Abstract
Purpose
A novel platform was developed that fuses pre-biopsy magnetic resonance imaging with real-time transrectal ultrasound imaging to identify and biopsy lesions suspicious for prostate cancer. The cancer detection rates for the first 101 patients are reported.
Materials and Methods
This prospective, single institution study was approved by the institutional review board. Patients underwent 3.0 T multiparametric magnetic resonance imaging with endorectal coil, which included T2-weighted, spectroscopic, dynamic contrast enhanced and diffusion weighted magnetic resonance imaging sequences. Lesions suspicious for cancer were graded according to the number of sequences suspicious for cancer as low (2 or less), moderate (3) and high (4) suspicion. Patients underwent standard 12-core transrectal ultrasound biopsy and magnetic resonance imaging/ultrasound fusion guided biopsy with electromagnetic tracking of magnetic resonance imaging lesions. Chi-square and within cluster resampling analyses were used to correlate suspicion on magnetic resonance imaging and the incidence of cancer detected on biopsy.
Results
Mean patient age was 63 years old. Median prostate specific antigen at biopsy was 5.8 ng/ml and 90.1% of patients had a negative digital rectal examination. Of patients with low, moderate and high suspicion on magnetic resonance imaging 27.9%, 66.7% and 89.5% were diagnosed with cancer, respectively (p <0.0001). Magnetic resonance imaging/ultrasound fusion guided biopsy detected more cancer per core than standard 12-core transrectal ultrasound biopsy for all levels of suspicion on magnetic resonance imaging.
Conclusions
Prostate cancer localized on magnetic resonance imaging may be targeted using this novel magnetic resonance imaging/ultrasound fusion guided biopsy platform. Further research is needed to determine the role of this platform in cancer detection, active surveillance and focal therapy, and to determine which patients may benefit.
Keywords: prostatic neoplasms, biopsy, magnetic resonance imaging, ultrasonography, early detection of cancer
Prostate cancer is the leading cause of cancer and the second most common cause of cancer related death in men.1 The current standard of care practice for an initial prostate biopsy involves taking 10 to 14 cores and has a cancer detection rate of 27% to 40.3%.2–6 Saturation biopsy has also been explored as an initial procedure, but it does not significantly improve cancer detection compared to standard biopsy.7
MRI has been proposed as an alternative to ultrasound to increase cancer detection. MRI has the benefit of increased resolution, superior imaging of anatomical structures and extraprostatic involvement, functional assessment, and the ability to potentially assign tumor grade.8–11 Prostate biopsies under real-time MRI guidance are conducted at several centers. However, this technique may be cumbersome, time-consuming, costly and impractical since the entire procedure is conducted in the MRI gantry.12–15 Thus, MRI/US fusion based systems have been developed to address these issues.16–19
An innovative approach to prostate biopsy was developed and translated from phantom to animal and then to patient.20 Clinical trial patients first underwent 3.0 T multiparametric MRI with endorectal coil to identify lesions suspicious for prostate cancer. If suspicious lesions were identified, patients were enrolled in this biopsy protocol. Patients in the trial underwent a standard of care 12-core TRUS biopsy and MRI/US fusion guided biopsy in the same setting. The MRI/US fusion guided biopsy involved sampling lesion(s) suspicious for prostate cancer that were identified on pre-biopsy MRI. Lesion locations from pre-biopsy MRI were fused onto real-time TRUS imaging by software developed in collaboration with Philips. The operator was guided to these specific locations on TRUS imaging by tracking the position of the biopsy needle with passive EM sensors. This novel platform takes the benefits of detecting prostate cancer with MRI out of the gantry and into an office based procedure room. The cancer detection rates are reported for the first 101 patients.
MATERIALS AND METHODS
Patients
This prospective study was approved by the institutional review board of the National Cancer Institute of the National Institutes of Health. Patients eligible for this study were consented and informed appropriately of the potential harms and benefits. Study enrollment began in 2007.
Multiparametric MRI
Patients underwent multiparametric imaging using a 3.0 T MRI scanner (Achieva, Philips Healthcare, Best, The Netherlands) combined with a 6-channel cardiac surface coil (SENSE, Philips Healthcare) positioned over the pelvis and an endorectal coil (BPX-30, Medrad, Pittsburgh, Pennsylvania). Tri-planar T2-weighted, 3-dimensional point resolved spatially localized spectroscopy, axial dynamic contrast enhanced, and axial diffusion weighted imaging with apparent diffusion coefficient mapping MRI sequences were conducted according to protocol. Details of these imaging sequences have been described previously.11,21 The criterion for a positive lesion on T2-weighted and diffusion weighted imaging was a well circumscribed, round-ellipsoid, low signal intensity lesion.11 A positive lesion on dynamic contrast enhanced imaging was the presence of foci showing early and intense enhancement, and rapid washout. A positive lesion on spectroscopy was an area where the choline-to-citrate ratio was 3 or more standard deviations above the mean healthy value.21
Two radiologists (PLC, BT) identified and graded lesions suspicious for cancer according to the number of MRI sequences suspicious for cancer, as low (2 or less), moderate (3) and high (4) suspicion sequences. MRI data sets were assessed in consensus between the 2 radiologists. Both radiologists were blinded to pre-imaging serum PSA values, prior biopsy status and previous histopathological findings. Each MRI sequence was evaluated independently and separately.
MRI/US Fusion Guided Biopsy
Patients with lesion(s) suspicious for prostate cancer on MRI entered the prostate biopsy protocol. Before biopsy the patients were given a cleansing Fleet® enema and antibiotic prophylaxis per American Urological Association guidelines. All patients underwent monitored anesthesia care for the procedure.
Patients first underwent a standard of care 12-core TRUS sextant biopsy of the medial and lateral margins of the right and left apex, mid gland and base of the prostate. For the 12-core TRUS biopsy the operator was blinded to the location of suspicious lesions identified on pre-biopsy MRI. At the same setting patients then underwent MRI/US fusion guided biopsy under EM tracking of suspicious lesions identified on MRI. An EM field generator (Northern Digital Inc., Ontario, Canada) was placed above the pelvis, which allowed for real-time tracking of a custom biopsy probe embedded with a passive EM tracking sensor (Traxtal Inc., A Philips Healthcare Company, Toronto, Ontario, Canada). A 2-dimensional TRUS sweep of the prostate was performed in the axial plane to render a 3-dimensional ultrasound image that was then registered and fused to the pre-biopsy MRI. Lesions suspicious for cancer identified on MRI were semiautomatically superimposed on the real-time TRUS image. At least 2 biopsy cores were taken for each lesion, 1 in the axial and 1 in the sagittal plane.
Statistical Analysis
Descriptive statistics were used to describe patient characteristics including age, PSA, DRE and previous biopsy data. A statistician (JHS) performed all calculations for this study. The results of the fusion biopsies were stratified according to the preoperative MRI scoring system (low, moderate and high). Chi-square analysis was used to determine if there was a correlation between cancer suspicion on MRI and cancer detected on biopsy. The within cluster resampling technique was used to account for the correlation between repeated measures of multiple lesions for each patient. For each degree of MRI suspicion the cancer detection rates per biopsy core were compared with standard 12-core TRUS biopsy alone vs MRI/US fusion guided biopsy alone.
RESULTS
Patient and biopsy characteristics are described in table 1. This initial cohort included 101 patients with a mean age of 63 years (range 41 to 82). Median PSA at biopsy was 5.8 ng/ml (range 0.2 to 103) and 90 of 101 (90.1%) patients had a negative DRE. The trial included patients with no prior, prior negative and prior positive biopsy histories. On average 2.6 lesions (range 1 to 7) per patient were identified with some degree of suspicion for prostate cancer on MRI. On average 5.8 cores per patient were taken during MRI/US fusion guided biopsy. The overall cancer detection rate in the clinical trial cohort was 54.4% (55 of 101 patients).
Table 1.
Patient and biopsy characteristics
| Mean pt age (range) | 63 (41–82) |
| Mean ng/ml PSA (range) | 8.3 (0.2–103) |
| Median, ng/ml PSA | 5.8 |
| No. biopsy history: | |
| No prior | 36 |
| Prior neg | 29 |
| Prior pos | 36 |
| Mean lesions suspicious for Ca on MRI (range) | 2.6 (1–7) |
| Mean biopsies/lesion (range) | 2.2 (1–8) |
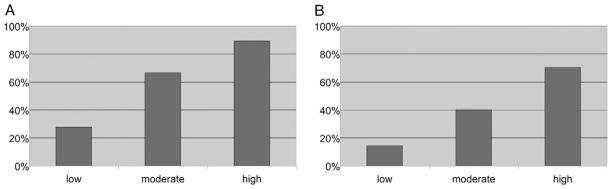
Chi-square analysis was used to determine if there was a correlation between suspicion of prostate cancer on MRI and cancer detected on MRI/US fusion guided biopsy for patients. Cancer was detected in 12 of 43 (27.9%), 26 of 39 (66.7%) and 17 of 19 (89.5%) patients with low, moderate and high suspicion, respectively (fig. 1, A). The within cluster resampling technique was performed to determine if there was a correlation between suspicion of prostate cancer on MRI and cancer detected on MRI/US fusion guided biopsy for lesions. This method takes into account repeated measures in each patient. Cancer was detected in 23 of 158 (14.6%), 29 of 72 (40.3%) and 24 of 34 (70.6%) lesions with low, moderate and high suspicion for cancer (fig. 1, B). Both tests were statistically significant (p <0.0001).
Figure 1.
Cancer detection rates for patients (A) and lesions (B) were correlated with suspicion on MRI. Chi-square analysis was conducted for patients (p 30.0001). Within cluster resampling analysis was conducted for lesions (p 30.0001).
MRI/US fusion guided biopsy detected more cancer per core than standard 12-core TRUS biopsy alone for all levels of suspicion combined (20.6% vs 11.7%, respectively). For low, moderate and high suspicion on MRI 4.8% vs 3.8%, 20.7% vs 12.3% and 53.8% vs 29.9% of cores were positive for cancer on MRI/US fusion guided biopsy and standard 12-core TRUS biopsy, respectively (fig. 2).
Figure 2.
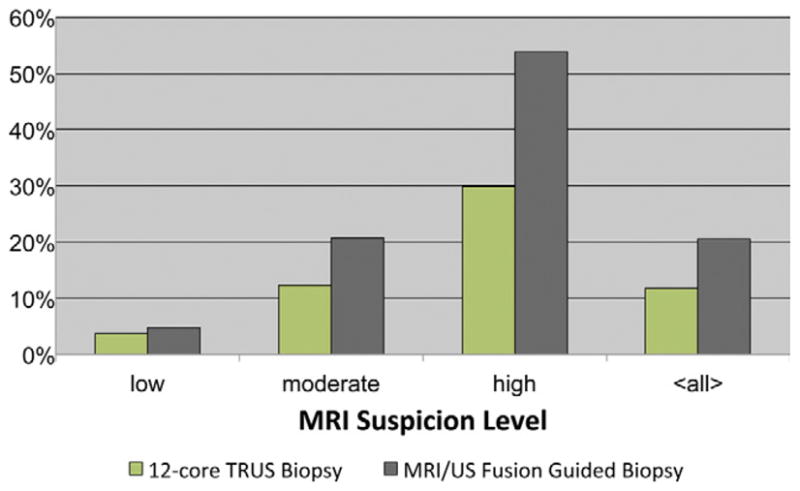
Cancer detection rates for biopsy cores were compared between standard 12-core TRUS biopsy alone and MRI/US fusion guided biopsy alone.
DISCUSSION
Although prostate biopsies are becoming less random and more systematic, cancer is still being missed. The current standard of care practice for an initial biopsy involves taking 10 to 14 cores and detects prostate cancer 27% to 40.3% of the time.2–6 Some physicians are moving toward saturation biopsy techniques with the hope of improving cancer detection. Studies have shown that there is no difference between the cancer detection rate of standard and saturation biopsies in biopsy naïve patients.7,22 Although the number of cores may be increased, biopsies are still not directed toward cancer. Prostate cancer remains the only tumor sampled with the hope of hitting tumor. However, saturation biopsies may have a role in detecting cancer at a rate of 29% to 41% in patients with a suspicion for cancer and prior negative biopsies.23–25 A saturation biopsy is often defined as taking 20 or more cores, with some reports taking more than 70 cores.2,26 In this series a biopsy protocol (combination of MRI/US fusion guided biopsy and 12-core TRUS) averaged 17.8 cores and detected cancer in 54.4% (55 of 101) of trial patients.
Statistically significant associations existed between the degree of suspicion on MRI and cancer detected on fusion biopsy for patients and lesions (p <0.0001). When stratified by cancer suspicion on MRI, fusion biopsy detected cancer in 27.9%, 66.7% and 89.5% of patients with low, moderate and high suspicion, respectively. Cancer detection has traditionally been assessed by patient. However, with the development of targeted biopsy techniques and the exploration of focal therapy, cancer detection may be better assessed by lesion or biopsy core. In this study MRI/US fusion guided biopsy vs standard 12-core TRUS biopsy alone detected more cancer per core for all suspicion levels.
These findings emphasize the potential value of MRI/US fusion guided biopsy. This platform allows for the detection of cancer on high resolution imaging, the stratification of patients and lesions by cancer suspicion, and the ability to detect cancer at a higher rate per core than conventional random biopsy. These are all potential benefits that are not available with current biopsy techniques. Further research is warranted to develop recommendations for or against the use of MRI/US fusion biopsy alone or in addition to a standard 12-core TRUS biopsy.
The technique of MRI in guiding prostate biopsies continues to evolve.12,14,27 Hambrock et al recently reported a cancer detection rate of 59% in a series of 68 patients who underwent in-gantry MRI guided biopsy.13 The authors focused on a population with a median PSA of 13 ng/ml (range 4 to 243), of which 97.2% had a negative DRE. A similar overall detection rate of 55.4% was seen in this current trial in a patient population with a median PSA of 5.8 ng/ml (range 0.2 to 103), of which 90.1% had a negative DRE. This platform may be an alternative to MRI guided biopsies, allowing for office based biopsies. Positive biopsy rates in any study will be highly influenced by the patient selection bias inherent to practice patterns and screening or study population.
This platform may benefit patients enrolled in active surveillance or focal therapy protocols. Patients on active surveillance could be followed by prostate MRI. If new lesions were identified or suspicion of a previous lesion increased, MRI/US fusion could be used to target specific areas at risk. This platform tracks the locations of lesions as well as the trajectory and path of needle biopsies digitally, allowing prior targets to be sampled and monitored. MRI/US fusion guided imaging with EM tracking may also have a role in focal therapy for prostate cancer. This platform could be modified to incorporate focal therapies such as cryotherapy, high intensity focused ultrasound ablation or brachytherapy to provide image guidance and EM tracking of therapeutic instruments.
This study has several limitations. Without the evaluation of whole mount prostatectomy specimens the difference in accuracy between MRI/US fusion guided and 12-core standard TRUS cannot be determined. Furthermore, whether cancer was missed on initial MRI vs MRI/US fusion guided biopsy cannot be assessed. MRI is currently limited to identifying cancers greater than 3 mm. For lesions greater than 3 mm a previous histopathological correlation of peripheral tumors with multiparametric MRI showed sensitivities of 94%, 56% and 39%, and specificities of 83%, 96% and 98% for T2-weighted, dynamic contrast enhanced and spectroscopy, respectively.21 In a phantom, non-living model the mean spatial accuracy of this MRI/US fusion biopsy platform was 2.4 mm.20 This work demonstrated that this platform has the ability to guide biopsy needles to selected targets within an adequate margin of error. Only histopathological correlations will allow us to address these limitations conclusively. Despite these limitations cancer was detected at a higher rate than previously reported for other biopsy techniques, although overall rates are highly dependent upon the patient population.
Of the 55 patients in whom prostate cancer was found 10 had detection on 12-core TRUS biopsy alone, 10 on MRI/US fusion guided biopsy alone and 35 with both methods. Of the 10 patients with disease detected on 12-core TRUS biopsy alone 5, 4 and 1 had low, moderate and high suspicion of prostate cancer on MRI, respectively. Of the 10 patients with cancer detected on MRI/US fusion guided biopsy alone 3, 3 and 4 had low, moderate and high suspicion on MRI, respectively. Of the 35 patients with disease detected using either biopsy strategy 3, 19 and 12 had low, moderate and high suspicion on MRI, respectively. Thus, MRI/US fusion biopsy may aid in the detection of higher risk prostate disease compared to standard TRUS biopsy alone. However, further research is necessary to suggest why patients had disease detected on MRI/US fusion alone or TRUS biopsy alone.
CONCLUSIONS
A novel platform was developed and deployed in clinic that fuses pre-biopsy MRI and real-time TRUS imaging to identify and target lesions suspicious for prostate cancer under EM tracking. These results indicated that localized prostate cancer may be identified on multiparametric MRI and targeted using this novel MRI/US fusion guided biopsy platform. There were statistically significant associations between the degree of suspicion on MRI and the incidence of cancer detected for patients and targets (p <0.0001), and MRI/US fusion guided biopsy detected more cancerous cores than standard 12-core TRUS biopsy alone. Further research is needed to determine the role of this platform in cancer detection, active surveillance and focal therapy, and to determine which patients may benefit.
Acknowledgments
Supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, Center for Interventional Oncology, and a Cooperative Research and Development Agreement between the National Institutes of Health and Philips Healthcare.
Abbreviations and Acronyms
- DRE
digital rectal examination
- EM
electromagnetic
- MRI
magnetic resonance imaging
- PSA
prostate specific antigen
- TRUS
transrectal ultrasound
- US
ultrasound
Footnotes
The National Institutes of Health and Philips have intellectual property in related fields.
Study received institutional review board approval.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Patel AR, Jones JS. Optimal biopsy strategies for the diagnosis and staging of prostate cancer. Curr Opin Urol. 2009;19:232. doi: 10.1097/mou.0b013e328329a33e. [DOI] [PubMed] [Google Scholar]
- 3.Presti JC, Jr, O’Dowd GJ, Miller MC, et al. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol. 2003;169:125. doi: 10.1016/S0022-5347(05)64051-7. [DOI] [PubMed] [Google Scholar]
- 4.Babaian RJ, Toi A, Kamoi K, et al. A comparative analysis of sextant and an extended 11-core multi-site directed biopsy strategy. J Urol. 2000;163:152. [PubMed] [Google Scholar]
- 5.Eskew LA, Bare RL, McCullough DL. Systematic 5 region prostate biopsy is superior to sextant method for diagnosing carcinoma of the prostate. J Urol. 1997;157:199. [PubMed] [Google Scholar]
- 6.Naughton CK, Miller DC, Yan Y. Impact of transrectal ultrasound guided prostate biopsy on quality of life: a prospective randomized trial comparing 6 versus 12 cores. J Urol. 2001;165:100. doi: 10.1097/00005392-200101000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Jones JS, Patel A, Schoenfield L, et al. Saturation technique does not improve cancer detection as an initial prostate biopsy strategy. J Urol. 2006;175:485. doi: 10.1016/S0022-5347(05)00211-9. [DOI] [PubMed] [Google Scholar]
- 8.Turkbey B, Albert PS, Kurdziel K, et al. Imaging localized prostate cancer: current approaches and new developments. AJR Am J Roentgenol. 2009;192:1471. doi: 10.2214/AJR.09.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turkbey B, Pinto PA, Choyke PL. Imaging techniques for prostate cancer: implications for focal therapy. Nat Rev Urol. 2009;6:191. doi: 10.1038/nrurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Mazaheri Y, Zhang J, et al. Assessment of biologic aggressiveness of prostate cancer: correlation of MR signal intensity with Gleason grade after radical prostatectomy. Radiology. 2008;246:168. doi: 10.1148/radiol.2461070057. [DOI] [PubMed] [Google Scholar]
- 11.Turkbey B, Shah VP, Pang Y, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011;258:488. doi: 10.1148/radiol.10100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beyersdorff D, Winkel A, Hamm B, et al. MR imaging-guided prostate biopsy with a closed MR unit at 1. 5 T: initial results. Radiology. 2005;234:576. doi: 10.1148/radiol.2342031887. [DOI] [PubMed] [Google Scholar]
- 13.Hambrock T, Somford DM, Hoeks C, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol. 2010;183:520. doi: 10.1016/j.juro.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Susil RC, Krieger A, Derbyshire JA, et al. System for MR image-guided prostate interventions: canine study. Radiology. 2003;228:886. doi: 10.1148/radiol.2283020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh AK, Krieger A, Lattouf JB, et al. Patient selection determines the prostate cancer yield of dynamic contrast-enhanced magnetic resonance imaging-guided transrectal biopsies in a closed 3-Tesla scanner. BJU Int. 2008;101:181. doi: 10.1111/j.1464-410X.2007.07219.x. [DOI] [PubMed] [Google Scholar]
- 16.Rastinehad AR, Baccala AA, Jr, Chung PH, et al. D’Amico risk stratification correlates with degree of suspicion of prostate cancer on multiparametric magnetic resonance imaging. J Urol. 2011;185:815. doi: 10.1016/j.juro.2010.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan I, Oldenburg NE, Meskell P, et al. Real time MRI-ultrasound image guided stereotactic prostate biopsy. Magn Reson Imaging. 2002;20:295. doi: 10.1016/s0730-725x(02)00490-3. [DOI] [PubMed] [Google Scholar]
- 18.Miyagawa T, Ishikawa S, Kimura T, et al. Real-time Virtual Sonography for navigation during targeted prostate biopsy using magnetic resonance imaging data. Int J Urol. 2010;17:855. doi: 10.1111/j.1442-2042.2010.02612.x. [DOI] [PubMed] [Google Scholar]
- 19.Schlaier JR, Warnat J, Dorenbeck U, et al. Image fusion of MR images and real-time ultrasonography: evaluation of fusion accuracy combining two commercial instruments, a neuronavigation system and a ultrasound system. Acta Neurochir (Wien) 2004;146:271. doi: 10.1007/s00701-003-0155-6. [DOI] [PubMed] [Google Scholar]
- 20.Xu S, Kruecker J, Turkbey B, et al. Real-time MRI-TRUS fusion for guidance of targeted prostate biopsies. Comput Aided Surg. 2008;13:255. doi: 10.1080/10929080802364645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection–histopathologic correlation. Radiology. 2010;255:89. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Descazeaud A, Rubin M, Chemama S, et al. Saturation biopsy protocol enhances prediction of pT3 and surgical margin status on prostatectomy specimen. World J Urol. 2006;24:676. doi: 10.1007/s00345-006-0134-7. [DOI] [PubMed] [Google Scholar]
- 23.Patel AR, Jones JS, Rabets J, et al. Parasagittal biopsies add minimal information in repeat saturation prostate biopsy. Urology. 2004;63:87. doi: 10.1016/j.urology.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 24.Rabets JC, Jones JS, Patel A, et al. Prostate cancer detection with office based saturation biopsy in a repeat biopsy population. J Urol. 2004;172:94. doi: 10.1097/01.ju.0000132134.10470.75. [DOI] [PubMed] [Google Scholar]
- 25.Walz J, Graefen M, Chun FK, et al. High incidence of prostate cancer detected by saturation biopsy after previous negative biopsy series. Eur Urol. 2006;50:498. doi: 10.1016/j.eururo.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 26.Onik G, Miessau M, Bostwick DG. Three-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer management. J Clin Oncol. 2009;27:4321. doi: 10.1200/JCO.2008.20.3497. [DOI] [PubMed] [Google Scholar]
- 27.D’Amico AV, Tempany CM, Cormack R, et al. Transperineal magnetic resonance image guided prostate biopsy. J Urol. 2000;164:385. [PubMed] [Google Scholar]