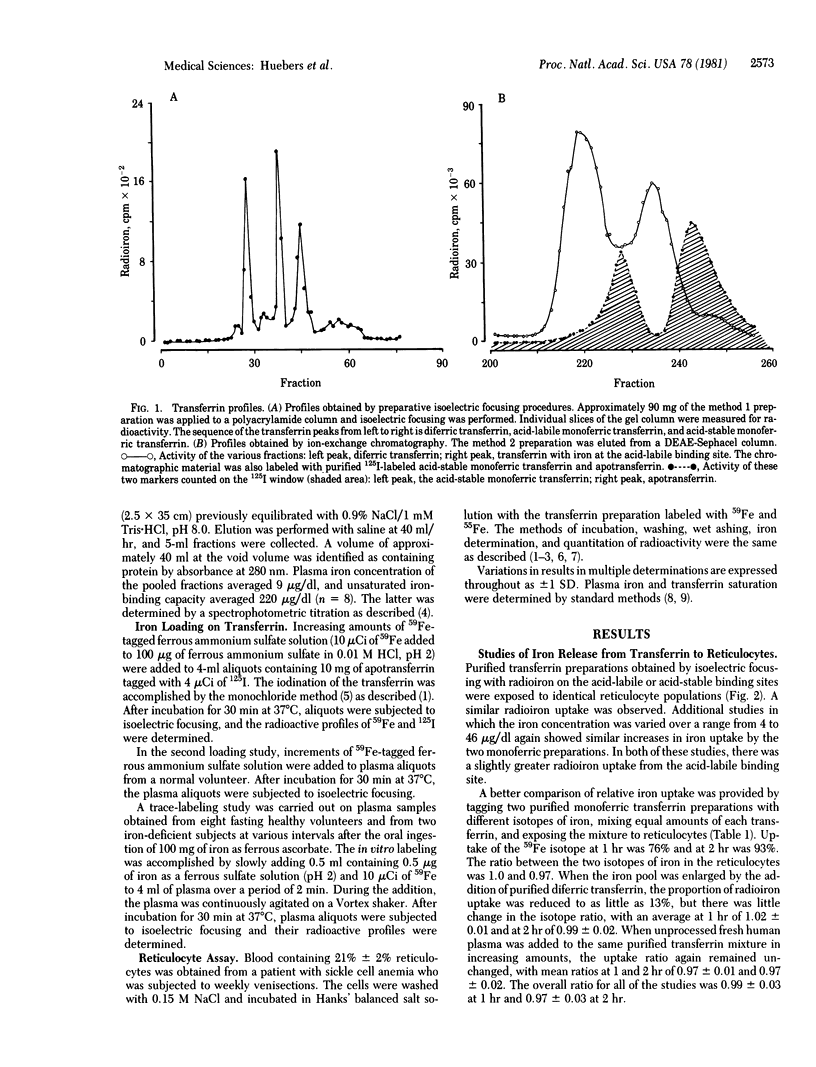
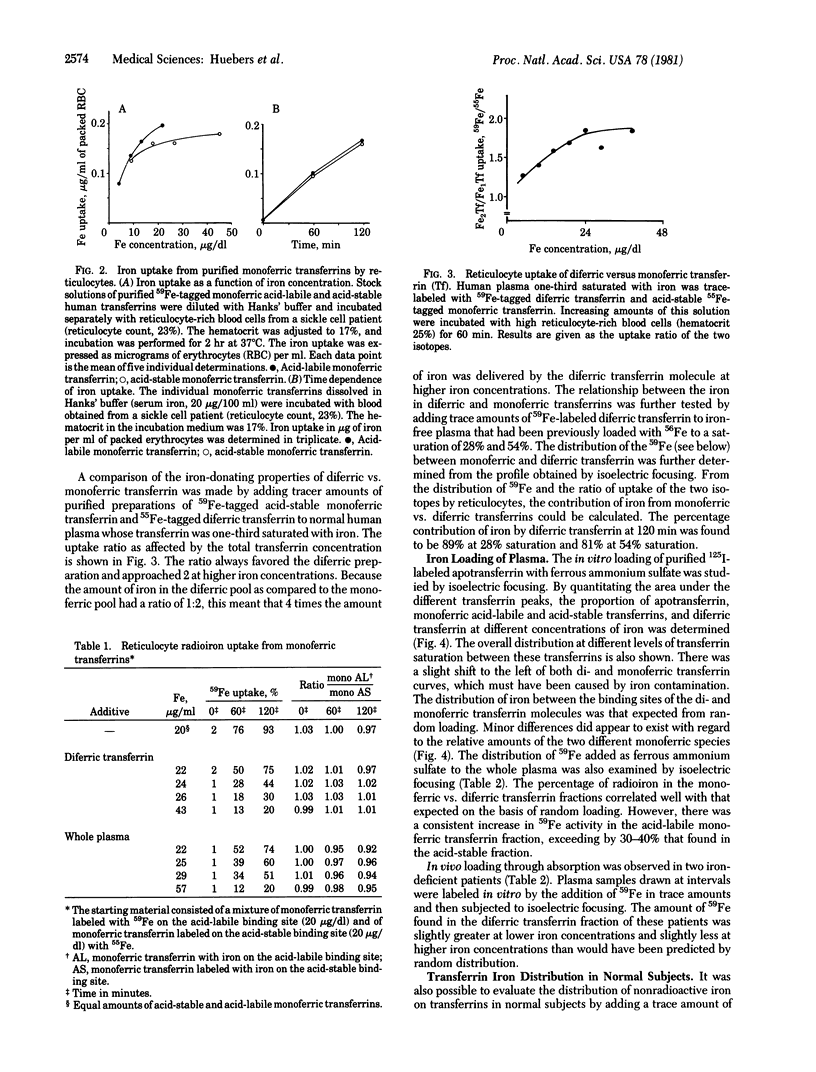
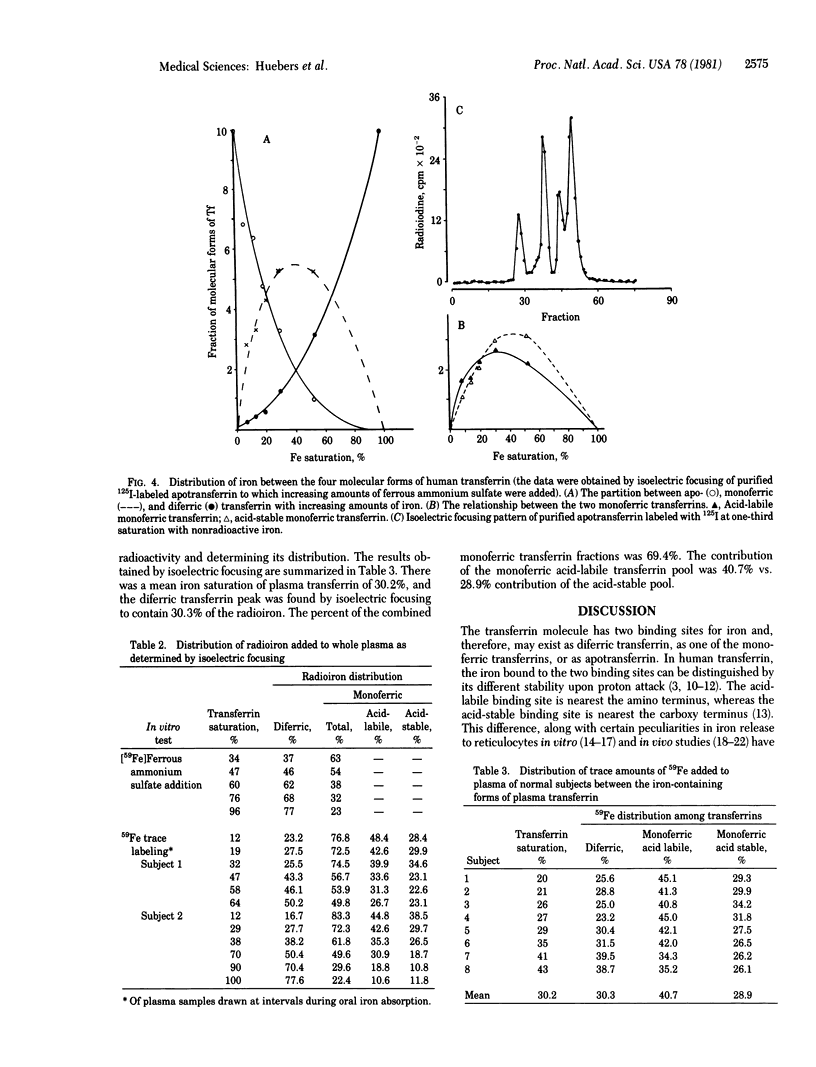
Purified fractions of human apotransferrin, monoferric transferrins with iron on the acid-labile binding site and on the acid-stable binding site, and diferric transferrin have been prepared. The iron loading and unloading behavior of these preparations has been examined by isoelectric focusing. Iron release from the two monoferric transferrin preparations to human reticulocytes was of similar magnitude. In a mixture containing equal amounts of diferic and monoferric iron, approximately 4 times the amount of iron delivered by the monoferric species was delivered by the diferric species. Iron loading of transferrin in vitro showed a random distribution between monoferric and diferric transferrin. Among the monoferric transferrins, loading of the acid-labile binding sites was greater than that of the acid-stable binding sites. In vivo iron distribution in normal subjects, as evaluated by in vitro-added 50Fe, gave similar results. Absorption of a large dose of orally administered iron in iron-deficient subjects resulted in a somewhat greater amount of diferric transferrin at low saturation and a somewhat smaller amount of diferric transferrin at higher saturations than would have been anticipated by random loading. These data would indicate that in the human, iron loading of transferrin may be considered essentially random. Unloading from the two monoferric transferrin species is of similar magnitude but far less than that delivered by diferric transferrin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown E. B., Okada S., Awai M., Chipman B. In vivo evidence for the functional heterogeneity of transferrin-bound iron. III. Studies of transferrin at high and low iron saturation. J Lab Clin Med. 1975 Oct;86(4):576–585. [PubMed] [Google Scholar]
- Christensen A. C., Huebers H., Finch C. A. Effect of transferrin saturation on iron delivery in rats. Am J Physiol. 1978 Jul;235(1):R18–R22. doi: 10.1152/ajpregu.1978.235.1.R18. [DOI] [PubMed] [Google Scholar]
- Cook J. D. An evaluation of adsorption methods for measurement of plasma iron-binding capacity. J Lab Clin Med. 1970 Sep;76(3):497–506. [PubMed] [Google Scholar]
- Evans R. W., Williams J. Studies of the binding of different iron donors to human serum transferrin and isolation of iron-binding fragments from the N- and C-terminal regions of the protein. Biochem J. 1978 Aug 1;173(2):543–552. doi: 10.1042/bj1730543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch C. A., Deubelbeiss K., Cook J. D., Eschbach J. W., Harker L. A., Funk D. D., Marsaglia G., Hillman R. S., Slichter S., Adamson J. W. Ferrokinetics in man. Medicine (Baltimore) 1970 Jan;49(1):17–53. doi: 10.1097/00005792-197001000-00002. [DOI] [PubMed] [Google Scholar]
- Fletcher J., Huehns E. R. Function of transferrin. Nature. 1968 Jun 29;218(5148):1211–1214. doi: 10.1038/2181211a0. [DOI] [PubMed] [Google Scholar]
- Ganzoni A. M., Hahn D., Späti B. Plasma iron transport: absence of an uniform system. Blut. 1972 May;24(5):269–273. doi: 10.1007/BF01642011. [DOI] [PubMed] [Google Scholar]
- Grahm G., Bates G. W. Approaches to the standardization of serum unsaturated iron-binding capacity. J Lab Clin Med. 1976 Sep;88(3):477–486. [PubMed] [Google Scholar]
- Hahn D. Functional behaviour of transferrin. Eur J Biochem. 1973 Apr;34(2):311–316. doi: 10.1111/j.1432-1033.1973.tb02760.x. [DOI] [PubMed] [Google Scholar]
- Hahn D., Ganzoni A. M. Functional heterogeneity of the transport iron compartment II. In vivo differences between transferrin iron binding sites, and in vitro interbinding site iron exchange. Acta Haematol. 1975;53(6):321–328. doi: 10.1159/000208200. [DOI] [PubMed] [Google Scholar]
- Harris D. C., Aisen P. Functional equivalence of the two iron-binding sites of human transferrin. Nature. 1975 Oct 30;257(5529):821–823. doi: 10.1038/257821a0. [DOI] [PubMed] [Google Scholar]
- Huebers H., Bauer W., Huebers E., Csiba E., Finch C. The behavior of transferrin iron in the rat. Blood. 1981 Feb;57(2):218–228. [PubMed] [Google Scholar]
- Huebers H., Csiba E., Josephson B., Huebers E., Finch C. Interaction of human diferric transferrin with reticulocytes. Proc Natl Acad Sci U S A. 1981 Jan;78(1):621–625. doi: 10.1073/pnas.78.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebers H., Huebers E., Csiba E., Finch C. A. Iron uptake from rat plasma transferrin by rat reticulocytes. J Clin Invest. 1978 Nov;62(5):944–951. doi: 10.1172/JCI109223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibman A., Aisen P. Distribution of iron between the binding sites of transferrin in serum: methods and results in normal human subjects. Blood. 1979 Jun;53(6):1058–1065. [PubMed] [Google Scholar]
- Lestas A. N. The effect of pH upon human transferrin: selective labelling of the two iron-binding sites. Br J Haematol. 1976 Mar;32(3):341–350. doi: 10.1111/j.1365-2141.1976.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Makey D. G., Seal U. S. The detection of four molecular forms of human transferrin during the iron binding process. Biochim Biophys Acta. 1976 Nov 26;453(1):250–256. doi: 10.1016/0005-2795(76)90270-1. [DOI] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Morgan E. H., Huebers H., Finch C. A. Differences between the binding sites for iron binding and release in human and rat transferrin. Blood. 1978 Dec;52(6):1219–1228. [PubMed] [Google Scholar]
- Morgan E. H. The role of plasma transferrin in iron absorption in the rat. Q J Exp Physiol Cogn Med Sci. 1980 Jul;65(3):239–252. doi: 10.1113/expphysiol.1980.sp002510. [DOI] [PubMed] [Google Scholar]
- Okada S., Chipman B., Brown E. B. In vivo evidence for the functional heterogeneity of transferrin-bound iron. IV. Selective uptake by erythroid precursors of radioiron from portal vein plasma transferrin during intestinal iron absorption. J Lab Clin Med. 1977 Jan;89(1):51–64. [PubMed] [Google Scholar]
- Okada S., Jarvis B., Brown E. B. In vivo evidence for the functional heterogeneity of transferrin-bound iron. V. Isotransferrins: an explanation of the Fletcher-Huehns phenomenon in the rat. J Lab Clin Med. 1979 Feb;93(2):189–198. [PubMed] [Google Scholar]
- Pootrakul P., Christensen A., Josephson B., Finch C. A. Role of transferrin in determining internal iron distribution. Blood. 1977 Jun;49(6):957–966. [PubMed] [Google Scholar]
- Zapolski E. J., Princiotto J. V. Binding of iron from nitrilotriacetate analogues by human transferrin. Biochemistry. 1980 Jul 22;19(15):3599–3603. doi: 10.1021/bi00556a028. [DOI] [PubMed] [Google Scholar]
- Zapolski E. J., Princiotto J. V. Preferential utilization in vitro of iron bound to diferric transferrin by rabbit reticulocytes. Biochem J. 1977 Aug 15;166(2):175–179. doi: 10.1042/bj1660175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Baarlen J., Brouwer J. T., Leibman A., Aisen P. Evidence for the functional heterogeneity of the two sites of transferrin in vitro. Br J Haematol. 1980 Nov;46(3):417–426. doi: 10.1111/j.1365-2141.1980.tb05988.x. [DOI] [PubMed] [Google Scholar]
- van Eijk H. G., van Noort W. L., Kroos M. J., van der Heul C. Isolation of the two monoferric human transferrins by preparative isoelectric focussing. J Clin Chem Clin Biochem. 1980 Sep;18(9):563–566. doi: 10.1515/cclm.1980.18.9.563. [DOI] [PubMed] [Google Scholar]


