Abstract
The immunosuppressive state of tumour-bearing hosts is attributable, at least in part, to myeloid-derived suppressor cells (MDSC). However, the role of MDSC in physiological conditions and diseases other than cancer has not been addressed. As the liver is a tolerogenic organ, the present study attempted to localize and assess functions of hepatic MDSC in a normal liver and in a murine model of chronic hepatitis B virus (HBV) infection. MDSC was identified in the liver of normal mice and HBV transgenic mice (TM) as CD11b+ Gr1+ cells by dual-colour flow cytometry. Highly purified populations of MDSC and their subtypes were isolated by fluorescence-activated cell sorting. The functions of MDSC and their subtypes were evaluated in allogenic mixed lymphocyte reaction (MLR) and hepatitis B surface antigen (HBsAg)-specific T cell proliferation assays. Normal mice-derived liver MDSC, but not other myeloid cells (CD11b+ Gr1-), suppressed T cell proliferation in allogenic MLR in a dose-dependent manner. Alteration of T cell antigens and impaired interferon-γ production seems to be related to MDSC-induced immunosuppression. In HBV TM, the frequencies of liver MDSC were about twice those of normal mice liver (13·6 ± 3·2% versus 6·05 ± 1·21%, n = 5, P < 0·05). Liver-derived MDSC from HBV TM also suppressed proliferative capacities of allogenic T cells and HBsAg-specific lymphocytes. Liver MDSC may have a critical role in maintaining homeostasis during physiological conditions. As liver MDSC had immunosuppressive functions in HBV TM, they may be a target of immune therapy in chronic HBV infection.
Keywords: hepatic immunity, hepatitis B virus, homeostasis, immunosuppression, myeloid-derived suppressor cells
Introduction
Myeloid-derived suppressor cells (MDSC) are a phenotypically heterogeneous cell population that includes mature myeloid cells as well as immature myelomonocytic precursors [1]. Excessive numbers of MDSC have been detected in the blood of patients with head and neck squamous cell carcinoma [2] and mice with lung tumours [3]. These cells have been detected in bone marrow, spleen and peripheral blood, within primary and metastatic solid tumours and in lymph nodes in tumour-bearing mice [1,4–6]. Functionally, MDSC suppresses the function of T cells, block natural killer (NK) cell cytotoxicity [7], modulate macrophages to an immunosuppressive M2 phenotype [8,9] and induce the development of regulatory T cells [10] in tumour-bearing hosts.
Although considerable insight has been developed into the immunosuppressive functions of MDSC in cancers, little is known about these cells in physiological conditions. However, many parenchymal organs of the body maintain an immune tolerogenic state in physiological conditions. The liver is a typical example of a tolerogenic organ in physiological condition. Different food products, inflammatory substances, allergens and drug metabolites constantly enter the liver through the gut or bloodstream. Under physiological conditions, the liver induces immunological tolerance to these substances to prevent detrimental immune reactions [11,12]. The inherent tolerogenic property of the liver is attributable to a unique hepatic microenvironment. However, unexplored immunocytes such as MDSC may have a role in this regard.
This study was performed to detect MDSC in normal mice liver by dual-colour flow cytometry. The subtypes of liver MDSC and expressions of surface antigens on different liver MDSC subtypes were elucidated. Functional assessment was accomplished to assess whether MDSC are immunosuppressive in normal mice. Also, the extents of immunosuppressive potentials of two subtypes of liver MDSC were compared. Finally, we evaluated functions of liver MDSC from hepatitis B virus (HBV) transgenic mice (TM), an animal model of virus-induced immunosuppression [13], to determine the clinical implications of MDSC in chronic viral infections.
Materials and methods
Mice
Seven-week-old male C57BL/6JJcl and C3H/HeNJcl mice were purchased from Clea Japan, Inc. (Tokyo, Japan). HBV-TM (official designation: l.2HB-BS10) were produced by microinjecting a partial tandem duplication of the complete HBV genome into fertilized eggs of C57BL/6 mice [14]. HBV TM produced hepatitis B surface antigen (HBsAg), hepatitis B e antigen, and HBV DNA in sera. HBV-related mRNAs were expressed in the liver, kidney and testis [14]. Normal C57BL/6JJcl (7-week-old male) mice were immunized twice with intraperitoneal HBsAg (10 µg, Heptavax-II, subtype adw; Banyu Pharmaceutical, Osaka, Japan) at an interval of 4 weeks to induce HBsAg-specific lymphocytes. Normal mice and HBV TM were housed separately in polycarbonate cages in a temperature-controlled room (23 ± 1°C) with a 12-h light/dark cycle in a pathogen-free animal housing facility at Ehime University Graduate School of Medicine. All animals received humane care, and study protocols were in compliance with the institution's guidelines. An animal experimental board of Ehime University approved the study.
Isolation of spleen cells and liver non-parenchymal cells (NPCs)
To produce a single cell suspension from the spleen, spleens were cut into pieces and passed through a 40-µm pore-size nylon filter (BD Falcon, Durham, NC, USA); the resulting cells were collected and suspended in a culture medium (RPMI-1640 medium; Gibco® Invitrogen, Carlsbad, CA, USA) plus 10% fetal bovine serum (Gibco® Invitrogen) [15,16].
To retrieve liver NPCs, liver tissues were cut into pieces, homogenized, passed through 70-µm pore-size steel meshes (Morimoto Yakuhin Co., Matsuyama, Japan) and suspended in 35% Percoll (Sigma Chemical, St Louis, MO, USA). After centrifugation for 15 min at 450 g at room temperature, a high-density cell pellet was collected and suspended in a culture medium [15,16].
Isolation of T lymphocytes and dendritic cells (DC)
T lymphocytes and DC were isolated from mouse spleen, as described previously [15,16]. T lymphocytes were isolated from C3H/He mice spleen single-cell suspension by a negative selection column method using a mouse pan T isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). DC were isolated from C57BL/6J mice spleen by positive selection column method using a mouse CD11c Microbeads (Miltenyi Biotec) based on the manufacturer's instructions.
Flow cytometry and cell sorting
To identify MDSC and their subtypes, allophycocyanin (APC) anti-mouse Gr-1 (clone RB6-8C5) and phycoerythrin (PE) anti-mouse CD11b (clone M1/70) were used (BD Biosciences, San Jose, CA, USA). In order to assess the expressions of surface antigens on subtypes of MDSC, fluorescein isothiocyanate (FITC) anti-mouse Ly-6G (clone 1A8), Ly-6C (clone AL-21), CD31 (clone 390) were purchased from BD Biosciences, and F4/80 (clone BM8) from eBioscience (San Diego, CA, USA). PE anti-mouse Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) (clone UC10-4F10-11), PD-1 (clone J43), CD62L (clone MEl-14) and CD40L (clone MR1) (BD Biosciences) were used to evaluate expressions of activation/exhaustion markers on T cells. For intracellular cytokine staining, cells were lysed using Fixation and Permeabilization Kit (Invitrogen, Carlsbad, CA, USA) based on the manufacturer's instructions, and stained with APC anti-mouse interferon (IFN)-γ (clone XMG 1·2) (eBioscience). The corresponding isotype antibodies were used with all the samples as controls. Flow cytometry was performed on a Becton Dickinson fluorescence activated cell sorter (FACS)Calibur using CellQuest Software (Becton Dickinson, Franklin Lakes, NJ, USA). Data analysis was performed by using FlowJo software (TreeStar Corporation, Ashland, OR, USA).
To isolate MDSC and MDSC subtypes, spleen cells and liver NPCs were stained with monoclonal antibodies to CD11b and Gr1 and were sorted with the BD FACSAria™ Cell Sorting System (Becton Dickinson). CD11b+ Gr1- cells were also sorted from liver NPCs and spleen cells suspensions by similar methods. All sorted cells were of purity above 98%.
The expressions of different surface antigens on MDSC and T cells were shown as relative frequencies among total cell populations or mean fluorescence intensity (MFI).
T cell suppression assay
C3H/HeN spleen T lymphocytes were mixed with C57BL/6J spleen DC and co-cultured in the absence or presence of sorted MDSC at different ratios to evaluate the suppressive function of MDSC in allogenic mixed leucocyte reaction (MLR). Spleen cells were also stimulated with concanavalin A (ConA, 1 µg/ml; Sigma). Spleen cells from HBsAg-injected C57BL/6J mice were cultured with or without HBsAg in the absence or presence of MDSC to assess the role of MDSC on HBsAg-specific lymphocyte proliferation. The culture conditions are described in detail elsewhere [15–17]. All cultures were performed in 96-well U-bottomed plates (Corning Inc., New York, NY, USA). [3H]-thymidine (1·0 µCi/ml; Amersham Biosciences, Little Chalfont, Buckinghamshire, UK) was diluted in sterile RPMI-1640 and added to the cultures for the last 16 h and harvested automatically by a multiple cell harvester (Labo Mash; Futaba Medical, Osaka, Japan) onto a filter paper (Labo Mash 101–10; Futaba Medical). The levels of incorporation of [3H]-thymidine were determined in a liquid scintillation counter (Beckman LS 6500; Beckman Instruments, Inc., Fullerton, CA, USA). The levels of T cell proliferation were enumerated as counts per minute (cpm). The level of cpm in culture containing only T cells was considered background proliferation and expressed as a stimulation index (SI) of 1·0. The levels of T cell proliferation in allogenic MLR were estimated by dividing the cpm in cultures containing T cells with DC or MDSC or other myeloid cells with the cpm of control cultures containing only T cells. The levels of proliferation of HBsAg-specific lymphocytes were estimated by dividing the cpm in cultures containing lymphocytes/HBsAg or MDSC with the cpm of control cultures containing T cells and an irreverent antigen, pyruvate dehydrogenase complex (Sigma Aldrich Corporation, St Louis, MO, USA) [17].
Statistical analysis
Data were expressed as mean ± standard deviation (s.d.). In all statistical analysis, data of two groups were analysed by Student's t-tests if they were normally distributed and by the Mann–Whitney rank-sum test if they were skewed. Differences were considered significant at P < 0·05 between two groups.
Results
Enumeration of MDSC and their subtypes
MDSC was detected in the liver and spleen of normal mice and HBV TM as cells expressing both CD11b and Gr1 (Fig. 1a). In normal mice, the proportions of liver MDSC were significantly higher than in the spleen (6·05 ± 1·21% versus 1·33 ± 0·50%, respectively; P < 0·05, n = 5). MDSC consisted of two main subtypes: CD11b+ Gr1high (shown by circle in Fig. 1a) and CD11b+ GR1dim (shown by square in Fig. 1a). In the spleen, the proportions of both subtypes of MDSC were almost comparable (Fig. 1b). However, the main subtype of MDSC in the liver was CD11b+ Gr1dim (Fig. 1c). This was seen in both normal mice and HBV TM (Fig. 1c).
Fig. 1.
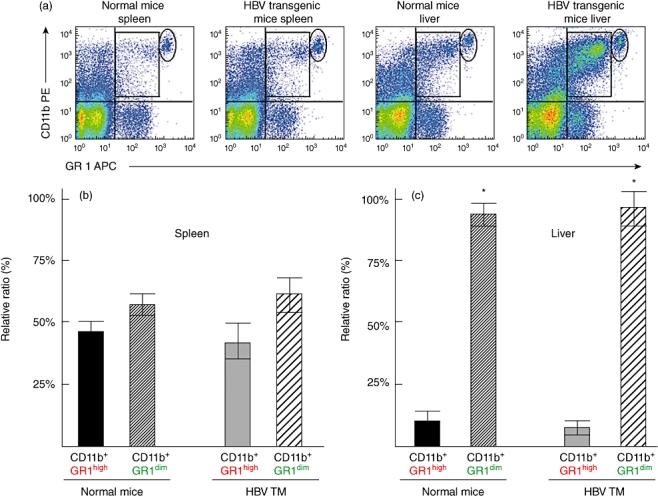
Dual-colour flow cytometry shows myeloid-derived suppressor cells (MDSC) in the spleen and liver of normal mice and hepatitis B virus (HBV) transgenic mice (HBV TM) (a). MDSC in the upper right quadrant expressed both CD11b and Gr1 antigens. Depending on the expression of Gr1 antigen, two subtypes of MDSC were detected: one expressing high levels of Gr1 (CD11b+Gr1high) (shown by circle) and another expressing low levels of Gr1 (CD11b+Gr1dim) (shown by square). (b) Comparable frequencies of CD11b+Gr1high MDSC and CD11b+Gr1dim MDSC in the spleen of normal mice and HBV TM. (c) Significantly higher frequencies of CD11b+Gr1dim MDSC compared to CD11b+Gr1high MDSC in the liver of normal mice and HBV TM (c). *P < 0·05, compared to CD11b+Gr1high MDSC.
The expression of different surface antigens showed considerable variations between MDSC subtypes of the liver (Fig. 2). The relative ratio of different surface antigens on two subtypes of liver MDSC was shown in Fig. 2b. Ly6G was expressed in most CD11b+ Gr1high MDSC, whereas CD31 and F4/80 were detected mainly in CD11b+ Gr1dim MDSC (Fig. 2b). The levels of expression of Ly6G (assessed by MFI) were significantly higher in CD11b+ Gr1high MDSC than in CD11b+ Gr1dim MDSC (Fig. 2c). Conversely, the levels of expression of Ly6C were significantly higher in CD11b+ Gr1dim MDSC compared to CD11b+ Gr1high MDSC (Fig. 2c).
Fig. 2.
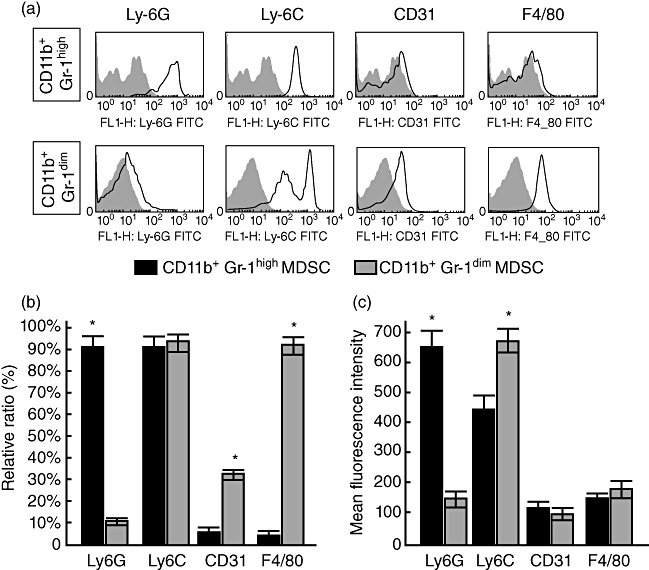
Phenotypic analyses of myeloid-derived suppressor cells (MDSC) subtypes of normal mice liver. (a) Representative histograms of phenotypic profiles of CD11b+Gr1high MDSC and CD11b+Gr1dim MDSC. (b) Proportions of Ly6G, Ly6C, CD31 and F4/80 in CD11b+Gr1high MDSC and CD11b+Gr1dim MDSC of liver from normal mice. (c) Levels of expression of Ly6G, Ly6C, CD31 and F4/80 [shown by mean fluorescence intensity (MFI) on CD11b+Gr1high MDSC and CD11b+Gr1dim MDSC. *P < 0·05, compared to other subtypes.
Immunosuppressive capacities of normal mouse liver-derived MDSC, but not by non-MDSC myeloid cells
MDSC from normal liver suppressed T cell proliferation in allogenic MLR in a dose-dependent manner. In allogenic MLR, the levels of T cell proliferation were 87·0 ± 5·3 SI (n = 5). As shown in Fig. 3a, when 1 × 104 MDSC were added to the culture, the levels of blastogenesis decreased to 73·4 ± 7·8 SI (n = 5) (P < 0·05). The levels of blastogenesis decreased further to 60·7 ± 5·6 SI (n = 5) (P < 0·05) and 45·3 ± 2·6 SI (n = 5) (P < 0·05) when 2 × 104 and 3 × 104 MDSC were added to the cultures, respectively (Fig. 3a). However, increased proliferations of T cells were seen in allogenic MLR when non-MDSC myeloid cells (CD11b+Gr1- cells) from normal mouse liver were added to the cultures (Fig. 3a).
Fig. 3.
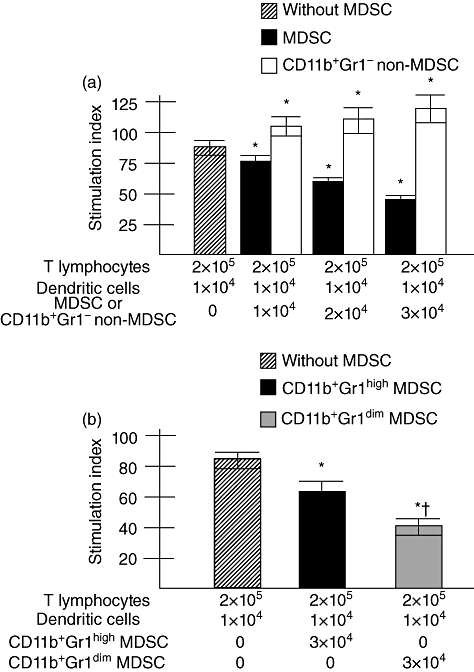
(a) Suppression of T cells proliferation by liver myeloid-derived suppressor cells (MDSC), but not by liver non-MDSC. T cells (2 × 105) and dendritic cells (1 × 104) from spleen of normal mice were cultured in allogenic mixed leucocyte reaction (MLR). MDSC or CD11b+Gr- non-MDSC were added to allogenic MLR, as mentioned. The levels of T cell proliferation are shown as the stimulation index (SI), as described in the Methods. The levels of SI in allogenic MLR without MDSC are shown as hatched bar and those in presence of MDSC and CD11b+Gr- non-MDSC are shown as black bar and clear bar, respectively. Data of five separate experiments are shown, with means and standard deviations. *P < 0·05 compared to levels of T cell proliferation in allogenic MLR without MDSC. (b) Increased T cell suppressive capacity of CD11b+Gr1dim MDSC compared to CD11b+Gr1high MDSC in allogenic MLR. The experimental conditions of allogenic MLR are similar to that described in Fig. 3a. *P < 0·05 compared to levels of T cell proliferation in allogenic MLR without MDSC. †P < 0·05 compared to levels of T cell proliferation in allogenic MLR containing CD11b+Gr1high MDSC.
As MDSC from mouse liver suppressed T cell proliferation, it was important to assess if there is any difference in T cell suppressive capacity between MDSC subtypes. In allogenic MLR, the levels of T cell proliferation were 86·0 ± 8·7 SI (n = 5) when 2 × 105 T cells from C3H/HeN mice were cultured with 1 × 104 DC from C57BL/6J mice. When 3 × 104 CD11b+ Gr1high MDSC were added to the culture, the levels of blastogenesis decreased to 62·1 ± 9·2 SI (n = 5) (P < 0·05, compared to cultures without MDSC). The levels of blastogenesis decreased to 40·2 ± 4·1 SI (n = 5) when 3 × 104 CD11b+ Gr1dim MDSC were added to the culture (P < 0·05, compared to cultures containing CD11b+ Gr1high MDSC). Taken together, CD11b+ Gr1dim MDSC exhibited significantly higher T cell suppressive capacities than CD11b+ Gr1high MDSC (Fig. 3b).
Mechanism of T cell suppression by MDSC
To develop insights into the mechanism of MDSC-induced T cell suppression, we checked the expressions of CD40L, CD62L, CTLA-4 and PD-1 on T cells cultured without or with MDSC (Fig. 4a). The frequencies of T cells expressing CD40L were decreased and those expressing CTLA-4 were increased due to culture with MDSC (n = 5, P < 0·05) (Fig. 4b,c). However, the frequencies of CD62L and PD-1 were not altered significantly due to cultures with MDSC (Fig. 4b). The levels of expression of CTLA-4 and PD1 increased significantly on T cells due to culture with MDSC (n = 5, P < 0·05). In addition, the frequencies of IFN-γ-producing T cells among total T cells were decreased significantly due to the addition of MDSC in T cell cultures compared to cultures without MDSC (1·81 ± 0·49% versus 6·11 ± 2·21%, respectively; n = 5, P < 0·05) (Fig. 4d,e).
Fig. 4.
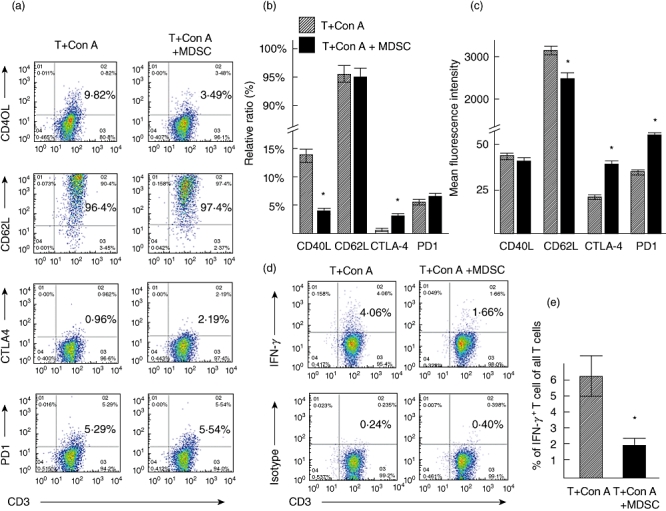
(a) Representative dot-plots of CD40L, CD62L, CTLA-4 and PD1 antigens on T lymphocytes cultured without or with myeloid-derived suppressor cells (MDSC). (b) The ratio of T cells expressing CD40L, CD62L, CTLA-4 and PD1 antigens cultured without or with MDSC. (c) The levels of expression (shown by mean fluorescence intensity) of CD40L, CD62L, CTLA-4 and PD1 antigens cultured without or with MDSC. (d) A representative staining of intracellular interferon (IFN)-γ in T cells among all T cells cultured without or with MDSC. (e) Data of five separate experiments about intracellular IFN-γ production by T cells among all T cells are shown. n = 5, *P < 0·05, compared to without MDSC.
Immunosuppressive function of liver MDSC in a murine model of chronic HBV infection
To assess the immunosuppressive capacity of MDSC in chronic viral infection, we checked the frequencies and functions of MDSC in HBV TM, a murine model of chronic HBV carrier state [15]. The proportions of liver MDSC were significantly higher in HBV TM compared to normal mice (13·6 ± 3·2% versus 6·05 ± 1·21%, respectively; P < 0·05, n = 5). In functional analyses, liver MDSC from HBV TM suppressed T cell proliferation in allogenic MLR in a dose-dependent manner (Fig. 5a).
Fig. 5.
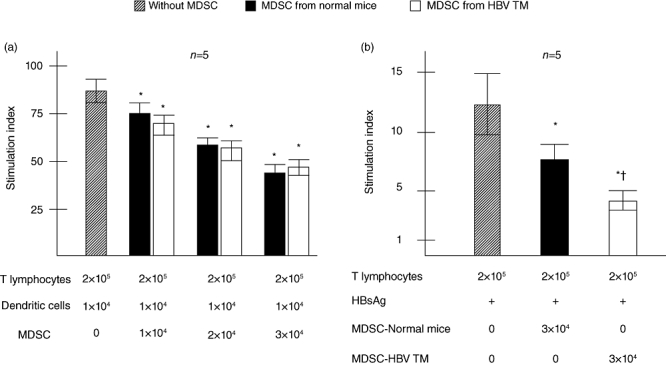
T cell suppressive capacity of hepatitis B virus transgenic mice (HBV TM)-derived MDSC. (a) T cell suppression by HBV TM-derived MDSC in allogenic MLR. (b) Suppression of antigen-specific lymphocyte proliferation by liver MDSC. T cells (2 × 105) from hepatitis B surface antigen (HBsAg)-injected mice were cultured with or without HBsAg. MDSC from normal mice and HBV TM were added to the cultures. The levels of T cell proliferation are shown as the stimulation index (SI), as described in the Methods. The levels of SI in lymphocyte proliferation without MDSC are shown as hatched bar and those in presence of MDSC from normal mice and HBV TM are shown as black bar and clear bar, respectively. Data of five separate experiments are shown, with means and standard deviations. *P < 0·05 compared to levels of HBsAg-specific lymphocyte proliferation without MDSC. †P < 0·05 compared to levels of T cell proliferation in HBsAg-specific lymphocyte proliferation containing normal mice-derived MDSC.
To assess the role of MDSC on antigen-specific immune responses, lymphocytes from HBsAg-immunized normal C57BL/6J mice were stimulated by HBsAg without or with MDSC. As shown in Fig. 5b, MDSC from both normal mice and HBV TM suppressed proliferation of HBsAg-specific lymphocytes. Moreover, the levels of suppression were significantly higher in HBV TM-derived MDSC compared to normal mice-derived MDSC (n = 5, P < 0·05) (Fig. 5b).
Discussion
Under physiological conditions, the liver maintains a state of immunological tolerance to various noxious substances to prevent extreme and detrimental immune reactions, although the liver harbours abundant amounts of immunocytes capable of inducing inflammation and cell damage. The immunosuppressive properties of the normal liver are regulated by several suppressor immunocytes, such as regulatory DC [18], regulatory NK cells [19] and regulatory T cells [20]. Interestingly, these suppressor cells of the liver also have immunogenic counterparts; immunogenic DC for regulatory DC, immunogenic T cells for regulatory T cells and immunogenic NK cells for regulatory NK cells. It seems that comprehensive functions of immunosuppressive immunocytes and immunogenic immunocytes maintain normal homeostasis under physiological conditions.
In this regard, there is a paucity of information about hepatic CD11b+ myeloid cells and their functional implications. Generally, it is assumed that CD11b+ myeloid cells are capable of producing inflammatory cytokines and play a role as immunogenic cells. Recently, it has been shown that MDSC, a subpopulation of CD11b+ myeloid cells that express both CD11b and Gr1 antigens, are endowed with immunosuppressive properties in cancer patients and in mice models of different cancers [1,2,4,5]. However, there is a paucity of information about localization, frequencies and functions of MDSC in the normal liver.
The studies presented here showed that MDSC were present in the liver of normal mice and suppressed non-antigen-specific (Fig. 3) as well as antigen-specific T cell proliferation (Fig. 5). To be more confident about the immunosuppressive properties of liver MDSC, we compared the functional capacities of liver MDSC and non-MDSC myeloid cells of the liver (cells expressing CD11b+Gr1-) in the same run. The data showed conclusively that MDSC, but not other myeloid cells, were immunosuppressive (Fig. 3).
We detected two subtypes of MDSC in the liver on the basis of expression of Gr1 antigen. Further analyses revealed that these two subtypes showed significant differences regarding expressions of Ly-6G, Ly-6C, CD31 and F4/80 antigens (Fig. 2). In addition, the magnitudes of T cell suppressive capacities of CD11b+ Gr1dim MDSC were significantly higher than CD11b+ Gr1high MDSC, the clinical implications of which should be assessed in a future study. Although both subtypes are now regarded as MDSC, further studies would be required to gain more insight into their roles in hepatic immunity in normal as well as in pathological conditions.
Various tumour-derived factors as well as arginase, nitric oxide and reactive oxygen species play a role in MDSC accumulation and their immunosuppressive functions in cancers [7–10]. In addition, MDSC-mediated immunosuppression is mediated, at least in part, through the regulation of functions of NK cells and DC [21,22] in cancers. Using a model of Con-A-induced T cell proliferation, this study pointed that MDSC may have some effects on expression of T cell antigen (Fig. 4). However, this should be confirmed in other models of T cell proliferation, especially in antigen-specific T cell proliferation. Addition of MDSC in T cell cultures down-regulated production of IFN-γ in T cells (Fig. 4).
In addition to exploring the functional capacities of MDSC in normal mice liver, we also checked MDSC function in a pathological condition other than cancer. The proportions of MDSC in the liver were significantly higher in HBV TM compared with those in the liver of normal mice. MDSC from HBV TM suppressed T cell proliferation in allogenic MLR. In addition, MDSC from HBV TM revealed significantly increased the capacity to suppress proliferation of HBsAg-specific lymphocytes compared to normal mice-derived MDSC (Fig. 5).
Immunosuppressive activity of MDSC in HBV TM, especially suppression of antigen-specific T cell proliferation by MDSC, is worthy of further study because manipulation of MDSC may have therapeutic implications. It has been shown that treatment that reduces MDSC levels, such as antibody depletion of Gr1 cells, or treatments that down-regulate MDSC, such as chemotherapeutic drugs or retinoic agents, improve the efficacy of cancer vaccines or other immunotherapy in vivo[23–27]. At present, there is no curative therapy against chronic HBV infection [28]. Immune therapy has been accomplished in patients with chronic hepatitis B, but an effective immune therapeutic regimen has yet to be developed. The therapeutic effects of different agents that deplete MDSC in HBV TM remain to be elucidated for the development of novel therapeutic approaches against chronic HBV infection.
In conclusion, this is one of the first reports to show that MDSC are present in the liver of normal mice. In addition, these cells were shown to suppress T cell immunity. We also showed that in contrast to CD11b+ Gr1high MDSC, CD11b+ Gr1dim MDSC were significantly higher in the liver and had increased immunosuppressive functions. Furthermore, we provided credible evidence about a role of MDSC in chronic HBV infection. Further studies into liver MDSC and their subtypes would provide more insight into the maintenance of hepatic homeostasis in the normal liver and information about immunosuppression after infection with hepatotrophic viruses. Finally, it may be possible to develop novel therapeutic strategies against these diseases by targeting MDSC.
Acknowledgments
We thank the Integrated Center for Science, Shigenobu Station, Ehime University for animal management and cell sorting.
Disclosures
The authors declare that there are no conflicts of interest related to the publication of this manuscript.
References
- 1.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34-cells which suppress immune functions within cancers that secrete granulocyte–macrophage colony-stimulating factor. Clin Cancer Res. 1995;1:95–103. [PubMed] [Google Scholar]
- 3.Young MR, Kolesiak K, Wright MA, Gabrilovich DI. Chemoattraction of femoral CD34-progenitor cells by tumor-derived vascular endothelial cell growth factor. Clin Exp Metastasis. 1999;17:881–8. doi: 10.1023/a:1006708607666. [DOI] [PubMed] [Google Scholar]
- 4.Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-associated CD8-T cell tolerance induced by bone marrow-derived immature myeloid cells. J Immunol. 2005;175:4583–92. doi: 10.4049/jimmunol.175.7.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte–macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–43. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 6.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–75. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C, Yu S, Kappes J, et al. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109:4336–42. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–45. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 9.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–83. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 10.Huang B, Pan PY, Li Q, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 11.Mehal WZ, Azzaroli F, Crispe IN. Immunology of the healthy liver: old questions and new insights. Gastroenterology. 2001;120:250–60. doi: 10.1053/gast.2001.20947. [DOI] [PubMed] [Google Scholar]
- 12.Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard or killing field? Immunol Rev. 2000;174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- 13.Akbar SM, Onji M, Inaba K, Yamamura K-I OY. Low responsiveness of hepatitis B virus transgenic mice in antibody response to T-cell-dependent antigen: defect in antigen presenting activity of dendritic cells. Immunology. 1993;78:468–75. [PMC free article] [PubMed] [Google Scholar]
- 14.Araki K, Miyazaki J, Hino O, et al. Expression and replication of hepatitis B virus genome in transgenic mice. Proc Natl Acad Sci USA. 1989;86:207–11. doi: 10.1073/pnas.86.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasebe A, Akbar SM, Furukawa S, Horiike N, Onji M. Impaired functional capacities of liver dendritic cells from murine hepatitis B virus (HBV) carriers. Clin Exp Immunol. 2005;139:35–42. doi: 10.1111/j.1365-2249.2005.02676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida O, Akbar F, Miyake T, et al. Impaired dendritic cell functions because of depletion of natural killer cells disrupt antigen-specific immune responses in mice. Clin Exp Immunol. 2008;152:174–81. doi: 10.1111/j.1365-2249.2008.03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyake T, Akbar SM, Yoshida O, et al. Impaired dendritic cell functions disrupt antigen-specific adaptive immune responses in mice with nonalcoholic fatty liver disease. J Gastroenterol. 2010;45:859–67. doi: 10.1007/s00535-010-0218-4. [DOI] [PubMed] [Google Scholar]
- 18.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–8. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida O, Akbar SM, Chen S, et al. Regulatory natural killer cells in murine liver and their immunosuppressive capacity. Liver Int. 2010;30:906–12. doi: 10.1111/j.1478-3231.2010.02253.x. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunogenic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 21.Hoechst B, Voigtlaender T, Ormandy L, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng P, Corzo CA, Luetteke N, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–49. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ugel S, Delpozzo F, Desantis G, et al. Therapeutic targeting of myeloid-derived suppressor cells. Curr Opin Pharmacol. 2009;9:470–81. doi: 10.1016/j.coph.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 25.Kusmartsev S, Cheng F, Yu B, et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63:4441–9. [PubMed] [Google Scholar]
- 26.Pan PY, Wang GX, Yin B, et al. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–28. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirza N, Fishman M, Fricke I, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shamliyan TA, MacDonald R, Shaukat A, et al. Antiviral therapy for adults with chronic hepatitis B: a systemic review for a National Institute of Health consensus development conference. Ann Intern Med. 2009;150:111–24. doi: 10.7326/0003-4819-150-2-200901200-00101. [DOI] [PubMed] [Google Scholar]


