Abstract
Rationale
VEGF impacts angiogenesis, atherosclerosis and cancer. Although the heritability of circulating VEGF levels is high, little is known about its genetic underpinnings.
Objective
Our aim was to identify genetic variants associated with circulating VEGF levels using an unbiased genome-wide approach and explore their functional significance with gene expression and pathway analysis.
Methods and results
We undertook a genome-wide association study (GWAS) of serum VEGF levels in 3,527 participants of the Framingham Heart Study (FHS), with pre-planned replication in 1,727 participants from two independent samples, the STANISLAS Family Study (SFS) and the Prospective Investigation of the Vasculature in Uppsala Seniors study (PIVUS). One hundred and forty SNPs reached genome-wide significance (p<5×10−8). We found evidence of replication for the most significant associations in both replication datasets. In a conditional GWAS 4 SNPs mapping to 3 chromosomal regions were independently associated with circulating VEGF levels: rs6921438 and rs4416670 (6p21.1, p=6.11×10−506 and p=1.47×10−12), rs6993770 (8q23.1, p=2.50×10−16) and rs10738760 (9p24.2, p=1.96×10−34). A genetic score including these four SNPs explained 48% of the heritability of serum VEGF levels. Six of the SNPs that reached genome-wide significance in the GWAS were significantly associated with VEGF mRNA levels in PBMCs. Ingenuity pathway analyses showed found plausible biological links between VEGF and 2 novel genes in these loci (ZFPM2 and VLDLR).
Conclusions
Genetic variants explaining up to half the heritability of serum VEGF levels were identified. These new insights provide important clues to the pathways regulating circulating VEGF levels.
Keywords: growth factors, genome-wide association study, gene expression, pathway analysis
Vascular endothelial growth factor (VEGF, also referred to as VEGFA in contrast to other members of the VEGF family) is pivotal in many physiological and pathological processes.1 It is primarily known for its key role in the stimulation of angiogenesis, with a potent mitogenic effect on vascular endothelial cells from arteries, veins and lymphatics.2 VEGF also promotes vasodilatation by inducing the production of nitric oxide and prostacyclin by endothelial cells.3 In addition, VEGF is involved in hematopoietic development and chemotaxis of monocytes, regulation of osteoclast differentiation, stimulation of surfactant production,1 and has neurotrophic and neuroprotective effects on neuronal and glial cells.4 Elevated circulating VEGF levels have been observed in vascular disease (ischemic heart disease,5, 6 heart failure,7 stroke8), and in various other disorders, including diabetes,9 cognitive decline and dementia,10, 11 reproductive,12–14 immune-inflammatory disorders,15,16 and neoplastic diseases.17, 18 Administration of VEGF promotes angiogenesis in patients with critical leg ischemia, as well as in animal models of coronary and limb ischemia.19 VEGF inhibitors such as bevacizumab and sorafenib have been successfully used to inhibit angiogenesis in several tumors,20, 21 in macular degeneration22 and in rheumatoid arthritis.23 However, despite the considerable toxicity associated with VEGF inhibitor drugs,24 there have been no pharmacogenomic studies to identify potential subgroups of responders partly because the genetic determinants of VEGF concentrations remain poorly understood. Indeed, although the heritability of circulating VEGF levels is very high, ranging between 60 and 80%,25–27 few studies have assessed the relation between circulating VEGF levels and genetic variants, yielding inconsistent results. The aim of the present study was to identify genetic variants associated with circulating VEGF levels using an unbiased genome-wide approach in a large community-based sample.
METHODS
Study populations
The Framingham Heart Study (FHS)
The FHS, initiated in 1948, is a three-generation, community-based, prospective cohort study conducted in Framingham, MA, USA.33–35 Serum VEGF levels were measured in third generation cohort participants (2002–2005) and genome-wide genotyping was performed on these individuals at Affymetrix (Santa Clara, CA) through an NHLBI funded SNP-Health Association Resource (SHARe) project. We chose not to include participants with cardiovascular disease, as the latter may influence VEGF levels. After excluding participants who had prevalent cardiovascular disease, which may influence their VEGF levels, or failed to meet quality control standards, 3,527 participants were enrolled.
The STANISLAS Family Study (SFS)
The SFS is a 10-year longitudinal survey involving 1,006 volunteer families from Vandoeuvre-lès-Nancy, France, whose members were free of chronic disease (cardiovascular or cancer) between 1993–1995.28 Plasma VEGF levels were measured at the second examination cycle (1998–2000) in a randomly selected subsample; of these 859 persons from 217 families, who also had DNA and met genotyping quality control criteria, were included.
Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study
The PIVUS study is a population-based study that enrolled 1,016 70-year old individuals living in the community of Uppsala, Sweden (2001–2004).29 Of these, 999 persons provided DNA for genetic studies and after exclusions for prevalent cardiovascular disease and inadequate genotyping quality, 868 participants were eligible.
Further details of the study samples are available in the Supplemental Methods, section 1.
Laboratory measurements of VEGF levels
VEGF levels were measured in serum for the FHS and PIVUS and plasma for the SFS (Supplemental Methods, section 2).
Genotyping
FHS
Genome-wide genotyping in the FHS was performed on the Affymetrix GeneChip Human Mapping 500K Array Set® and 50K Human Gene Focused Panel®. Genotyping, quality control and imputation methods are detailed in the Supplemental Methods, section 3.
SFS
The SNPs were genotyped by Genoscreen© (http://genoscreen.fr) using a Sequenom® iPLEX Gold assay – Medium Throughput Genotyping Technology.30
PIVUS study
The SNPs were genotyped as part of a 96-plex assay at the SNP technology platform in Uppsala University (http://www.genotyping.se/) using the Illumina BeadXpress system from Illumina Inc.31 Genotyping calls were done with Illumina BeadStudio software.
Statistics
VEGF levels were natural log-transformed to normalize their distribution.
Genome-wide association analysis in the FHS
A linear mixed effects model accounting for familial relatedness was used to evaluate the association of each SNP with VEGF levels.32 An additive genetic model with one degree of freedom was used. In a first step (model A), analyses were adjusted for age, sex, and the ninth principal component (Supplemental Methods, section 4). In a second step designed to explore potential mechanisms, we additionally adjusted our most significant associations for covariates previously found to be associated with serum VEGF levels:25 compared to model A, model B was additionally adjusted for hypertension; model C for smoking; model D for central obesity and model E for the presence of a metabolic syndrome (Supplemental Methods, section 5).
Genetic association study in the SFS and the PIVUS study
In order to confirm our findings in the FHS, we genotyped 25 SNPs in two independent samples. To select a parsimonious number of SNPs for replication we used criteria of strength of association (p-value), whether the SNP was genotyped or imputed, linkage disequilibrium (LD) between SNPs and functionality (Supplemental Methods, section 6). A linear regression model using the same covariates and analytic strategy as in the FHS was implemented.
Joint analysis of the FHS, SFS and PIVUS study
For SNPs that were successfully genotyped in the SFS and the PIVUS study we performed a meta-analysis of the SNP-phenotype associations, using a fixed effects inverse-variance meta-analysis technique for the combination of results from the FHS and the PIVUS study (which had both measured VEGF levels in the serum) and an effective sample size weighted meta-analysis for the combination of results from all three studies, to account for the different scales of VEGF levels in serum and plasma (Supplemental Methods, section 7).
Genetic score
The methods used for computing a genetic score are detailed in the Supplemental Methods, section 8, and Online Table I. The phenotypic variance explained by this genetic score was separately calculated in the FHS, the SFS and the PIVUS study, using regression models that included age and sex as covariates.
VEGF gene expression analysis in peripheral blood mononuclear cells (PBMCs)
Sample preparation and quantification of the PBMC messenger RNA (mRNA) of VEGF spliced forms and statistical analyses of these data are described in the Supplemental Methods (section 10).
Biological pathway analysis
Methods for the biological pathway analysis are provided in the Supplemental Methods, section 9.
RESULTS
Characteristics of the 5,273 study participants are presented in Table 1.
Table 1.
Characteristics of Study Participants
| Characteristics | FHS | SFS† | PIVUS Study |
|---|---|---|---|
| Number of participants | 3,527 | 859 | 868 |
| Mean circulating VEGF level (ng/L), median (IQR) * | 280 (294.7) | 27.4 (28.2) | 187.5 (210.6) |
| Mean age (SD) at VEGF measurement, mean (SD) | 40.0 (8.7) | 29.83 (14.5) | 70.2 (0.2) |
| Women (%) | 1890 (53.2) | 428 (49.8) | 454 (52.3) |
| Cardiovascular risk factor at VEGF measurement | |||
| Systolic blood pressure, mean (SD) | 116.7 (14.0) | 120.3 (12.8) | 149.6 (22.7) |
| Hypertension (%) | 561 (15.9) | 23 (2.7) | 606 (69.8) |
| Diabetes mellitus (%) | 89 (2.5) | 0 | 68 (7.8) |
| Current smoker (%) | 544 (15.3) | 188 (21.9) | 93 (10.7) |
| Central obesity (%) | 1315 (37.2) | 37 (44.3) | 266 (30.6) |
| Metabolic syndrome (%) | 693 (19.6) | 19 (2.2) | 198 (22.8) |
IQR: Inter-Quartile Range; SD: Standard Deviation;
Serum levels for the FHS and the PIVUS study, and plasma levels for the SFS;
by design, SFS participants were free of chronic disorders (cardiovascular or cancer) and had no personal history of cardiovascular disease at the time of inclusion (VEGF levels and covariates for the present analysis were measured during the second examination cycle in 1998 – 2000); all individuals with CVD (cardiovascular disease), defined in the FHS as presence of stroke, congestive heart failure, coronary heart disease or intermittent claudication, were excluded before analyses in FHS and PIVUS
GWAS of VEGF levels in the FHS
The quantile-quantile plot showed an excess of extreme p-values but no evidence of systematic inflation of the genomic control inflation factor (λ=1.02) (Online Figure I). The genome-wide plot of p-values for the individual SNPs against their genomic position is shown in Online Figure II. A total of 140 SNPs cleared the threshold for genome-wide significance at 5×10−8 (Supplemental Table II). These were located in three chromosomal regions: 6p21.1, 8q23.1, 9p24.2 (Table 2). The most significant association was found with rs6921438 on chromosome 6p21.1 (p=6.11×10−506), at 171 kb downstream of the VEGF gene, and close to the mitochondrial ribosomal protein L14 gene (MRPL14) and the MCG45491 gene (C6orf223), encoding an uncharacterized protein. Sixty-seven other SNPs on chromosome 6p21.1 were also associated with VEGF levels at p<5×10−8 (Figure 1a). When running a conditional GWAS adjusting for rs6921438, one other SNP in 6p21.1 (rs4416670) still yielded a genome-wide significant association, suggesting that two variants in this region independently modulate VEGF levels. In the 8q23.1 region the SNP yielding the most significant association with VEGF levels (rs6993770, p=2.50×10−16) is located in the zinc finger protein, multitype 2 (ZFPM2) gene and 980.4 kb away from the low-density lipoprotein receptor-related protein 12 gene (LRP12). Forty-three SNPs in LD with rs6993770 were also associated with VEGF levels at p<5×10−8 (Figure 1b). A conditional GWAS adjusting for rs6993770, rs6921438 and rs4416670 did not yield any other genome-wide significant association in chromosome 8q23.1. The most significant association on 9p24.2 was observed with rs10738760 (p=1.96×10−34), located close to the very low density lipoprotein receptor (VLDLR) and potassium voltage-gated channel subfamily V, member 2 (KCNV2) genes. Twenty-nine SNPs in LD with rs10738760 were also associated with VEGF levels at p<5×10−8 (Figure 1c). None reached genome-wide significance in a conditional GWAS adjusted for rs6921438, rs4416670, rs6993770 and rs10738760. We computed a genetic score including the four SNPs yielding genome-wide significant associations with VEGF levels in the conditional GWAS (Online Table I). This score explained 47.6% of serum VEGF variability (p=2.19×10−644).
Table 2.
Genome-wide significant single nucleotide polymorphism (SNP)-phenotype associations in genome-wide association analysis of circulating VEGF levels (p<5×10−8)
| SNP | Chr | Position* | Function | CAF | Coded Allele | strand | beta† (FHS) | SE (FHS) | p (FHS)|| | p (PIVUS)|| | p (SFS)|| | Dir | Meta-p (FHS+PIVUS)‡ | Meta-p (all)§ | Gene1 | Distance (kb) | Gene2 | Distance (kb) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs6921438 | 6 | 44033585 | intergenic | 0.51 | G | + | 0.72 | 0.01 | 6.11×10−506 | NA | 2.93×10−39 | -?- | 6.11×10−506 | 4.44×10−524 | MGC45491 | 42.7 | MRPL14 | 155.8 |
| rs4513773 | 6 | 44033504 | intergenic | 0.47 | G | + | −0.70 | 0.01 | 2.08×10−482 | 6.23×10−139 | NA | ++? | 4.45×10−619 | 1.10×10−584 | MGC45491 | 42.8 | MRPL14 | 155.8 |
| rs9472159 | 6 | 44027673 | intergenic | 0.50 | C | + | 0.76 | 0.02 | 4.30×10−452 | 3.27×10−109 | 4.11×10−35 | 1.61×10−557 | 8.16×10−553 | MGC45491 | 48.6 | MRPL14 | 161.7 | |
| rs9369434 | 6 | 44026385 | intergenic | 0.53 | C | + | 0.84 | 0.02 | 2.15×10−442 | 1.43×10−63 | 5.31×10−28 | 1.43×10−490 | 1.21×10−496 | MGC45491 | 49.9 | MRPL14 | 163.0 | |
| rs1776717 | 6 | 44059314 | intergenic | 0.21 | A | + | −0.23 | 0.02 | 8.10×10−20 | 3.74×10−4 | 9.75×10−6 | 3.28×10−22 | 1.07×10−26 | MGC45491 | 17.0 | MRPL14 | 130.0 | |
| rs1776721 | 6 | 43998961 | intronic | 0.31 | T | + | −0.18 | 0.02 | 1.52×10−19 | 3.43×10−8 | 0.02 | 5.38×10−26 | 4.23×10−26 | MGC45491 | 77.3 | VEGF | 136.8 | |
| rs1886979 | 6 | 44012879 | 3′UTR | 0.41 | G | + | 0.17 | 0.02 | 3.71×10−19 | 3.23×10−6 | 0.01 | 6.55×10−24 | 1.70×10−24 | MGC45491 | 63.4 | VEGF | 150.7 | |
| rs9472155 | 6 | 44005705 | intronic | 0.22 | T | + | −0.20 | 0.02 | 4.45×10−19 | 3.93×10−9 | 0.01 | 2.50×10−26 | 1.51×10−26 | MGC45491 | 70.6 | VEGF | 143.5 | |
| rs844294 | 6 | 44008685 | intronic | 0.52 | C | + | −0.15 | 0.02 | 1.19×10−14 | 2.25×10−5 | 0.09 | +++ | 1.41×10−18 | 2.46×10−18 | MGC45491 | 67.6 | VEGF | 146.5 |
| rs4416670 | 6 | 44058431 | intergenic | 0.55 | T | + | 0.13 | 0.02 | 1.47×10−12 | 0.10 | 2.87×10−4 | +++ | 1.44×10−12 | 2.08×10−15 | MGC45491 | 17.9 | MRPL14 | 130.9 |
| rs910611 | 6 | 44058829 | intergenic | 0.08 | C | + | −0.26 | 0.04 | 2.61×10−10 | 6.36×10−6 | 0.11 | +++ | 9.92×10−15 | 1.94×10−14 | MGC45491 | 17.5 | MRPL14 | 130.5 |
| rs6993770 | 8 | 106650704 | intronic | 0.32 | T | + | −0.17 | 0.02 | 2.50×10−16 | 3.99×10−8 | 0.02 | +++ | 2.60×10−22 | 4.71×10−23 | ZFPM2 | 0 | LRP12 | 980.4 |
| rs16873402 | 8 | 106658423 | intronic | 0.33 | T | + | −0.15 | 0.02 | 1.97×10−14 | 9.49×10−9 | 0.16 | 1.10×10−20 | 5.32×10−20 | ZFPM2 | 0 | LRP12 | 988.1 | |
| rs16873365 | 8 | 106627411 | intronic | 0.22 | T | + | −0.16 | 0.02 | 5.65×10−12 | 2.09×10−6 | 0.37 | 4.10×10−16 | 2.27×10−15 | ZFPM2 | 0 | LRP12 | 957.1 | |
| rs7013321 | 8 | 106662734 | intronic | 0.49 | A | + | −0.14 | 0.02 | 6.75×10−12 | NA | 0.01 | -?- | 6.75×10−12 | 4.49×10−13 | ZFPM2 | 0 | LRP12 | 992.4 |
| rs6993696 | 8 | 106650460 | intronic | 0.46 | A | + | −0.13 | 0.02 | 8.54×10−12 | 1.49×10−4 | 0.05 | 6.18×10−15 | 2.12×10−15 | ZFPM2 | 0 | LRP12 | 980.1 | |
| rs16873291 | 8 | 106597206 | intronic | 0.31 | T | + | −0.13 | 0.02 | 5.30×10−11 | 7.65×10−7 | 0.07 | 1.12×10−15 | 4.75×10−16 | ZFPM2 | 0 | LRP12 | 926.9 | |
| rs1349319 | 8 | 106625810 | intronic | 0.39 | A | + | 0.11 | 0.02 | 3.59×10−8 | 1.32×10−3 | 0.05 | +++ | 1.99×10−10 | 3.53×10−11 | ZFPM2 | 0 | LRP12 | 955.5 |
| rs10738760 | 9 | 2681186 | intergenic | 0.49 | A | + | 0.28 | 0.02 | 1.96×10−34 | 1.12×10−8 | 0.03 | +++ | 4.46×10−41 | 9.93×10−40 | KCNV2 | 26.3 | VLDLR | 36.7 |
| rs6475920 | 9 | 2663933 | intergenic | 0.36 | A | + | −0.24 | 0.02 | 3.76×10−32 | 2.40×10−8 | 0.02 | 6.11×10−39 | 7.93×10−38 | VLDLR | 19.4 | KCNV2 | 43.6 | |
| rs4741756 | 9 | 2658187 | intergenic | 0.28 | C | + | −0.25 | 0.02 | 2.95×10−31 | 8.64×10−5 | 0.09 | +++ | 4.45×10−34 | 3.41×10−32 | VLDLR | 13.7 | KCNV2 | 49.3 |
| rs2375980 | 9 | 2682622 | intergenic | 0.42 | G | + | −0.25 | 0.02 | 1.30×10−27 | 2.25×10−8 | 0.02 | +++ | 2.55×10−34 | 1.01×10−33 | KCNV2 | 24.9 | VLDLR | 38.1 |
| rs10122587 | 9 | 2681951 | intergenic | 0.28 | T | + | −0.22 | 0.02 | 3.02×10−24 | NA | 0.02 | -?- | 3.02×x10−24 | 4.67×10−24 | KCNV2 | 25.6 | VLDLR | 37.5 |
| rs10967492 | 9 | 2671175 | intergenic | 0.21 | A | + | −0.22 | 0.02 | 1.02×10−21 | NA | 0.10 | -?- | 1.02×10−21 | 1.25×10−20 | VLDLR | 26.7 | KCNV2 | 36.3 |
| rs10967470 | 9 | 2665698 | intergenic | 0.24 | G | + | −0.22 | 0.02 | 1.17×10−21 | NA | 0.04 | +?+ | 1.17×10−21 | 2.79×10−21 | VLDLR | 21.2 | KCNV2 | 41.8 |
CAF: Coded Allele Frequency; Chr: chromosome; Dir: Direction of association in FHS, PIVUS, SFS; Gene1: closest referenced gene; Gene2: second closest referenced gene; Meta-p: meta-analysis p-value; SNP: Single Nucleotide Polymorphism; CSE: Standard Error;
genome build 36.3;
effect estimate for the minor allele;
inverse variance meta-analysis;
effective sample size weighted meta-analysis;
model A: adjusted for age and gender, as well as for the ninth principal component in FHS
Figure 1.
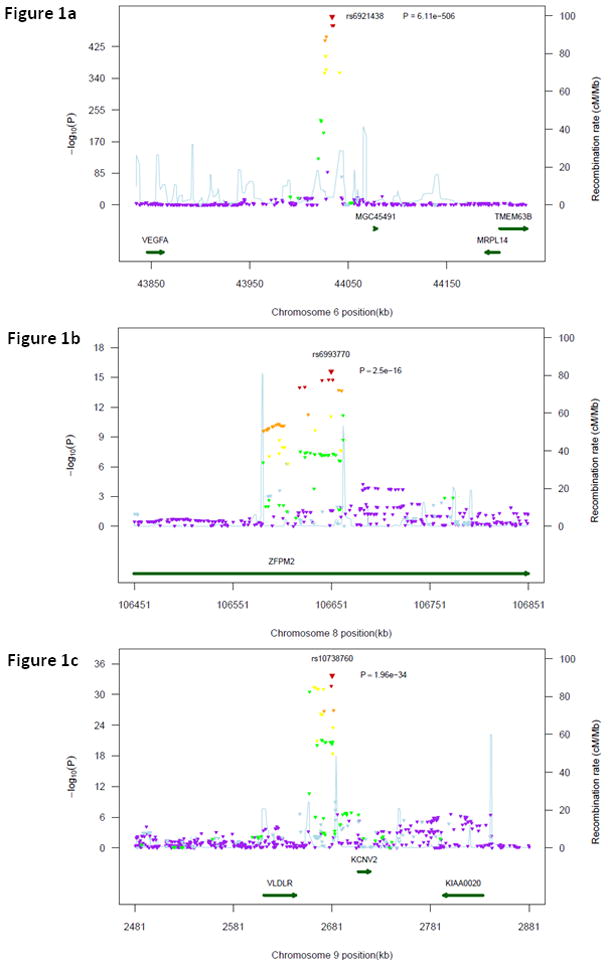
Regional plot for associations in region centered on rs6921438 (1a), rs6993770 (1b) and rs10738760 (1c). All SNPs (triangles) are plotted with their meta-analysis p-values against their genomic position. The color of the triangles represents the linkage disequilibrium between each of the SNPs in the region with rs6921438: purple: r2 ≤0.05, light blue: 0.05<r2 ≤0.10, green: 0.10<r2≤0.30, yellow: 0.30<r2≤0.60, orange: 0.60<r2≤0.80, red: r2>0.80. Light blue line represents estimated recombination rates. Genes are shown as dark green arrows. LD and recombination rates were drawn from Hapmap (release #22).
Replication studies
We sought to replicate our most significant results in two independent cohorts. Of the 25 SNPs selected for replication, 24 were successfully genotyped in the SFS and 20 in the PIVUS study (Table 2). Of these, 17 and 20 respectively reached nominal significance in association with VEGF levels, with the same direction of effect (Table 2). When meta-analyzing the results of the FHS and the PIVUS study, which both used serum VEGF levels, for the 19 SNPs genotyped in both studies, all 19 SNPs were associated with VEGF levels at p<0.05 (Table 2). The joint meta-analysis of results from all three studies, using an effective sample size weighted meta-analysis, is displayed in Table 2. There was statistically significant heterogeneity between studies for a few but not all SNPs in each locus, due to differences in effect size, but not in direction of effects (Supplemental Table III). The genetic score explained 16.6% (p=1.75×10−36) of observed plasma VEGF variability in the SFS and 48.4% (p=3.31×10−180) of observed serum VEGF variability in the PIVUS study. The observed associations remained unchanged in each of the three cohorts after adjusting for hypertension, current smoking, central obesity and metabolic syndrome (Online Table IV).
VEGF gene expression analysis
In order to better characterize the functional role of the SNPs identified in the GWAS we quantified mRNA expression of the two splice variants corresponding to the diffusible isoforms of VEGF, VEGF121 and VEGF165, in PBMCs of 220 SFS participants. The association of VEGF mRNA levels with the 24 SNPs successfully genotyped in the SFS was assessed.
At the nominal significance level, 1 SNP on chromosome 6p21.1, 4 SNPs on chromosome 8q23.1 and 1 SNP on chromosome 9p24.2 were associated with VEGF121 mRNA levels (Table 3).
Table 3.
Significant associations between SNPs and VEGF transcripts
| phenotype | SNP | Chr | position | CA | CAF | beta † | SE | p | h2q (%) |
|---|---|---|---|---|---|---|---|---|---|
| mRNA_121 | rs16873365 | 8 | 106627411 | T | 0.22 | 22.71 | 7.22 | 0.002 | 4.73 |
| mRNA_121 | rs16873402 | 8 | 106658423 | T | 0.33 | 12.15 | 5.10 | 0.017 | 2.84 |
| mRNA_121 | rs6993770 | 8 | 106650704 | T | 0.32 | 12.06 | 5.23 | 0.021 | 2.82 |
| mRNA_121 | rs16873291 | 8 | 106597206 | T | 0.31 | 11.95 | 5.37 | 0.026 | 2.47 |
| mRNA_121 | rs2375980 | 9 | 2682622 | G | 0.42 | 10.15 | 4.75 | 0.032 | 2.03 |
| mRNA_121 | rs910611 | 6 | 44058829 | C | 0.08 | −19.47 | 9.49 | 0.040 | 2.13 |
log-transformed;
effect estimate for the minor allele; CAF: Coded Allele Frequency; Chr: chromosome; h2q: variance explained; SE: standard error
Biological pathway analysis
Using the Ingenuity Pathway Analysis software (IPA, Ingenuity Systems, www.ingenuity.com) we explored functional relationships between VEGF and the genes closest to the SNPs on chromosome 8q23.1 and 9p24.2 that were significantly associated with circulating VEGF levels. In each case we selected the genes closest to the identified SNPs, as in Table 2, to identify plausible biological pathways. We selected five focus genes: VEGF, ZFPM2, LRP12, VLDLR, KCNV2. The IPA network analysis identified relationships among three of these five focus genes (VEGF, ZFPM2 and VLDLR) as part of a larger network of 35 genes. The probability of finding 3 or more focus genes in a set of 35 genes randomly selected from the Global Molecular Network was p=10−8, suggesting that the presence of three of our five focus genes in this network was unlikely to occur by chance. In Figure 2 we present a subset of this network, including only interactions between VEGF and the two other focus genes in the network, with 2 or fewer intermediate nodes.
Figure 2.
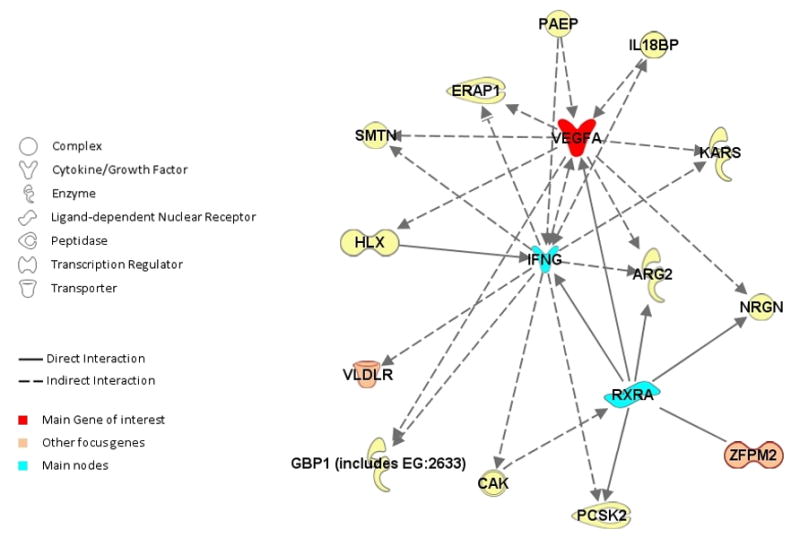
Putative Gene Network based on Ingenuity Path Analysis. Edges are displayed with labels describing the nature of the relationship between the nodes. The lines between genes represent known interactions and the nodes are displayed using various shapes which represent the functional class of the gene product (legend).
DISCUSSION
Principal findings
In this first GWAS of circulating VEGF levels undertaken in 3,527 community individuals of European descent, we identified novel genetic associations: 140 SNPs reached genome-wide significance. Of these, 4 SNPs were independently associated with VEGF levels (rs6921438 and rs4416670 on chromosome 6p21.1, rs6993770 on chromosome 8q23.1 and rs10738760 on chromosome 9p24.2). We found evidence of replication for selected SNPs in 1,727 individuals of European descent from two independent community-based samples. The SNPs are located close to the VEGF and MRPL14 genes (chromosome 6p21.1), within the ZFPM2 gene (chromosome 8q23.1), and between the VLDLR and KCNV2 genes (chromosome 9p24.2). In a subset of participants we found that 6 of 25 selected SNPs yielding genome-wide significant associations with circulating VEGF levels were also associated with VEGF mRNA levels (VEGF121 splice variant) in PBMCs.
In the context of the current literature
Genetic association study
Candidate gene studies exploring associations between VEGF polymorphisms and circulating VEGF levels have yielded controversial results (Supplemental Methods, section 11; Online Table V). Eight studies have found significant associations with candidate polymorphisms (rs699947, rs1570360, rs833061, rs2010963, rs3025039 and -2549 18bp I/D) in the promoter, 5′ and 3′ untranslated region of the VEGF gene.33–40 However, several other studies did not identify any association with these and other VEGF SNPs (Online Table V). Using a hypothesis-free genome-wide approach, the present study revealed novel associations with 140 SNPs. Of these, 68 SNPs are located on chromosome 6 approximately 150 kb downstream from the 3′ end of the VEGF gene, far from previously tested candidate SNPs. Although we do replicate previously described associations of 2 VEGF promoter polymorphisms (rs699947 and rs833061) with serum VEGF levels at p<5×10−7 (Supplemental Methods, section 11; Online Table V; Online Figure III), none of the SNPs that reached genome-wide significance in our analysis, on chromosome 6p21.1, 8q23.1 and 9p24.2, had been examined previously in relation with circulating VEGF levels.
Transcriptomic analysis
While several studies have examined the association of candidate genetic variants with VEGF gene expression in pathological tissues,41, 42 little is known about the genetic variants influencing VEGF expression in normal cells. Our data suggest that six of the SNPs associated with circulating VEGF levels in our GWAS also modulate the expression of the VEGF121 splice variant in PBMCs of community-based persons. The diffusible VEGF isoforms, VEGF165 and VEGF121, are released by a variety of tumor and normal cells, including PBMCs.43 VEGF121 lacks a heparin-binding domain and has a higher migration but lower mitogenic potency than VEGF165.44
Potential mechanisms mediating observed genetic associations
Our data suggest that almost half the inherited component of circulating VEGF levels is explained by genetic variants located downstream from the VEGF gene on chromosome 6p21.1. The conditional GWAS suggests that this region could harbor at least two distinct loci that are independently associated with circulating VEGF levels. Although located relatively far from the VEGF gene, results from our transcriptomic analysis indicate that this region could indeed contain functional variants modulating VEGF gene expression. Genome-wide significant associations with circulating VEGF levels were also identified for SNPs located on chromosome 8q23.1 and 9p24.2. Although these trans effects explain a much smaller proportion of the heritability of VEGF levels, they provide important clues about the pathways involved in the regulation of VEGF expression. The SNPs on chromosome 8q23.1 are located in introns 4 and 5 of the ZFPM2 gene. This gene encodes a widely expressed member of the Friend of GATA family of transcription factors that modulate the activity of the GATA family proteins, which are important regulators of embryogenesis and also seem to play a significant role in endothelial cell biology.45–47 The second closest gene to the SNPs identified on chromosome 8q23.1 is LRP12, encoding a low-density lipoprotein receptor-related protein that interacts with proteins related to signal transduction pathways and is differentially expressed in many cancer cells. The SNPs on chromosome 9p24.2 are located between the VLDLR and KCNV2 genes. VLDLR encodes a lipoprotein receptor involved in the metabolism of apolipoprotein-E-containing triacylglycerol-rich lipoproteins. Like VEGF, VLDLR appears to modify the risk of developing age-related macular degeneration,48 and recent data suggest that VLDLR could play a central role in a network of interacting angiogenic genes activated in response to hypoxia.49 KCNV2 encodes a member of the potassium voltage-gated channel subfamily V involved in regulation of neurotransmitter release, neuronal excitability and heart rate. While the present data does not permit us to formally determine which of these genes underlie the observed SNP associations with circulating VEGF levels, our in silico biological pathway analysis suggests that ZFPM2 and VLDLR are the most likely candidates. Further research is needed to explore the mechanisms underlying the associations of cis and trans acting genetic variants with circulating VEGF levels, such as modulation of gene expression, differential splicing or mRNA degradation.
Strengths and limitations
The findings from this first GWAS of circulating VEGF levels emphasize the importance of screening for genetic variation modulating biomarker levels not only within and in close proximity to the gene encoding the protein under investigation, but also in more distant potentially regulatory regions, including on other chromosomes. The strength of the observed associations and the fact that we were able to replicate our findings in two independent cohorts suggest that these associations are real. This is further supported by the association of several of these genetic variants with VEGF gene expression in PBMCs. Our study also had several limitations. Whereas focusing on white populations of European descent has the advantage of minimizing potential population stratification issues, our findings cannot be generalized to other ethnic groups. We may not have identified the true causal variants but merely SNPs in LD with the latter. Plasma levels of VEGF were measured in one of the replication cohorts (SFS), while serum VEGF levels had been measured in the discovery cohort. Serum VEGF concentrations are higher than plasma concentrations due to the release of VEGF from platelets during the clotting process.50 Although the vast majority of associations found in the FHS did replicate in the SFS, suggesting an important overlap between genetic susceptibility factors of serum and plasma VEGF levels, the lower proportion of VEGF variability explained by the genetic score in the SFS compared to the FHS and PIVUS study may be related to differences in plasma and serum VEGF concentrations. Finally, although our transcriptomic analysis does provide some support for a functional role of SNPs associated with mRNA levels of VEGF121, these results are exploratory and were not corrected for multiple testing. We may have been underpowered for this analysis due to limited sample size and also because VEGF expression was measured on PBMCs only, which are not the sole contributors to circulating VEGF levels. Further studies looking at the association of genetic markers with VEGF expression in other cell types, including endothelial cells, would be of great value.
Clinical Implications
VEGF plays a key role in various diseases including atherosclerosis, inflammatory and neurodegenerative disorders, and cancer.1 Anti-VEGF and proangiogenic VEGF based treatments have recently been developed for several therapeutic indications.19–23 The identification of polymorphisms linked to VEGF levels could help in identifying patients who are more likely to respond favorably to such treatments. These therapies can have major side-effects,24 and optimizing the risk-benefit ratio of their administration could lead to substantial improvements in patient care. The discovery of trans-acting genetic variants influencing VEGF levels could also spur the discovery of new molecular targets for pro- or anti-angiogenic therapies.
Conclusions
In a large population-based sample of European ancestry we identified novel genetic variants associated with circulating VEGF levels, on chromosome 6p21.1, 8q23.1 and 9p24.2, which explain almost half of the observed phenotypic variation.
Supplementary Material
Novelty and Significance.
What is known?
Circulating level of Vascular Endothelial Growth Factor (VEGF) is a heritable trait (60–80% heritability). The underlying genetic variants have not been identified.
VEGF has important physiologic and pathophysiological roles in promoting and supporting angiogenesis, as a vasodilator and neurotrophic factor. It also promotes atherosclerosis.
VEGF antagonists are used to treat macular degeneration and certain cancers.
VEGF agonists may help treat limb ischemia.
What new information does this article contribute?
Four independent novel loci are identified, using an unbiased genome-wide association study (GWAS) approach combined with mRNA expression and Ingenuity pathway analysis.
The novel loci explain nearly half the observed variation in serum VEGF levels, in population-based samples of European descent, who were free of cardiovascular disease.
Two novel loci are downstream of the VEGFA gene on chromosome 6 and may be transcription regulators.
Two other novel ‘trans’ loci are located on chromosomes 8q23.1 and 9p24.2. Potential candidate genes at these loci are zinc finger protein multitype 2 (ZFPM2) and very low density lipoprotein receptor (VLDLR).
VEGF has a pathophysiologic role in atherosclerosis, inflammatory and neurodegenerative disorders, and cancer. Circulating VEGF levels are strongly heritable trait but the underlying genetic variants are unknown. Understanding the genetic determinants of VEGF levels is of potential pharmacogenomic importance as VEGF antagonists (with inter-individual differences in toxicity and efficacy) are used to treat macular degeneration, colon cancer and other conditions. We undertook GWAS of serum VEGF levels in 3527 community-based, Framingham Heart Study participants and replicated our findings in two other healthy samples, the Stanislas Family Study and the Prospective Investigation of Vasculature in Uppsala Seniors. We identified 4 novel loci across 3 chromosomes: 6p21.1, 8q23.1 and 9p24.2 that together explain 48% of the observed variability in serum VEGF. In exploratory analyses 6 of 24 SNPs studied were associated with PBMC mRNA expression of VEGF. Further Ingenuity pathway analyses revealed that genes adjacent to the two trans loci, ZFPM2 on chromosome 8 and VLDLR on chromosome 9 are linked to VEGF along plausible biological pathways These findings highlight the potential importance of distant regulatory regions in determining biomarker levels. These results might help target VEGF based treatments and spur the discovery of new molecular targets for pro- or anti-angiogenic therapies.
Acknowledgments
Sources of Funding
Framingham Heart Study: This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. N01-HC-25195) and its contract with Affymetrix, Inc for genotyping services (Contract No. N02-HL-6-4278). A portion of this research utilized the Linux Cluster for Genetic Analysis funded by the Robert Dawson Evans Endowment of the Department of Medicine at Boston University School of Medicine and Boston Medical Center. Analyses reflect intellectual input and resource development from the Framingham Heart Study investigators participating in the SNP Health Association Resource (SHARe) project. This study was also supported by grants from the National Heart Lung and Blood Institute (HL-077477, HL093029, HL-K24-04334), the National Institute of Neurological Disorders and Stroke (NS17950) and the National Institute of Aging (AG08122, AG033193, AG031287, AG033040, P30AG013846). Dr. Debette received an award from the Bettencourt-Schueller Foundation.
STANISLAS Family Study: The STANISLAS Family study samples and data used here are part of the Biological Resources Bank (BRC) “Interactions Gène-Environnement en Physiopathologie CardioVasculaire” (IGE-PCV) in Nancy, France. The STANISLAS Family Study, as part of the BRC, was supported by the “Caisse Nationale d’Assurance Maladies des Travailleurs Salariés” (CNAM), the “Institut National de la Santé et de la Recherche Médicale” (INSERM), the “Région Lorraine”, the “Communauté Urbaine du Grand Nancy,” and the “Henri Poincaré” University of Nancy I. We are deeply grateful to the cooperation of the families participating in the STANISLAS Cohort. We thank the staff of the “Centre de Médecine Préventive” of Vandoeuvre-lès-Nancy(France) for their involvement in the recruitment of the STANISLAScohort. This work was also funded through the Collaborative BioIntelligence Program.
Prospective Investigation of the Vasculature in Uppsala Seniors Study: Genotyping was performed by the SNP Technology Platform in Uppsala (www.genotyping.se). We thank Tomas Axelsson, Ann-Christine Wiman and Caisa Pöntinen for their excellent assistance with genotyping. The SNP Technology Platform is supported by Uppsala University and the Knut and Alice Wallenberg Foundation. E.I. is supported by grants from the Swedish Research Council, the Swedish Foundation for Strategic Research, and the Royal Swedish Academy of Science.
Non-standard Abbreviations and Acronyms
- FHS
Framingham Heart Study
- GWAS
Genome-Wide Association Study
- IPA
Ingenuity Pathway Analysis
- KCNV2
Potassium voltage-gated Channel subfamily V, member 2
- LD
Linkage Disequilibrium
- LRP12
Low-density lipoprotein Receptor-related Protein 12
- mRNA
messenger RNA
- MRPL14
Mitochondrial Ribosomal Protein L14
- PBMS
Peripheral Blood Mononuclear Cells
- PIVUS
Prospective Investigation of the Vasculature in Uppsala Seniors study
- SFS
STANISLAS Family Study
- SHARe
SNP-Health Association Resource
- SNP
Single Nucleotide Polymorphism
- VEGF
Vascular Endothelial Growth Factor
- VLDLR
Very Low Density Lipoprotein Receptor
- ZFPM2
Zinc Finger Protein, Multitype 2
Footnotes
Disclosures: None
References
- 1.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 3.Wei W, Jin H, Chen ZW, Zioncheck TF, Yim AP, He GW. Vascular endothelial growth factor-induced nitric oxide- and PGI2-dependent relaxation in human internal mammary arteries: a comparative study with KDR and Flt-1 selective mutants. J Cardiovasc Pharmacol. 2004;44:615–621. doi: 10.1097/00005344-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Zachary I. Neuroprotective role of vascular endothelial growth factor: signalling mechanisms, biological function, and therapeutic potential. Neurosignals. 2005;14:207–221. doi: 10.1159/000088637. [DOI] [PubMed] [Google Scholar]
- 5.Hojo Y, Ikeda U, Zhu Y, Okada M, Ueno S, Arakawa H, Fujikawa H, Katsuki T, Shimada K. Expression of vascular endothelial growth factor in patients with acute myocardial infarction. J Am Coll Cardiol. 2000;35:968–973. doi: 10.1016/s0735-1097(99)00632-4. [DOI] [PubMed] [Google Scholar]
- 6.Blann AD, Belgore FM, McCollum CN, Silverman S, Lip PL, Lip GY. Vascular endothelial growth factor and its receptor, Flt-1, in the plasma of patients with coronary or peripheral atherosclerosis, or Type II diabetes. Clin Sci (Lond) 2002;102:187–194. [PubMed] [Google Scholar]
- 7.Chin BS, Chung NA, Gibbs CR, Blann AD, Lip GY. Vascular endothelial growth factor and soluble P-selectin in acute and chronic congestive heart failure. Am J Cardiol. 2002;90:1258–1260. doi: 10.1016/s0002-9149(02)02848-5. [DOI] [PubMed] [Google Scholar]
- 8.Slevin M, Krupinski J, Slowik A, Kumar P, Szczudlik A, Gaffney J. Serial measurement of vascular endothelial growth factor and transforming growth factor-beta1 in serum of patients with acute ischemic stroke. Stroke. 2000;31:1863–1870. doi: 10.1161/01.str.31.8.1863. [DOI] [PubMed] [Google Scholar]
- 9.Wirostko B, Wong TY, Simo R. Vascular endothelial growth factor and diabetic complications. Prog Retin Eye Res. 2008;27:608–621. doi: 10.1016/j.preteyeres.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 11.Del Bo R, Scarlato M, Ghezzi S, Martinelli Boneschi F, Fenoglio C, Galbiati S, Virgilio R, Galimberti D, Galimberti G, Crimi M, Ferrarese C, Scarpini E, Bresolin N, Comi GP. Vascular endothelial growth factor gene variability is associated with increased risk for AD. Ann Neurol. 2005;57:373–380. doi: 10.1002/ana.20390. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N, Frantz G, LeCouter J, Dillard-Telm L, Pham T, Draksharapu A, Giordano T, Peale F. Differential expression of the angiogenic factor genes vascular endothelial growth factor (VEGF) and endocrine gland-derived VEGF in normal and polycystic human ovaries. Am J Pathol. 2003;162:1881–1893. doi: 10.1016/S0002-9440(10)64322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peitsidis P, Agrawal R. Role of vascular endothelial growth factor in women with PCO and PCOS: a systematic review. Reprod Biomed Online. 2010;20:444–452. doi: 10.1016/j.rbmo.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Smith GC, Wear H. The perinatal implications of angiogenic factors. Curr Opin Obstet Gynecol. 2009;21:111–116. doi: 10.1097/GCO.0b013e328328cf7d. [DOI] [PubMed] [Google Scholar]
- 15.Distler JH, Gay S, Distler O. Angiogenesis and vasculogenesis in systemic sclerosis. Rheumatology (Oxford) 2006;45(Suppl 3):iii26–27. doi: 10.1093/rheumatology/kel295. [DOI] [PubMed] [Google Scholar]
- 16.Paleolog EM. The vasculature in rheumatoid arthritis: cause or consequence? Int J Exp Pathol. 2009;90:249–261. doi: 10.1111/j.1365-2613.2009.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura R, Nagao K, Miyayama H, Matsuda M, Baba K, Yamashita H, Fukuda M. Higher plasma vascular endothelial growth factor levels correlate with menopause, overexpression of p53, and recurrence of breast cancer. Breast Cancer. 2003;10:120–128. doi: 10.1007/BF02967636. [DOI] [PubMed] [Google Scholar]
- 18.Moon HS, Kim SC, Ahn JJ, Woo BH. Concentration of vascular endothelial growth factor (VEGF) and transforming growth factor-beta1 (TGF-beta1) in the serum of patients with cervical cancer: prediction of response. Int J Gynecol Cancer. 2000;10:151–156. doi: 10.1046/j.1525-1438.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 19.Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, Rosenfield K, Razvi S, Walsh K, Symes JF. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet. 1996;348:370–374. doi: 10.1016/s0140-6736(96)03361-2. [DOI] [PubMed] [Google Scholar]
- 20.Yeung YA, Wu X, Reyes AE, 2nd, Vernes JM, Lien S, Lowe J, Maia M, Forrest WF, Meng YG, Damico LA, Ferrara N, Lowman HB. A Therapeutic Anti-VEGF Antibody with Increased Potency Independent of Pharmacokinetic Half-life. Cancer Res. 2010;70:3269–3277. doi: 10.1158/0008-5472.CAN-09-4580. [DOI] [PubMed] [Google Scholar]
- 21.Hasskarl J. Sorafenib. Recent Results Cancer Res. 184:61–70. doi: 10.1007/978-3-642-01222-8_5. [DOI] [PubMed] [Google Scholar]
- 22.Dixon JA, Oliver SC, Olson JL, Mandava N. VEGF Trap-Eye for the treatment of neovascular age-related macular degeneration. Expert Opin Investig Drugs. 2009;18:1573–1580. doi: 10.1517/13543780903201684. [DOI] [PubMed] [Google Scholar]
- 23.Schoettler N, Brahn E. Angiogenesis inhibitors for the treatment of chronic autoimmune inflammatory arthritis. Curr Opin Investig Drugs. 2009;10:425–433. [PubMed] [Google Scholar]
- 24.Wagner AD, Arnold D, Grothey AA, Haerting J, Unverzagt S. Anti-angiogenic therapies for metastatic colorectal cancer. Cochrane Database Syst Rev. 2009:CD005392. doi: 10.1002/14651858.CD005392.pub3. [DOI] [PubMed] [Google Scholar]
- 25.Lieb W, Safa R, Benjamin EJ, Xanthakis V, Yin X, Sullivan LM, Larson MG, Smith HM, Vita JA, Mitchell GF, Sawyer DB, Vasan RS. Vascular endothelial growth factor, its soluble receptor, and hepatocyte growth factor: clinical and genetic correlates and association with vascular function. Eur Heart J. 2009;30:1121–1127. doi: 10.1093/eurheartj/ehp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pantsulaia I, Trofimov S, Kobyliansky E, Livshits G. Heritability of circulating growth factors involved in the angiogenesis in healthy human population. Cytokine. 2004;27:152–158. doi: 10.1016/j.cyto.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Berrahmoune H, Herbeth B, Lamont JV, Masson C, Fitzgerald PS, Visvikis-Siest S. Heritability for plasma VEGF concentration in the Stanislas family study. Ann Hum Genet. 2007;71:54–63. doi: 10.1111/j.1469-1809.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- 28.Visvikis-Siest S, Siest G. The STANISLAS Cohort: a 10-year follow-up of supposed healthy families. Gene-environment interactions, reference values and evaluation of biomarkers in prevention of cardiovascular diseases. Clin Chem Lab Med. 2008;46:733–747. doi: 10.1515/CCLM.2008.178. [DOI] [PubMed] [Google Scholar]
- 29.Lind L, Fors N, Hall J, Marttala K, Stenborg A. A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Arterioscler Thromb Vasc Biol. 2005;25:2368–2375. doi: 10.1161/01.ATV.0000184769.22061.da. [DOI] [PubMed] [Google Scholar]
- 30.Ehrich M, Bocker S, van den Boom D. Multiplexed discovery of sequence polymorphisms using base-specific cleavage and MALDI-TOF MS. Nucleic Acids Res. 2005;33:e38. doi: 10.1093/nar/gni038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan JB, Oliphant A, Shen R, Kermani BG, Garcia F, Gunderson KL, Hansen M, Steemers F, Butler SL, Deloukas P, Galver L, Hunt S, McBride C, Bibikova M, Rubano T, Chen J, Wickham E, Doucet D, Chang W, Campbell D, Zhang B, Kruglyak S, Bentley D, Haas J, Rigault P, Zhou L, Stuelpnagel J, Chee MS. Highly parallel SNP genotyping. Cold Spring Harb Symp Quant Biol. 2003;68:69–78. doi: 10.1101/sqb.2003.68.69. [DOI] [PubMed] [Google Scholar]
- 32.Chen MH, Yang Q. GWAF: an R package for genome-wide association analyses with family data. Bioinformatics. 2009;26:580–581. doi: 10.1093/bioinformatics/btp710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steffensen KD, Waldstrom M, Brandslund I, Jakobsen A. The relationship of VEGF polymorphisms with serum VEGF levels and progression-free survival in patients with epithelial ovarian cancer. Gynecol Oncol. 2010;117:109–116. doi: 10.1016/j.ygyno.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Kamoun M, Houman MH, Hamzaoui A, Hamzaoui K. Vascular endothelial growth factor gene polymorphisms and serum levels in Behcet’s disease. Tissue Antigens. 2008;72:581–587. doi: 10.1111/j.1399-0039.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhai R, Gong MN, Zhou W, Thompson TB, Kraft P, Su L, Christiani DC. Genotypes and haplotypes of the VEGF gene are associated with higher mortality and lower VEGF plasma levels in patients with ARDS. Thorax. 2007;62:718–722. doi: 10.1136/thx.2006.069393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrante M, Pierik M, Henckaerts L, Joossens M, Claes K, Van Schuerbeek N, Vlietinck R, Rutgeerts P, Van Assche G, Vermeire S. The role of vascular endothelial growth factor (VEGF) in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:870–878. doi: 10.1097/01.mib.0000235095.01608.10. [DOI] [PubMed] [Google Scholar]
- 37.Krippl P, Langsenlehner U, Renner W, Yazdani-Biuki B, Wolf G, Wascher TC, Paulweber B, Haas J, Samonigg H. A common 936 C/T gene polymorphism of vascular endothelial growth factor is associated with decreased breast cancer risk. Int J Cancer. 2003;106:468–471. doi: 10.1002/ijc.11238. [DOI] [PubMed] [Google Scholar]
- 38.Awata T, Inoue K, Kurihara S, Ohkubo T, Watanabe M, Inukai K, Inoue I, Katayama S. A common polymorphism in the 5′-untranslated region of the VEGF gene is associated with diabetic retinopathy in type 2 diabetes. Diabetes. 2002;51:1635–1639. doi: 10.2337/diabetes.51.5.1635. [DOI] [PubMed] [Google Scholar]
- 39.Renner W, Kotschan S, Hoffmann C, Obermayer-Pietsch B, Pilger E. A common 936 C/T mutation in the gene for vascular endothelial growth factor is associated with vascular endothelial growth factor plasma levels. J Vasc Res. 2000;37:443–448. doi: 10.1159/000054076. [DOI] [PubMed] [Google Scholar]
- 40.Petrovic MG, Korosec P, Kosnik M, Osredkar J, Hawlina M, Peterlin B, Petrovic D. Local and genetic determinants of vascular endothelial growth factor expression in advanced proliferative diabetic retinopathy. Mol Vis. 2008;14:1382–1387. [PMC free article] [PubMed] [Google Scholar]
- 41.Cacev T, Loncar B, Seiwerth S, Spaventi S, Kapitanovic S. Vascular endothelial growth factor polymorphisms -1154 G/A and -460 C/T are not associated with VEGF mRNA expression and susceptibility to sporadic colon cancer. DNA Cell Biol. 2008;27:569–574. doi: 10.1089/dna.2008.0756. [DOI] [PubMed] [Google Scholar]
- 42.Cosin R, Gilabert-Estelles J, Ramon LA, Espana F, Gilabert J, Romeu A, Estelles A. Vascular endothelial growth factor polymorphisms (-460C/T, +405G/C, and 936C/T) and endometriosis: their influence on vascular endothelial growth factor expression. Fertil Steril. 2009;92:1214–1220. doi: 10.1016/j.fertnstert.2008.08.079. [DOI] [PubMed] [Google Scholar]
- 43.Visvikis-Siest S, Marteau JB, Samara A, Berrahmoune H, Marie B, Pfister M. Peripheral blood mononuclear cells (PBMCs): a possible model for studying cardiovascular biology systems. Clin Chem Lab Med. 2007;45:1154–1168. doi: 10.1515/CCLM.2007.255. [DOI] [PubMed] [Google Scholar]
- 44.Keyt BA, Berleau LT, Nguyen HV, Chen H, Heinsohn H, Vandlen R, Ferrara N. The carboxyl-terminal domain (111–165) of vascular endothelial growth factor is critical for its mitogenic potency. J Biol Chem. 1996;271:7788–7795. doi: 10.1074/jbc.271.13.7788. [DOI] [PubMed] [Google Scholar]
- 45.Tevosian SG, Deconinck AE, Tanaka M, Schinke M, Litovsky SH, Izumo S, Fujiwara Y, Orkin SH. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell. 2000;101:729–739. doi: 10.1016/s0092-8674(00)80885-5. [DOI] [PubMed] [Google Scholar]
- 46.Song H, Suehiro J, Kanki Y, Kawai Y, Inoue K, Daida H, Yano K, Ohhashi T, Oettgen P, Aird WC, Kodama T, Minami T. Critical role for GATA3 in mediating Tie2 expression and function in large vessel endothelial cells. J Biol Chem. 2009;284:29109–29124. doi: 10.1074/jbc.M109.041145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Kodolitsch Y, Ito WD, Franzen O, Lund GK, Koschyk DH, Meinertz T. Coronary artery anomalies. Part I: Recent insights from molecular embryology. Z Kardiol. 2004;93:929–937. doi: 10.1007/s00392-004-0152-7. [DOI] [PubMed] [Google Scholar]
- 48.Haines JL, Schnetz-Boutaud N, Schmidt S, Scott WK, Agarwal A, Postel EA, Olson L, Kenealy SJ, Hauser M, Gilbert JR, Pericak-Vance MA. Functional candidate genes in age-related macular degeneration: significant association with VEGF, VLDLR, and LRP6. Invest Ophthalmol Vis Sci. 2006;47:329–335. doi: 10.1167/iovs.05-0116. [DOI] [PubMed] [Google Scholar]
- 49.Loewen N, Chen J, Dudley VJ, Sarthy VP, Mathura JR., Jr Genomic response of hypoxic Muller cells involves the very low density lipoprotein receptor as part of an angiogenic network. Exp Eye Res. 2009;88:928–937. doi: 10.1016/j.exer.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 50.Verheul HM, Hoekman K, Luykx-de Bakker S, Eekman CA, Folman CC, Broxterman HJ, Pinedo HM. Platelet: transporter of vascular endothelial growth factor. Clin Cancer Res. 1997;3:2187–2190. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


