Abstract
Phage display technology has been utilized to select target molecules against circulating antibodies. The aims of this study were to isolate a peptide that binds with serum from Crohn's disease (CD) patients and to examine its diagnostic and pathogenic significance. A phage display library was constructed using cDNA from Caco-2 cells. Affinity selection using this cDNA library and serum samples from patients with CD was then performed. Phage clones that specifically reacted with the CD sera were then selected using a phage enzyme-linked immunosorbent assay (ELISA). After the DNA sequences of the selected phages were determined and converted to amino acid sequences, the synthesized peptides were examined using an ELISA. The effect of the synthesized peptides on cytokine release from cultured blood mononuclear cells was investigated. An ELISA analysis for TCP-353 demonstrated that while 61·7% of the samples from CD patients were seroreactive, seroreactivity was less common among patients with ulcerative colitis (7·3%), acute colitis (0%) or colon cancer (11·4%) and among normal subjects (2·8%). The induction of interleukin (IL)-1β, IL-6 and tumour necrosis factor (TNF)-α release, but not IL-10 release, in response to TCP-353 peptide was enhanced in CD mononuclear cells only. We isolated a novel peptide that specifically binds to CD sera and stimulates the proinflammatory responses of CD mononuclear cells. TCP-353 may have diagnostic, pathogenic and therapeutic significance with regard to the treatment of CD.
Keywords: biomarker, Crohn's disease, phage display
Introduction
Crohn's disease (CD), a form of inflammatory bowel disease, is a disorder of unclear aetiology characterized by transmural inflammation that can occur throughout the gastrointestinal tract. Although the cause of the disease is unknown, recent evidence suggests that antigens present within the intestinal lumen may trigger and drive an aberrant immune response, resulting in intestinal inflammation in genetically susceptible individuals [1,2]. In fact, strong evidence of humoral immune responses to extrinsic microbial and food agents has been reported, including antibodies to the Escherichia coli outer-membrane porin C (OmpC) [3,4], the Pseudomonas fluorescence-associated sequence I2 [5–7], bacterial flagellin [8,9] and anti-saccharomyces cerevisiae antibody (ASCA) [10,11], as well as intrinsic host cell antigens such as perinuclear antineutrophil cytoplasmic antibodies [11–13] and anti-colon antibodies [14]. However, the predominant immune targets have not been identified. For these reasons, the value and potential role of these antibodies and antigens in the evaluation of patients with CD have been increasingly appreciated, and their potential applications to studies on disease pathogenesis, disease diagnosis, clinical stratification and strategies for treatment have been examined [15,16]. However, anti-colon antibodies are more predominant among patients with ulcerative colitis (UC) than among patients with CD, suggesting that some unidentified proteins from Caco-2 cells may exist that are specific to CD [14].
Phage display technology has emerged as a powerful tool for the isolation and characterization of peptides that bind to target molecules, such as antibodies and receptors [17,18]. This approach is very useful for identifying ligands for disease-specific antibodies, as it requires only a phage display random peptide library and sera samples from normal individuals and patients. Thus, this method is particularly suitable for the study of diseases in which the aetiological agents and pathological antigens are largely unknown. In fact, several studies using phage display peptide libraries have been performed for autoimmune diseases such as rheumatoid arthritis [19] and autoimmune thrombocytopenia [20].
In the present study, we coincidentally identified a novel immunoreactive peptide that specifically binds to sera from CD patients while examining autoantigens from a Caco-2 cell library. We then examined the role of this peptide in the diagnosis and pathogenesis of CD.
Patients and methods
Study populations
Sera or peripheral blood mononuclear cells (PBMC) from Japanese patients with CD, UC, acute colitis and colon cancer were used in this study. Each patient's diagnosis was confirmed based on the clinical history, endoscopic and radiological examinations and histopathological findings. The acute colitis samples included sera from patients with infectious colitis and ischaemic colitis. The colon cancer patient samples included sera from patients with Dukes grade B or C. The normal control group was a collection of environmental controls composed of sera from individuals with no symptoms or signs of disease. Ethical approval for the human studies was obtained from the institution review board at Kurume University School of Medicine, and informed consent was obtained from all the individuals prior to enrolment in the study.
Cell lines
The human colon carcinoma cell line Caco-2 was obtained from the Riken Cell Bank (Ibaraki, Japan) and was maintained in Dulbecco's modified Eagle's medium (Life Technologies, Rockville, MD, USA) supplemented with 10% (vol/vol) fetal calf serum (FCS), 100 U/ml penicillin, 100 µg/ml streptomycin and 2 mm l-glutamine in an atmosphere of 5% CO2/95% air.
cDNA library construction
We constructed a phage display cDNA library using the bacteriophage T7Select System (Novagen, Madison, WI, USA). Total RNA was prepared from ∼108 Caco-2 cells using an RNeasy kit (Qiagen, Valencia, CA, USA), according to the manufacturer's protocol; poly(A)+ RNA was selected using oligo-dT affinity chromatography. The first strand of cDNA was synthesized using Omni-RT reverse transcriptase (Qiagen) and a random hexamer; the second strand was then synthesized using RNase H and DNA polymerase I (Takara, Shiga, Japan), as described previously. The cDNA library was constructed using a T7Select 10–3 cloning Kit (Merck KGaA, Darmstadt, Germany), according to the manufacturer's protocol. After in vitro packaging, a small aliquot was used to titrate the cDNA library. The remaining cDNA library was amplified prior to biopanning.
Affinity selection of cDNA library
Microtitre wells (Immulon 2; Dynatec Laboratory, Chantilly, VA, USA) were coated overnight at 4°C with 50 µl of 20 µg/ml protein G (Sigma, St Louis, MO, USA) in phosphate-buffered saline (PBS) containing 0·05% sodium azide. After blocking with Tris-buffered saline (TBS) containing 0·1% Tween 20 and 5% skimmed milk (TBS-TM), CD patient serum diluted 1/100 in PBS was added and incubated for 2 h at room temperature to capture the serum immunoglobulin (Ig)G. Unbound serum proteins were removed by washing the wells three times with TBS-TM. Next, the serum IgG-coated wells were incubated for 3 h at room temperature with ∼1 × 1011 phages in TBS-TM. Unbound phages were removed by washing the wells six times with TBS-TM, followed by TBS; the remaining phages were eluted with PBS containing 0·5% sodium dodecyl sulphide (SDS). An overnight culture of BLT5615 was infected with an aliquot of the eluted phages, and plated on an lysogeny broth (LB) agar plate containing 50 µg/ml of ampicillin and 1 mm isopropyl β-D-1-thiogalactopyranoside (IPTG). After 3 h of incubation at 37°C, the plaques that formed on the plate were lifted onto a nitrocellulose membrane (GE Water & Process Technologies, Trevose, PA, USA) and incubated overnight at 4°C with CD patient serum diluted to 1/500 in TBS-TM. The membrane was washed with TBS-TM, then incubated with 1:5000 diluted alkaline phosphatase-conjugated mouse anti-human IgG (Sigma) for 1 h at room temperature. After washing with TBS-TM, the membrane was immersed in the substrate solution [0·33 mg/ml nitro-blue tetrazolium chloride (NBT), 0·165 mg/ml 5-bromo-4-chloro-3′-indolylphosphatase p-toluidine salt (BCIP) in AP buffer (100 mm Tris-HCl, pH 9·5/100 mm NaCl/5 mM MgCl2)] and colour developed. Phage was picked up from the immunostained plaque, and resuspended in LB media containing 50 µg/ml ampicillin as a selected phage.
Phage enzyme-linked immunosorbent assay (ELISA)
Microtitre wells were coated overnight at 4°C with rabbit polyclonal anti-T7 phage antibody at 1 µg/ml in PBS containing 0·05% sodium azide. Meanwhile, selected phages were grown with BLT5615 until complete lysis to express the foreign proteins/peptides on the particle surface. These phage cultures were diluted 1:5 with TBS-TM, and incubated for 2 h at 4°C in microtitre wells preblocked with TBS-TM. After washing the wells three times, patient sera diluted 1:250 in TBS-TM were added to the wells and incubated for 1 h at room temperature. After washing the wells six times, the secondary antibody, alkaline phosphatase-conjugated mouse anti-human IgG (Sigma) diluted 1:5000 in TBS-TM was added to the wells and incubated for 1 h at room temperature. After the wells were washed four times and then twice with AP buffer, substrate solution containing 1 mg/ml p-nitrophenyl phosphate (p-NPP; Sigma) in AP buffer was added to the wells and incubated for 40 min at room temperature. Colour development was stopped by adding 2 m NaOH. Optical density (OD) was measured at 405 nm using a microplate reader (Vmax, Sunnyvale, CA, USA). This measurement was performed using vector phage as a negative control. The phage ELISA value was calculated such that the mean OD value using a selected phage was subtracted from the mean OD values of the negative control.
DNA sequence
The DNA sequences of the selected phage were determined using the dideoxynucleotide chain termination method. The deduced DNA sequences were converted to amino acid sequences.
Peptide synthesis
The TCP-47 and TCP-353 peptides were synthesized by Sigma Aldrich Japan Inc. (Tokyo, Japan).
Peptide ELISA
Microtitre wells were coated overnight at 4°C with the synthesized peptide at 2 µg/ml in PBS containing 0·05% sodium azide. After blocking with PBS containing 2% bovine serum albumin (BSA; Sigma), 5% sucrose and 0·05% sodium azide, serum samples diluted 1:100 or 1:400 in PBS containing 1% BSA and 0·1% Tween 20 (PBS-BT) were added and incubated for 1 h at room temperature. After washing the wells six times with PBS containing 0·1% Tween 20 (PBST), horseradish peroxidase-conjugated anti-human IgG (Dako Cytomation, Denmark) diluted 1:4000 in PBST was added to the wells and incubated for 1 h at room temperature. After washing the wells eight times with PBS-BT, substrate solution [tetramethylbenzidine (TMB)+; Dako] was added to the wells and incubated for 30 min at room temperature. Colour development was stopped by adding 1 M H2SO4. The optical OD was measured at 450 to 650 nm using a microplate reader. For the quantitative assay a series of dilutions of the standard, which was prepared from positive serum samples, was added to each plate. The antibody titre was calculated using a standard curve.
ASCA ELISA
ASCA-IgG was measured using a commercially available ELISA kit (Genosis Diagnostics, Ely, UK) according to the manufacturer's instructions.
PBMC isolation and cytokine ELISA
PBMCs were isolated from the heparinized blood by density centrifugation through Ficoll-Hypaque. These cells were washed twice in PBS and then incubated with 100 µg/ml of TCP-353 or control peptide, which consisted of the same amino acids in a different sequence from that of TCP-353, at a density of 106 cells/ml in a complete medium consisting of RPMI-1640 (Nissui Pharmaceutical, Tokyo, Japan), 10% FCS, 100 U/ml penicillin, 100 µg/ml streptomycin and 2 mm/l l-glutamine. Then, the cells were incubated for 24 h in a humidified environment at 37°C and an atmosphere containing 5% CO2. The supernatants were collected and stored at −70°C until use in ELISAs for tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and IL-10 (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
Statistical analysis was performed using Student's t-test or Mann–Whitney U-test using the Statview program (Abacus Concepts Inc., Berkeley, CA, USA). Results were considered significant at P < 0·05.
Results
cDNA library screening
We constructed a Caco-2 cDNA library using a T7Select10-3b vector. The library consisted of 1·3 × 107 independent recombinants with insert sizes ranging from 100 to 3000 base pairs (bp) (600 bp on average). The cDNA library was searched for antigens recognized by CD sera. As probes, serum samples from 21 individual patients or seven pooled serum mixtures consisting of samples from three of the 21 patients were used to select clones from the cDNA library. For affinity selection, approximately ∼1 × 1011 bacteriophage plaque-forming unit (pfu) phages from a library grown with BLT5615 were applied to microtitre wells coated with the serum samples. After removing unbound phages from the wells, bound phages were eluted. After selection, the eluted phages were plated on agar and the phage plaques were immunostained with the serum used above after blotting onto nitrocellulose filters.
Phage ELISA
We succeeded in isolating more than 400 phage clones that were reactive to CD sera. To rescreen these phages, we performed phage ELISA using sera from CD, UC and normal subjects (n = 6 for each group) and obtained several phage clones that were reactive to CD. From these clones, we selected four more specific clones, CD#47, CD#336, CD#353 and CD#355, using additional samples (Fig. 1). As a result, CD#47 and CD#353 showed a high reactivity with CD sera, compared with UC sera and normal samples, while CD#336 and CD#355 showed a high reactivity with CD sera compared with UC sera but not normal samples. Thus, we synthesized two peptides, TCP-47 and TCP-353, from the CD#47 and CD#353 clones, respectively.
Fig. 1.

Association of antibodies against Crohn's disease (CD) phages in human subjects. Human sera from patients with CD or ulcerative colitis (UC) and normal control (NC) subjects were tested using an enzyme-linked immunosorbent assay (ELISA) for reactivity to CD#47 (a), CD#336 (b), CD#353 (c) and CD#355 (d). Reactivity was expressed as the actual optical density (OD) at 405 nm. Each dot represents the value for a single patient. Each value is the mean of triplicate measurements. The P-values were determined using Student's t-test; n.s.: not significant.
DNA sequences
The nucleotide sequences for the DNA inserted into these phage clones were determined using purified phage DNA as a template and were converted to amino acid sequences. The inserted DNA of these phages matched the human genome sequences, but the mRNA only resulted in the expression of short peptides, not the whole protein. The amino acid sequences displayed on the four phages are shown in Table 1. A Basic Local Alignment Search Tool (BLAST) search of the Swiss Protein Database revealed that partial homology was found with a variety of proteins from different organisms. However, no significant homology with any human protein or pathogen was observed.
Table 1.
Amino acid sequences expressed on phage clones selected
| Clone name | Amino acid sequence expressed | Length |
|---|---|---|
| CD#47 | NSVKNEVEEVTFTKHTQCLGCFKSGFS | 27 |
| CD#336 | VIPALSEAEAGGSPEVRSSRPAWPIW | 26 |
| CD#353 | MIRGLFPN | 8 |
| CD#355 | PDGSGGGGWGRGE | 13 |
Peptide ELISAs
Based on the phage ELISA results, we performed an ELISA with TCP-47 and TCP-353 and determined the reactivity to CD as well as UC and normal sera (n = 32 for each group). As shown in Fig. 2a,b, TCP-47 and TCP-353 reacted strongly with the CD sera, compared with the UC and normal samples. The positive rate was 40·6% for the CD sera, 0% for the UC sera and 9·4% for the normal sera in the TCP-47 ELISA and 53·1% for the CD sera, 3·1% for the UC sera and 6·3% for the normal sera in the TCP-353 ELISA. Therefore, we selected the TCP-353 ELISA because of its higher sensitivity and lower cut-off value.
Fig. 2.

Association of antibodies against TCP peptides in human subjects. Human sera from patients with Crohn's disease (CD) or ulcerative colitis (UC), and normal control (NC) subjects were tested using an an enzyme-linked immunosorbent assay (ELISA) for reactivity to TCP-47 (a) and TCP-353 (b). Reactivity was expressed as the actual optical density (OD) at 450–650 nm. Each cut-off value, defined as the mean OD of normal subjects plus two standard deviations, is indicated as a dotted line (0·449 for the TCP-47 ELISA and 0·112 for the TCP-353 ELISA). Each dot represents the value for a single patient. Each value is the mean of triplicate measurements. The P-values were determined using the Mann–Whitney U-test.
TCP-353 ELISA
We finally tested the presence and magnitude of the antibody response towards TCP-353 using an ELISA and serum samples from a relatively large number of subjects. As expected, the antibody titres for TCP-353 were significantly higher in the CD sera than in sera from UC, acute colitis or colon cancer patients or normal subjects (Fig. 3a). The positive rate was 61·7% for CD, 7·3% for UC, 0% for acute colitis, 11·4% for colon cancer and 2·8% for normal subjects; the sensitivity was 61·7% and the specificity was 93·5%. No significant correlation between the antibody titre and disease activity or treatment was observed (data not shown). Figure 3b shows the results of a concurrent ELISA for ASCA, the most widely studied marker for CD. The positive rate was 31·7% for CD, 8·3% for UC, 9·1% for acute colitis, 5·7% for colon cancer and 8·5% for normal subjects; the sensitivity was 31·7% and the specificity was 90·9%. The diagnostic accuracy of the markers was evaluated using a receiver operator characteristics (ROC) curve analysis generated by plotting sensitivity versus 1-specificity to differentiate the CD patients from the healthy subjects (Fig. 4). The ROC curve for the TCP-353 ELISA was better than that for the ASCA ELISA. No correlation in the CD sera antibody titres was observed between the TCP-353 and ASCA ELISAs (r = 0·229).
Fig. 3.

Antibody titres in sera samples from patients with Crohn's disease (CD, n = 60, 34 men and 26 women, mean age 33·3), ulcerative colitis (UC, n = 109, 62 men and 47 women, mean age 34·9), acute colitis (AC, n = 11, six men and five women, mean age 31·3) or colon cancer (CC, n = 35, 25 men and 10 women, mean age 49·4) and normal control subjects (NC, n = 71, 24 men and 47 women, mean age 30·7) measured using a TCP-353 an enzyme-linked immunosorbent assay (ELISA) (b) and an anti-saccharomyces cerevisiae antibody (ASCA) ELISA (b). Each cut-off value is indicated as a dotted line (54 U/ml for the TCP-353 ELISA and 20 U/ml for the ASCA ELISA). The cut-off value was defined as three standard deviations above the mean titre for the normal subjects for the TCP-353 ELISA (a) and as the point on a receiver operating characteristics curve with the best sensitivity and with a specificity of more than 90% for the ASCA ELISA (b). Each dot represents the value for a single patient. Each value is the mean of triplicate measurements. The P-values were determined using the Mann–Whitney U-test.
Fig. 4.

Receiver operating characteristics (ROC) curves for the TCP-353 an enzyme-linked immunosorbent assay (ELISA) and the anti-saccharomyces cerevisiae antibody (ASCA) ELISA. The ROC curves for the TCP-353 ELISA and the ASCA ELISA were obtained by plotting the sensitivity versus the specificity. The sensitivity versus the specificity was calculated using the results for 60 Crohn's disease patients and 71 healthy subjects for each ELISA.
PBMC response to TCP-353
To investigate the underlying pathophysiological mechanism related to this antigenic response, the effect of TCP-353 peptide on cytokine production was examined in PBMCs (Fig. 5). Interestingly, in PBMCs from CD patients, TCP-353 induced the proinflammatory cytokines IL-1β, IL-6 and TNF-α, but not the anti-inflammatory cytokine IL-10. In striking contrast, this induction was not observed in PBMCs from UC patients and normal subjects. The stimulatory effect of TCP-353 on PBMCs was specific because a control peptide containing the same amino acids but in a scrambled sequence from that of TCP-353 did not produce a similar effect. In addition, no correlation was found between the TCP-353-induced cytokine response and the serum antibody titres of TCP-353 in patients with CD.
Fig. 5.
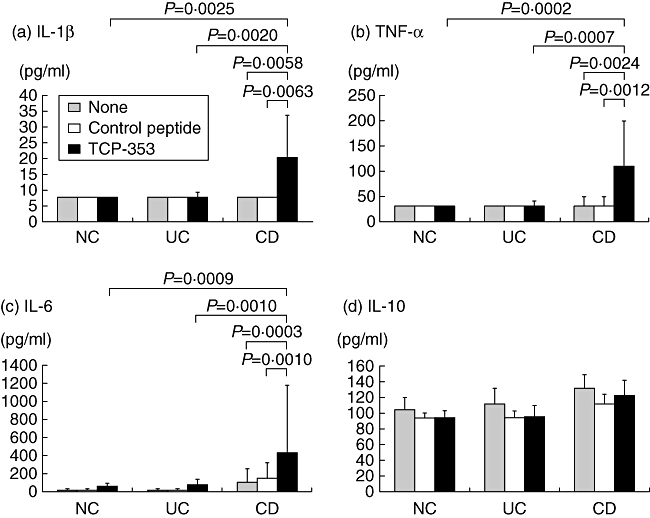
Effect of TCP-353 on cytokine production from peripheral blood mononuclear cells (PBMCs). Cells isolated from patients with Crohn's disease (CD, n = 20) or ulcerative colitis (UC, n = 18) and normal control subjects (NC, n = 19) were treated with 100 µg/ml of TCP-353 or the control peptide. After 24 h, the supernatants were collected for the an enzyme-linked immunosorbent assay (ELISA) to determine the concentration of tumour necrosis factor (TNF)-α (a), IL-1β (b), IL-6 (c) and interleukin (IL)-10 (d). The data represent the cytokine concentrations in supernatants from triplicate cultures [expressed as the median and interquartile range (IQR)]. Note that TCP-353 enhanced the production of TNF-α, IL-1β and IL-6 but not IL-10 in PBMCs from patients with CD. A P-value was determined using the Mann–Whitney U-test.
Discussion
We used a phage display technique to identify autoantigens from Caco-2 cells, but coincidentally isolated predominant phage clones that expressed short peptides relevant to CD. While we have not succeeded in isolating any cognate antigens from Caco-2 cells, we speculated that smaller peptides are prone to bind with greater avidity to the antibodies. In general, peptides selected by biopanning phage display libraries with antibodies are mimics, rather than replicas, of the cognate antigen. Therefore, the TCP-353 peptide has no significant homology to any human protein and it is likely to be a mimic. From among these phage clones, we selected two clones, synthesized their peptides and then performed several types of ELISAs. Finally, an ELISA using the TCP-353 peptide was selected because of its highly favourable diagnostic accuracy.
In this series of experiments, the CD sera samples reacted more strongly on the peptide ELISA than on the phage ELISA. One possible explanation for this difference was that the T7Select10-3b phage can display fewer than 15 copies of foreign peptides on its surface; thus, the total peptide molecules are estimated to be in the order of fewer than 1 pmol (making them insufficient as antigens for ELISA); meanwhile, synthetic peptides in the order of 100 pmol can be coated directly onto the microtitre wells.
The recognition of the TCP-353 peptide by serum antibodies was shown to be a specific serological phenomenon for CD, as the detection of significant antibody titres in control cohorts of patients with other intestinal diseases, such as UC, acute colitis or colon cancer, was rare. Furthermore, the present results showed that the sensitivity was approximately twice as high and the specificity was slightly higher for the TCP-353 ELISA than for the ASCA ELISA. Previous work has shown that ASCA is found in 60% to 70% of CD patients among western populations [10,11], but among only 30–45% of CD patients in the Japanese population [21], similar to the present data. Thus, the lower sensitivity and specificity of ASCA ELISA seen in this study may be due to the difference in the study populations. Whether the seroreactivity of TCP-353 is correlated with recently described biomarkers for CD, such as anti-OmpC [3,4], anti-I2 [5–7] or anti-flagellin antibody [8,9], will be interesting to determine.
From a clinical perspective, a limitation of our ELISA results is that our study comprised a single-centre analysis of Japanese patients mainly with inflammatory bowel disease. Although this cohort was chosen intentionally to eliminate common confounding variables and comorbid conditions, it will be of considerable interest to determine how the reactivity against TCP-353 peptide is correlated with genetic determinants, clinical phenotypes and responses to treatment in studies across multiple populations of patients and ethnic groups and, more importantly, in prospective studies. Such studies are currently under way to address these issues.
We assessed the antigenic targets of TCP-353. The BLAST search revealed that the TCP-353 peptide showed no significant homology to any human protein or pathogen. However, partial homology was observed for a variety of proteins from different organisms, such as bacteria, fungi, zebra fish and Oryza sativa (Table 2). Among these organisms, high serum antibody titres for Caenorhabditis elegans[22,23] and Mycobacterium sp. [24] have been reported in inflammatory bowel disease. Interestingly, TCP-353 might also represent the rice antigen O. sativa, considering the dietary habits of Japanese subjects. Also, we cannot rule out the possibility that the TCP-353 peptide may derive from a novel microorganism or perhaps a well-known enteric microorganism that has not yet been characterized for this gene segment. We are continuing our efforts to identify the antigen that is associated with this peptide.
Table 2.
Homology search result of TCP-353 peptide
| Protein | Sequence | Source |
|---|---|---|
| TCP-353 (8 a.a.) | MIRGLFPN | |
| Putative chloride channel protein | 783 789 | Oryza sativa japonica Gr. |
| GYIRGLFPNVLRE | ||
| Major facilitator superfamily transporter | 118 125 | Mycobacterium gilvum |
| ALIRGLFPDQR | ||
| Nicotinamide–nucleotide adenylyltransferase | 1 7 | Pyrobaculum arsenaticum |
| MVRGLFPGRF | ||
| Heat shock protein 14 | 347 354 | Danio rerio |
| QMIRDLFPDVE | ||
| Cytochrome P450 | 46 52 | Laccaria bicolor |
| FMLRGLFPPLR | ||
| Aconitate hydratase | 692 669 | Rhizobium sp. |
| VMVRGLFTNKT | ||
| Dynein heavy chain | 2146 2153 | Leishmania major |
| GLIRSLFPNLD | ||
| DNA PRImase homologue | 270 275 | Caenorhabditis elegans |
| NMIRGLFDDDY |
The expression of antibodies to TCP-353 not only distinguishes CD from other diseases, but also presents a unique opportunity to investigate the underlying pathophysiological mechanism related to this antigenic response. Of note, TCP-353 is capable of stimulating the production of proinflammatory cytokines TNF-α, IL-1β and IL-6, but not anti-inflammatory cytokine IL-10 in PBMCs; these findings were observed only in CD patients. These results may provide a potentially important link for this peptide with innate immunity, rather than adaptive immunity. Furthermore, the CD-specific hyperresponsiveness of PBMCs leads us to speculate that TCP-353 may play a role in mucosal immune responses.
The present findings suggest some possibilities: (i) TCP-353 may play a modulating role in patients with CD, aggravating the ongoing inflammation without being responsible for its initiation; (ii) TCP-353 may share an antigenetic similarity with some other, as yet unidentified, pathogen, leading to a cross-reaction in the immune system; and (iii) lastly and most interestingly, TCP-353 may play a crucial role in precipitating CD. Further experiments are currently under way to answer these possibilities.
Saito et al. succeeded in isolating four CD-associated peptides from Japanese patients using the phage display technique with a M13 filamentous phage and synthesized multiple antigenic peptides (MAPs) [21]. They showed that an ELISA with a mixture of these MAPs (known as a cocktail MAP ELISA) had a sensitivity of 56·5% and a specificity of 95·7%, providing a similar diagnostic accuracy to that of TCP-353. However, the number of antigenic peptides used in these assays differed, with four multiple peptides used in the cocktail MAP ELISA and a single, linear peptide used in the TCP-353 ELISA. Furthermore, their report did not examine the functional role of the four peptides in the cocktail MAP.
In conclusion, we obtained a novel peptide that binds specifically to CD sera and also stimulates proinflammatory responses in CD PBMCs. The TCP-353-expressing antigen may be of diagnostic, pathogenic and therapeutic significance to CD.
Disclosure
None.
References
- 1.Schreiber S, Rosenstiel P, Albrecht M, Hampe J, Krawczak M. Genetics of Crohn disease, an archetypal inflammatory barrier disease. Nat Rev Genet. 2005;6:376–88. doi: 10.1038/nrg1607. [DOI] [PubMed] [Google Scholar]
- 2.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–40. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 3.Landers CJ, Cohavy O, Misra R, et al. Selected loss of tolerance evidenced by Crohn's disease-associated immune responses to auto- and microbial antigens. Gastroenterology. 2002;123:689–99. doi: 10.1053/gast.2002.35379. [DOI] [PubMed] [Google Scholar]
- 4.Mow WS, Vasiliauskas EA, Lin YC, et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn's disease. Gastroenterology. 2004;126:414–24. doi: 10.1053/j.gastro.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Wei B, Huang T, Dalwadi H, Sutton CL, Bruckner D, Braun J. Pseudomonas fluorescens encodes the Crohn's disease-associated I2 sequence and T-cell superantigen. Infect Immun. 2002;70:6567–75. doi: 10.1128/IAI.70.12.6567-6575.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalwadi H, Wei B, Kronenberg M, Sutton CL, Braun J. The Crohn's disease-associated bacterial protein I2 is a novel enteric T cell superantigen. Immunity. 2001;15:149–58. doi: 10.1016/s1074-7613(01)00164-9. [DOI] [PubMed] [Google Scholar]
- 7.Sutton CL, Kim J, Yamane A, et al. Identification of a novel bacterial sequence associated with Crohn's disease. Gastroenterology. 2000;119:23–31. doi: 10.1053/gast.2000.8519. [DOI] [PubMed] [Google Scholar]
- 8.Sitaraman SV, Klapproth JM, Moore DA, III, et al. Elevated flagellin-specific immunoglobulins in Crohn's disease. Am J Physiol Gastrointest Liver Physiol. 2005;288:G403–6. doi: 10.1152/ajpgi.00357.2004. [DOI] [PubMed] [Google Scholar]
- 9.Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Main J, McKenzie H, Yeaman GR, et al. Antibody to Saccharomyces cerevisiae (bakers' yeast) in Crohn's disease. BMJ. 1988;297:1105–6. doi: 10.1136/bmj.297.6656.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinton JF, Sendid B, Reumaux D, et al. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788–91. doi: 10.1136/gut.42.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rump JA, Scholmerich J, Gross V, et al. A new type of perinuclear anti-neutrophil cytoplasmic antibody (p-ANCA) in active ulcerative colitis but not in Crohn's disease. Immunobiology. 1990;181:406–13. doi: 10.1016/S0171-2985(11)80509-7. [DOI] [PubMed] [Google Scholar]
- 13.Saxon A, Shanahan F, Landers C, Ganz T, Targan S. A distinct subset of antineutrophil cytoplasmic antibodies is associated with inflammatory bowel disease. J Allergy Clin Immunol. 1990;86:202–10. doi: 10.1016/s0091-6749(05)80067-3. [DOI] [PubMed] [Google Scholar]
- 14.Lee JC, Cevallos AM, Naeem A, Lennard-Jones JE, Farthing MJ. Detection of anti-colon antibodies in inflammatory bowel disease using human cultured colonic cells. Gut. 1999;44:196–202. doi: 10.1136/gut.44.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaven SW, Abreu MT. Biomarkers in inflammatory bowel disease. Curr Opin Gastroenterol. 2004;20:318–27. doi: 10.1097/00001574-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Sandborn WJ. Serologic markers in inflammatory bowel disease: state of the art. Rev Gastroenterol Disord. 2004;4:167–74. [PubMed] [Google Scholar]
- 17.Santi E, Capone S, Mennuni C, et al. Bacteriophage lambda display of complex cDNA libraries: a new approach to functional genomics. J Mol Biol. 2000;296:497–508. doi: 10.1006/jmbi.1999.3471. [DOI] [PubMed] [Google Scholar]
- 18.Niwa M, Maruyama H, Fujimoto T, Dohi K, Maruyama IN. Affinity selection of cDNA libraries by lambda phage surface display. Gene. 2000;256:229–36. doi: 10.1016/s0378-1119(00)00348-6. [DOI] [PubMed] [Google Scholar]
- 19.Fakhfakh F, Ayadi H, Bahloul Z, Jarraya A, Sioud M, Zouali M. Antibody epitopes probed by immunoselected phage-display library peptides in members of a family with various rheumatic manifestations. Clin Exp Rheumatol. 1996;14:607–11. [PubMed] [Google Scholar]
- 20.Gevorkian G, Manoutcharian K, Almagro JC, Govezensky T, Dominguez V. Identification of autoimmune thrombocytopenic purpura-related epitopes using phage-display peptide library. Clin Immunol Immunopathol. 1998;86:305–9. doi: 10.1006/clin.1997.4502. [DOI] [PubMed] [Google Scholar]
- 21.Saito H, Fukuda Y, Katsuragi K, et al. Isolation of peptides useful for differential diagnosis of Crohn's disease and ulcerative colitis. Gut. 2003;52:535–40. doi: 10.1136/gut.52.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oshitani N, Hato F, Kitagawa S, et al. Distinct elevation of levels of anti-Caenorhabditis elegans antibody in sera of patients with inflammatory bowel disease. Clin Diagn Lab Immunol. 2003;10:856–61. doi: 10.1128/CDLI.10.5.856-861.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oshitani N, Hato F, Kitagawa S, et al. Analysis of intestinal HLA-DR bound peptides and dysregulated immune responses to enteric flora in the pathogenesis of inflammatory bowel disease. Int J Mol Med. 2003;11:99–104. [PubMed] [Google Scholar]
- 24.Elsaghier A, Prantera C, Moreno C, Ivanyi J. Antibodies to Mycobacterium paratuberculosis-specific protein antigens in Crohn's disease. Clin Exp Immunol. 1992;90:503–8. doi: 10.1111/j.1365-2249.1992.tb05874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


