Abstract
Objectives
We sought to evaluate placental growth factor (PlGF) and soluble fms-like tyrosine kinase 1 (sFlt-1) as clinical biomarkers in chronic heart failure (HF).
Background
Vascular remodeling is a crucial compensatory mechanism in chronic HF. The angiogenic ligand PlGF and its target receptor fms-like tyrosine kinase 1 (Flt-1) modulate vascular growth and function, but their relevance in human HF is undefined.
Methods
We measured plasma PlGF and sFlt-1 in 1,403 patients from the Penn Heart Failure Study, a multi-center cohort of chronic systolic HF. Subjects were followed for death, cardiac transplantation, or ventricular assist device placement over a median follow-up of 2 years.
Results
sFlt-1 was independently associated with measures of HF severity, including NYHA Class (p<0.01) and BNP (p<0.01). Patients in the 4th quartile of sFlt-1 (>379pg/ml) had a 6.17-fold increased risk of adverse outcomes (p<0.01). This association was robust, even after adjustment for the Seattle Failure Model (HR 2.54, 95%CI 1.76–2.27, p<0.01) and clinical confounders including heart failure etiology (HR 1.67, 95%CI 1.06–2.63, p=0.03). Combined assessment of sFlt-1 and BNP exhibited high predictive accuracy at 1-year (AUC 0.791, 95%CI 0.752–0.831), that was greater than either marker alone (p<0.01 and p=0.03, respectively). In contrast, PlGF was not an independent marker of disease severity or outcomes.
Conclusions
Our findings support a role for sFlt-1 in the biology of human heart failure. With additional study, circulating sFlt-1 may emerge as a clinically useful biomarker to assess the influence of vascular remodeling on clinical outcomes.
Keywords: heart failure, soluble Flt-1, placental growth factor
Background
Although heart failure (HF) is primarily a disorder of the myocardium, abnormal vascular function has a major impact on HF progression and cardiac remodeling (1, 2). Angiogenic growth factors, such as the vascular endothelial growth factor (VEGF) family of proteins, govern numerous aspects of vessel homeostasis (3, 4). In the setting of ischemic heart disease, alterations in angiogenic growth factors contribute to endothelial cell dysfunction and impaired revascularization (5). Even in the setting of nonischemic heart disease, angiogenic factors regulate myocardial capillary density as the heart hypertrophies (6,7), exert antiapoptoic and protrophic effects in dilated cardiomyopathy (8), and influence peripheral vascular load (9).
Placental growth factor (PlGF) is a member of the VEGF family of angiogenic proteins and is expressed in placental, cardiac, and lung tissue (10–12). PlGF activates the Fms-like tyrosine kinase receptor 1 (Flt-1), and is expressed in numerous cell types including endothelial cells, monocytes, and renal mesangial cells. The Flt-1 receptor has affinity for PlGF, VEGF-A, and VEGF-B. In animal models, these growth factors exert pleiotrophic effects, including potentially beneficial effects, such as the promotion of angiogenesis, and potentially harmful pro-inflammatory effects, such as the promotion of atherogenesis (10–12). Hence, the overall effect of PlGF/Flt-1 signaling in cardiovascular disorders may vary according to disease state and comorbidities.
To better understand PlGF/Flt-1 signaling in the setting of human disease, investigators have capitalized on the observations that both PlGF and the circulating form of the Flt-1 receptor, soluble Flt-1 (sFlt-1), can be easily quantified, providing a method to conveniently gauge overall PlGF and sFlt-1 activity in patients with cardiovascular disorders. During pregnancy, changes in circulating PlGF and sFlt-1 reflect impaired endothelial and glomerular function and predict preeclamptic risk (13,14). In patients with chest pain and acute coronary syndromes, higher PlGF levels are seen in those with myocardial infarction (MI) and are associated with an increased risk of short and long-term adverse outcomes (12,15,16). Studies of circulating sFlt-1 have demonstrated conflicting results, with some studies noting higher levels during acute MI compared to control patients (11), and others noting lower plasma levels in patients during the acute phase of MI compared to controls (17,18).
Although PlGF and sFlt-1 may be important disease modifiers in chronic human HF, neither factor has been comprehensively studied in this population. The largest published experience on PlGF was a cross-sectional study of 98 patients that showed a positive relationship between PlGF levels and New York Heart Association (NYHA) class in ischemic HF, but not in nonischemic disease (19). Circulating sFlt-1 has not been studied in human HF.
The purpose of our study was to evaluate circulating PlGF and sFlt-1 as clinical biomarkers in a multi-center cohort of 1,403 ambulatory HF outpatients. Our goals were 1) to determine the factors that independently affect baseline levels of PlGF and sFlt-1 in chronic HF and 2) to test the hypotheses that PlGF and sFlt-1 predict the combined outcome of ventricular assist device (VAD) placement, cardiac transplantation, or death.
Methods
Study Population
The Penn Heart Failure Study (PHFS) is a multi-center prospective cohort study of outpatients with primarily chronic systolic HF recruited from referral centers at the University of Pennsylvania (Philadelphia, PA), Case Western University (Cleveland, OH), and the University of Wisconsin (Madison, WI) (20). The primary inclusion criterion is a clinical diagnosis of HF. Participants are excluded if they have a non-cardiac condition resulting in an expected mortality of less than 6 months as judged by the treating physician, or if they were unable or unwilling to provide informed consent.
At time of study entry, detailed clinical data were obtained using a standardized questionnaire administered to the patient and physician, with verification via medical records. Venous blood samples were obtained at enrollment, processed, and stored at −80°C until time of assay. Follow-up events including all-cause mortality, cardiac transplantation, and VAD placement were prospectively ascertained every 6 months.
All participants provided written, informed consent, and the PHFS protocol was approved by participating Institutional Review Boards.
Biomarkers Assays
All biomarkers were measured from the same aliquot from a banked plasma sample that was obtained at time of study entry. PlGF and sFlt-1 were measured using prototype ARCHITECT immunoassays (Abbott Laboratories, Abbott Park, IL). The sFlt-1 immunoassay measures both free and bound sFlt-1, with an assay range of 15 to 50,000pg/ml. The intra- and interassay coefficients of variation (CV) ranged from 1.3% to 5.2% and 1.9% to 5.9%, respectively. The PlGF immunoassay measures the free, not bound PlGF-1 with approximately 20% cross-reactivity with the PlGF-2 isoform, with an assay range from 1 to 1,500pg/ml. The intra- and interassay CV ranged from 1.4% to 6.7% and 1.8% to 6.7%, respectively.
BNP was measured using the ARCHITECT® BNP chemiluminescent microparticle immunoassay (Abbott Laboratories, Abbott Park, IL) as previously described (21). The assay range was from 10 to 5,000pg/ml. The intra- and interassay CV ranged from 0.9% to 5.6% and 1.7% to 6.7%, respectively.
Statistical Methods
Baseline characteristics were summarized for the entire cohort using standard descriptive statistics. Independent determinants of sFlt-1 and PlGF levels were ascertained using a multivariable linear regression model for each marker. Adjustment variables were selected from a saturated model that included all variables listed in Supplementary Tables 2 and 3. The inclusion of adjustment variables was based on a stepwise model-selection procedure that chose the subset of variables that minimized the Akaike information criterion (AIC). Based on clinical/biologic judgment, the following variables were retained regardless of their impact on AIC: age, gender, race, NYHA class, cardiomyopathy etiology, estimated glomerular filtration rate (eGFR), and BNP.
Cox regression models were used to determine the unadjusted association between sFlt-1 and PlGF and time to the combined outcome of all-cause death, cardiac transplantation, or VAD placement. Biomarkers were modeled as continuous variables and according to quartiles. Adjusted models included covariates based on statistical evidence for confounding and clinical judgment. Statistical evidence was defined by a univariable association with the combined outcome at a significance level<0.10 and a >10% change in the estimated regression coefficient for each biomarker. Covariates that met these criteria were: age, gender, race, NYHA class, history of hypertension, history of diabetes, tobacco use, cardiomyopathy etiology, cardiac resynchronization, defibrillator, ACE inhibitor/angiotensin receptor blocker use, aldosterone use, aspirin use, beta blocker use, HMG CoA reductase inhibitor use, and body mass index. Age exhibited non-proportional hazards and was thus adjusted for using a time-varying covariate, which was obtained by multiplying age by a linear term for time. Adjustment for NYHA class was achieved by stratifying the baseline hazard function.
Clinical judgment included the consideration of candidate mediators of the observed association between biomarker and outcome based upon the known biology of these vascular growth factors. We decided a priori to not adjust for peripheral vascular disease, ejection fraction, pulse pressure, eGFR, and sodium given the concern that each of these measures might represent causal pathway mediators of the association between vascular growth factors and adverse outcomes. These hypotheses were based upon the established biologic effects of sFlt-1 and PlGF on renal dysfunction, vascular disease, and cardiac remodeling (7,8,22–26). We fit additional multivariable models to comprehensively assess the independence and predictive value of our observed associations in the context of validated clinical models by adjusting for the Seattle Heart Failure Model (SHFM) score, a standard risk prediction algorithm in HF (27).
The joint effects of sFlt-1 and BNP were evaluated by dividing the cohort into groups based on the median level of each marker. In addition, time-dependent receiver operating characteristic (ROC) curves were used to compare the ability of ln-transformed sFlt-1 and BNP to classify patients with regard to death, cardiac transplantation, or VAD placement at 1 year (28). Confidence intervals for the area under the ROC curve (AUC) were obtained from 1,000 bootstrapped samples, and AUCs were compared using Wald tests. All statistical analyses were completed using R 2.11.0, including the MASS, survival, and survival ROC packages (29–32).
Results
Baseline Characteristics
Biomarker data were available for 1,535 subjects. Twenty-four subjects whose sFlt-1 or PlGF was greater than the 99th percentile were excluded a priori from all analyses given the levels in these patients are most likely to be indicative of the influence of non-HF disease states (e.g. pregnancy, infection, inflammation, lupus, recent surgery, or cancer) (13,33–38). Of these 24 patients, there were 6 without an identifiable non-HF cause of highly elevated biomarker levels. Inclusion of these 6 patients did not substantially change the results. Of the remaining 1,511 patients, complete data on all baseline characteristics and outcomes were available for 1,403 (93%) subjects. For each characteristic with any missing data, the amount of missingness averaged 1.5% and was no more than 1.7%. Those patients with any missing data did not differ systematically from the remainder of the cohort (Supplementary Table 1).
The clinical characteristics of the 1,403 patients with complete data are shown in Table 1. The majority of the patients were male (67%) and Caucasian (74%), with a mean age across the cohort of 56 years. There were 423 patients (30%) with an ischemic cause of HF, 397 (28%) patients with a history of diabetes, and 817 (58%) with a history of hypertension.
Table 1.
Baseline Characteristics for the Entire Cohort and by sFlt-1 Quartiles
| Cohort | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p value* | |
|---|---|---|---|---|---|---|
| n = 1403 | n = 351 | n = 353 | n = 348 | n = 351 | ||
| Demographic Characteristics | ||||||
| Age, years | 56 (14) | 53 (14) | 55 (14) | 58 (15) | 58 (14) | < 0.01 |
| Male, n (%) | 939 (67) | 228 (65) | 238 (67) | 231 (66) | 242 (69) | 0.78 |
| Race, n (%) | < 0.01 | |||||
| Caucasian | 1034 (74) | 310 (88) | 263 (75) | 238 (68) | 223 (64) | |
| African American | 320 (23) | 30 (9) | 72 (20) | 101 (29) | 117 (33) | |
| Other | 49 (3) | 11 (3) | 18 (5) | 9 (3) | 11 (3) | |
| Medical History and Risk Factors | ||||||
| History of hypertension, n (%) | 817 (58) | 165 (47) | 191 (54) | 234 (67) | 227 (65) | < 0.01 |
| History of diabetes, n (%) | 397 (28) | 71 (20) | 91 (26) | 112 (32) | 123 (35) | < 0.01 |
| Any peripheral vascular disease, n (%) | 182 (13) | 23 (7) | 51 (14) | 56 (16) | 52 (15) | < 0.01 |
| Tobacco use, n (%) | 0.03 | |||||
| Never | 518 (37) | 154 (44) | 124 (35) | 120 (34) | 120 (34) | |
| Current | 129 (9) | 26 (7) | 40 (11) | 26 (7) | 37 (11) | |
| Former | 756 (54) | 171 (49) | 189 (54) | 202 (58) | 194 (55) | |
| Hypercholesterolemia, n (%) | 887 (63) | 201 (57) | 218 (62) | 236 (68) | 232 (66) | 0.02 |
| Heart Failure Characteristics | ||||||
| NYHA classification, n (%) | < 0.01 | |||||
| I | 227 (16) | 94 (27) | 81 (23) | 41 (12) | 11 (3) | |
| II | 649 (46) | 189 (54) | 174 (49) | 180 (52) | 106 (30) | |
| III | 421 (30) | 63 (18) | 84 (24) | 108 (31) | 166 (47) | |
| IV | 106 (8) | 5 (1) | 14 (4) | 19 (5) | 68 (19) | |
| Ischemic heart failure, n (%) | 423 (30) | 87 (25) | 94 (27) | 127 (36) | 115 (33) | < 0.01 |
| Ejection fraction | 33 (17) | 37 (16) | 34 (16) | 33 (17) | 28 (17) | < 0.01 |
| Cardiac resynchronization therapy, n (%) | 354 (25) | 63 (18) | 67 (19) | 108 (31) | 116 (33) | < 0.01 |
| Defibrillator, n (%) | 593 (42) | 105 (30) | 135 (38) | 164 (47) | 189 (54) | < 0.01 |
| Medication Use | ||||||
| ACE inhibitors or ARBs, n (%) | 1224 (87) | 315 (90) | 323 (92) | 310 (89) | 276 (79) | 0.54 |
| Aldosterone antagonists, n (%) | 477 (34) | 103 (29) | 104 (29) | 123 (35) | 147 (42) | < 0.01 |
| Aspirin, n (%) | 762 (54) | 190 (54) | 199 (56) | 195 (56) | 178 (51) | 0.41 |
| Beta-blockers, n (%) | 1225 (87) | 309 (88) | 317 (90) | 310 (89) | 289 (82) | 0.01 |
| Clinical Measures | ||||||
| Body mass index, kg/m2 | 30 (7) | 29 (5) | 30 (7) | 32 (8) | 30 (8) | < 0.01 |
| Pulse pressure, mmHg | 45 (15) | 45 (13) | 45 (15) | 47 (16) | 42 (16) | < 0.01 |
| eGFR, ml/min/1.73 m2 | 69 (25) | 77 (21) | 73 (24) | 64 (26) | 59 (26) | < 0.01 |
| Sodium | 139 (3) | 140 (3) | 139 (3) | 140 (3 | 138 (4) | < 0.01 |
| Biomarkers or Clinical Risk Scores | ||||||
| BNP, pg/ml, median (IQR) | 175 (48, 531) | 57 (21, 117) | 124 (39, 282) | 229 (80, 520) | 766 (250, 1295) | < 0.01 |
| sFlt-1, pg/ml | 348 (181) | 227 (26) | 282 (14) | 336 (21) | 546 (266) | |
| PlGF, pg/ml | 19.4 (6.2) | 18.0 (4.9) | 19.4 (5.8) | 20.4 (5.9) | 20.0 (7.4) | < 0.01 |
| SHFM score | −0.07 (1.0) | −0.60 (0.8) | −0.33 (0.8) | 0.03 (0.9) | 0.62 (1.0) | < 0.01 |
sFlt-1 quartiles: ≤ 258; 258 to 308; 308 to 379, > 379 pg/ml
Summaries presented as mean (standard deviation) unless otherwise noted as n (%) or median (inter-quartile range; IQR)
Based on ANOVA for symmetric continuous variables; Kruskal-Wallis test for non-symmetric continuous variables; χ2 for categorical variables
Independent Determinants of Baseline sFlt-1 and PlGF Levels
Across the cohort, the distributions of sFlt-1 and PlGF were approximately normal, with slightly heavier positive tails than would be expected if their distributions were truly normal. sFlt-1 levels ranged from 115 to 2012pg/ml, with a mean±SD of 348±181pg/ml. The median and interquartile range were 308 (258,379)pg/ml. PlGF levels ranged from 0.7 to 42.3pg/ml, with a mean±SD of 19.4±6.2pg/ml. The median and interquartile range were 18.6 (15.0,22.7) pg/ml.
To establish the independent determinants of either biomarker, we first assessed the univariable associations between baseline levels of either biomarker and clinical characteristics (Table 1, Supplementary Tables 2 and 3). We then utilized multivariable models to determine clinical factors that independently influenced baseline levels of each biomarker. African American race, higher NYHA class, hypercholesterolemia, and higher plasma BNP were each independently associated with higher levels of sFlt-1 (Table 2). Increasing age, aspirin use, beta blocker use, higher eGFR, and sodium were each independently associated with lower levels of sFlt-1. Given the positive skew of sFlt-1, we considered a sensitivity analysis in which we modeled ln-transformed sFlt-1 levels as the outcome, rather than the non-transformed levels. The direction and magnitude of independent associations with ln-transformed sFlt-1 were similar to those provided in Table 2, with the exception that pulse pressure was independently associated with a significant decrease in sFlt-1 levels (1.4% decrease in sFlt-1 per 10 mmHg increase in pulse pressure, p=0.01).
Table 2.
Independent Determinants of Baseline sFlt-1 Levels
| Difference in sFlt-1* | 95% CI | p value | |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (10 year difference) | −7.9 | (−15, −0.66) | 0.02 |
| African American (vs Caucasian) | +70 | (49, 91) | < 0.01 |
| Heart Failure Characteristics | |||
| NYHA functional classification | |||
| II (vs I) | +7.2 | (−17, 32) | 0.56 |
| III (vs I) | +53 | (26, 80) | < 0.01 |
| IV (vs I) | +220 | (180, 250) | < 0.01 |
| Medical History and Risk Factors | |||
| Hypercholesterolemia (vs none) | +21 | (1.0, 40) | 0.04 |
| Medication Use | |||
| Beta-blockers (vs none) | −30 | (−55, −4.6) | 0.02 |
| Aspirin (vs none) | −24 | (−43, −6.3) | 0.01 |
| Clinical Measures | |||
| eGFR (10 ml/min/1.73 m2 difference) | −5.0 | (−8.8, −1.2) | < 0.01 |
| Sodium (1 unit difference) | −5.1 | (−7.7, −2.6) | <0.01 |
| BNP (multiplicative difference of 2) | +17 | (13, 21) | < 0.01 |
This column denotes the β coefficient from a multivariable linear regression model for sFlt-1, and represents the difference in mean sFlt-1 (pg/ml) between each group for categorical or continuous variables. The mean±SD of sFlt-1 was 348±181 pg/ml.
For PlGF, increasing age, male gender, history of diabetes, higher pulse pressure, use of cardiac resynchronization, and higher BNP were each associated with higher levels (Table 3). African American race, angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) use, and higher eGFR were each associated with lower levels of PlGF. Interestingly, ischemic etiology was not independently associated with either biomarker (p=0.36 for sFlt-1; p= 0.19 for PlGF). Comparing Tables 2 and 3, it is apparent that sFlt-1 levels were associated more strongly with measures of HF severity (e.g. NYHA Class, BNP) compared to PlGF.
Table 3.
Independent Determinants of Baseline PlGF Levels
| Difference in PlGF* | 95% CI | p value | |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (10 year difference) | +0.57 | (0.31, 0.84) | < 0.01 |
| Male (vs female) | +0.98 | (0.34, 1.6) | < 0.01 |
| African American (vs Caucasian) | −3.0 | (−3.8, −2.3) | < 0.01 |
| Medical History and Risk Factors | |||
| History of diabetes (vs none) | +1.1 | (0.40, 1.8) | < 0.01 |
| Heart Failure Characteristics | |||
| Cardiac resynchronization therapy (vs none) | +0.88 | (0.17, 1.6) | 0.02 |
| Medication Use | |||
| ACE inhibitors or ARBs (vs none) | −1.1 | (−2.0, −0.21) | 0.02 |
| Clinical Measures | |||
| Pulse pressure (10 mmHg difference) | +0.43 | (0.23, 0.64) | < 0.01 |
| eGFR (10 ml/min/1.73 m2 difference) | −0.26 | (−0.39, −0.11) | < 0.01 |
| BNP (multiplicative difference of 2) | +0.21 | (0.065, 0.35) | < 0.01 |
This column denotes the β coefficient from a multivariable linear regression model for PlGF, and represents the difference in mean PlGF (pg/ml) between each group for categorical or continuous variables. The mean±SD of PlGF was 19.4±6.2 pg/ml
sFlt-1 is Independently Associated with Adverse Outcomes in Chronic HF
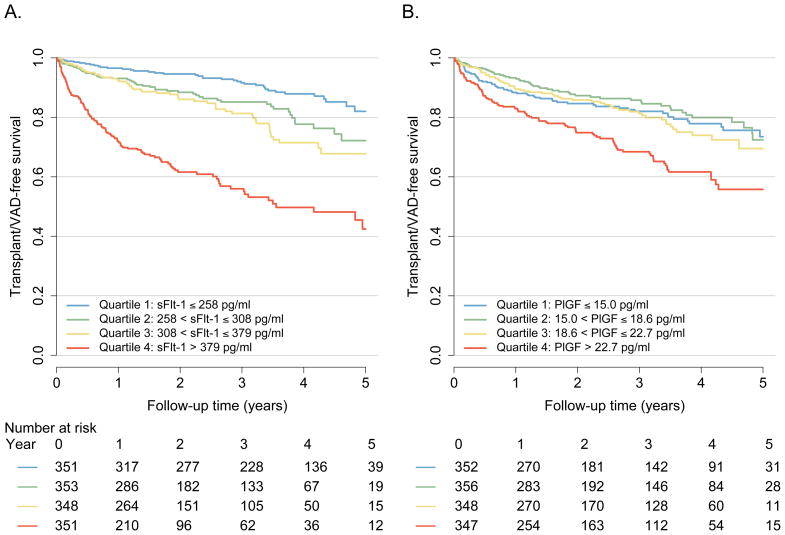
Over a median follow-up time of 2 years, there were 175 deaths, 103 transplants, and 27 VADs implanted. In unadjusted Cox models comparing the 4th versus 1st quartile, those patients with a circulating sFlt-1 level >379pg/ml had a 6.17-fold increased risk of adverse outcomes (p<0.01) (Table 4, Figure 1A). After adjustment for demographics, HF characteristics, and clinical measures including BNP, this association was attenuated in magnitude but remained statistically significant (HR 1.67, 95%CI 1.06–2.63, p=0.03 comparing 4th quartile to 1st quartile). After adjustment for established clinical risk scores such as the Seattle Heart Failure Model, this association was attenuated to a lesser degree (HR 2.54, 95%CI 1.76–2.27, p<0.01). Similar results were obtained when sFlt-1 was modeled as a continuous variable. In contrast, patients in the highest quartile of PlGF (>22.7pg/ml) had only a 1.89-fold increased risk, which did not remain significant in adjusted models (Table 4, Figure 1B). As in our cross-sectional analyses, these findings support a role for sFlt-1 as an independent biomarker of HF severity, whereas PlGF had no independent associations with outcomes.
Table 4.
Association of sFlt-1 and PlGF with Risk of All-cause Death, Cardiac Transplantation, or VAD Placement
| sFlt-1 HR (95% CI) | p value | PlGF HR (95% CI) | p value | |
|---|---|---|---|---|
| Model 1 | ||||
| Quartile 1 | Referent | Referent | ||
| Quartile 2 | 1.76 (1.16, 2.66) | 0.01 | 0.83 (0.58, 1.19) | 0.31 |
| Quartile 3 | 2.21 (1.47, 3.31) | < 0.01 | 1.06 (0.75, 1.50) | 0.76 |
| Quartile 4 | 6.17 (4.30, 8.86) | < 0.01 | 1.89 (1.39, 2.58) | < 0.01 |
| Per SD increase | 1.46 (1.37, 1.54) | < 0.01 | 1.31 (1.18, 1.46) | < 0.01 |
| Model 2 | ||||
| Quartile 1 | Referent | Referent | ||
| Quartile 2 | 1.42 (0.93, 2.19) | 0.10 | 0.84 (0.58, 1.23) | 0.38 |
| Quartile 3 | 1.53 (1.00, 2.33) | 0.05 | 0.93 (0.64, 1.35) | 0.71 |
| Quartile 4 | 2.61 (1.72, 3.96) | < 0.01 | 1.39 (0.98, 1.97) | 0.07 |
| Per SD increase | 1.19 (1.10, 1.28) | < 0.01 | 1.10 (0.98, 1.23) | 0.10 |
| Model 3 | ||||
| Quartile 1 | Referent | Referent | ||
| Quartile 2 | 1.24 (0.81, 1.90) | 0.33 | 0.93 (0.64, 1.36) | 0.72 |
| Quartile 3 | 1.16 (0.75, 1.80) | 0.50 | 0.94 (0.65, 1.37) | 0.76 |
| Quartile 4 | 1.67 (1.06, 2.63) | 0.03 | 1.36 (0.96, 1.93) | 0.08 |
| Per SD increase | 1.14 (1.05, 1.24) | < 0.01 | 1.11 (0.99, 1.25) | 0.05 |
| Model 4 | ||||
| Quartile 1 | Referent | Referent | ||
| Quartile 2 | 1.40 (0.93, 2.13) | 0.11 | 0.72 (0.50, 1.03) | 0.07 |
| Quartile 3 | 1.36 (0.90, 2.07) | 0.14 | 0.76 (0.53, 1.08) | 0.12 |
| Quartile 4 | 2.54 (1.76, 2.27) | <0.01 | 1.04 (0.75, 1.42) | 0.83 |
| Per SD increase | 1.26 (1.17, 1.36) | <0.01 | 1.05 (0.94, 1.17) | 0.44 |
HR = hazard ratio; CI = confidence interval; SD = standard deviation
Model 1: Unadjusted
Model 2: Adjusted for age, gender, race, NYHA functional classification, history of diabetes, tobacco use, ischemic cardiomyopathy etiology, internal cardiac defibrillator, cardiac resynchronization therapy, ACE inhibitor/angiotensin receptor blocker use, aldosterone antagonist use, beta blocker use, HMG CoA reductase inhibitor use, body mass index, and clinical site
Model 3: Adjusted for Model 2 covariates and log2-transformed BNP
Model 4: Adjusted for SHFM score
Figure 1. Transplant and VAD-Free Survival According to Levels of sFlt-1 (A) and PlGF (B).
Kaplan-Meier plots illustrating the incidence of all-cause death, cardiac transplantation, or ventricular assist device (VAD) placement according to baseline quartiles of sFlt-1 (A) and PlGF (B) (p<0.01 by log rank test for each panel)
Associations between vascular growth factors and outcomes might differ based upon the underlying etiology of HF, with prior literature indicating increased relevance in ischemic disease (11,19). To explore these possibilities, we performed secondary analyses that included interaction terms between biomarker levels (modeled continuously) and HF etiology (ischemic or nonischemic). In contrast to previously published reports, there were no significant interactions by HF etiology on the associations between either marker and outcome in our adjusted models (interaction p=0.18 for sFlt-1; p=0.41 for PlGF).
Combined Use of sFlt-1 and BNP in Predicting Outcomes
We explored the effects of joint assessment of sFlt-1 and the clinically used biomarker BNP in predicting adverse outcomes. There was a moderate correlation between levels of sFlt-1 and BNP (R=0.54, p<0.01), and their combined use was important in risk assessment (Table 5, Supplementary Figure 1). Compared to the referent group of patients with levels of both markers less than the median, patients with elevations in both sFlt-1 and BNP had a markedly elevated risk than either marker alone, and this association remained significant in multivariable adjusted models (HR 2.87, 95%CI 1.96–4.21, p<0.01). Furthermore, in the group of patients with high BNP levels, the combination of a high sFlt-1 level was associated with a 1.5 to 2 fold increase in risk compared to those patients with low sFlt-1 levels (p<0.01 unadjusted, p=0.04 adjusted).
Table 5.
Joint Effects of sFlt-1 and BNP on Risk of All-Cause Death, Cardiac Transplantation, or VAD Placement
| sFlt-1* | BNP* | n | HR (95% CI) | p value | HR (95% CI) | p value |
|---|---|---|---|---|---|---|
| Low | Low | 482 | Referent | Referent | ||
| High | Low | 219 | 1.44 (0.86, 2.40) | 0.16 | 1.21 (0.71, 2.14) | 0.48 |
| Low | High | 219 | 2.78 (1.84, 4.19) | < 0.01 | 1.99 (1.30, 3.05) | < 0.01 |
| High | High | 483 | 6.08 (4.36, 8.50) | < 0.01 | 2.87 (1.96, 4.21) | < 0.01 |
HR = hazard ratio; CI = confidence interval
Low/high sFlt-1 defined as below/above median (308 pg/ml)
Low/high BNP defined as below/above median (175 pg/ml)
Adjusted for all covariates listed in Table 4, Model 2
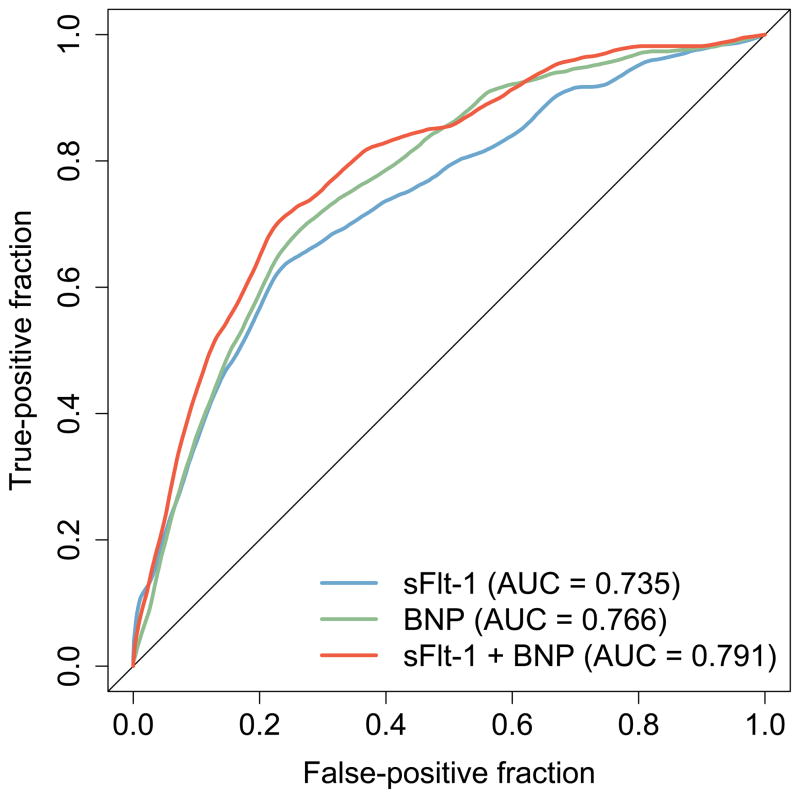
In ROC analysis at 1-year (Figure 2), sFlt-1 and BNP in combination (AUC=0.791, 95% CI 0.752–0.831) showed greater accuracy in classifying patients who died, required heart transplantation, or VAD placement than sFlt-1 alone (AUC=0.735, 95% CI 0.689–0.781, p<0.01) or BNP alone (AUC=0.766, 95% CI 0.726–0.807, p=0.03). These findings illustrate an improved ability to discern high- and low-risk HF patients at 1 year using both sFlt-1 and BNP compared to BNP alone.
Figure 2. ROC Curves for sFlt-1 and BNP at 1-Year.
Area under the receiver operating characteristic (ROC) curves comparing the ability of baseline sFlt-1 and BNP levels to classify patients who died, required heart transplantation, or VAD placement at 1-year (p<0.01 and p=0.03, respectively for area under the curve (AUC) comparing sFlt-1 and BNP versus sFlt-1 or BNP alone)
Discussion
We report the first comprehensive assessment of PlGF and sFlt-1 as biomarkers in chronic HF. Our results indicate that sFlt-1 is strongly associated with adverse outcomes across a broad spectrum of disease, even after adjusting for existing standards such as the Seattle Heart Failure Model and natriuretic peptide levels. Furthermore, combined use of sFlt-1 and BNP may be superior to classifying patient risk than either biomarker alone. These findings support a role for sFlt-1 in the biology of human HF and suggest that, with additional study, circulating sFlt-1 may emerge as a clinically useful biomarker to assess the influence of vascular remodeling on clinical outcomes. In contrast, we found no evidence to support a role for circulating PlGF in chronic HF.
Studies in animal models suggest several potential mechanisms through which VEGF/Flt-1 signaling might modify the severity and course of HF. sFlt-1 opposes angiogenesis by binding to and sequestering salutary VEGF ligands in the circulation, resulting in endothelial dysfunction and vascular rarefaction that increases mechanical load on the heart (39–41). Excess sFlt-1 may also increase myocardial fibrosis and decrease myocardial capillary density, thereby directly affecting myocardial structure and function (7,26). sFlt-1 also impairs glomerular function and may contribute to unfavorable cardiorenal interactions. Exogenous administration of sFlt-1 to both pregnant and nonpregnant animals induces widespread endothelial dysfunction, hypertension, and renal dysfunction (13,23). Consistent with these observations, we found that sFlt-1 was independently associated with renal dysfunction (22). Discerning which of the effects of sFlt-1 is responsible for our observed clinical associations will require additional laboratory work.
Of note, we found that sFlt-1 was significantly associated with disease severity and clinical outcomes independent of HF etiology. These results provide human data supporting a role for angiogenic growth factors even in nonischemic disease, which has been observed in animal models. For example, Izumiya, et al. have shown that inhibiting angiogenic factors in mice subjected to pressure overload impairs cardiac growth and accelerates the transition to HF (7). Similarly, VEGF signaling maintains endothelial cell homeostasis (2) and exerts anti-apoptotic effects in pacing-induced cardiomyopathy (8). Taken together with our findings, these results emphasize the importance of vascular growth remodeling in diverse forms of HF.
Consistent with our findings in HF, studies of sFlt-1 in other cardiovascular diseases have associated elevated sFlt-1 with worse outcomes. sFlt-1 is elevated in patients with acute MI who subsequently develop HF (11) and in pregnant patients who subsequently develop preeclampsia (13,14). As noted above, a plausible interpretation of these observations is that increased sFlt-1 reflects an underlying pathogenic process that accelerates endothelial dysfunction, renal dysfunction, vascular disease, and adverse remodeling by sequestering VEGF and associated ligands. However, Kodama, et al. have shown elevated sFlt-1 levels in response to atorvastatin treatment in a post-infarction study, and in this setting, a serial increase in sFlt-1 is associated with an improvement in LV function (18). These findings suggest that the relationship between circulating sFlt-1 and outcomes may be complicated by the presence of acute ischemia and also by pharmacologic therapy with statins. While the associations we identified in chronic HF were independent of pharmacotherapies, we cannot comment on the impact of acute ischemia, which was not present in our study.
Although PlGF levels were associated with a variety of clinical factors in our cohort, including higher levels in renal dysfunction, hypertension, and diabetes, PlGF was not an independent marker of HF severity or adverse outcomes in adjusted models. This stands in contrast to other disease phenotypes in which PlGF is a predictor of disease outcomes, such as preeclampsia, acute coronary syndromes, and cancer (12,13,16,42). Thus, PlGF may be more relevant to disease states that are primarily vascular in etiology, with no current data to support a role for PlGF as a marker of risk in chronic HF.
We acknowledge several limitations. The strong and independent associations between sFlt-1, NYHA Class, and risk of adverse outcomes observed in our study support sFlt-1 as a HF biomarker, but whether sFlt-1 itself plays a causal role in HF progression cannot be determined from our observational study. Intervention studies are necessary to rigorously test this hypothesis and test the biologic basis of our findings. Although the assays utilized were of high quality, we quantified biomarker levels from peripheral plasma, and there may be differences according to sampling site (11). Our results may also not be generalizable to populations of acute HF, but represent chronic HF patients in a tertiary referral setting that includes a substantial representation of more severe disease. We currently do not have complete data on transplant urgency, exercise capacity, other biomarkers such as troponin, or quantitative cardiac remodeling data and as such are unable to determine the relationship between these parameters and sFlt-1 or PlGF. Finally, as this work is the first to assess the relevance of circulating sFlt-1 in chronic human HF, additional studies are necessary to validate this work and further define clinical effectiveness (43,44).
In conclusion, sFlt-1 is robustly associated with adverse outcomes in chronic HF, supporting a role for Flt-1 signaling in human HF progression. With further study, assessment of sFlt-1 may emerge as a useful clinical biomarker to enhance our ability to stratify patient risk above and beyond currently used approaches.
Supplementary Material
Kaplan-Meier plots illustrating the incidence of all-cause death, cardiac transplantation, or ventricular assist device (VAD) placement according to median sFlt-1 and BNP Levels
Acknowledgments
Funding/Support: Dr Ky was supported by the NIH/Clinical and Translational Science Award KL1 RR024132, NIH K23 HL095661-01, and the Heart Failure Society of America Research Fellowship Award. This work was also supported by NIH HL088577 (Dr. Cappola). Assay support was provided by Abbott Diagnostics. Neither the funding organizations nor Abbott Diagnostics had any role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Abbreviation List
- AUC
Area under the curve
- CV
Coefficients of variation
- eGFR
estimated Glomerular Filtration Rate
- HF
Heart Failure
- HR
Hazard ratio
- MI
Myocardial infarction
- NYHA
New York Heart Association
- PlGF
Placental growth factor
- ROC
Receiver operating characteristic
- sFlt-1
soluble Fms-like tyrosine kinase receptor 1
Footnotes
Disclosures: Dr. Cappola reports receiving research support from Abbott Diagnostics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oka T, Komuro I. Molecular mechanisms underlying the transition of cardiac hypertrophy to heart failure. Circ J. 2008;72(Suppl A):A13–6. doi: 10.1253/circj.cj-08-0481. [DOI] [PubMed] [Google Scholar]
- 2.Lip GY, Chung I. Vascular endothelial growth factor and angiogenesis in heart failure. J Card Fail. 2005 May;11:285–7. doi: 10.1016/j.cardfail.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh PC, Davis ME, Lisowski LK, Lee RT. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu Rev Physiol. 2006;68:51–66. doi: 10.1146/annurev.physiol.68.040104.124629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helotera H, Alitalo K. The VEGF family, the inside story. Cell. 2007 Aug 24;130:591–2. doi: 10.1016/j.cell.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005 Dec 15;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 6.Abraham D, Hofbauer R, Schafer R, et al. Selective downregulation of VEGF-A(165), VEGF-R(1), and decreased capillary density in patients with dilative but not ischemic cardiomyopathy. Circ Res. 2000 Oct 13;87:644–7. doi: 10.1161/01.res.87.8.644. [DOI] [PubMed] [Google Scholar]
- 7.Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension. 2006 May;47:887–93. doi: 10.1161/01.HYP.0000215207.54689.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pepe M, Mamdani M, Zentilin L, et al. Intramyocardial VEGF-B167 gene delivery delays the progression towards congestive failure in dogs with pacing-induced dilated cardiomyopathy. Circ Res. 2010 Jun 25;106:1893–903. doi: 10.1161/CIRCRESAHA.110.220855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Q, Ishibashi M, Hiasa K, Tan C, Takeshita A, Egashira K. Essential role of vascular endothelial growth factor in angiotensin II-induced vascular inflammation and remodeling. Hypertension. 2004 Sep;44:264–70. doi: 10.1161/01.HYP.0000138688.78906.6b. [DOI] [PubMed] [Google Scholar]
- 10.Luttun A, Tjwa M, Carmeliet P. Placental growth factor (PlGF) and its receptor flt-1 (VEGFR-1): Novel therapeutic targets for angiogenic disorders. Ann NY Acad Sci. 2002 Dec;979:80–93. doi: 10.1111/j.1749-6632.2002.tb04870.x. [DOI] [PubMed] [Google Scholar]
- 11.Onoue K, Uemura S, Takeda Y, et al. Usefulness of soluble fms-like tyrosine kinase-1 as a biomarker of acute severe heart failure in patients with acute myocardial infarction. Am J Cardiol. 2009 Dec 1;104:1478–83. doi: 10.1016/j.amjcard.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Heeschen C, Dimmeler S, Fichtlscherer S, et al. Prognostic value of placental growth factor in patients with acute chest pain. JAMA. 2004 Jan 28;291:435–41. doi: 10.1001/jama.291.4.435. [DOI] [PubMed] [Google Scholar]
- 13.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004 Feb 12;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 14.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010 Aug 3;122:478–87. doi: 10.1161/CIRCULATIONAHA.109.895458. [DOI] [PubMed] [Google Scholar]
- 15.Iwama H, Uemura S, Naya N, et al. Cardiac expression of placental growth factor predicts the improvement of chronic phase left ventricular function in patients with acute myocardial infarction. J Am Coll Cardiol. 2006 Apr 18;47:1559–67. doi: 10.1016/j.jacc.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 16.Lenderink T, Heeschen C, Fichtlscherer S, et al. Elevated placental growth factor levels are associated with adverse outcomes at four-year follow-up in patients with acute coronary syndromes. J Am Coll Cardiol. 2006 Jan 17;47:307–11. doi: 10.1016/j.jacc.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 17.Belgore FM, Blann AD, Lip GY. sFlt-1, a potential antagonist for exogenous VEGF. Circulation. 2000 Oct 10;102:E108–9. doi: 10.1161/01.cir.102.15.e108. [DOI] [PubMed] [Google Scholar]
- 18.Kodama Y, Kitta Y, Nakamura T, et al. Atorvastatin increases plasma soluble fms-like tyrosine kinase-1 and decreases vascular endothelial growth factor and placental growth factor in association with improvement of ventricular function in acute myocardial infarction. J Am Coll Cardiol. 2006 Jul 4;48:43–50. doi: 10.1016/j.jacc.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Funayama H, Kubo N, et al. Elevation of plasma placental growth factor in the patients with ischemic cardiomyopathy. Int J Cardiol. 2009 Jan 9;131:186–91. doi: 10.1016/j.ijcard.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 20.Ky B, Kimmel SE, Safa RN, et al. Neuregulin-1beta is associated with disease severity and adverse outcomes in chronic heart failure. Circulation. 2009;120:310–317. doi: 10.1161/CIRCULATIONAHA.109.856310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeo KT, Wu AH, Apple FS, et al. Multicenter evaluation of the roche NT-proBNP assay and comparison to the biosite triage BNP assay. Clin Chim Acta. 2003 Dec;338:107–15. doi: 10.1016/j.cccn.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Di Marco GS, Reuter S, Hillebrand U, et al. The soluble VEGF receptor sFlt1 contributes to endothelial dysfunction in CKD. J Am Soc Nephrol. 2009 Oct;20:2235–45. doi: 10.1681/ASN.2009010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003 Mar;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onoue K, Uemura S, Takeda Y, et al. Reduction of circulating soluble fms-like tyrosine kinase-1 plays a significant role in renal dysfunction-associated aggravation of atherosclerosis. Circulation. 2009 Dec 15;120:2470–7. doi: 10.1161/CIRCULATIONAHA.109.867929. [DOI] [PubMed] [Google Scholar]
- 25.Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004 Jun;65:2003–17. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 26.Giordano FJ, Gerber HP, Williams SP, et al. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc Natl Acad Sci USA. 2001 May 8;98:5780–5. doi: 10.1073/pnas.091415198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy WC, Mozaffarian D, Linker DT, et al. The seattle heart failure model: Prediction of survival in heart failure. Circulation. 2006 Mar 21;113:1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 28.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000 Jun;56:337–44. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 29.Heagerty PJ, Saha P. R package version 1.0.0. 2006. Survival ROC: Time-dependent ROC curve estimation from censored survival data. [Google Scholar]
- 30.R Development Core Team. R; A language and environment for statistical computing. 2009. [Google Scholar]
- 31.Therneau T, Lumley T. R package version 2.35–4. 2009. Survival: Survival analysis, including penalized likelihood. [Google Scholar]
- 32.Venables WN, Risch NJ. Modern applied statistics with S. 4. New York: Springer; 2002. [Google Scholar]
- 33.Pradhan AD, Manson JE, Rossouw JE, et al. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: Prospective analysis from the women’s health initiative observational study. JAMA. 2002 Aug 28;288:980–7. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- 34.Denizot Y, Leguyader A, Cornu E, et al. Release of soluble vascular endothelial growth factor receptor-1 (sFlt-1) during coronary artery bypass surgery. J Cardiothorac Surg. 2007 Sep 21;2:38. doi: 10.1186/1749-8090-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aref S, El Sherbiny M, Goda T, Fouda M, Al Askalany H, Abdalla D. Soluble VEGF/sFLt1 ratio is an independent predictor of AML patient out come. Hematology. 2005 Apr;10:131–4. doi: 10.1080/10245330500065797. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro NI, Yano K, Okada H, et al. A prospective, observational study of soluble FLT-1 and vascular endothelial growth factor in sepsis. Shock. 2008 Apr;29:452–7. doi: 10.1097/shk.0b013e31815072c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rini BI, Michaelson MD, Rosenberg JE, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol. 2008 Aug 1;26:3743–8. doi: 10.1200/JCO.2007.15.5416. [DOI] [PubMed] [Google Scholar]
- 38.Robak E, Sysa-Jedrzejewska A, Robak T. Vascular endothelial growth factor and its soluble receptors VEGFR-1 and VEGFR-2 in the serum of patients with systemic lupus erythematosus. Mediators Inflamm. 2003 Oct;12:293–8. doi: 10.1080/09629350310001619726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005 Feb 15;65:550–63. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Belgore FM, Blann AD, Li-Saw-Hee FL, Beevers DG, Lip GY. Plasma levels of vascular endothelial growth factor and its soluble receptor (SFlt-1) in essential hypertension. Am J Cardiol. 2001 Mar 15;87:805–7. A9. doi: 10.1016/s0002-9149(00)01512-5. [DOI] [PubMed] [Google Scholar]
- 41.Walsh K, Shiojima I. Cardiac growth and angiogenesis coordinated by intertissue interactions. J Clin Invest. 2007 Nov;117:3176–9. doi: 10.1172/JCI34126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: A multidisciplinary phase II study. J Clin Oncol. 2009 Jun 20;27:3020–6. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: A scientific statement from the american heart association. Circulation. 2009 May 5;119:2408–16. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrow DA, de Lemos JA. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation. 2007 Feb 27;115:949–52. doi: 10.1161/CIRCULATIONAHA.106.683110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan-Meier plots illustrating the incidence of all-cause death, cardiac transplantation, or ventricular assist device (VAD) placement according to median sFlt-1 and BNP Levels