Abstract
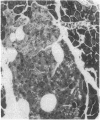
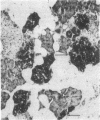
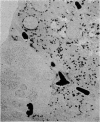
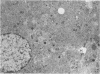
Differentiation is the process by which multicellular organisms achieve the specialized functions necessary for adaptation and survival. An in vivo model in the Syrian golden hamster is described in which regenerating pancreatic cells are converted into hepatocyte-like cells, as evidenced by the presence of albumin, peroxisomes, and a variety of morphological markers. These cells are stable after the conversion is triggered by a single dose of the carcinogen N-nitrosobis(2-oxopropyl)amine administered during the S phase in regenerating pancreatic cells. This suggests that, given the proper stimulus, regenerating cells in adult pancreas can be redirected into a totally different pathway of differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britten R. J., Davidson E. H. Gene regulation for higher cells: a theory. Science. 1969 Jul 25;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Dustin P., Jr Cell differentiation and carcinogenesis: a critical review. Cell Tissue Kinet. 1972 Nov;5(6):519–533. doi: 10.1111/j.1365-2184.1972.tb00389.x. [DOI] [PubMed] [Google Scholar]
- FITZGERALD P. J. The problem of the precursor cell of regenerating pancreatic acinar epithelium. Lab Invest. 1960 Jan-Feb;9:67–85. [PubMed] [Google Scholar]
- Farber E. Biochemistry of carcinogenesis. Cancer Res. 1968 Sep;28(9):1859–1869. [PubMed] [Google Scholar]
- Frei J. V., Harsono T. Increased susceptibility to low doses of a carcinogen of epidermal cells in stimulated DNA synthesis. Cancer Res. 1967 Aug;27(8):1482–1484. [PubMed] [Google Scholar]
- GROBSTEIN C. CYTODIFFERENTIATION AND ITS CONTROLS. Science. 1964 Feb 14;143(3607):643–650. doi: 10.1126/science.143.3607.643. [DOI] [PubMed] [Google Scholar]
- Gross P. R. Biochemistry of differentiation. Annu Rev Biochem. 1968;37:631–660. doi: 10.1146/annurev.bi.37.070168.003215. [DOI] [PubMed] [Google Scholar]
- Hand A. R. Morphologic and cytochemical identification of peroxisomes in the rat parotid and other exocrine glands. J Histochem Cytochem. 1973 Feb;21(2):131–141. doi: 10.1177/21.2.131. [DOI] [PubMed] [Google Scholar]
- Hollander C. F., Bentvelzen P. Enhancement of urethan induction of hepatomas in mice by prior partial hepatectomy. J Natl Cancer Inst. 1968 Dec;41(6):1303–1306. [PubMed] [Google Scholar]
- Holtzer H., Weintraub H., Mayne R., Mochan B. The cell cycle, cell lineages, and cell differentiation. Curr Top Dev Biol. 1972;7:229–256. doi: 10.1016/s0070-2153(08)60073-3. [DOI] [PubMed] [Google Scholar]
- Kimbrough R. D. Pancreatic-type tissue in livers of rats fed polychlorinated biphenyls. J Natl Cancer Inst. 1973 Aug;51(2):679–681. [PubMed] [Google Scholar]
- MARGOLIASH E., NOVOGRODSKY A., SCHEJTER A. Irreversible reaction of 3-amino-1:2:4-triazole and related inhibitors with the protein of catalase. Biochem J. 1960 Feb;74:339–348. doi: 10.1042/bj0740339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff A. B., Goldfischer S. Visualization of peroxisomes (microbodies) and mitochondria with diaminobenzidine. J Histochem Cytochem. 1969 Oct;17(10):675–680. doi: 10.1177/17.10.675. [DOI] [PubMed] [Google Scholar]
- Otten J., Jonckheer M., Dumont J. E. Thyroid albumin. II. In vitro synthesis of a thyroid albumin by normal human thyroid tissue. J Clin Endocrinol Metab. 1971 Jan;32(1):18–26. doi: 10.1210/jcem-32-1-18. [DOI] [PubMed] [Google Scholar]
- Pour P., Althoff J., Krüger F. W., Mohr U. A potent pancreatic carcinogen in Syrian hamsters: N-nitrosobis(2-oxopropyl)amine. J Natl Cancer Inst. 1977 May;58(5):1449–1453. doi: 10.1093/jnci/58.5.1449. [DOI] [PubMed] [Google Scholar]
- Pour P., Mohr U., Althoff J., Cardesa A., Kmoch N. Spontaneous tumors and common diseases in two colonies of Syrian hamsters. III. Urogenital system and endocrine glands. J Natl Cancer Inst. 1976 May;56(5):949–961. doi: 10.1093/jnci/56.5.949. [DOI] [PubMed] [Google Scholar]
- QUASTLER H., SHERMAN F. G. Cell population kinetics in the intestinal epithelium of the mouse. Exp Cell Res. 1959 Jun;17(3):420–438. doi: 10.1016/0014-4827(59)90063-1. [DOI] [PubMed] [Google Scholar]
- Scarpelli D. G., Rao M. S., Subbarao V., Beversluis M. Regeneration of Syrian golden hamster pancreas and covalent binding of N-nitroso-2,6-[3H]dimethylmorpholine. Cancer Res. 1981 Mar;41(3):1051–1057. [PubMed] [Google Scholar]
- Takahashi M., Pour P. Spontaneous alterations in the pancreas of the aging Syrian golden hamster. J Natl Cancer Inst. 1978 Feb;60(2):355–364. doi: 10.1093/jnci/60.2.355. [DOI] [PubMed] [Google Scholar]
- Terao K., Nakano M. Cholangiofibrosis induced by short-term feeding of 3'-methyl-4-(dimethylamino)azobenzene: an electron microscopic observation. Gan. 1974 Jun;65(3):249–260. [PubMed] [Google Scholar]
- Uriel J. Cancer, retrodifferentiation, and the myth of Faust. Cancer Res. 1976 Nov;36(11 Pt 2):4269–4275. [PubMed] [Google Scholar]