Abstract
Dendritic cell (DC) function is believed to be of critical importance for the pathogenesis of inflammatory bowel disease (IBD). To date, most research in animal models and the few human data available is restricted to myeloid DC, while plasmacytoid DC (pDC) capable of controlling both innate and adaptive immune responses have not yet been investigated systematically in human Crohn's disease (CD) or ulcerative colitis (UC). CD11c-, CD303+/CD304+ and CD123+ pDC from peripheral blood (n = 90), mucosal tissue (n = 28) or mesenteric lymph nodes (n = 40) (MLNs) of patients with UC and CD or controls were purified and cultured. Thereafter, pDC were enumerated, phenotyped and cytokine secretion measured by flow cytometry (FACS), immunohistochemistry and/or cytometric bead array, respectively. Interferon (IFN)-α secretion following cytosine phosphatidyl guanine (CpG) A oligodeoxynucleotide (ODN) 2216 (5′-GGGGGACGATCGTCGGGGGG-3′) stimulation was assessed by enzyme-linked immunosorbent assay (ELISA). We found a significantly higher frequency of pDC in the inflamed colonic mucosa and MLN of IBD patients. Moreover, the fraction of CD40 and CD86 expressing cultured peripheral blood pDC was significantly higher in flaring UC and CD patients and their secretion of tumour necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8 were increased significantly compared with controls. In contrast, the IFN-α secretion of peripheral blood pDC isolated from flaring IBD, particularly in UC patients, was reduced significantly compared with controls. Our data suggest an aberrant distribution and function of pDC in IBD, contrary to their generally implicated role as inducers of tolerance. We speculate that the impaired IFN-α secretion may relate to the hypothesized defect in innate immunity in IBD and could also impact upon the generation of regulatory T cells (Treg).
Keywords: antigen presentation, Crohn's disease, dendritic cells, ulcerative colitis
Introduction
Crohn's disease (CD) and ulcerative colitis (UC) are two debilitating, progressive, systemic inflammatory illnesses targeting mainly the gastrointestinal tract in genetically susceptible individuals [1]. Dendritic cells (DC) are thought to be involved critically in the breakdown of immunological tolerance towards indigenous microbiota and the resulting inflammatory T cell polarization and activation known to cause these two disorders [2]. To date, research on DC in human inflammatory bowel disease (IBD) is limited to the investigation of myeloid DC (mDC), monocyte generated DC (moDC) and animal work with DC derived from rodent models or in vitro systems, while plasmacytoid DC (pDC) have not yet been studied systematically in human IBD [3]. However, based on the available literature, pDC are thought to be of key importance for the regulation of tolerance, considered to be inductors of regulatory T cells and thus key to our understanding of the aetiology and pathophysiology of IBD [4–6]. Our group has recently demonstrated a striking correlation between pDC and disease activity [7]. Here, we present the first systematic analysis of primary human pDC from IBD patients.
Materials and methods
Patients and tissues
Peripheral blood was obtained from patients with UC (n = 39) or CD (n = 37) seen at our Inflammatory Bowel Disease Center. Control samples (n = 14) were obtained from healthy volunteers or blood bank leucocyte filters [for the interferon (IFN)-α experiments]. For tissue investigations, mucosal tissue (n = 28) or mesenteric lymph nodes (MLNs) (n = 40) from IBD patients or patients undergoing surgery for non-inflammatory (diverticulosis or irritable bowel syndrome) conditions were collected. Samples were analysed exclusively from carefully selected patients off steroids, biologics, immunomodulators or immunosuppressants. The study protocol was approved by Charité's institutional review board. All patients and volunteers gave informed consent to the study. Two validated instruments, the modified Truelove Witts Severity Index (MTWSI) for UC and the Harvey Bradshaw Severity Index (HBSI) for CD were used to assess disease activity [8,9]. UC patients who scored ≥ 10 on the MTWSI and CD patients who scored ≥ 7 on the HBSI were categorized to have active disease [flare-up (FU)]; all others as remission (RM). Table 1 summarizes the demographic data.
Table 1.
Demographic data of patients and controls for peripheral blood plasmacytoid dendritic cells (pDC) experiments
| (n) | % | Mean | Min | Max | |
|---|---|---|---|---|---|
| Number | 76 | ||||
| Ethnicity | |||||
| Caucasian | 74 | 97·4 | |||
| Jewish | 2 | 2·6 | |||
| Crohn's disease | 37 | 41 | |||
| Male | 7 | 18·9 | |||
| Female | 30 | 81·1 | |||
| Age (years) | 35 | 19 | 73 | ||
| Disease duration (years) | 10 | 0 | 41 | ||
| Age at diagnosis (years) | 26 | 10 | 63 | ||
| Harvey Bradshaw Severity Index remission | 1·5 | 0 | 5·5 | ||
| Harvey Bradshaw Severity Index flare-up | 8·2 | 7 | 18 | ||
| Ulcerative colitis | 39 | 51·3 | |||
| Male | 17 | 45·6 | |||
| Female | 22 | 56·4 | |||
| Age (years) | 48 | 19 | 85 | ||
| Disease duration (years) | 12 | 0 | 39 | ||
| Age at diagnosis (years) | 36 | 14 | 65 | ||
| Modified Truelove Witts Index remission | 4·5 | 0 | 9 | ||
| Modified Truelove Witts Index flare-up | 11 | 10 | 13 | ||
| Healthy controls | 14 | ||||
| Male | 6 | ||||
| Female | 8 | ||||
| Age (years) | 30 | 26 | 37 |
Purification of pDC from peripheral blood
Peripheral blood mononuclear cells (PBMC) were obtained from whole blood as described previously [7]. pDC were isolated and purified by magnetic cell separation from PBMCs using CD304 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) as published previously [7]. Viability and purity of the isolated pDC population was checked by fluorescence activated cell sorter (FACS) and samples with less than 95% pDC discarded.
Purification of CD45+ mucosal cells
CD45+ cells were isolated from surgical specimens of mucosal tissue using magnetic separation with commercial kits (Miltenyi Biotech), as described previously [10]. Viability and purity of the isolated lymphocyte population were checked as described above for blood.
Analysis of pDC from MLNs
MLNs were homogenized and washed in magnetic affinity cell sorting (MACS) buffer. The cell suspension was filtered through a sterile gauze and passed through a 0·25-mm nylon mesh. Finally, cells were washed again and labelled with CD123 and CD303 (BDCA-2) for further analysis as described above for blood.
Culture of pDC
pDC were cultured as described previously [7]. Briefly, 5 × 104 freshly isolated pDC were resuspended in 150 µl RPMI-1640 plus l-glutamine medium with 1% penicillin/streptomycin, 1% sodium pyruvate (all Gibco, New York, USA) and 10% human serum type AB (Cambrex, Charles City, VA, USA) supplemented with interleukin (IL)-3 at 10 ng/ml (R&D Systems, Wiesbaden, Germany) (to prevent apoptosis) and incubated for 21 h at 37°C and 5% CO2.
Cytometric bead array (CBA)
Secretion of TNF-α, IL-6 and IL-8 in culture supernatants was measured by CBA analysis according to the manufacturer's guidelines (BD Biosciences, Heidelberg, Germany). After acquisition of sample data using FACS, the cytokine concentrations were calculated using the proprietary FCAP™ analysis software (BD Biosciences) [11].
IFN-α ELISA
To assess IFN-α secretion, pDC were isolated from peripheral blood from patients and healthy volunteers or fresh blood bank leucocyte filters (collected within the hour of blood donation). The cells were cultured as described above and stimulated with 3 µg/ml cytosine phosphatidyl guanine (CpG) A [oligodeoxynucleotide (ODN) 2216] 5′-GGGGGACGATCGTCGGGGGG-3′ (TIB Molbiol, Berlin, Germany). Side-by-side experiments of volunteer- and filter-derived pDC did not find any differences regarding purity and IFN-α secretion (data on file). Supernatants were harvested and analysed with a human IFN-α ELISA kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions. The IFN-α concentration was calculated with KCjunior™ software (Wolf Laboratories, York, UK), version 1.41.4.
Antibodies
All monoclonal antibodies [CD40, CD45, CD83, CD86, CD123 and CD197 (CCR7)] were obtained as fluorochrome conjugates from BD Biosciences. CD303 was purchased from Miltenyi Biotech.
For immunofluorescence staining anti-CD3-phycoerythrin (PE) (Caltag, San Diego, CA, USA) and biotinylated anti-CD303 (Miltenyi Biotech) were used as primary antibodies. As a secondary antibody antibio-Alexa488 was used (Invitrogen, Darmstadt, Germany). To exclude unspecific binding, biotinylated mouse immunoglobulin (Ig)G1 (Caltag) was used as an isotype control.
Flowcytometric analysis
Three- or four-colour flow cytometric analysis (FACS) was used to identify and enumerate pDC as described previously [7,12].
Immunohistochemistry
MLNs were embedded in O.C.T. compound (Tissue-Tek, Zoeterwoude, Netherlands), frozen in liquid nitrogen and stored at −80°C. Specimens were sectioned at 7 µm in a cryostat at −25°C on glass slides. Cryosections were circled using a Pap-Pen (Dako, Glostrup, Denmark) prior to staining. All incubation steps were performed in a moisture chamber at room temperature. To block unspecific protein binding of IgG molecules prior to the exposure of the primary antibodies, tissue sections were preincubated with blocking buffer. Slides were incubated for 1 h with the primary antibodies at a final concentration of 16·5 µg/ml to 20 µg/ml before washing them on a shaker 3× for 10 min. Fluorochrome-conjugated secondary antibodies, at a final concentration of 5 µg/ml, were added for 1 h. Slides were washed for 3 × 10 min in washing buffer followed by water. Finally, slides were fixed with ethanol, mounted and examined with a confocal laser scanning microscope (LSM510; Zeiss, Jena, Germany). Confocal images were processed using Zeiss LSM Image Browser software.
Statistical analysis
Data are expressed as mean ± standard error of the mean (s.e.m.). Comparisons were performed by a Student's t-test with statistical significance accepted for P < 0·05.
Results
Increased frequency of pDC in the mucosa and MLNs
It is conceivable that circulating DCs in active IBD migrate to secondary lymphatic organs and sites of inflammation, as suggested by previous work by our group [7]. We decided to corroborate this hypothesis further by evaluating pDC in two other spaces – the colonic mucosa and MLNs.
Colonic mucosa
We found a significantly higher frequency of pDC in the inflamed mucosa of UC patients [0·56 ± 0·24% of CD45 + lamina propria mononuclear cells (LPMC)] and CD patients (0·19 ± 0·04% of CD45+ LPMC) compared with controls (0·03 ± 0·01% of CD45 + LPMC) (Fig. 1a).
Fig. 1.
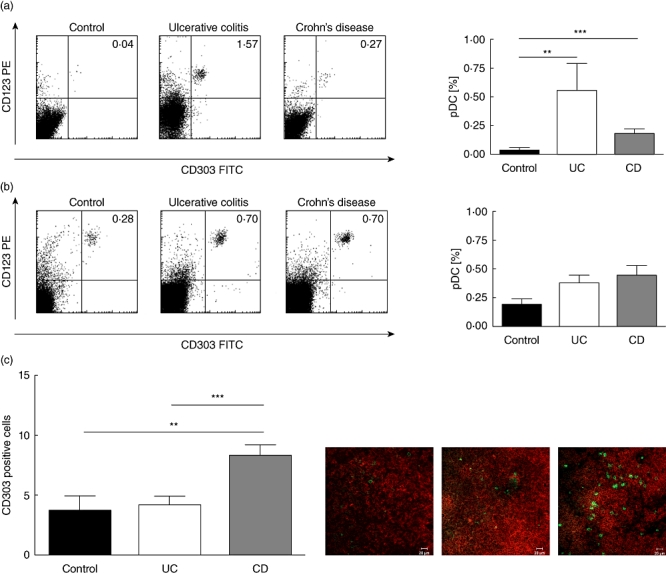
Increased frequency of plasmacytoid dendritic cells (pDC) in mesenteric lymph nodes and colonic mucosa of flaring inflammatory bowel disease (IBD) patients. (a) Colonic mucosa: FACS plots from representative experiments. Quadrant thresholds were placed determined according to isotype controls. Bar graphs summarize data from all experiments with control mucosa (n = 16) or tissue from ulcerative colitis (UC) (n = 6) and Crohn's disease (CD) (n = 6) patients. (b) Mesenteric lymph node: FACS plots from representative experiments. Quadrant thresholds were placed determined according to isotype controls. Bar graphs summarize data from all experiments with control lymph node (n = 6) or tissue from UC (n = 14) and CD (n = 20) patients. (c) Mesenteric lymph node: sample immunofluorescence images from control, UC and CD. Asterisks denote statistical significance: **P < 0·01; ***P < 0·001.
MLNs
The results from the mucosal space matched with the MLN. An increased frequency was found of pDC in MLNs of UC (0·39 ± 0·06%) and CD patients (0·45 ± 0·09%) compared with controls (0·19 ± 0·06%) (Fig. 1b). These data were corroborated by immunohistochemistry. Here we counted a higher number of pDC on immunohistochemical sections of both patients with UC (4·20 ± 0·77) and even more in those suffering from CD (8·34 ± 0·89) compared with non-IBD controls (3·67 ± 1·28) (Fig. 1c).
Distinct phenotype of peripheral blood pDC in CD and UC
Because we hypothesize that DC from IBD patients may induce and/or perpetuate inflammation, we investigated their native phenotype. We were particularly interested in their expression of co-stimulatory molecules. Expression of CD40 and CD86, two co-stimulatory molecules known to be up-regulated on mature, activated DC and critical for T cell activation was studied [13,14].
CD40
In UC, the fraction of CD40 expressing pDC was higher in UC patients in RM (16·83 ± 7·07%) and reached statistical significance in acute FUs (15·16 ± 0·58%, P < 0·001) compared with controls (4·22 ± 1·17%). In CD, the fraction of CD40 expressing pDC was comparable between controls and CD patients in RM (3·67 ± 2·38%). However, the fraction of CD40 expressing pDC was significantly higher in flaring patients (11·60 ± 3·4%) compared with controls (P < 0·05) (Fig. 2a).
Fig. 2.
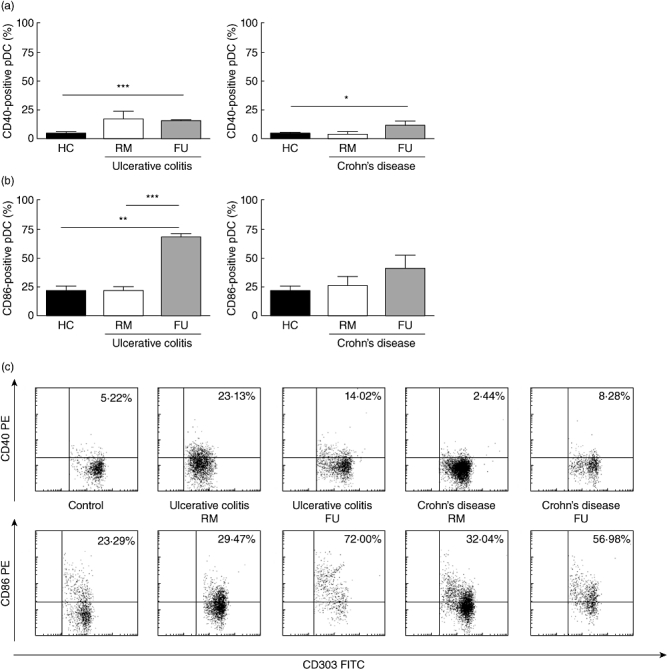
Distinct phenotype of peripheral blood plasmacytoid dendritic cells (pDC) in Crohn's disease (CD) and ulcerative colitis. (a) CD40: increased fraction of CD40 expressing pDC in flaring UC [remission (RM) n = 6, flare-up (FU) n = 3] and CD (RM n = 4, FU n = 3) patients compared with controls (n = 6). (b) CD86: increased fraction of CD86 expressing pDC in flaring UC (RM n = 3, FU n = 3) and CD (RM n = 4, FU n = 4) patients compared with controls (n = 5). (c) FACS plots from representative experiments. Quadrant thresholds were placed determined according to isotype controls. Asterisks denote statistical significance: **P < 0·01; ***P < 0·001.
CD86
In UC, the fraction of CD86 expressing pDC was comparable between controls (21·78 ± 4·48%) and UC patients in RM (22·83 ± 2·82%). However, the fraction of CD86 expressing pDC was significantly higher in flaring patients (69·13 ± 2·87%) compared with controls (P < 0·01) and RM (P < 0·001). In CD, the fraction of CD86 expressing pDC was higher in patients in RM (26·51 ± 8·17%) and acute FUs (41·48 ± 11·68%) compared with controls (Fig. 2b).
CD83 and CD197 (CCR7)
Overall, the fractions of CD83 and CD197 expressing pDC were very low and did not allow meaningful statistical comparisons (data not shown).
Increased secretion of key inflammatory cytokines by peripheral blood pDC
Because our previous studies suggested not only migration but also an activated phenotype of DC in active IBD, we then studied functional aspects of pDC, such as the native (unstimulated) secretion of key inflammatory cytokines such as TNF-α, IL-6 and IL-8.
TNF-α
Cultured pDC from UC patients (UC RM 24·11 ± 2·40 versus UC FU 32·94 ± 27·27 pg/ml) (Fig. 3a) and CD (CD RM 30·54 ± 9·18 pg/ml versus CD FU 34·81 ± 9·33 pg/ml) secreted more TNF-α than controls (15·90 ± 3·44 pg/ml). This effect was most apparent in flaring Crohn's patients, where it also reached statistical significance (P < 0·05).
Fig. 3.
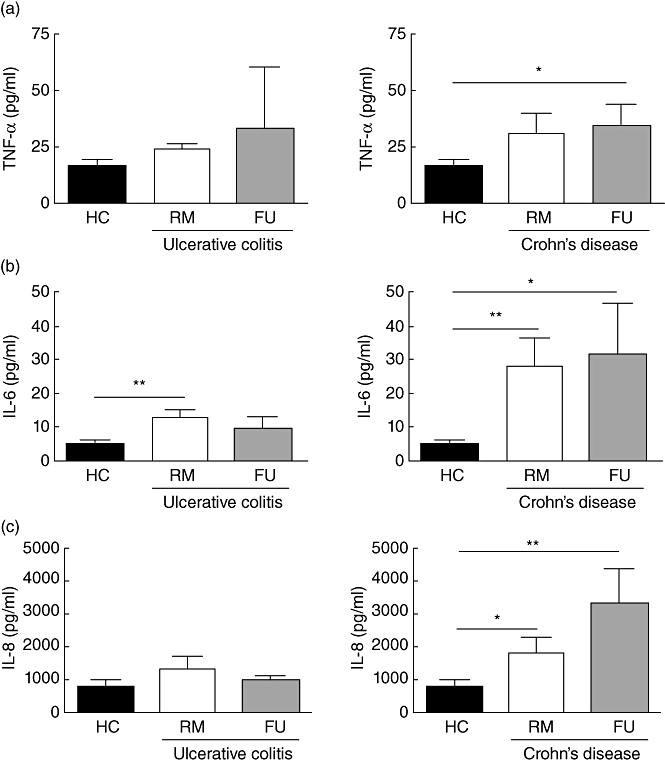
Increased secretion of key inflammatory cytokines by peripheral blood plasmacytoid dendritic cells (pDC) from inflammatory bowel disease (IBD) patients. (a) Tumour necrosis factor (TNF)-α: cultured pDC from ulcerative colitis [remission (RM) n = 7, flare-up (FU) n = 3] and Crohn's disease (CD) (RM n = 8, FU n = 10) patients secrete more tumour necrosis factor (TNF)-α than healthy controls (n = 14). (b) Interleukin (IL)-6: cultured pDC from UC (RM n = 7, FU n = 3) and CD (RM n = 8, FU n = 9) patients secrete more IL-6 than healthy controls (n = 13). (c) IL-8: cultured pDC from UC (RM n = 6, FU n = 3) and CD (RM n = 9, FU n = 9) patients secrete more IL-8 than healthy controls (n = 14). Asterisk denotes statistical significance *P < 0·05; **P < 0·01.
IL-6
Surprisingly, cultured pDC from UC patients in RM secreted more IL-6 (UC RM 12·90 ± 2·25 pg/ml versus UC FU 9·56 ± 3·53 pg/ml) than flaring patients and both more than healthy controls (4·88 ± 1·39 pg/ml) (Fig. 3b). In CD, however, both patients in RM and during FUs (CD RM 28·24 ± 8·56 pg/ml versus CD FU 31·75 ± 15·20 pg/ml) secreted significantly more IL-6, than controls (P < 0·01 and P < 0·05), respectively.
IL-8
As already observed for IL-6, cultured pDC from UC patients in RM secreted more IL-8 (UC RM 1312 ± 385·6 pg/ml versus UC FU 964·1 ± 143·7 pg/ml) than flaring patients and both more than healthy controls (740·7 ± 234 pg/ml). However, these differences did not reach statistical significance (Fig. 3c). In CD, both patients in RM and during FUs (CD RM 1777 ± 481·9 pg/ml versus CD FU 3301 ± 1039 pg/ml) secreted significantly more IL-8, than controls (P < 0·05 and P < 0·01), respectively.
Impaired secretion of IFN-α by pDC
Because native (unstimulated) pDC from both IBD patients and controls do not secrete any IFN-α (data not shown), we had to use a stimulant for these experiments. Cultured and CpG ODN 2216-stimulated pDC from UC patients (UC RM 154·9 ± 61·08 and UC FU 87·29 ± 83·51 ng/ml) secreted significantly less IFN-α than control pDC (585·6 ± 72·18 ng/ml, P < 0·01). In CD the significantly decreased secretion was restricted to flaring patients (CD RM 707·2 ± 117·9 ng/ml versus CD FU 195·9 ± 107·8 ng/ml, P < 0·01) (Fig. 4).
Fig. 4.
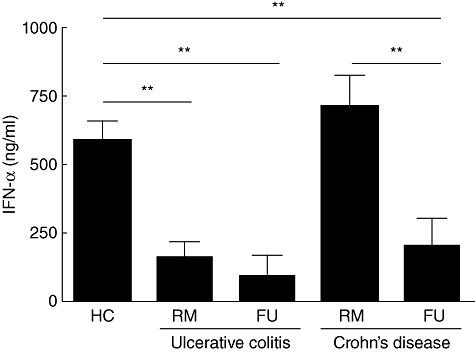
Impaired secretion of interferon (IFN)-α by peripheral blood plasmacytoid dendritic cells (pDC) from inflammatory bowel disease (IBD) patients. Cytosine–phosphatidyl guanine (CpG) A oligodeoxynucleotide (ODN) 2216-stimulated pDC from flaring ulcerative colitis (UC) (RM n = 6, FU n = 3) and CD (RM n = 6, FU n = 6) patients secrete significantly less IFN-α than healthy controls (n = 14). Asterisks denote statistical significance: **P < 0·01.
Discussion
To date, DC research in human IBD has focused mainly on mDC [10,15–19]. However, while the occasionally confusing classification of DC subsets in man and mice is still ongoing, there is largely agreement that mDC and pDC originate from different progenitors alongside different lineages and thus have distinct properties and functions within the immune system [20,21].
pDC develop in the bone marrow from a continuum of CD135+(Flt3+), CD117+(c-kitlow) lymphoid and common DC progenitors (CDP) and proceed through the putative committed pDC precursor and immature pDC (both still in the bone marrow) towards mature peripheral pDC [22]. The main function of pDC is their recognition of foreign (normally bacterial and viral) nucleic acids through their endosomal Toll-like receptors TLR-7 and TLR-9, resulting in the secretion of large amounts of type I IFNs, but also IBD typical inflammatory cytokines such as TNF-α and IL-12 (in animals) that recruit additional immune cells such as natural killer (NK) cells and thereby link the innate and adaptive immune responses [23,24]. Their frequency is rare, at approximately 0·1–2%, and renders their study in human disease somewhat difficult [7,25]. Therefore, it is perhaps not surprising that this is the first systematic analysis of their role in human CD and UC.
For this project we have exclusively analysed highly purified pDC from IBD patients in RM and acute FUs off immunomodulating, immunosuppressive or biological drugs to avoid any influence on their function, which we consider a major strength of our work. Some patients were on mesalamine, which is not known to alter DC function.
Our data show an increased frequency of IBD pDC in both the mucosal space and MLNs that, taken together by our previously reported drop in the peripheral circulation, suggest their migration to areas where they could potentially encounter their cognate antigens, e.g. foreign (bacterial) or foreign-thought nucleic acids [7]. Our data corroborate observations in murine adoptive transfer models that previously reported an accumulation of pDC in the inflamed mucosa [26] and demonstrated the CCR9-dependent homing of pDC to the intestine under inflammatory conditions [27,28].
Interestingly, the frequency of CD40 and CD86, i.e. co-stimulatory molecule expressing unstimulated peripheral blood pDC from flaring IBD patients, is already higher than in controls. This activated phenotype suggests a premature activation of pDC that may contribute to the well-established overactivation of T cells in IBD.
This interpretation is supported further by an increased secretion of the inflammatory cytokines IL-6, IL-8 and TNF-α by pDC isolated from flaring IBD patients. In other words, the observed phenotypic differences are paralleled by functional consequences. An increased secretion of these prototypical inflammatory cytokines polarizes T cells towards a proinflammatory phenotype [T helper type 1 (Th1)] that contributes to the perpetuation of inflammation known to occur in IBD [29]. Approved and investigational drugs individually targeting TNF and IL-6 are available and clinically effective, emphasizing their importance in IBD [30,31]. Conversely, CpG ODN 2216-stimulated secretion of the pDC hallmark cytokine IFN-α is reduced significantly in flaring UC and CD patients. IFN-α links both adaptive and innate immunity and recent IBD research has pointed to defects in the innate immune response [32,33]. IFN-α protects from and ameliorates experimental colitis in animal models [34,35], and has been used successfully to treat human UC [36]. While the exact role of IFN-α in chronic (intestinal) inflammation remains unclear, it appears there is a dichotomy of effects mediated through different pathways. On one hand, IFN-α–TLR-9 engagement could activate the NF-κB pathway [37], known to be critically important in IBD [2]. This mechanism has been demonstrated recently with pDC in lupus patients and contributes to the perpetuation of inflammation [38]. However, IFN-α has also been demonstrated to indirectly [39] and directly [40] induce regulatory T cells (Treg) and thereby attenuate Th1 responses. Our research demonstrates a diminished IFN-α secretion of predominantly UC pDC in both RM and FUs. This provides a new explanation for the known lack of IFN-α in human UC and the reported mixed efficacy in preclinical models and patients with UC [41,42].
Chemokine receptor 7 (CCR7, CD197), which contributes to both immunity and tolerance, was essentially not expressed by unstimulated pDC [43]. The functional dichotomy of CCR7 is reflected by CCR7-deficient mice that exhibit a phenotype with inflammatory lymphoid structures of the gastrointestinal mucosa and in turn its requirement for proper Treg function [44,45]. The low expression of CCR7 clearly does not appear to impact on homing of human pDC in human IBD shown by us in this study, and previously in animal models [26–28].
Two other studies have attempted to characterize pDC in human mucosal tissue and MLNs from IBD patients [46,47]. The first study was limited by critical methodological flaws. Here the authors claim that fascin would be a global marker for human DC including pDC. In immunohistochemical studies using triple staining with fascin, CD123, CD303, they did not find an increased frequency of pDC in mucosal and lymph node samples of CD patients. Neither fascin nor CD123 are specific markers for human pDC [22]. However, the combined staining may have masked the epitope for proper CD303 binding and thus caused misleading results. As our data are not based exclusively on immunohistochemistry but flow cytometry of complete mucosal tissue, not simply sections, we are confident that they reflect the true situation in the human gut. Moreover, CD patients were not phenotyped according to the Montreal classification in that study, and their disease activity was not measured with validated instruments in this study [8,9,48]. This may have contributed to a failure in the proper detection of pDC.
The second study reported larger MLNs in CD and increased total number of DCs, and thus generally supports our findings [47]. However, their panel to identify pDC, CD11c-, CD123+ and major histocompatibility complex (MHC)-II+ does not identify pDC unequivocally in humans [22].
Taken together, these data suggest that pDC differ substantially in their distribution, phenotype and function in human IBD. Their animal model-derived role as inducers of oral tolerance and Treg in inflammatory conditions of the intestine may be impaired in human inflammatory bowel disease [49,50]. Further research into additional molecular mechanisms appears warranted.
Acknowledgments
This work was supported by research grants fron the Eli & Edythe L. Broad Foundation, Los Angeles, CA, USA and Deutscher Akademischer Austauschdienst (DAAD), Bonn Germany as well as a Charité Medical School Bonus Research Grant to D. C. B. The authors thank the patients, medical, laboratory and nursing staff of the Charité Inflammatory Bowel Disease Center for their support.
Disclosure
None.
References
- 1.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–57. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 2.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–40. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 3.Kelsall B. Recent progress in understanding the phenotype and function of intestinal dendritic cells and macrophages. Mucosal Immunol. 2008;1:460–9. doi: 10.1038/mi.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matta BM, Castellaneta A, Thomson AW. Tolerogenic plasmacytoid DC. Eur J Immunol. 2010;40:2667–76. doi: 10.1002/eji.201040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reizis B. Regulation of plasmacytoid dendritic cell development. Curr Opin Immunol. 2010;22:206–11. doi: 10.1016/j.coi.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–62. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumgart DC, Metzke D, Schmitz J, et al. Patients with active inflammatory bowel disease lack immature peripheral blood plasmacytoid and myeloid dendritic cells. Gut. 2005;54:228–36. doi: 10.1136/gut.2004.040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–86. doi: 10.1053/j.gastro.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 9.Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology. 2002;122:512–30. doi: 10.1053/gast.2002.31072. [DOI] [PubMed] [Google Scholar]
- 10.Baumgart DC, Thomas S, Przesdzing I, et al. Exaggerated inflammatory response of primary human myeloid dendritic cells to lipopolysaccharide in patients with inflammatory bowel disease. Clin Exp Immunol. 2009;157:423–36. doi: 10.1111/j.1365-2249.2009.03981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carson RT, Vignali DA. Simultaneous quantitation of 15 cytokines using a multiplexed flow cytometric assay. J Immunol Methods. 1999;227:41–52. doi: 10.1016/s0022-1759(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 12.Baumgart DC, Olivier WA, Reya T, Peritt D, Rombeau JL, Carding SR. Mechanisms of intestinal epithelial cell injury and colitis in interleukin 2 (IL2)-deficient mice. Cell Immunol. 1998;187:52–66. doi: 10.1006/cimm.1998.1307. [DOI] [PubMed] [Google Scholar]
- 13.McLellan AD, Sorg RV, Williams LA, Hart DN. Human dendritic cells activate T lymphocytes via a CD40: CD40 ligand-dependent pathway. Eur J Immunol. 1996;26:1204–10. doi: 10.1002/eji.1830260603. [DOI] [PubMed] [Google Scholar]
- 14.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell SJ, Rigby R, English N, et al. Migration and maturation of human colonic dendritic cells. J Immunol. 2001;166:4958–67. doi: 10.4049/jimmunol.166.8.4958. [DOI] [PubMed] [Google Scholar]
- 16.Hart AL, Al-Hassi HO, Rigby RJ, et al. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50–65. doi: 10.1053/j.gastro.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda Y, Akbar SM, Matsui H, Onji M. Antigen-presenting dendritic cells in ulcerative colitis. J Gastroenterol. 2002;37(Suppl 14):53–5. doi: 10.1007/BF03326414. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda Y, Akbar F, Matsui H, Onji M. Characterization of antigen-presenting dendritic cells in the peripheral blood and colonic mucosa of patients with ulcerative colitis. Eur J Gastroenterol Hepatol. 2001;13:841–50. doi: 10.1097/00042737-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Vuckovic S, Florin TH, Khalil D, et al. CD40 and CD86 upregulation with divergent CMRF44 expression on blood dendritic cells in inflammatory bowel diseases. Am J Gastroenterol. 2001;96:2946–56. doi: 10.1111/j.1572-0241.2001.04686.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu K, Victora GD, Schwickert TA, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–7. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol. 2011;29:163–83. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 24.Krug A, French AR, Barchet W, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–19. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–20. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 26.Karlis J, Penttila I, Tran TB, et al. Characterization of colonic and mesenteric lymph node dendritic cell subpopulations in a murine adoptive transfer model of inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:834–47. doi: 10.1097/00054725-200411000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Wendland M, Czeloth N, Mach N, et al. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci USA. 2007;104:6347–52. doi: 10.1073/pnas.0609180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wurbel MA, McIntire MG, Dwyer P, Fiebiger E. CCL25/CCR9 interactions regulate large intestinal inflammation in a murine model of acute colitis. PLoS One. 2011;6:e16442. doi: 10.1371/journal.pone.0016442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 30.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 31.Ito H, Takazoe M, Fukuda Y, et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology. 2004;126:989–96. doi: 10.1053/j.gastro.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Blumberg R. What are innate and acquired immunity, and why are they important in IBD? Inflamm Bowel Dis. 2008;14(Suppl 2):S93–4. doi: 10.1002/ibd.20689. [DOI] [PubMed] [Google Scholar]
- 33.Rakoff-Nahoum S, Bousvaros A. Innate and adaptive immune connections in inflammatory bowel diseases. Curr Opin Gastroenterol. 2010;26:572–7. doi: 10.1097/MOG.0b013e32833f126d. [DOI] [PubMed] [Google Scholar]
- 34.Louis NA, Robinson AM, MacManus CF, Karhausen J, Scully M, Colgan SP. Control of IFN-alphaA by CD73: implications for mucosal inflammation. J Immunol. 2008;180:4246–55. doi: 10.4049/jimmunol.180.6.4246. [DOI] [PubMed] [Google Scholar]
- 35.Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, Raz E. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madsen SM, Schlichting P, Davidsen B, et al. An open-labeled, randomized study comparing systemic interferon-alpha-2A and prednisolone enemas in the treatment of left-sided ulcerative colitis. Am J Gastroenterol. 2001;96:1807–15. doi: 10.1111/j.1572-0241.2001.03875.x. [DOI] [PubMed] [Google Scholar]
- 37.Yang CH, Murti A, Valentine WJ, Du Z, Pfeffer LM. Interferon alpha activates NF-kappaB in JAK1-deficient cells through a TYK2-dependent pathway. J Biol Chem. 2005;280:25849–53. doi: 10.1074/jbc.M413721200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guiducci C, Gong M, Xu Z, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–41. doi: 10.1038/nature09102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aman MJ, Tretter T, Eisenbeis I, et al. Interferon-alpha stimulates production of interleukin-10 in activated CD4+ T cells and monocytes. Blood. 1996;87:4731–6. [PubMed] [Google Scholar]
- 40.Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal MR, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol. 2001;166:5530–9. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 41.Wirtz S, Neurath MF. Illuminating the role of type I IFNs in colitis. J Clin Invest. 2005;115:586–8. doi: 10.1172/JCI24518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seow CH, Benchimol EI, Griffiths AM, Steinhart AH. Type I interferons for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2008;(3):CD006790. doi: 10.1002/14651858.CD006790.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Forster R, valos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–71. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 44.Hopken UE, Wengner AM, Loddenkemper C, et al. CCR7 deficiency causes ectopic lymphoid neogenesis and disturbed mucosal tissue integrity. Blood. 2007;109:886–95. doi: 10.1182/blood-2006-03-013532. [DOI] [PubMed] [Google Scholar]
- 45.Schneider MA, Meingassner JG, Lipp M, Moore HD, Rot A. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J Exp Med. 2007;204:735–45. doi: 10.1084/jem.20061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Middel P, Raddatz D, Gunawan B, Haller F, Radzun HJ. Increased number of mature dendritic cells in Crohn's disease: evidence for a chemokine mediated retention mechanism. Gut. 2006;55:220–7. doi: 10.1136/gut.2004.063008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakuraba A, Sato T, Kamada N, Kitazume M, Sugita A, Hibi T. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn's disease. Gastroenterology. 2009;137:1736–45. doi: 10.1053/j.gastro.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 48.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5–36. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 49.Dubois B, Joubert G, Gomez de AM, Gouanvic M, Goubier A, Kaiserlian D. Sequential role of plasmacytoid dendritic cells and regulatory T cells in oral tolerance. Gastroenterology. 2009;137:1019–28. doi: 10.1053/j.gastro.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 50.Goubier A, Dubois B, Gheit H, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–75. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


