Abstract
Hyper-immunoglobulin (Ig)E syndrome (HIES) is a primary immunodeficiency associated with mutations in STAT3 resulting in impaired development of T helper type 17 (Th17) lymphocytes. HIES patients with a reduced frequency of Th17 cells present with infections caused by Staphylococcus aureus and/or Candida strains. The same spectrum of pathogens is present in patients with chronic granulomatous disease (CGD).We analysed the characteristics of the Th17 compartment in HIES and CGD. HIES patients showed very low numbers of Th17 cells. By contrast, the frequency of Th17 cells and production of Th17-derived cytokines was significantly higher among CGD patients when compared to both control samples and HIES. Naive CD4+ cells in CGD patients had a normal capacity to differentiate into IL-17-producing cells and the numbers of Th17 cells in the CGD patients normalized following successful bone marrow transplantation. Our findings complement recent data on the importance of Th17 cells for elimination of infections with C. albicans and S. aureus.
Keywords: Th17 lymphocytes, hyper-IgE, chronic granulomatous disease, candida infections
Introduction
Following pathogen recognition, antigen-presenting cells (APCs) produce cytokines that drive the polarization of naive CD4+ T cells into different subsets, with distinct effector functions aimed at co-ordinating other components of the immune system to eventually clear the infection. In addition to interferon (IFN)-γ-producing T helper type 1 (Th1) cells and interleukin (IL)-4-producing Th2 cells, IL-17-producing Th17 lymphocytes with distinct effector functions have been described recently [1,2]. Th17 cells are characterized by the production of IL-17, IL-21 and IL-22. Among other functions, IL-17 increases the recruitment of neutrophils, IL-21 promotes the amplification and differentiation of Th17 cells and IL-22 induces the expression of anti-microbial peptides in epithelial cells [3].
Since their discovery in mice, efforts have been made to characterize human Th17 cells, to identify the factors involved in their differentiation and to elucidate the role Th17 cells play in protective immunity and immunopathology. Initial studies have described increased numbers of Th17 lymphocytes in various autoimmune diseases and Th17 cells have been identified as potent inducers of autoimmunity through the promotion of tissue inflammation [4,5].
Soon after their initial discovery, efforts have been made to characterize the role of Th17 cells in protective immunity in humans. With regard to the role of Th17 cells in protective immunity, the intriguing question has been which pathogens require the appropriate function of Th17 cells for elimination. Diverse pathogens have been reported to trigger a substantial Th17 response, including Propionibacterium acnes, Citrobacter rodentium, Klebsiella pneumonia, Bacteroides spp., Borrelia spp., Mycobacterium tuberculosis and Candida albicans[6–11]. However, because the effector mechanisms of the immune system are largely redundant, the fact that a pathogen induces a Th17 immune response does not necessarily mean that Th17 lymphocytes are critical for its elimination [12]. Recently, studies of primary immunodeficiencies (PIDs) involving genetic defects in signalling pathways important for the differentiation of Th17 cells have indicated the necessity of an intact Th17 response for the defence against C. albicans and Staphylococcus aureus. Th17-associated effector molecules are critical for protection against an array of pathogens, mainly through neutrophil recruitment and the induction of anti-microbial peptides. Th17 cells develop in a STAT3-dependent manner; therefore, patients with hyper-IgE syndrome (HIES), associated with dominant negative mutations in signal transduction and activator of transcription 3 (STAT3) gene, fail to generate Th17 cells [13,14]. HIES patients with a reduced frequency of Th17 cells typically present with abnormal susceptibility to infections with S. aureus and/or Candida strains [15]. Notably, the same spectrum of dominant pathogens is seen in patients with chronic granulomatous disease (CGD). CGD is a primary immunodeficiency resulting from a defect in the multi-component nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex, which is responsible for the production of bactericidal reactive oxygen species (ROS) in phagocytes. As a result, CGD patients have increased susceptibility to certain catalase-positive bacteria and fungi and Aspergillus species. The primary clinical features of CGD are recurrent infections and granuloma formation [16].
Thus, despite an identical spectrum of pathogens, HIES and CGD differ in terms of the cellular compartments affected: Th17 cells in HIES and phagocytes in CGD. In this study, we chose to analyse and compare the characteristics of the Th17 compartment in both HIES and CGD patients to elucidate the roles of Th17 cells and neutrophils in the control of Candida and Staphylococcus infections in humans.
Materials and methods
Patients
The study enrolled 10 healthy controls (six males, four females, age range 18–36 years), four HIES patients (one male, three females, age range 11–36 years) and seven CGD patients (seven males, age range 5–39 years). Blood samples were collected after obtaining informed consent and all patients were free of active systemic infections at the time of blood sampling. The project was approved by the institutional review board of the 2nd Medical School, Charles University, Prague, Czech Republic. Study subjects were diagnosed with HIES by experienced clinicians assisted by a diagnostic scoring system, and diagnoses were confirmed by the identification of STAT3 mutations in all cases. CGD patients were diagnosed by the absence of NADPH-oxidase activity in stimulated neutrophils by using one or more of the following tests: nitroblue tetrazolium (NBT) reduction, dihydrorhodamine oxidation and chemiluminescence. All cases of CGD were confirmed by mutation analysis of the CYBB gene.
In 2007 two of the CGD patients underwent successful allogeneic bone marrow transplantation.
Cell cultures and stimulation
Whole blood was collected from healthy donors and GCD or HIES patients. Peripheral blood mononuclear cells (PBMCs) were prepared by centrifugation using Ficoll-Paque (Sigma Aldrich, Seelze, Germany) and cultured in 24-well plates (1 × 106/ml) in complete medium. Cells were then stimulated for 6 h with 100 ng/ml phorbol myristate acetate (PMA) (Sigma Aldrich) and 750 ng/ml ionomycin (Sigma Aldrich), with 3 µg/ml of Brefeldin A (eBioscience, San Diego, CA, USA) added to the cultures after 2 h. The cells were then harvested, washed once in phosphate-buffered saline (PBS) and prepared for surface and intracellular staining.
An enzyme-linked immunosorbent assay (ELISA) was used for cytokine detection. Sampled PBMCs were stimulated for 24 h with PMA and ionomycin as described above and culture supernatants were collected.
Flow cytometry and ELISA
Stimulated and non-stimulated PBMCs were stained for surface markers using anti-CD4-PC7 (BD Biosciences, San Diego, CA, USA), anti-CD3-Alexa700 (Exbio, Prague, Czech Republic) and anti-CD8-PE-Dy590 (Exbio) monoclonal antibodies. After 30 min of incubation in the dark cells were washed once in PBS and stained intracellular cytokine expression, using a fixation and permeabilization kit (eBioscience), anti-IL-17A-Alexa 647 (eBioscience) and anti-IFN-γ-phycoerythrin (PE) (BD Pharmingen). Data were acquired using a fluorescence activated cell sorter (FACS) Aria (BD Biosciences) and analysed using FlowJo software (Tree Star, Ashland, OR, USA).
IL-17A was measured in culture supernatants using the human IL-17A ELISA Max-SET DeLuxe kit (Biolegend, San Diego, CA, USA), according to the manufacturer's instructions.
The secretion of IL-23 and IL-21 was measured using corresponding human ELISA Ready-Set-Go kits (eBioscience).
In vitro differentiation of naive CD4 T cells
For in vitro differentiation towards a Th17 phenotype, naive CD4+ T cells were isolated using an EasySep naive CD4+ T cell enrichment kit (Stemcell, Vancouver, Canada). A total of 100 000 purified naive CD4+ T cells were then placed into 96-well round-bottomed plates and cultured with beads coated with anti-CD2, anti-CD3 and anti-CD28 (Miltenyi Biotec, Bergisch Gladbach, Germany) together with 20 U/ml IL-2 alone (PeproTech EC, London, UK), or in the presence of 20 ng/ml IL-23 (eBioscience) and 10 ng/ml IL-1β (PeproTech EC).
Cells were then cultured in complete medium for 4 or 5 days. The culture was then split and the cells were placed in 20 U/ml of IL-2 for another 7 days. On day 12, the cells were harvested, washed and stimulated overnight with PMA and ionomycin as described above. After 24 h the supernatants were collected and ELISAs were performed.
Statistics
The Mann–Whitney U-test was used for statistical analysis, and P < 0·05 was considered to be statistically significant.
Results
Increased frequency of circulating Th17 cells in patients with CGD
Four patients from three families with HIES and seven patients with CGD with confirmed mutations in STAT3 and CYBB, respectively, were enrolled into this study. All patients shared a history of recurrent S. aureus and C. albicans infections.
We first analysed the frequency of Th17 cells in the peripheral blood of HIES and CGD patients after PBMC stimulation with PMA and calcium ionomycin (Fig. 1). In agreement with previous reports, STAT3-deficient HIES patients had very low numbers of Th17 cells. Interestingly, the frequency of IL-17+ CD4+ T cells was significantly higher in CGD patients. The number of Th17 cells in CGD patients was significantly higher when compared to both healthy controls and HIES patients (P < 0·05) (Fig. 1b). In contrast, CGD patients had significantly lower levels of IFN-γ+ CD4 T cells when compared to healthy controls (P < 0·05) and HIES (P = 0·05). Thus, CGD patients have a significantly higher ratio of Th17 to Th1 cells than healthy controls and HIES patients, by an average of 10-fold (Fig. 1c).
Fig. 1.
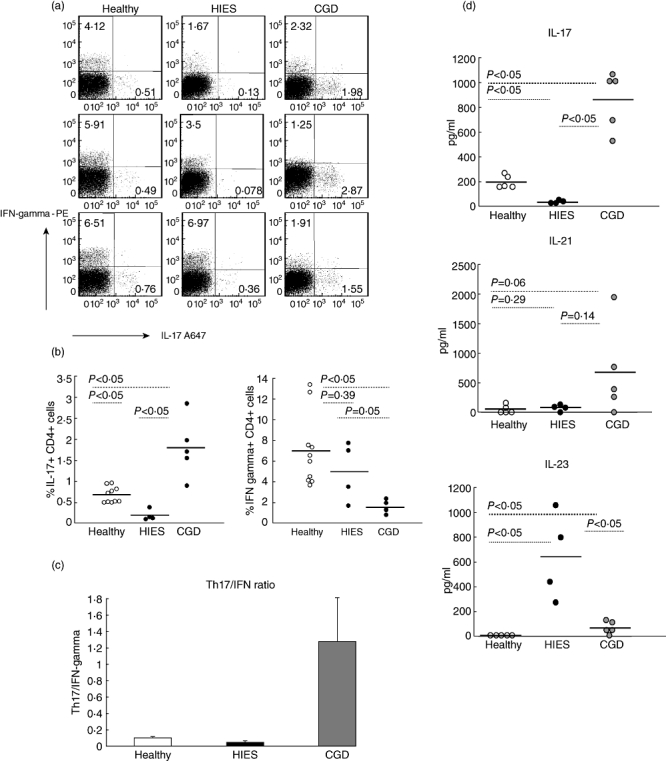
Chronic granulomatous disease (CGD) patients have a significantly increased frequency of T helper type 17 (Th17) lymphocytes and Th17-related cytokines in peripheral blood. (a) Peripheral blood mononuclear cells (PBMCs) from healthy controls, hyper-IgE syndrome (HIES) patients and CGD patients were stimulated for 6 h with phorbol myristate acetate (PMA) and calcium ionomycin and the frequency of interleukin (IL)-17 and interferon (IFN)-γ-producing CD4 T cells was determined by flow cytometry. Representative dot-plots (gated on CD4 T cells) are shown for three CGD patients, three HIES patients and three age-matched healthy controls. (b) Summary data on the frequency of IL-17+ CD4 T cells (left) and IFN-γ CD4 T cells (right) in healthy controls, CGD patients and HIES patients. Each symbol represents an individual patient and the horizontal line represents the mean value for the cohort. (c) Th17/IFN-γ ratio in healthy controls, HIES patients and CGD patients. (d) PBMCs were stimulated with PMA and calcium ionomycin. Cell culture supernatants were collected after 24 h, and the concentrations of IL-17, IL-21 and IL-23 were analysed by enzyme-linked immunosorbent assay. Each symbol represents an individual patient, and the horizontal bar indicates the mean value for all experiments.
PBMCs from CGD patients produce higher amounts of Th17-derived cytokines
We next tested the production of Th17-derived cytokines by activated PBMCs in HIES and CGD patients. STAT3-deficient HIES patients produced no IL-17 or IL-21, the major Th17-derived effector cytokines, consistent with the absence of Th17 T cells. Stimulation of PBMCs isolated from CGD patients resulted in significantly greater production of IL-17 (>fourfold) and IL-21 (>eightfold) than observed in healthy controls (Fig. 1d). We also investigated baseline production of IL-23, a cytokine important for the differentiation of Th17 lymphocytes. Whereas steady-state PBMCs from healthy controls produced virtually no IL-23, PBMCs from CGD and HIES patients produced detectable amounts of IL-23 at a significantly higher level than healthy controls (P < 0·05). The concentration of IL-23 produced by PBMCs from HIES patients, however, was five- to sevenfold higher than that observed for CGD patients (Fig. 1d).
Thus, CGD patients show significant expansion and activation of the Th17 compartment, reflected by the proportion of Th17 cells and the levels of Th17-derived cytokines.
Correction of Th17 compartment abnormalities in CGD after allogeneic haematopoietic stem cell transplantation (HSCT)
Two of the CGD patients had life-threatening infectious complications and underwent successful allogeneic HSCT. We repeatedly evaluated the characteristics of Th17 cells before HSCT and monitored the Th17 compartment regularly post-transplantation. Correction of the genetic defect in neutrophils in these CGD patients resulted in the normalization of IL-17+ CD4 T cells, an increase in the number of IFN-γ+ CD4 T lymphocytes and normalization of the Th17/Th1 ratio (Fig. 2a). In accordance with the normal numbers of Th17 cells, PBMCs from the transplanted CGD patients produced similar amounts of IL-17, IL-21 and IL-23 to those observed for healthy controls (Fig. 2b).
Fig. 2.
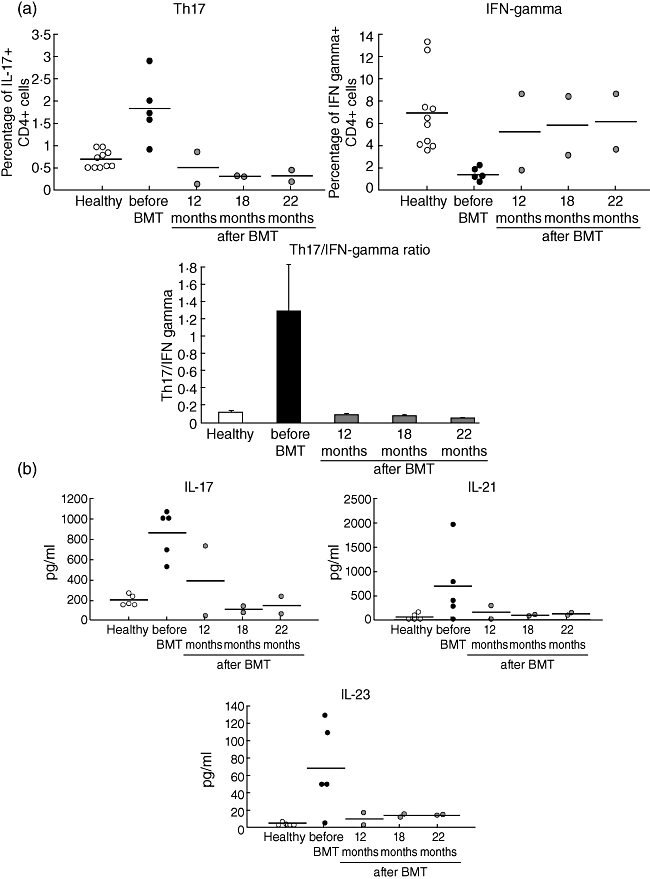
Allogeneic haematopoietic stem cell transplantation (HSCT) corrects the disturbances in T helper type 17 (Th17) homeostasis in chronic granulomatous disease (CGD) patients. (a) Frequency of interleukin (IL)-17+ CD4 T cells (top left) and interferon (IFN)-γ+ CD4 T cells (top right) in two CGD patients who underwent allogeneic HSCT and normalization of the Th17/IFN-γ ratio post-transplantation (bottom). (b) Phorbol myristate acetate (PMA) and ionomycin-induced production of IL-17 and IL-21 and baseline IL-23 production by peripheral blood mononuclear cells (PBMCs) from the transplanted CGD patients. Each symbol represents an individual patient at the indicated time-point after HSCT, and the horizontal bar shows the mean value for each time-point.
Naive CD4+ cells in CGD patients have a normal capacity to differentiate into IL-17-producing cells
To determine whether the increased number of Th17 cells observed in CGD patients reflects a primary defect in T cells or a secondary event driven by another mechanism, we evaluated the capacity of naive CD4+ T cells in CGD patients to differentiate into effector IL-17+ CD4 T cells. Sorted naive CD4 T cells were stimulated for a total of 12 days with anti-CD3/anti-CD28 beads, IL-2, IL-1β and IL-23. This combination resulted in the differentiation of comparable numbers of IL-17-producing CD4 cells in healthy controls and CGD patients (Fig. 3). By contrast, naive CD4 cells from STAT3-deficient HIES patients failed to differentiate into Th17 cells and to produce IL-17.
Fig. 3.
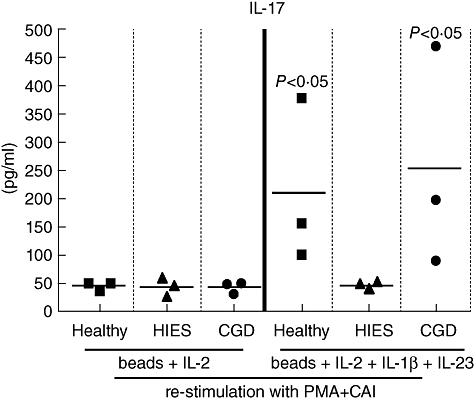
Naive CD4 T cells from chronic granulomatous disease (CGD) patients have a normal capacity to differentiate into T helper type 17 (Th17) CD4+ T cells in response to Th17-polarizing cytokines. Naive CD4+ T cells were cultured with beads coated with anti-CD2, anti-CD3 and anti-CD28, along with interleukin (IL)-2, IL-23 and IL-1β. Cells were then cultured in complete medium for 4 or 5 days. The cultures were then split, and the cells were placed in 20 U/ml of IL-2 for another 7 days. On day 12, the cells were harvested, washed and stimulated overnight with phorbol myristate acetate (PMA) and ionomycin. After 24 h, the culture supernatants were collected, and an IL-17 enzyme-linked immunosorbent assay was performed. Symbols represent values for healthy controls, CGD patients and hyper-IgE syndrome (HIES) patients and the horizontal bars show mean values.
Discussion
Primary immunodeficiencies (PIDs) represent an elegant model with which to study the roles of affected components of the immune system in the defence against specific infections.
Recently, studies of PIDs involving genetic defects in signalling pathways important for the differentiation of Th17 cells have indicated the necessity of an intact Th17 response for the defence against C. albicans and S. aureus. Two signalling pathways play a critical role in Th17 development. First, in APCs, recognition of the hyphal form of C. albicans, zymosan and b-glucan through C-type lectins (e.g. dectin-1, dectin-2 and MINCLE) results in downstream activation of Syk-CARD9 signalling, which leads to the activation of nuclear factor-κB [17,18]. This pathway promotes production of the key Th17-polarizing cytokines, IL-1, IL-6 and IL-23 [19]. Engagement of C-type lectins by pathogen components activates signalling pathways that converge on CARD9. CARD9-deficient mice show susceptibility to fungal infections, and fail to mount a Candida-specific Th17 response [18,20]. Consistent with the murine data, a homozygous CARD9 mutation has been reported very recently in a family with chronic mucocutaneous candidiasis [21]. That study lacked functional data on APCs and serum cytokines due to logistical constraints, but the affected family members showed very low numbers of Th17 lymphocytes in the peripheral blood.
Th17-polarizing cytokines act on naive CD4 T cells and drive their differentiation into Th17 cells in a STAT3-dependent manner. Eventually, Th17-polarizing cytokines induce the transcription programme which subsequently induces a set of effector molecules including IL-17, IL-21, IL-22 and IL-23R [1,22,23]. Thus, HIES patients, with inactivating mutations in STAT3 have a block in the development of Th17 cells and the production of Th17-effector cytokines [13,14]. The most prevalent infections in HIES patients are Candida and Staphylococcus infections, further supporting the role of Th17 cells in the elimination of these pathogens.
In the present study, we focused on characterization of the Th17 compartment in patients with CGD, a primary immunodeficiency that shares an identical spectrum of infections with HIES but involves a genetic defect that affects the function of neutrophils, the most important target cells of Th17 cells. Th17 effector cytokines regulate granulopoiesis, neutrophil chemotaxis and the production of anti-microbial peptides [24,25]. Our results show that CGD patients have profound disturbances in the homeostasis of the Th17 compartment, despite an apparently normal capacity of naive CD4 T cells to differentiate into Th17 effectors cells in the presence of Th17-polarizing cytokines. The inability of defective neutrophils to eliminate the persistent burden of Candida and S. aureus results in continuous stimulation of APCs, increased production of IL-23 and secondary expansion of Th17 cells. Thus, a simple analysis of Th17/IFN-γ-producing T cells can serve as a quick and reliable test in patients suspected of having HIES or CGD. The expanded Th17 compartment in CGD patients produces excessive amounts of effector Th17 cytokines, attracting dysfunctional neutrophils to sites of infection. This factor most probably plays a role in the pathogenesis of granulomas, a hallmark of the CGD presentation. Notably, the clinical presentation of CGD patients differs from that of patients with HIES and mucocutaneous candidiasis, as CGD patients are at increased risk of developing a broad spectrum of specific autoimmune diseases, consistent with the high autoimmune potential of Th17 cells [26]. Activation of the Th17 axis (overproduction of IL-23, amplification of Th17 cells and excessive production of Th17 effector cytokines) has been implicated in patients with rheumatoid arthritis [27], multiple sclerosis [28], inflammatory bowel disease [29] and psoriasis [30]. Secondary expansion and activation of the Th17 effector arm of the immune system could thus participate in the pathogenesis of Crohn-like inflammatory bowel disease seen in as many as 30% of CGD patients [31]. In additional 10% of CGD patients have other autoimmune disorders, such as rheumatoid arthritis [27] and systemic lupus [32]. Under the prevailing paradigm, inflammatory bowel disease in CGD patients have been viewed as part of a broad spectrum of granuloma formation. The identification of activated Th17 cells in CGD patients in the present study provides experimental evidence for a new paradigm proposed recently by De Ravin et al., which states that manifestations of autoimmune diseases in CGD patients should be regarded and treated as separate clinical entities according to the treatment protocols for autoimmunities [26].
We had a unique opportunity to monitor the kinetics of Th17 compartment reconstitution in two CGD patients who underwent a successful allogeneic HSCT. Correction of the NADPH deficiency resulted in the normalization of the frequency of Th17 cells and normalization of IL-17, IL-21 and IL-23 production, further indicating that the activation of Th17 cells in CGD patients is secondary to the neutrophil deficiency.
Taken together, studies of CARD9 deficiency, HIES patients and CGD patients with genetic defects at different levels in the Th17 arm of the immune system have helped to elucidate the role of Th17 lymphocytes in protective immunity against C. albicans and S. aureus and in co-ordinating anti-fungal immune reactions in humans. Figure 4 illustrates the consequences of genetic defects in the development of Th17 immune responses. CARD9 deficiency prevents the activation of APCs and abrogates the production of Th17-polarizing cytokines, resulting in a deficient number of Th17 cells and defective clearance of pathogens. Although it could be assumed that naive T cells in patients with CARD9 deficiency have a normal capacity to differentiate into Th17 cells, this point remains to be tested.
Fig. 4.
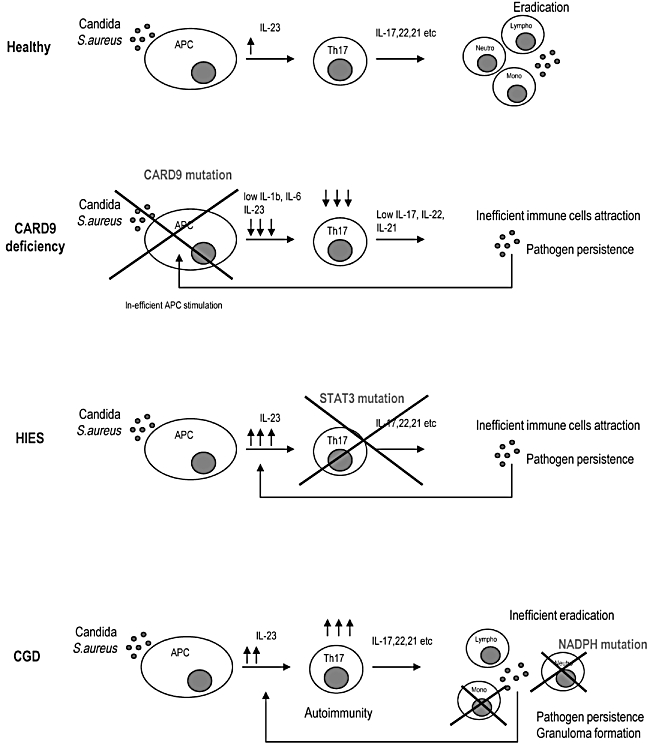
Disturbances of the T helper type 17 (Th17) compartment in primary immunodeficiency (PID) patients with chronic Candida albicans infections. CARD9 deficiency prevents the activation of antigen-presenting cells and abrogates the production of Th17-polarizing cytokines. This results in a deficient number of Th17 cells and defective clearance of pathogens. Although one would assume that naive T cells in patients with CARD9 deficiency have normal capacity to differentiate into Th17 cells, this point remains to be tested. In hyper-IgE syndrome (HIES) patients, signal transduction and activator of transcription 3 (STAT3) mutations affect the development of Th17 cells and the production of Th17 effector cytokines resulting in impaired attraction of neutrophils. Persistent infection drives the stimulation of antigen-presenting cells and excessive interleukin (IL)-23 production. In chronic granulomatous disease (CGD), defective neutrophils fail to eliminate candical and staphylococcal infections, despite normal function of recognition mechanisms in antigen-presenting cells and normal function of Th17 lymphocytes. Persistent infection drives overactivation of the Th17 axis, as documented by increased levels of IL-23, Th17 lymphocytes and Th17 effector cytokines. Excessive activation of the Th17 axis potentiates granuloma formation and probably plays a role in the pathogenesis of autoimmune disorders in CGD patients.
In HIES patients, STAT3 mutations affect the development of Th17 cells and the production of Th17 effector cytokines, resulting in impaired attraction of neutrophils. Persistent infection drives the stimulation of APCs and excessive IL-23 production, as shown in the HIES patients tested in the present study. However, excessive levels of IL-23 in HIES patients do not induce inflammatory bowel disease indicating that, at least in humans, Th17 cells must be present for IL-23 to participate in the pathogenesis of inflammatory bowel disease. Impaired STAT3 signalling in non-immune cells might account for other clinical features in HIES patients, such as skeletal abnormalities.
In CGD patients, defective neutrophils fail to eliminate Candida and Staphylococcus infections, despite normal function of recognition mechanisms in APCs and normal function of Th17 lymphocytes. Persistent infection drives over-activation of the Th17 axis as documented by increased levels of IL-23, Th17 lymphocytes and Th17 effector cytokines. Excessive activation of the Th17 axis potentiates granuloma formation and probably plays a role in the pathogenesis of autoimmune disorders in CGD patients, notably inflammatory bowel disease.
From a therapeutic viewpoint, HIES and CARD9 deficient patients could benefit from approaches that potentiate the Th17 responses. In CGD patients this approach would be inefficient and potentially harmful. It is conceivable that CGD patients might benefit from strategies that inhibit Th17 cells to alleviate autoimmunity. Infusions of functional granulocytes also represent a transient benefit, as they can participate in pathogen elimination and lead to the decrease in Th17 activation. HSCT performed in eligible patients is, however, currently the only curative treatment, as it corrects the genetic defect. Interestingly, the use of IFN-γ in CGD patients has long been discussed. IFN-γ, a Th1-specific cytokine, has been shown to inhibit the expansion of Th17 cells.
Taken together, by analysing patients with CGD, our findings complement recent human data on the importance of Th17 cells for successful elimination of infections with C. albicans and S. aureus. Similar to patients with CARD9 deficiency and HIES, CGD patients have disturbances in the homeostasis of the Th17 compartment. In CGD patients, the expansion of Th17 cells and Th17-effector cytokines is secondary to the dysfunction of neutrophils and can be corrected by allogeneic HSCT. Overproduction of IL-23 and Th17-effector cytokines probably plays a role in the pathogenesis of autoimmune diseases seen frequently in CGD patients. Studies of human PIDs characterized by persistent infections with C. albicans and S. aureus have revealed the critical role of Th17 immune responses for the elimination of these pathogens. The concerted action of all components of the Th17 axis, including APCs, Th17 cells and neutrophils, is a prerequisite for protective immunity against both C. albicans and S. aureus.
Conclusions
Our findings complement recent human data on the importance of Th17 cells for successful elimination of infections with C. albicans and S. aureus. Overproduction of IL-23 and Th17-effector cytokines probably plays a role in the pathogenesis of autoimmune diseases seen frequently in CGD patients.
Acknowledgments
This study was supported by grant IGA NS10489-3/2009 from the Ministry of Health Czech Republic and project MSM 0021620812 from The Czech Ministry of Education.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang XO, Pappu BP, Nurieva R, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 5.Chabaud M, Durand JM, Buchs N, et al. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–70. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 6.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mangan PR, Harrington LE, O'Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 8.Chung DR, Kasper DL, Panzo RJ, et al. CD4+ T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism. J Immunol. 2003;170:1958–63. doi: 10.4049/jimmunol.170.4.1958. [DOI] [PubMed] [Google Scholar]
- 9.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–15. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 10.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 11.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–31. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 12.O'Connor W, Jr, Zenewicz LA, Flavell RA. The dual nature of T(H)17 cells: shifting the focus to function. Nat Immunol. 2010;11:471–6. doi: 10.1038/ni.1882. [DOI] [PubMed] [Google Scholar]
- 13.Ma CS, Chew GY, Simpson N, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–7. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milner JD, Brenchley JM, Laurence A, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–6. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckley RH. Primary immunodeficiency diseases: dissectors of the immune system. Immunol Rev. 2002;185:206–19. doi: 10.1034/j.1600-065x.2002.18517.x. [DOI] [PubMed] [Google Scholar]
- 16.Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine. 2000;79:170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178:3107–15. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- 18.Gross O, Gewies A, Finger K, et al. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature. 2006;442:651–6. doi: 10.1038/nature04926. [DOI] [PubMed] [Google Scholar]
- 19.Leibundgut-Landmann S, Osorio F, Brown GD, Reis e Sousa C. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood. 2008;112:4971–80. doi: 10.1182/blood-2008-05-158469. [DOI] [PubMed] [Google Scholar]
- 20.Hsu YM, Zhang Y, You Y, et al. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol. 2007;8:198–205. doi: 10.1038/ni1426. [DOI] [PubMed] [Google Scholar]
- 21.Glocker EO, Hennigs A, Nabavi M, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–35. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–46. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 23.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–94. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Ivanov BS, II, McKenzie L, Zhou CE, et al. Littman. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 26.De Ravin SS, Naumann N, Cowen EW, et al. Chronic granulomatous disease as a risk factor for autoimmune disease. J Allergy Clin Immunol. 2008;122:1097–103. doi: 10.1016/j.jaci.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee BW, Yap HK. Polyarthritis resembling juvenile rheumatoid arthritis in a girl with chronic granulomatous disease. Arthritis Rheum. 1994;37:773–6. doi: 10.1002/art.1780370524. [DOI] [PubMed] [Google Scholar]
- 28.Lock C, Hermans G, Pedotti R, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–8. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 29.Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–7. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 31.Marciano BE, Rosenzweig SD, Kleiner DE, et al. Gastrointestinal involvement in chronic granulomatous disease. Pediatrics. 2004;114:462–8. doi: 10.1542/peds.114.2.462. [DOI] [PubMed] [Google Scholar]
- 32.Schmitt CP, Scharer K, Waldherr R, Seger RA, Debatin KM. Glomerulonephritis associated with chronic granulomatous disease and systemic lupus erythematosus. Nephrol Dial Transplant. 1995;10:891–5. [PubMed] [Google Scholar]


