Abstract
Alloreactive T cells that infiltrate the graft after lung transplantation (LTx) play a role in chronic rejection. Chemokines such as thymus and activation-regulated chemokine (TARC), macrophage-derived chemokine (MDC) and monocyte chemotactic protein-1 (MCP-1) are produced locally in the lung and attract T cells via chemokine receptor 4 (CCR4). In a TARC gradient, cells expressing CCR4++ migrate more efficiently than CCR4+-expressing cells. In this study, we compared the CCR4 expression of T cells in blood from 20 lung transplant recipients to healthy controls. We then examined whether CCR4 expression is associated with the occurrence of chronic rejection. The CCR4++ expression was decreased on CD4 T cells from LTx patients (P < 0·0001) when compared to healthy controls. The analysis of CD4 T cell subsets showed that this decrease was present on central memory, effector memory and terminally differentiated T cells (P = 0·0007, P < 0·0001 and P = 0·05, respectively), while a trend was found for naive CD4 T cells (P = 0·06). Also, the expression of CCR4+ on regulatory T cells (Tregs) was decreased in LTx patients when compared to healthy controls (P = 0·02). Interestingly, the CCR4++ expression on CD4 effector memory T cells was decreased in patients developing chronic rejection sometimes more than a year before the clinical diagnosis when compared to patients who did not (P = 0·04). The analysis of CD8 T cell subsets only showed the CCR4+ expression to be increased significantly on effector memory and terminally differentiated CD8 T cells (P = 0·02, P = 0·03, respectively) in LTx patients, but no relation was found in chronic rejection. In conclusion, the expression of CCR4 on T cell subsets was altered after LTx and appears to be related to chronic rejection.
Keywords: BOS, CCR4, lung transplantation, TARC, T cell subsets
Introduction
Lung transplantation (LTx) is a final treatment option for end-stage lung diseases, even though the success of LTx is still restrained by occurrence of chronic rejection. The pathogenesis of chronic rejection, also known as the bronchiolitis obliterans syndrome (BOS), is unclear. BOS is characterized by the influx of leucocytes, including alloreactive T cells, that leads to fibrosis and obliteration of the airways [1,2].
In order to translocate T cells to transplanted lungs, chemoattractive signals must be provided. The chemokine receptor type 4 (CCR4) expressed on T cells ligates to a number of locally produced chemokines in the lungs, such as thymus and activation-regulated chemokine (TARC/CCL17), monocyte chemotactic protein-1 (MCP-1/CCL2) and macrophage-derived chemokine (MDC/CCL22). TARC, MCP-1 and MDC have all been studied in relation to the development of BOS. Concentrations of TARC present in the circulation 1 month after LTx were decreased in patients who eventually developed BOS [3]. MCP-1 was elevated prior to BOS [4], while high MDC levels in BALF at 6 months post-transplantation were speculated to be predictive for BOS development [5,6]. The up-regulation of these chemokines at the site of inflammation might stimulate the migration of leucocytes from the periphery into the allograft.
CCR4 is expressed on T cell subsets infiltrating the skin, synovial fluid and on bronchial T cells. Less CCR4 expression is seen on interstitial T cells and T cells in the tonsils. CCR4 expression was not observed in the intestine [7]. It has been shown that the CCR4 expression of infiltrating T cells varies between different tissues. T cells infiltrating the skin have a higher expression of CCR4 (CCR4++) than cells found within the bronchial cavity or the synovial fluid (CCR4+) [7]. Additionally, it was shown that cells expressing CCR4++ migrate more efficiently towards a TARC gradient [7]. In lung transplant patients, T cells with CCR4 expression have been described to be present in the lungs at time of rejection [8]. Regulatory T cells (Tregs) in BALF expressing CCR4 did not influence the development of BOS. TARC may not be critical for maintenance of Tregs in the allograft because it can selectively recruit other T cells expressing CCR4 [9–11].
The finding that high levels of TARC in early post-transplant were associated with freedom of BOS led us to speculate that this chemokine may attract CCR4++ T cells, which are not involved in (or prevent) the pathogenesis of BOS. In this study, we explored the relationship between systemic TARC levels and the expression of CCR4 on T cells. In addition, we examined whether CCR4 expression on T cell subsets, including its relation with BOS, was affected by lung transplantation.
Materials and methods
Patients and controls
Included in this study were 20 LTx patients who survived more than 3 months and who received transplants between September 2003 and March 2008 at the Heart Lung Center in Utrecht, the Netherlands. Five patients developed BOS during follow-up. BOS was defined as a decline in forced expiratory volume in 1 s (FEV1) from post-operative baseline at two distinctive time-points, of more than 20% in the absence of infection or other aetiologies. Standard immunosuppressive therapy consisted of basiliximab, tacrolimus, mycophenolate-mofetil and prednisone. Furthermore, blood obtained at one time-point from 11 healthy controls was included in the measurements.
The study design was approved by the Medical Ethical Committee. Informed consent was obtained from each patient.
Sampling
The blood samples from these 20 patients, taken at approximately 5 months after LTx (range 4·2–6·1 months), were used in a cross-sectional study. Peripheral blood mononuclear cells (PBMCs) were isolated from 40 ml of heparinized whole blood by Ficoll Paque Plus (GE Healthcare, Uppsala, Sweden). No absolute cell counts were performed on fresh blood. All samples were frozen and preserved in liquid nitrogen until measurement. During a longitudinal study including 10 patients, samples were collected once prior to LTx and monthly up to 12 months after LTx, as described above. In parallel, serum samples were also collected.
Flow cytometry
Cryopreserved PBMCs were thawed rapidly in a 37°C water bath and added to a medium containing 10% fetal calf serum (FCS); the cells were then centrifuged and resuspended in phosphate-buffered saline (PBS). Two million PBMCs were incubated with either relevant antibodies or isotype controls for 30 min on ice in the dark, which was then followed by washing and measurement.
Data acquisition and analysis were performed on a BD fluorescence activated cell sorter (FACS) Canto II with 8-colour detection (BD Bioscience, San Jose, CA, USA). Each recipient sample measured had approximately 450 000 events (range 70 000–900 000 events) in the lymphocyte gate, which was defined by CD45+ and side-scatter (SSC) and analysed with FACS diva software (BD Bioscience).
Antibodies and gating strategy
Lymphocytes were identified with a scatter dot of CD45– and sidewards scatter. Within this lymphocyte gate, both CD4 and CD8 T cells were distinguished with CD4– and CD8–. CD45RO-Pe Cy7 (Becton Dickinson) and CD27-peridinin chlorophyll (PerCP) (BioLegend, San Diego, CA, USA) were used to discriminate the naive T cells (N), central memory T cells (CM), effector memory T cells (EM) and terminally differentiated T cells (TD) (Fig. 1). Tregs were defined on CD4+CD25+and CD127– expression by the use of CD25–and CD127–. For their CCR4+ and CCR4++ expression, all subtypes were analysed with CCR4-PerCP Cy5·5 (BioLegend).
Fig. 1.
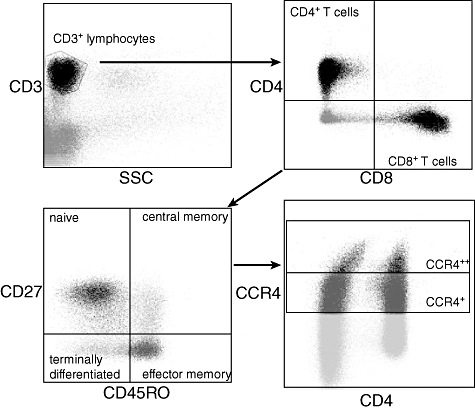
Gating strategy. CD3+ lymphocytes were selected on sidewards scatter (SSC) and CD3+ expression. T cells were divided for both CD4+ and CD8+ expression. Both subtypes were typed further for CD27 and CD45RO expression. CD27+CD45RO- are naive, CD27+CD45RO+ are central memory, CD27-CD45RO+ are effector memory and CD27-CD45RO- are terminally differentiated T cells. All subtypes were analysed for CCR4+ and CCR4++ expression.
Enzyme-linked immunosorbent assay (ELISA)
As described previously [12], serum TARC levels were measured in duplicate. Ninety-six-well ELISA-plates (Becton Dickinson, Franklin Lakes, NJ, USA) were coated with a murine monoclonal capturing antibody directed against anti-human TARC (MAB364; R&D Systems, Abingdon, UK). Diluted human serum (1:2) was added and standard concentrations (range: 4000 to 16 pg/ml) were prepared with recombinant human TARC (364-DN; R7D Systems) in PBS containing 1% bovine serum albumin (BSA). Goat polyclonal biotinylated anti-human TARC antibody (BAF364, R&D Systems) was used as the detecting antibody. According to the manufacturer's manual, horseradish peroxidise (HRP)–streptavidin conjugate (Zymed, San Francisco, CA, USA) and substrate [3,3′,5,5′-tetramethylbenzidine (TMB) substrate; Pierce, Rockford, IL, USA] were used. A Thermo Labsystems Multiskan RC plate reader measured optical densities at 450 nm. The minimal detectable concentration of TARC was 16 pg/ml.
Statistics
A P-value below or equal to 0·05 was considered significant. The differences of CCR4 expression between LTx patients and healthy controls were analysed using the Mann–Whitney rank-sum test (MW). Correlations were tested using Spearman's rank correlation and were significant when the P-value was below or equal to 0·05 and the R-value was greater than (−) 0·4.
Results
Twenty LTx patients were included in this study, five of whom developed BOS. Patient characteristics are displayed in Table 1. A total of three patients died during follow-up. Two of these patients died as result of BOS, and one patient due to heart failure. None of the patients with cystic fibrosis (CF) in this study developed BOS. The distribution of primary disease was significantly different (P = 0·018) between the groups with and without BOS. No CF patients who eventually developed BOS were included in the group. The non-BOS group consisted mainly of patients with CF and only one patient with emphysema. The age of the non-BOS group was slightly younger than the BOS group (44 years versus 56 years). This was probably associated with the presence of CF patients, who are often transplanted at a younger age, in the non-BOS group. Overall, the differences between both groups did not contribute to the outcome, BOS development. As shown previously for our cohort, the development of BOS was not correlated with human leucocyte antigen (HLA) antibodies because they are scarcely present at both pre- and post-transplant [13].
Table 1.
Patient characteristics
| BOS | Non-BOS | |
|---|---|---|
| Total number | 5 | 15 |
| Deceased | 2 (40%) | 1 (7%) |
| Age, years (range) | 56·2 (48–62) | 43·8 (19–63) |
| Follow-up time | ||
| Months (range) | 35·8 (9–65) | 42·6 (29–66) |
| Gender | ||
| Male | 2 (40%) | 10 (66%) |
| Female | 3 (60%) | 5 (34%) |
| Primary disease | ||
| Cystic fibrosis | 0 (0%) | 8 (53%) |
| Emphysema | 3 (60%) | 1 (7%) |
| Fibrotic disease | 2 (40%) | 6 (40%) |
| BOS onset | ||
| Months (range) | 24 (9–41) | n.a. |
| BOS grade | ||
| I | 2 (40%) | |
| II | 2 (40%) | |
| III | 1 (40%) |
BOS: bronchiolitis obliterans syndrome; n.a.: not available.
CCR4 expression differs after transplantation on T cells (subsets) from healthy controls
To examine whether CCR4 expression on T cells and T cell subsets was altered due to LTx, blood from 20 LTx patients, taken approximately 5 months after LTx, was analysed and compared to results found in 11 healthy controls. As shown in Fig. 2a, CD4+ T cells from LTx patients have a decreased expression of CCR4++ (P < 0·0001, MW) when compared to healthy controls (HC). CCR4+ expression was not different between these groups. This decrease in CCR4 expression was present in patient groups developing BOS or not (P = 0·002 and P < 0·0001, respectively, data not shown). For CD8+ T cells, no differences were observed in CCR4 expression between LTx patients and HC, as shown in Fig. 2b.
Fig. 2.
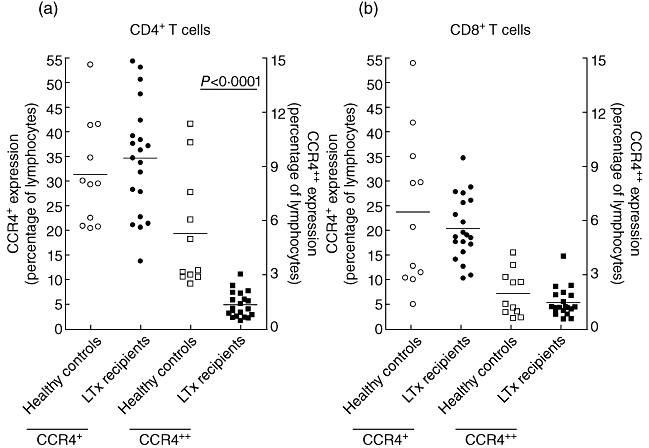
Expression of CCR4+ (left y-axis) and CCR4++ (right y-axis) on peripheral CD4+ (a) and CD8+ (b) T cells in healthy controls and lung transplant (LTx) patients. Expression of CCR4++ on CD4+ T cells was decreased in LTx patients (P = 0·002). No differences were observed for CCR4 expression on CD8+ T cells between healthy controls and LTx patients.
It was then determined whether this decrease in CCR4++ expression on CD4+ T cells contributed to a specific T cell subset, or whether all subsets contributed equally. This analysis also examined subsets of CD8+ T cells and Tregs. As shown in Fig. 3, CCR4 expression was examined on naive N, CM, effector memory EM and terminally differentiated TD CD4+ and CD8+ T cells. The CCR4++ expression was decreased on CM, EM and TD effector CD4+ T cells (P = 0·0007, P < 0·0001 and P = 0·05, respectively, MW) in lung transplant recipients compared to HC. Meanwhile, a trend was found for naive CD4+ T cells (P = 0·06, MW), which indicated that all CD4+ T cell subsets contributed to decreased CCR4++ expression on patient CD4+ T cells. Although CCR4+ expression on CD4+ T cell subsets was not different between patients and controls, it was increased on EM CD8+ T cells and TD CD8+ T cells of LTx patients (P = 0·02 and P = 0·03 respectively, MW). There was no difference between CCR4++ expression in patients and controls on CD8+ T cell subsets.
Fig. 3.
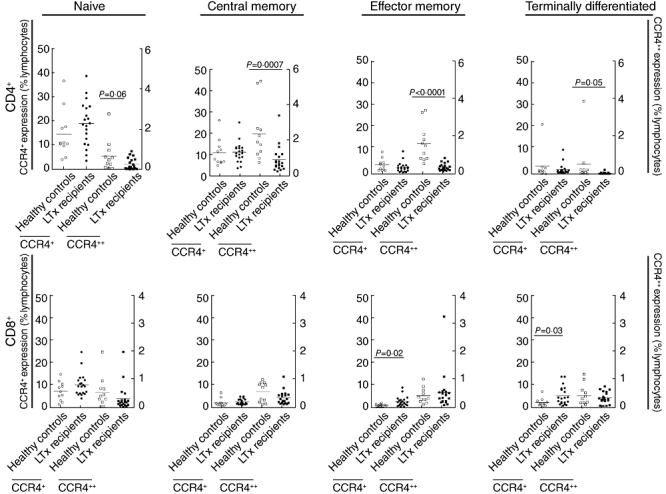
CCR4+ (left y-axis) and CCR4++ (right y-axis) expression was affected at 5 months after lung transplantation (LTx). For CD4+ T cell subsets, CCR4+ expression did not differ between healthy controls and LTx patients; however, CCR4++ expression (right y-axis) was decreased in LTx patients for all subtypes (P = 0·0007 CM, P = 0·0001 effector memory T cells (EM), P = 0·05 terminally differentiated T cells (TD), and a trend was found for naive P = 0·06). In CD8+ T cells, CCR4+ expression (left y-axis) was increased in LTx patients when compared to healthy controls on effector memory and terminally differentiated effector T cells (P = 0·02 and P = 0·03, respectively).
Analysis of Tregs (CD4+CD25+CD127–) showed a significant decrease in CCR4+ expression on T cells from LTx patients when compared to HC (P = 0·02, MW), as shown in Fig. 4. CCR4++ expression was not different between patients and controls.
Fig. 4.
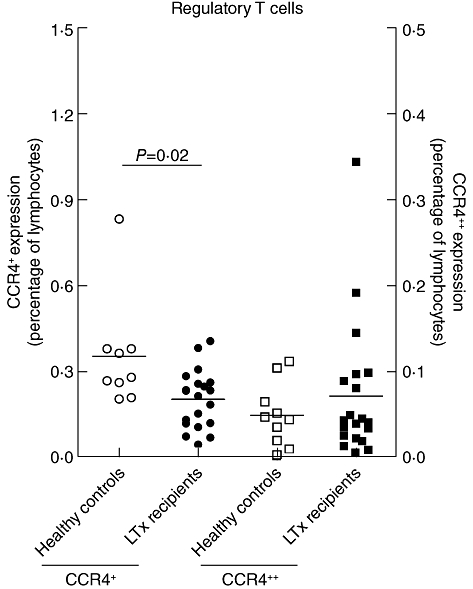
CCR4+ expression (left y-axis) on regulatory T cells (Tregs) was decreased 5 months after lung transplantation (LTx) in LTx patients when compared to healthy controls (P = 0·02). CCR4++ expression (right y-axis) was not affected.
Correlation between CCR4 expression, TARC and BOS
To study the correlation between the CCR4 receptor and its ligand TARC, a longitudinally sampled patient cohort was analysed for both CCR4 expression on CD4+ T cells and serum TARC concentration, in order to circumvent the influences of immune suppressives. Blood samples from 10 patients were analysed. These samples were taken during the first 12 months (range 9–12, on average 10·6 months) after LTx, which resulted in a total of 61 samples (6·1 samples per patient). Serum TARC was measured at the same time-points. CCR4+ or CCR4++ expression on lymphocytes, CD4+ T cells, CD4+CD25+ T cells or Tregs was not correlated with the levels of TARC in the serum samples of LTx patients (data not shown). In order to investigate a possible role of T cell migration via CCR4 and TARC in the development of BOS, the level of CCR4 expression in patients with BOS was compared to the levels in patients without BOS. There was no difference in CCR4+ expression levels on lymphocytes, CD4+ and CD8+ T cells, their subsets and Tregs between patients with or without BOS. However, CCR4++ expression levels on CD4+ EM T cells was decreased for patients with BOS when compared to patients without BOS (P = 0·04), as shown in Fig. 5. CCR4++ expression in other investigated cells, such as lymphocytes, CD4+ and CD8+ T cells, their subsets and Tregs did not differ between patients with and without BOS.
Fig. 5.
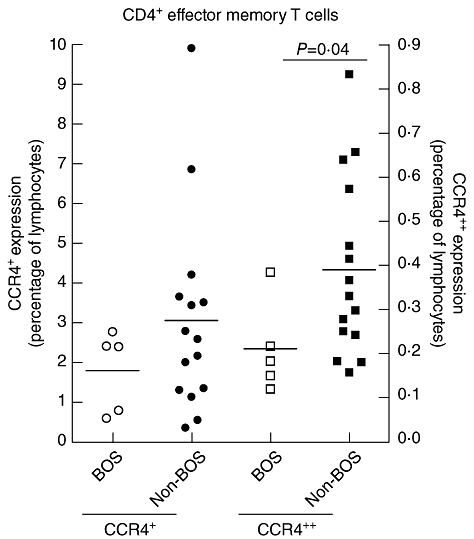
CCR4++ expression (right y-axis) on CD4+ effector memory T cells was decreased 5 months after lung transplantation (LTx) in patients developing bronchiolitis obliterans syndrome (BOS) when compared to patients without BOS (P = 0·04). CCR4+ expression (left y-axis) was not affected.
Discussion
The reported association between high serum concentrations of TARC 1 month after LTx and freedom from BOS thereafter stimulated the analysis of whether expression of its ligand CCR4 was also related to BOS. In this study, lung transplantation was shown to affect CCR4 expression on specific T cell subsets 5 months after LTx. A decrease in CCR4++ expression on CD4+ T cells from LTx patients was present in all CD4+ subsets. The finding that a higher percentage of CM CD4 T cells express CCR4++ in the circulation of patients remaining BOS-free when compared to those who develop BOS suggested that CCR4++ expression and TARC production are somehow linked during the pathogenesis of chronic rejection.
The difference in expression of CCR4++ on CD4+ T cells between LTx patients and healthy controls was reflected in all four subsets. It is unknown whether the decreased CCR4 expression affects the tissue-specific homing and chemotaxis of CD4+ T cells from LTx patients, although it has been suggested that small numbers of CCR4 molecules per cell may be insufficient to allow adhesion to endothelial cells under shear [7]. Furthermore, there is no information available indicating whether there is a difference in cytokine profile between CCR4+versus CCR4++ CD4+ T cells. In CD8+ T cells, only the EM and TD subsets were different between LTx patients and HC, which indicated that the immune suppressive regimen did not specifically change CCR4 expression on all T cells. This difference in expression on T cell subsets between healthy controls and LTx patients might partly reflect the alloresponse. Furthermore, it is important to consider the T cells' developmental stage after LTx. The expression of CCR4 in early stadia did not correlate with its functionality. In the later stadia of T helper type 2 (Th2) differentiation, this correlation was present [14]. The CCR4+ expression on Tregs was decreased in LTx patients when compared to healthy controls (P = 0·02). The response of Tregs was potent and efficient, but was less specific to gradients of TARC and MDC. This CCR4 expression was not restricted to Tregs, as was CCR8. Blood-borne Tregs preferentially express both CCR4 and CCR8, but as mature dendritic cells (DCs) produce TARC and MDC and almost no CCR8 chemokines, the main route of Treg attraction is via CCR4 [15]. This supports the hypothesis that decreased levels of TARC produced by mature DC in the lungs at time of inflammation would result in reduced attraction of Tregs, thereby contributing to ongoing inflammation leading to BOS development [3].
TARC not only functioned as a chemoattractant for CCR4 expressing Th2 cells but its production by immature DCs also polarized Th2 development from CD4+ CM cells. Th2 cells shed CD30 upon activation. In several studies, associations were found between circulating concentrations of soluble CD30 and BOS occurrence after lung transplantation [16–19]. In our study, no correlation was found between serum concentrations of TARC, sCD30 and expression of CCR4 on peripheral T cells in a longitudinal analysis (data not shown). Although the immune suppressive regimen used after transplantation pointed mainly to T cell suppression, it has been described that TARC levels are also suppressed by immune suppressive regimens [20]. Therefore, it is possible that a relationship between CCR4 expression and TARC concentrations was affected by the medication prescribed after LTx. In addition, TARC and CCR4 expression on T cells are both measured in the periphery, which does not reflect the ongoing situation at the local inflammation within the lungs. Unfortunately, we did not have BAL or biopsies available from the patients investigated to determine local concentrations of TARC. In the lung, TARC is expressed by DCs and activated airway epithelial cells (AEC) [21], and both cell types are targeted by alloresponses. It can be expected that higher levels of locally produced TARC is present in lungs remaining free from BOS versus those suffering from apoptosis of AEC, a process which takes place during chronic allograft rejection leading to BOS [2]. A comparison between patients who eventually did or did not develop BOS showed that a higher expression level of CCR4 on EM CD4+ T cells was related to freedom from BOS after LTx. Thus, it was speculated that sufficient TARC production by lung AEC or DCs attracted benign EM TH2 CD4+, which expressed high levels of CCR4 towards the site of inflammation. It is unknown, however, why this difference between BOS and non-BOS patients was present specifically in the EM but not in other subsets of T cells.
In conclusion, expression of CCR4 on T cell subsets was altered after LTx and appeared to be related to chronic rejection. Patients developing BOS showed a lower percentage in CCR4++ CM T cells than patients remaining free from BOS. Although the difference found cannot be used to identify reliably patients at risk for developing BOS, the difference was present more than a year before FEV1 decline. The presence of these cells, in combination with high production of TARC, may reflect a process of local inflammation in the lung, not causing fibrosis and obliteration of the airways.
Acknowledgments
None.
Disclosure
None.
References
- 1.Burke CM, Theodore J, Dawkins KD, et al. Post-transplant obliterative bronchiolitis and other late lung sequelae in human heart–lung transplantation. Chest. 1984;86:824–9. doi: 10.1378/chest.86.6.824. [DOI] [PubMed] [Google Scholar]
- 2.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 3.Paantjens AW, Kwakkel-van Erp JM, van Ginkel WG, et al. Serum thymus and activation regulated chemokine levels post-lung transplantation as a predictor for the bronchiolitis obliterans syndrome. Clin Exp Immunol. 2008;154:202–8. doi: 10.1111/j.1365-2249.2008.03764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belperio JA, Keane MP, Burdick MD, et al. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest. 2001;108:547–6. doi: 10.1172/JCI12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meloni F, Solari N, Miserere S, et al. Chemokine redundancy in BOS pathogenesis. A possible role also for the CC chemokines: MIP3-beta, MIP3-alpha, MDC and their specific receptors. Transpl Immunol. 2008;18:275–80. doi: 10.1016/j.trim.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Bhorade SM, Chen H, Molinero L, et al. Decreased percentage of CD4+FoxP3+ cells in bronchoalveolar lavage from lung transplant recipients correlates with development of bronchiolitis obliterans syndrome. Transplantation. 2010;90:540–6. doi: 10.1097/TP.0b013e3181e8dabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunkel EJ, Boisvert J, Murphy K, et al. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am J Pathol. 2002;160:347–55. doi: 10.1016/S0002-9440(10)64378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregson AL, Hoji A, Palchevskiy V, et al. Protection against bronchiolitis obliterans syndrome is associated with allograft CCR7+ CD45RA– T regulatory cells. PLoS ONE. 2010;5:e11354. doi: 10.1371/journal.pone.0011354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vijayanand P, Durkin K, Hartmann G, et al. Chemokine receptor 4 plays a key role in T cell recruitment into the airways of asthmatic patients. J Immunol. 2010;184:4568–74. doi: 10.4049/jimmunol.0901342. [DOI] [PubMed] [Google Scholar]
- 10.Stolberg VR, Chiu BC, Schmidt BM, Kunkel SL, Sandor M, Chensue SW. CC Chemokine receptor 4 contributes to innate NK and chronic stage T helper cell recall responses during Mycobacterium bovis. Infection. 2011;178:233–44. doi: 10.1016/j.ajpath.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yogo Y, Fujishima S, Inoue T, et al. Macrophage derived chemokine (CCL22), thymus and activation-regulated chemokine (CCL17), and CCR4 in idiopathic pulmonary fibrosis. Respir Res. 2009;10:80. doi: 10.1186/1465-9921-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hijnen D, De Bruin-Weller M, Oosting B, et al. Serum thymus and activation-regulated chemokine (TARC) and cutaneous T cell-attracting chemokine (CTACK) levels in allergic diseases: TARC and CTACK are disease-specific markers for atopic dermatitis. J Allergy Clin Immunol. 2004;113:334–40. doi: 10.1016/j.jaci.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Paantjens AW, van de Graaf EA, van Ginkel WG, van den Bosch JM, Otten HG. Lung transplantation under a tacrolimus/mycophenolate mofetil-based immunosuppressive regimen results in low titers of HLA and MICA IgG antibodies which are not related to development of BOS. J Heart Lung Transplant. 2010;29:596. doi: 10.1016/j.healun.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Morimoto Y, Bian Y, Gao P, et al. Induction of surface CCR4 and its functionality in mouse Th2 cells is regulated differently during Th2 development. J Leukoc Biol. 2005;78:753–61. doi: 10.1189/jlb.0305139. [DOI] [PubMed] [Google Scholar]
- 15.Iellem A, Mariani M, Lang R, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–53. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauwens AM, van de Graaf EA, van Ginkel WG, van Kessel DA, Otten HG. Pre-transplant soluble CD30 is associated with bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2006;25:416–19. doi: 10.1016/j.healun.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Kwakkel-van Erp JM, Otten HG, Paantjens AW, et al. Soluble CD30 measured after lung transplantation does not predict bronchiolitis obliterans syndrome in a tacrolimus/mycophenolate mofetil-based immunosuppressive regimen. J Heart Lung Transplant. 2008;27:1172–5. doi: 10.1016/j.healun.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Fields RC, Bharat A, Steward N, et al. Elevated soluble CD30 correlates with development of bronchiolitis obliterans syndrome following lung transplantation. Transplantation. 2006;82:1596–601. doi: 10.1097/01.tp.0000241076.46033.4c. [DOI] [PubMed] [Google Scholar]
- 19.Golocheikine AS, Saini D, Ramachandran S, Trulock EP, Patterson A, Mohanakumar T. Soluble CD30 levels as a diagnostic marker for bronchiolitis obliterans syndrome following human lung transplantation. Transpl Immunol. 2008;18:260–3. doi: 10.1016/j.trim.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwakkel-van Erp JM, Haeck IM, Paantjens AW, et al. Differential usefulness of biomarkers thymus and activation-regulated chemokine and soluble CD30 during enteric coated mycophenolate sodium and cyclosporine therapy in atopic dermatitis. J Am Acad Dermatol. 2010;63:e70–2. doi: 10.1016/j.jaad.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Berin MC, Eckmann L, Broide DH, Kagnoff MF. Regulated production of the T helper 2-type T-cell chemoattractant TARC by human bronchial epithelial cells in vitro and in human lung xenografts. Am J Respir Cell Mol Biol. 2001;24:382–9. doi: 10.1165/ajrcmb.24.4.4360. [DOI] [PubMed] [Google Scholar]


