Abstract
Activation of the oxidative burst and failure of CD4+CD25+ cell regulation have been implicated in idiopathic nephrotic syndrome (iNS). The intimate mechanism is, however, unknown and requires specifically focused studies. We investigated reactive oxygen species (ROS) generation [di-chlorofluorescein-diacetate (DCFDA)] fluorescence assay and the regulatory adenosine 5′-triphosphate (ATP) pathways in the blood of 41 children with iNS, utilizing several agonists and antagonists of nucleotide/nucleoside receptors, including the addition of soluble apyrase. The CD4+CD25+CD39+/CD73+ expression was determined in vivo in parallel during disease activity. Overall, we found that the percentage of CD39+CD4+CD25+ was reduced markedly in iNS by 80% (3·43 ± 0·04% versus 13·14 ± 0·07% of total lymphocytes, P < 0·001). In these patients, reactive oxygen species (ROS) generation by polymorphonuclear neutrophils (PMN) at rest was a function of apyrase (CD39) expressed by CD4+CD25+, with higher rates in patients with very low CD39+CD4+CD25+ levels (< 7·5%). Addition of apyrase reduced ROS generation by 40% in both iNS and controls and was mainly effective in patients. The quota of ROS surviving ATP elimination was higher still in iNS. In vitro studies to limit ROS generation with adenosine analogues (2′-chloroadenosine and 5′-N-ethylcarboxamidoadenosine) produced minor effects. At variance, antagonizing ATP efflux with carbenoxolone or by antagonizing ATP effects (Brilliant Blue G, KN62 and A437089) reduced ROS generation comparable to apyrase. These results confirm a key role of ATP in the regulation of innate immunity and minimize the effect of adenosine. Decreased CD39+CD4+CD25+ expression in iNS highlights an impairment of ATP degradation in this pathology. However, high ROS surviving ATP consumption implies a major role of other regulatory pathways.
Keywords: adenosine, ATP regulatory pathway, free radicals, innate immunity, nephrotic syndrome, oxidation burst, proteinuria
Introduction
Primary idiopathic nephrotic syndrome (iNS) is a frequent source of morbidity in children. It requires long-term therapies with steroids and often combinations of drugs associated with side effects. In a minority of cases, mutations in podocyte genes [1–4] explain proteinuria. Pathogenesis, however, is unknown for the major group of patients who do not present molecular defects, in which case a general problem has been proposed linked to T cell immunity [4–8].
Multiple independent observations point to the involvement of free radicals of oxygen (ROS) in proteinuria deriving from an altered regulation by regulatory T cells (Tregs) of polymorphonuclear neutrophil (PMN) burst. In fact, ROS production by PMN in children with iNS is increased 10-fold and correlates with proteinuria [9]. Secondly, there is evidence that plasma proteins are massively oxidized in iNS, representing a hidden abnormal ROS activity in plasma [10–12]. Thirdly, it is well known that oxidants are toxic for the kidney in humans and in animals, and when oxidant production overcomes the intra- and extracellular defences, renal damage is generated [13,14]. Infusion of oxidants in animals produces a unique alteration, a disease that mimics focal segmental glomerulosclerosis, the most serious variant of iNS in children [15–21].
In general, anomalies of ROS production derive from leucocytes activated by immunological triggers (i.e. complement, immune complexes or ANCA) [22,23], but may also occur in the presence of mitochondrial defects or of an inherited defect of co-enzyme Q synthesis [24].
There is now increasing evidence that the nucleotide/nucleoside pathway regulates the immune system as a highly co-ordinated network in which adenosine deriving from adenosine 5′-triphosphate (ATP) degradation by ectoenzymes present on several blood cells functions as a repressor, while ATP is a prostimulatory molecule that promotes ROS production [25,26]. This pathway regulates ROS production mainly by neutrophils and represents [27], in terms of rapidity, the first immune response against pathogens. Purine nucleosides and nucleotides are released mainly from injured cells and modulate their extracellular action by specific receptors, among which P2X7 plays a major role. P2X7R is expressed in mammalian immune cells and epithelia with immunomodulatory function [28–30]. In this paper we address the main question of ROS regulation by the nucleotide/nucleoside pathway in patients with iNS by determining the deputed enzyme equipment and its functionality in vivo and then trying to modify their response by specific activator/inhibitors in vitro.
Materials and methods
Patients
Overall, 41 children with iNS were enrolled into this study (Table 1). Control blood samples were obtained from the Gaslini staff (22–45 years) and from children with hypercalciuria (2–16 years) who were followed at our department. Patients with iNS showed no familiarity or presented relevant mutations of slit-diaphragm genes (NPHS2, CD2AP2, WT1). Blood samples from children with iNS for ROS determination were collected and analysed repeatedly during different phases of the disease, i.e. recurrence and remission or partial remission of proteinuria. Criteria for the definition of nephrotic syndrome, renal histology and response to therapies utilized for clinical purposes have been detailed in a previous companion paper [31]. Informed consent to the study was obtained by parents' prior consent.
Table 1.
Clinical data relative to patient categories involved in the study. Renal function was graduated according to creatinine levels as 1 (serum creatinine < 1 mg%), 2 (serum creatinine between 1 and 2 mg%), 3 (serum creatinine > 2 mg%). Activity (+) indicates the presence of an increased proteinuria (Pu/Cu > 1)
| Patients | n | Sex (male/female) | Age (years) | Age at onset (years) | U prot (yes/no) | Renal function (1/2/3) | Serum C3 | Autoantibodies | Therapy (St/Csa/ACEi/other) |
|---|---|---|---|---|---|---|---|---|---|
| FSGS | 5 | 5/0 | 9 | 6 | 5/0 | 2/2/1 | Normal | No | 8/12/15/0 |
| IgM | 2 | 1/1 | 6 | 3 | 2/0 | 2/0/0 | Normal | No | 2/3/3/0 |
| MCN | 8 | 7/1 | 10 | 2 | 5/3 | 8/0/0 | Normal | No | 1/0/0/0 |
| No biopsy | 26 | 17/9 | 8 | 6 | 14/12 | 26/0/0 | Normal | No | 16/2/10/0 |
| Total | 41 | 30/11 | 6 | 3 | 30/11 | 38/2/1 | |||
| 10–15 | 2–7 |
ACEi: angiotensin converting enzyme inhibitors; FSGS: focal segmental glomerulosclerosis; IgM: mesangial proliferation with immunoglobulin (Ig)M deposition; ANCA: anti-neutrophil antibodies; MGN: membranous glomerulonephritis; MPGN: membrano-proliferative glomerulonephritis; St: steroids; csa: cyclosporine.
Cell sorting and fractionation
Peripheral whole blood was obtained in the morning after an overnight fast. After collection in heparinized tubes, blood was fractionated for purifying PMNs and monucleated cells, as described previously [31]. For PMNs, the following procedures were utilized: (i) initial sedimentation with dextran solution (Plander 70 000; 30 g/500 ml) for 45 min at room temperature; (ii) centrifugation in Ficoll-Histopaque 1077a at 290 g for 30 min at room temperature. PMNs were collected in the pellet and contaminating red blood cells were lysed in hypotonic solution (PMN purity ≥ 95%, as assessed by CD15 monoclonal antibody); (iii) monucleated cells (lymphocytes and monocytes) were collected at the interfaces of Ficoll and washed with Hank's balanced salt solution (HBSS); cells were then resuspended at a concentration of 106 cells/ml for di-chlorofluorescein-diacetate (DCFDA) fluorescence assay (Molecular Probes, Eugene, OR, USA).
Highly purified (> 90%) CD4+ CD25+ lymphocytes (Treg cells) were obtained using CD4+ CD25+ Regulatory T Cells Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany), used according to the manufacturer's instructions. Briefly, after density gradient separation of mononuclear cells from peripheral blood, CD4+ T cells were first purified by negative selection and subsequently fractionated into CD25- and CD25+ by positive selection, using a leucocyte depletion (LD) column. To assess purity, cytofluorimetric staining with anti-CD4 fluorescein isothiocyanate (FITC) and anti-CD25 phycoerythrin (PE)-conjugated monoclonal antibodies (BD Pharmingen, San Diego, CA, USA) and fluorescence activated cell sorter (FACS) analysis were performed.
ROS production
ROS generation was determined by the DCFDA assay, as described previously [31]. Intracellular fluorescence (excitation 492 nm, emission 527 nm) was measured using a Becton Dickinson FACSCalibur instrument (Franklin Lakes, NJ, USA) equipped with CellQuest software. Results are given as mean fluorescence intensity (MFI).
CD39/CD73 expression studies
To compare ectoenzyme surface expression by regulatory (CD4+CD25+) and effector (CD15+) cells, aliquots of 5 × 105 freshly isolated cells from normal donors and iNS patients were resuspended in 200 µl phosphate-buffered saline (PBS) without Ca++ and Mg++, supplemented with 2 mM ethylenediamine tetraacetic acid (EDTA) and 0·5% human serum, and stained with anti-CD39 FITC and anti-CD73 PE-conjugated monoclonal antibodies (BD Pharmingen, San Diego, CA, USA) for 30 min at 4°C. The percentage of CD4+CD25+CD39+/CD4+CD25+CD73+ and CD39+CD15+/CD73+CD15+ cells were determined by FACS analysis.
ATP inhibition experiments
To evaluate the role of the ATP/adenosine pathway, ROS generation by PMN (CD15+ cells) was determined at rest and after incubation with adenosine receptor agonists, ATP antagonists and apyrase as follows: (i) the two adenosine analogues, 2′-chloroadenosine (which acts selectively at A1 receptors) and 5′-N-ethylcarboxamidoadenosine (which acts equally at A1 and A2 receptors); both substances were utilized at a concentration of 100 µM; (ii) three specific antagonists of P2X7 ATP receptors, i.e. Brilliant Blue G (1 µM), KN62 (10 µM) and A437089 (10 µM); (iii) the inhibitor of connexin hemichannels and gap junctions, carbenoxolone (50 µM), which is involved in ATP release from cells; (iv) a generic inhibitor of P2 receptors, i.e. PPADS (4-[[4-formyl-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-2-pyridinyl]azo]-1,3-benzenedisulphonic acid) at a concentration of 100 µM; and finally with (v) soluble apyrase (CD39) at a concentration of 10 U/ml. All these reagents were from Sigma-Aldrich (St Louis, MO, USA). In some circumstances soluble apyrase was added together with ATP receptors or gap junction inhibitors, to examine a potential synergistic effect. In all cases, agonists and inhibitors were added to PMN in the reaction mixture 20 min at 37°C before ROS evaluation with the DCFDA assay.
Determination of ATP levels
The ability of apyrase to hydrolyse ATP released from PMN after 15 min and 45 min at 37°C of incubation was assessed with the adenosine 5′-triphosphate bioluminescent assay kit (Sigma-Aldrich) [32], according to the manufacturer's instructions. ATP concentrations were calculated using a standard calibration curve. Determinations were made in triplicate for each sample tested.
Statistical analysis
The one-way analysis of variance was used for comparison of ROS levels in various conditions and during in vitro experiments. Results were give as mean ± standard error of the mean (s.e.m.).
Results
CD39/CD73 expression by lymphocytes
Positivity for CD39 and CD73 identifies those blood cells expressing two ectoenzymes that transform ATP into adenosine. The former, i.e. apyrase (CD39), transforms ATP into ADP and AMP; the other, i.e. ecto-5′-nucleotidase (CD73), transforms AMP into adenosine [33–35]. Differential expression of CD39 and CD25 can be used to identify three cell categories among CD4+ cells [36–38]: (i) CD39+CD4+CD25+, corresponding to a Treg subset; (ii) CD39-CD4+CD25+, denoting a population with Th17 potential; and (iii) CD39+CD4+CD25-, that have a memory phenotype and may represent proinflammatory cells. In humans, CD73 seems not to be co-expressed with CD39 in regulatory subsets.
In iNS the percentage of total lymphocytes of CD4+CD25+ was decreased by 50% (14·1 ± 0·07 versus 23·9 ± 0·11, P < 0·005) (Fig. 1a). In parallel, the percentage of CD4+CD25+ expressing CD39 was decreased by another 50% in iNS compared to normal blood, giving an overall percentage of CD39+CD4+CD25+ reduced by 80% (3·43 ± 0·04% versus 13·14 ± 0·07%, P < 0·001) (Fig. 1b). Conversely, the percentage of CD4+CD25+ expressing CD73 was doubled in iNS compared to normal blood, giving a comparable overall amount of lymphocytes expressing CD73 (1·51 ± 0·01% versus 1·62 ± 0·01%) (Fig. 1c). Finally, the percentage of neutrophils (CD15+) expressing CD39 was not modified in iNS (Fig. S1).
Fig. 1.

Expression of apyrase (CD39) and ecto-5′-nucleotidase (CD73) by CD4+ CD25+ lymphocytes. Expression of CD39 and CD73 by circulating lymphocytes were evaluated in 12 children with active nephrotic syndrome [three/one with focal segmental glomerulosclerosis (FSGS), one with minimal change nephrosis (MCN), eight without pathology definition] and in 13 normal subjects. The following parameters were evaluated: (a) CD4+CD25+ as percentage of total lymphocytes. (b) CD39+ as percentage of CD4+CD25+ cell fraction. (c) CD73+ as percentage of CD4+CD25+ cell fraction. The percentage of CD4+CD25+ lymphocytes was reduced in idiopathic nephrotic syndrome (iNS) by 50% (14·1 ± 0·07% versus 23·9 ± 0·11%); the percentage of CD4+CD25+ co-expressing CD39 was still reduced in iNS, giving an overall final concentration of CD4+CD25+CD39+ reduced by 80% (3·43 ± 0·04% versus 13·14 ± 0·07%, P < 0·001). **P < 0·005; *P < 0·05.
ROS generation as a function of CD39/CD73
ROS generation was first evaluated in resting PMNs (overall, 41 nephrotic patients and 30 normal controls) utilizing the DCFDA fluorescence assay [39]. In 27 cases for which CD39+CD4+CD25+ had been tested previously, we determined ROS before and after apyrase addition in vitro. The results shown in Fig. 2a,b demonstrate that ROS generation by PMN at rest was a function of the amount of CD39 expressed by CD4+CD25+ in patients with iNS. If patients with a particularly low CD39+CD4+CD25+ level (< 7·5%) (Fig. 2b) are considered, ROS generation at rest was higher than the companion patients with higher CD39+CD4+CD25+ (> 7·5%), and the decrement was greater after addition of apyrase (Fig. 2b). In fact, when PMNs were co-incubated with apyrase in an amount sufficient to metabolize all the ATP of the medium (Table 2), ROS generation was reduced by 30–40%. This means that ATP levels play a key regulatory effect, but also suggests that ROS generation is regulated by other pathways besides apyrase. Finally, activity of the disease as defined by the presence of proteinuria had no influence (Fig. S2a,b). In normal blood cells, ROS generation was random (Fig. 2c) with respect to CD39+CD4+CD25+ and was comparably reduced with the addition of apyrase (Fig. 2d).
Fig. 2.
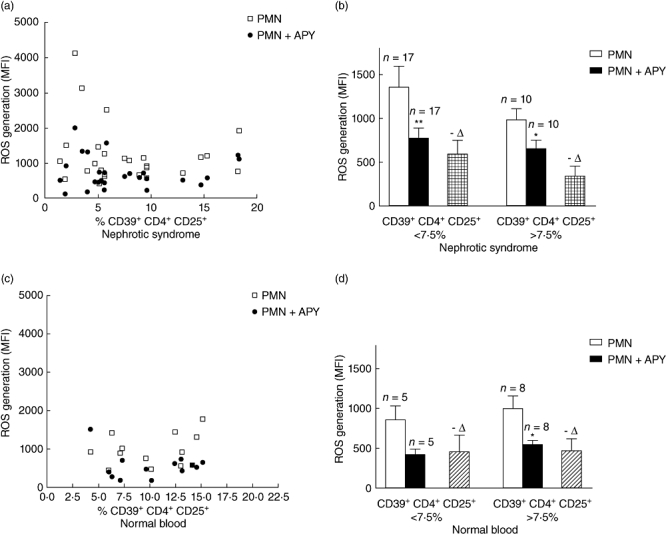
Reactive oxygen species (ROS) production by polymorphonuclear neutrophils (PMN) as a function of CD4+CD25+CD39+. ROS production in 27 children with active nephrotic syndrome. All patients presented proteinuria at the time of the analysis (proteinuria > 7 g/24 h in 11, 1–5 g/24 h in 5, 0·5–1/g 24 h in 11). Only nine had a pathology diagnosis [focal segmental glomerulosclerosis (FSGS) four cases, minimal change nephrosis (MCN) four cases, immunoglobulin (Ig)M one]. (a) ROS production as a function of CD4+CD25+CD39+ in children with idiopathic nephrotic syndrome (iNS) (open squares) and effects of exogenous apyrase (closed circles); (b) ROS production in iNS patients with different percentage of CD4+CD25+CD39+cells and modification produced by the addition of apyrase in vitro; (c) ROS production as a function of CD4+CD25+CD39+ in normal subjects (open squares) and effects of exogenous apyrase (closed circles); (d) ROS production in normal subjects with different percentage of CD4+CD25+CD39+cells and modification produced by the addition of apyrase in vitro. **P < 0·005; *P < 0·05.
Table 2.
Levels of adenosine 5′-triphosphate (ATP) released from normal and nephrotic polymorphonuclear neutrophils (PMN) in the presence and absence of apyrase. Measurements were made using a commercial ATP bioluminescent assay kit (Sigma) (Leach, 1986 #2010); concentrations are given as mean of four independent experiments ± standard error of the mean
| ATP conc. (µM) | 15′ | 15′+ apyrase (10 U/ml) | 45′ | 45′+ apyrase (10 U/ml) | |
|---|---|---|---|---|---|
| Normal controls | 0·095 ± 0·012 | 0·002 ± 0·001 | 0·124 ± 0·032 | 0·003 ± 0·003 | |
| n = 4 | |||||
| Nephrotic syndrome | 0·135 ± 0·017 | 0·001 ± 0·0005 | 0·160 ± 0·035 | 0·002 ± 0·001 | |
| n = 4 | |||||
| Standard ATP | 0·100 ± 0·015 | 0·018 ± 0·006 |
Considering all cases, ROS generation was not influenced by CD73+CD4+CD25+ (Fig. S3).
Overall, the results from this set of experiments demonstrated that CD39+CD4+CD25+ plays a regulatory role in ROS generation by effector cells and that this system is altered in iNS. This defect is specific for apyrase and does not involve ecto-5′-nucleotidase. Based on these results, further experiments were performed in order to define which nucleotide/nucleoside receptors are activated by extracellular apyrase-degraded ATP.
Inhibition of ROS generation by adenosine agonists
In the first approach, we utilized two adenosine analogues, i.e. 2′-chloroadenosine (which acts selectively at A1 receptors) and 5′-N-ethylcarboxamidoadenosine (which acts equally at A1 and A2 receptors) to define a direct inhibitory effect on ROS generation [40,41]. The results shown in Fig. 3a,b clearly show a lack of any relevant effect of these two molecules.
Fig. 3.

Reactive oxygen species (ROS) production in presence of adenosine agonists. ROS production was determined in 26 children with active nephrotic syndrome and in six patients in a remission phase. Oxidative burst was determined in the presence and absence of adenosine agonists. All patients presented proteinuria at the time of the analysis (proteinuria > 5 g/24 h in 11, 1–5 g/24 h in 8, 0·5–1/g 24 h in 13). Pathology characterization was available in 10 cases [focal segmental glomerulosclerosis (FSGS) four cases, minimal change nephrosis (MCN) five cases, immunoglobulin (Ig)M one case]. Failure of two adenosine analogues, i.e. 2′-chloroadenosine (which acts selectively at A1 receptors) and 5′-N-ethylcarboxamidoadenosine (which acts equally at A1 and A2 receptors) to modify ROS generation by PMN in children with idiopathic nephrotic syndrome (iNS) (a) and in normal subjects (b). *P < 0·05.
Inhibition of ROS generation by ATP antagonists
Experiments for antagonizing ATP were performed utilizing compounds with different effects: (i) a generic inhibitor of P2 receptors, i.e. PPADS; (ii) three specific antagonists of ATP P2X7R, i.e. Brilliant Blue G [42,43], KN62 [44] and A437089 [45]; and (iii) the inhibitor of panx1, carbenoxolone, which is involved in ATP release from cells [46]. The effects on ROS generation produced by these compounds in iNs were comparable, although less evident than apyrase (Fig. 4a,b), with the exception of Brilliant Blue G. With normal PMN, all ATP active compounds produced the same effect of apyrase.
Fig. 4.
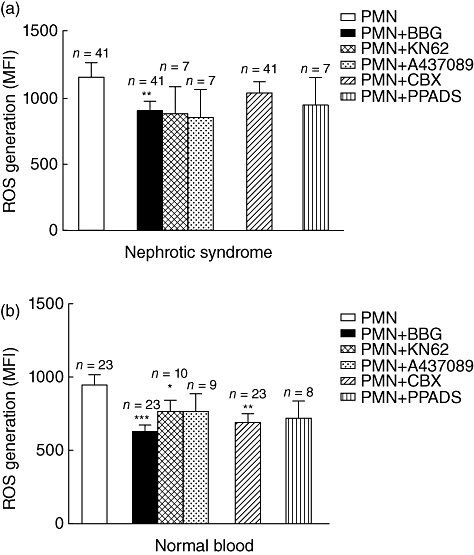
Reactive oxygen species (ROS) production in the presence of adenosine 5′-triphosphate (ATP) antagonists. ROS production was determined in 41 children (Table 1) in presence and in absence of ATP agonists. ROS generation by polymorphonuclear neutrophils (PMN) from nephritic patients (a) and normal donors (b). Several antagonists of ATP were utilized to reduce ROS generation: the list includes a generic inhibitor of P2 receptors, i.e. PPADS (4-[[4-Formyl-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-2-pyridinyl]azo]-1,3-benzenedisulphonic acid), three specific antagonists of P2X7 ATP receptor, i.e. Brilliant Blue G [43], KN62 [44] and A437089, and finally the inhibitor of connexin hemi-channels and gap junctions, carbenoxolone, which is involved in ATP release from cells. Reduction of ROS generation with apyrase is shown for comparison. ***P < 0·001; **P < 0·005; *P < 0·05.
In some circumstances, soluble apyrase was added together with ATP antagonists in order to verify a potential synergistic effect. As shown in Fig. 5a,b, the combination of apyrase with Brilliant Blue G and carbenoxolone produced only minor additive effects.
Fig. 5.
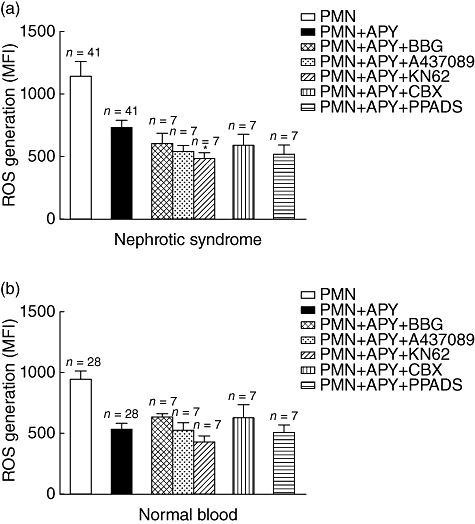
Additive effect of adenosine 5′-triphosphate (ATP) antagonists and apyrase on reactive oxygen species (ROS) generation by polymorphonuclear neutrophils (PMN) in vitro. In the same cases above (see Fig. 4a), soluble apyrase was added together with ATP antagonists in order to verify a potential synergistic effect on ROS production by polymorphonuclear neutrophils (PMN) obtained from idiopathic nephrotic syndrome (iNS) children (a) and from normal subjects (b). No additive effect was observed. The same patient cohort of the experiment above of (Fig. 4) was here utilized.
Discussion
Oxidants produced by activated phagocytes, i.e. neutrophils and monocytes, play a fundamental role in innate immunity. These cells generate superoxide (O2−), and its dismutation product hydrogen peroxide (H2O2) is converted by myeloperoxidase into the major oxidant, hypochlorous acid. All these metabolites are directly toxic for microorganisms in terms of milliseconds, and they are then buffered in plasma by physiological systems (for a review see [12,47,48]. However, living cells are also sensitive to the toxic effect of ROS, and organ damage is generated whenever their production overcomes intra- and extracellular defences [13,14]. It is clear that the importance of this system is essential for survival but requires rigid control to avoid recurrent cell damage.
ATP, and the nucleotide pathway in general, have developed over time as the most efficient and probably safe control of ROS production, linked to a double regulatory network that confers rapid activation of its effectors and their block [25,33,34,49]. ATP is, in fact, released upon cell damage (being one of the most represented intracellular substances) and is sensored by the purinergic ionotropic P2X 1–7 receptors [26,27,50] that promote, on activation, a proinflammatory response (increased ROS, perforin, cytokins, etc.). Conversely, ATP hydrolysis by ectoenzymes apyrase (CD39) and ecto-5′-nucleotidase (CD73) produces adenosine, which plays an immunosuppressive effect via the adenosine A1 and A2 receptors [34,51].
Overall, ATP metabolism mediates a critical balance between inflammatory activation and inhibition, and triggers or inhibits various immune responses, from direct killing of microorganisms to organization of the inflammasome. This equilibrium relies on a distinct and complex control (P2X receptors, CD39 and CD73, adenosine A2 receptors) that confers a type of independent lock.
In humans, the differential expression of CD39+ and CD25+ on CD4+ differentiates cell subsets, among which CD39+CD4+CD25+ correspond to Treg, while CD39-CD4+CD25+ identifies a population with T helper type 17 (Th17) activity [36–38]. Finally, CD39+CD4+CD25- is a memory phenotype that may represent proinflammatory cells.
Some new findings in this paper address the role of the nucleotide pathway as major determinant of ROS generation in vivo and point to the implication of ROS regulatory cells in iNS. The first point is related to the effective ROS quota, which is regulated by ATP in vivo and corresponds to 30–40% of total ROS production by PMN. A second main finding is the marked decrease of CD39+CD4+CD25+ in iNS children, correlating with the extent of ROS generation. Moreover, when apyrase was added to the reaction in an amount sufficient to convert all ATP of the medium in ADP/AMP, the decrease in ROS corresponded to 40% of the original level. In iNS the ROS generation quota after apyrase was still high (double than in normal blood), and explains the oxidative stress observed in these patients [9,11,52].
Addition of apyrase would have produced ADP/AMP but did not generate adenosine, as no ecto-5′-nucleotidase (i.e. CD73, required for adenosine generation) was added in parallel. Therefore, the fall in ROS generation after apyrase was due entirely to ATP consumption. The same conclusion is suggested by the observation that adenosine agonists played no effect when they were added to the medium. In agreement with the above conclusion, we also demonstrated that ATP-selective P2X7 antagonists (i.e. Brilliant Blue G) [42] reduced ROS generation to the same extent as apyrase. Overall, ATP regulates almost 50% of ROS generation in vivo, leaving the remainder under the control of other mechanisms that have not yet been characterized.
Therefore, these results strengthen the concept of ATP regulatory function on innate immunity and also confirm the key role of regulatory T CD39+CD4+CD25+. The observation that these cells are lower in nephrotic patients compared to normal controls may explain, to some extent, the high ROS generation that characterizes these patients [9]. However, the high quota of ROS generation that survives apyrase addition and the ATP block in vitro suggests that other pathways contribute to oxidative stress in iNS.
Overall, our data indicate some basic clues in the regulation of innate immunity in iNS that may be implicated in the pathogenesis of kidney lesions. They also present some therapeutic options utilizing specific P2X7 antagonists, such as Brilliant Blue G, or blockers of ATP escape, such as carbenoxolone. The low toxicity and high selectivity of Brilliant Blue G makes this compound an ideal candidate for blocking ATP-dependent ROS production in vivo, but chemical compounds such as carbenoxolone may also be considered as support treatments in nephrotic syndrome.
Experimental therapeutic approaches should, however, consider other pathways for ROS regulation as a target of selective intervention in this pathology.
Acknowledgments
This work was supported by the Italian Ministry of Health (Ricerca Finalizzata (Costituzione di un network multi regionale per la prevenzione della malattia renale e migliorare il management cronico del paziente nefropatico) and by the Renal Child Foundation. Authors also acknowledge Fondazione Mara Wilma e Bianca Querci for financial support to the project ‘Ruolo dello stress reticolare nella progressione del danno renale e tumorale’ and Fondazione La Nuova Speranza for supporting the project ‘Progetto integrato per la definizione dei meccanismi implicati nella glomerulo sclerosi focale. Dalla predisposizione genetica alla regolazione della produzione di fattori cellulari e circolanti’. Data were discussed critically with Professor Rosanna Gusmano. The authors also acknowledge the suggestions made by Professor Rosa Bacchetta.
Disclosure
None to declare.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Expression by CD15+ of apyrase (CD39) in 24 children with active nephrotic syndrome. Proteinuria at the time of the analysis was variable but present in all (proteinuria > 5 g/24 h in 8, 1–5 g/24 h in 8, 0·5–1 g/24 h in 8). Peripheral blood cells were stained with anti-CD39 FITC conjugated monoclonal antibodies and the percentage of CD15+CD39+ was determined by FACS analysis.
Fig. S2. ROS production by PMN as a function of CD4+CD25+CD39+ in patients with (a) and without (b) proteinuria. ROS production by PMNs at rest (open squares) and after the addition of exogenous apyrase (closed circles) was evaluated in the two cohorts as a function of CD4+CD25+CD39+ percentage.
Fig. S3. ROS production by PMN as a function of CD4+CD25+CD73+ in children with active iNS (open squares).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Caridi G, Bertelli R, Carrea A, et al. Prevalence, genetics, and clinical features of patients carrying podocin mutations in steroid-resistant nonfamilial focal segmental glomerulosclerosis. J Am Soc Nephrol. 2001;12:2742–6. doi: 10.1681/ASN.V12122742. [DOI] [PubMed] [Google Scholar]
- 2.Caridi G, Bertelli R, Di Duca M, et al. Broadening the spectrum of diseases related to podocin mutations. J Am Soc Nephrol. 2003;14:1278–86. doi: 10.1097/01.asn.0000060578.79050.e0. [DOI] [PubMed] [Google Scholar]
- 3.Caridi G, Perfumo F, Ghiggeri GM. NPHS2 (Podocin) mutations in nephrotic syndrome. Clinical spectrum and fine mechanisms. Pediatr Res. 2005;57:54R–61R. doi: 10.1203/01.PDR.0000160446.01907.B1. [DOI] [PubMed] [Google Scholar]
- 4.Caridi G, Trivelli A, Sanna-Cherchi S, Perfumo F, Ghiggeri GM. Familial forms of nephrotic syndrome. Pediatr Nephrol. 2010;25:241–52. doi: 10.1007/s00467-008-1051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagrue G, Xheneumont S, Branellec A, Weil B. Lymphokines and nephrotic syndrome [Letter] Lancet. 1975;1:271–2. doi: 10.1016/s0140-6736(75)91164-2. [DOI] [PubMed] [Google Scholar]
- 6.Koyama A, Fujisaki M, Kobayashi M, Igarashi M, Narita M. A glomerular permeability factor produced by human T cell hybridomas. Kidney Int. 1991;40:453–60. doi: 10.1038/ki.1991.232. [DOI] [PubMed] [Google Scholar]
- 7.Ghiggeri GM, Artero M, Carraro M, Perfumo F. Permeability plasma factors in nephrotic syndrome: more than one factor, more than one inhibitor. Nephrol Dial Transplant. 2001;16:882–5. doi: 10.1093/ndt/16.5.882. [DOI] [PubMed] [Google Scholar]
- 8.Vincenti F, Ghiggeri GM. New insights into the pathogenesis and the therapy of recurrent focal glomerulosclerosis. Am J Transplant. 2005;5:1179–85. doi: 10.1111/j.1600-6143.2005.00968.x. [DOI] [PubMed] [Google Scholar]
- 9.Bertelli R, Trivelli A, Magnasco A, et al. Failure of regulation results in an amplified oxidation burst by neutrophils in children with primary nephrotic syndrome. Clin Exp Immunol. 2010;161:151–8. doi: 10.1111/j.1365-2249.2010.04160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginevri F, Ghiggeri GM, Candiano G, et al. Peroxidative damage of the erythrocyte membrane in children with nephrotic syndrome. Pediatr Nephrol. 1989;3:25–32. doi: 10.1007/BF00859620. [DOI] [PubMed] [Google Scholar]
- 11.Musante L, Candiano G, Petretto A, et al. Active focal segmental glomerulosclerosis is associated with massive oxidation of plasma albumin. J Am Soc Nephrol. 2007;18:799–810. doi: 10.1681/ASN.2006090965. [DOI] [PubMed] [Google Scholar]
- 12.Candiano G, Petretto A, Bruschi M, et al. The oxido-redox potential of albumin methodological approach and relevance to human diseases. J Proteomics. 2009;73:188–95. doi: 10.1016/j.jprot.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Zhou LL, Hou FF, Wang GB, et al. Accumulation of advanced oxidation protein products induces podocyte apoptosis and deletion through NADPH-dependent mechanisms. Kidney Int. 2009;76:1148–60. doi: 10.1038/ki.2009.322. [DOI] [PubMed] [Google Scholar]
- 14.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–54. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 15.Bertani T, Poggi A, Pozzoni R, et al. Adriamycin-induced nephrotic syndrome in rats: sequence of pathologic events. Lab Invest. 1982;46:16–23. [PubMed] [Google Scholar]
- 16.Bertani T, Rocchi G, Sacchi G, Mecca G, Remuzzi G. Adriamycin-induced glomerulosclerosis in the rat. Am J Kidney Dis. 1986;7:12–19. doi: 10.1016/s0272-6386(86)80051-8. [DOI] [PubMed] [Google Scholar]
- 17.Raats CJ, Bakker MA, van den Born J, Berden JH. Hydroxyl radicals depolymerize glomerular heparan sulfate in vitro and in experimental nephrotic syndrome. J Biol Chem. 1997;272:26734–41. doi: 10.1074/jbc.272.42.26734. [DOI] [PubMed] [Google Scholar]
- 18.Bertelli R, Ginevri F, Gusmano R, Ghiggeri GM. Cytotoxic effect of adriamycin and agarose-coupled adriamycin on glomerular epithelial cells: role of free radicals. In Vitro Cell Dev Biol. 1991;27A:799–804. doi: 10.1007/BF02631246. [DOI] [PubMed] [Google Scholar]
- 19.Ginevri F, Gusmano R, Oleggini R, et al. Renal purine efflux and xanthine oxidase activity during experimental nephrosis in rats: difference between puromycin aminonucleoside and adriamycin nephrosis. Clin Sci. 1990;78:283–93. doi: 10.1042/cs0780283. [DOI] [PubMed] [Google Scholar]
- 20.Lebrecht D, Setzer B, Rohrbach R, Walker UA. Mitochondrial DNA and its respiratory chain products are defective in doxorubicin nephrosis. Nephrol Dial Transplant. 2004;19:329–36. doi: 10.1093/ndt/gfg564. [DOI] [PubMed] [Google Scholar]
- 21.Ghiggeri GM, Cercignani G, Ginevri F, et al. Puromycin aminonucleoside metabolism by glomeruli and glomerular epithelial cells in vitro. Kidney Int. 1991;40:35–42. doi: 10.1038/ki.1991.176. [DOI] [PubMed] [Google Scholar]
- 22.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–19. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah SV, Baliga R, Rajapurkar M, Fonseca VA. Oxidants in chronic kidney disease. J Am Soc Nephrol. 2007;18:16–28. doi: 10.1681/ASN.2006050500. [DOI] [PubMed] [Google Scholar]
- 24.Diomedi-Camassei F, Di Giandomenico S, Santorelli FM, et al. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol. 2007;18:2773–80. doi: 10.1681/ASN.2006080833. [DOI] [PubMed] [Google Scholar]
- 25.Haag F, Adriouch S, Brass A, et al. Extracellular NAD and ATP: partners in immune cell modulation. Purinergic Signal. 2007;3:71–81. doi: 10.1007/s11302-006-9038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeiffer ZA, Guerra AN, Hill LM, et al. Nucleotide receptor signaling in murine macrophages is linked to reactive oxygen species generation. Free Radic Biol Med. 2007;42:1506–16. doi: 10.1016/j.freeradbiomed.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suh BC, Kim JS, Namgung U, Ha H, Kim KT. P2X7 nucleotide receptor mediation of membrane pore formation and superoxide generation in human promyelocytes and neutrophils. J Immunol. 2001;166:6754–63. doi: 10.4049/jimmunol.166.11.6754. [DOI] [PubMed] [Google Scholar]
- 28.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–8. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 29.Di Virgilio F, Borea PA, Illes P. P2 receptors meet the immune system. Trends Pharmacol Sci. 2001;22:5–7. doi: 10.1016/s0165-6147(00)01574-1. [DOI] [PubMed] [Google Scholar]
- 30.Di Virgilio F. Purinergic signalling in the immune system. A brief update. Purinergic Signal. 2007;3:1–3. doi: 10.1007/s11302-006-9048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertelli R, Ginevri F, Caridi G, et al. Recurrence of focal segmental glomerulosclerosis after renal transplantation in patients with mutations of podocin. Am J Kidney Dis. 2003;41:1314–21. doi: 10.1016/s0272-6386(03)00364-0. [DOI] [PubMed] [Google Scholar]
- 32.Leach FR, Webster JJ. Commercially available firefly luciferase reagents. Methods Enzymol. 1986;133:51–70. doi: 10.1016/0076-6879(86)33055-6. [DOI] [PubMed] [Google Scholar]
- 33.Hyman MC, Petrovic-Djergovic D, Visovatti SH, et al. Self-regulation of inflammatory cell trafficking in mice by the leukocyte surface apyrase CD39. J Clin Invest. 2009;119:1136–49. doi: 10.1172/JCI36433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3:171–80. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borsellino G, Kleinewietfeld M, Di Mitri D, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–32. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 37.Fletcher JM, Lonergan R, Costelloe L, et al. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–10. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 38.Dwyer KM, Hanidziar D, Putheti P, et al. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant. 2010;10:2410–20. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walrand S, Valeix S, Rodriguez C, Ligot P, Chassagne J, Vasson MP. Flow cytometry study of polymorphonuclear neutrophil oxidative burst: a comparison of three fluorescent probes. Clin Chim Acta. 2003;331:103–10. doi: 10.1016/s0009-8981(03)00086-x. [DOI] [PubMed] [Google Scholar]
- 40.Kumar V, Sharma A. Adenosine: an endogenous modulator of innate immune system with therapeutic potential. Eur J Pharmacol. 2009;616:7–15. doi: 10.1016/j.ejphar.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Baraldi PG, Preti D, Tabrizi MA, et al. 6)-[(hetero)aryl/(cyclo)alkyl-carbamoyl-methoxy-phenyl]-(2-chloro)-5′-N- ethylcarboxamido-adenosines: the first example of adenosine-related structures with potent agonist activity at the human A(2B) adenosine receptor. Bioorg Med Chem. 2007;15:2514–27. doi: 10.1016/j.bmc.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 42.Peng W, Cotrina ML, Han X, et al. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci USA. 2009;106:12489–93. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol Pharmacol. 2000;58:82–8. [PubMed] [Google Scholar]
- 44.Michel AD, Chambers LJ, Clay WC, Condreay JP, Walter DS, Chessell IP. Direct labelling of the human P2X7 receptor and identification of positive and negative cooperativity of binding. Br J Pharmacol. 2007;151:103–14. doi: 10.1038/sj.bjp.0707196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donnelly-Roberts DL, Jarvis MF. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol. 2007;151:571–9. doi: 10.1038/sj.bjp.0707265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benfenati V, Caprini M, Nicchia GP, et al. Carbenoxolone inhibits volume-regulated anion conductance in cultured rat cortical astroglia. Channels. 2009;3:323–36. doi: 10.4161/chan.3.5.9568. [DOI] [PubMed] [Google Scholar]
- 47.Carr AC, Hawkins CL, Thomas SR, Stocker R, Frei B. Relative reactivities of N-chloramines and hypochlorous acid with human plasma constituents. Free Radic Biol Med. 2001;30:526–36. doi: 10.1016/s0891-5849(00)00495-0. [DOI] [PubMed] [Google Scholar]
- 48.Dalle-Donne I, Scaloni A, Giustarini D, et al. Proteins as biomarkers of oxidative/nitrosative stress in diseases: the contribution of redox proteomics. Mass Spectrom Rev. 2005;24:55–99. doi: 10.1002/mas.20006. [DOI] [PubMed] [Google Scholar]
- 49.Guerra AN, Gavala ML, Chung HS, Bertics PJ. Nucleotide receptor signalling and the generation of reactive oxygen species. Purinergic Signal. 2007;3:39–51. doi: 10.1007/s11302-006-9035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hewinson J, Moore SF, Glover C, Watts AG, MacKenzie AB. A key role for redox signaling in rapid P2X7 receptor-induced IL-1 beta processing in human monocytes. J Immunol. 2008;180:8410–20. doi: 10.4049/jimmunol.180.12.8410. [DOI] [PubMed] [Google Scholar]
- 51.Sitkovsky M, Lukashev D, Deaglio S, Dwyer K, Robson SC, Ohta A. Adenosine A2A receptor antagonists: blockade of adenosinergic effects and T regulatory cells. Br J Pharmacol. 2008;153(Suppl 1):S457–64. doi: 10.1038/bjp.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Musante L, Bruschi M, Candiano G, et al. Characterization of oxidation end product of plasma albumin in vivo. Biochem Biophys Res Commun. 2006;349:668–73. doi: 10.1016/j.bbrc.2006.08.079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


