Abstract
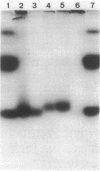
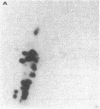
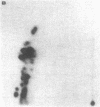
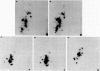
The genome of Bacillus phage phi 29 contains covalently linked protein at both ends. These DNA terminal proteins are essential for phi 29 DNA replication. We have isolated phi 29 terminal protein from each end separately and compared their two-dimensional peptide maps. Our results showed the two proteins to be identical. The DNAs of four phages examined (phi 15, Nf, M2Y, and GA-1) also contain protein at both ends of the DNA molecules. The chymotryptic peptide maps of these DNA terminal proteins have been compared with the map of the phi 29 terminal protein. Despite the similarities in molecular size, peptide maps of the terminal proteins show clear differences among the unrelated phages. These results are consistent with the idea that the terminal proteins are encoded by viral DNA rather than by the host chromosome. We have also determined the nucleotide sequences of the termini of four phage DNAs and compared them with the sequence of phi 29 DNA. The sequence data indicate that all of these phages DNA contain short inverted terminal repeats: 5'A-A-A-G-T-A for phi 29 and phi 15, 5' A-A-A-G-T-A-A-G for Nf and M2Y, and 5' A-A-A-T-A-G-A for GA-1.
Full text
PDF
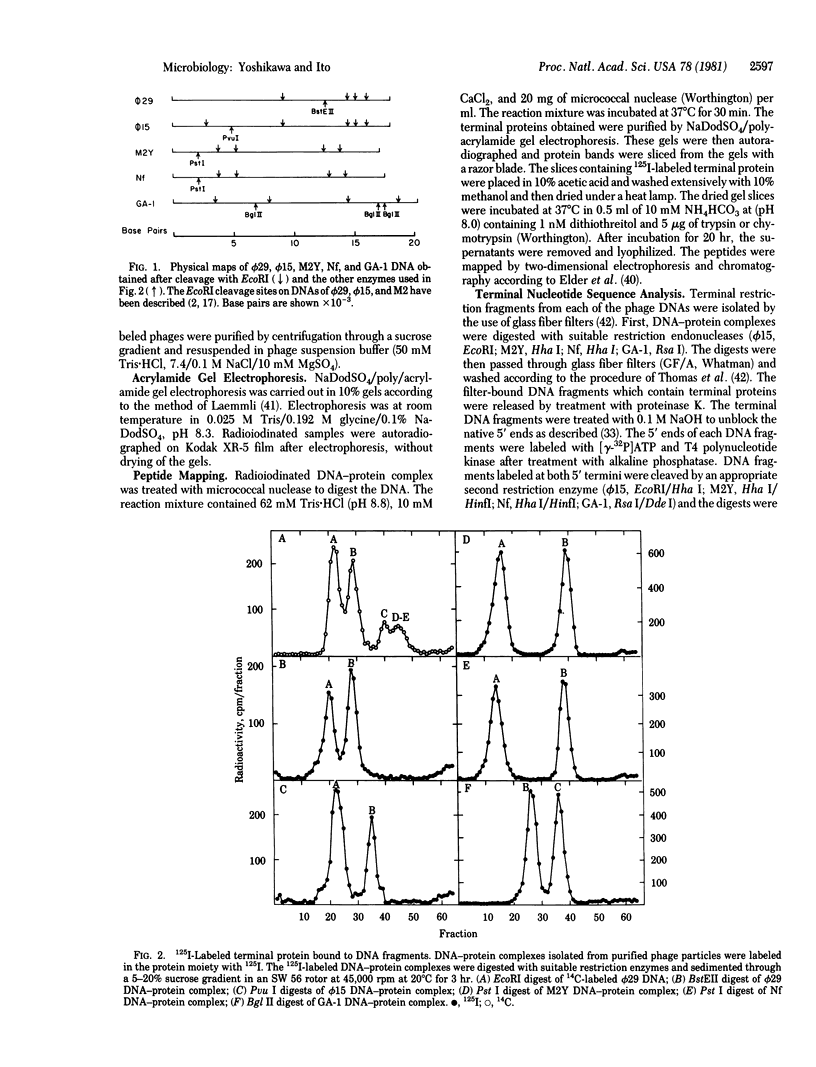
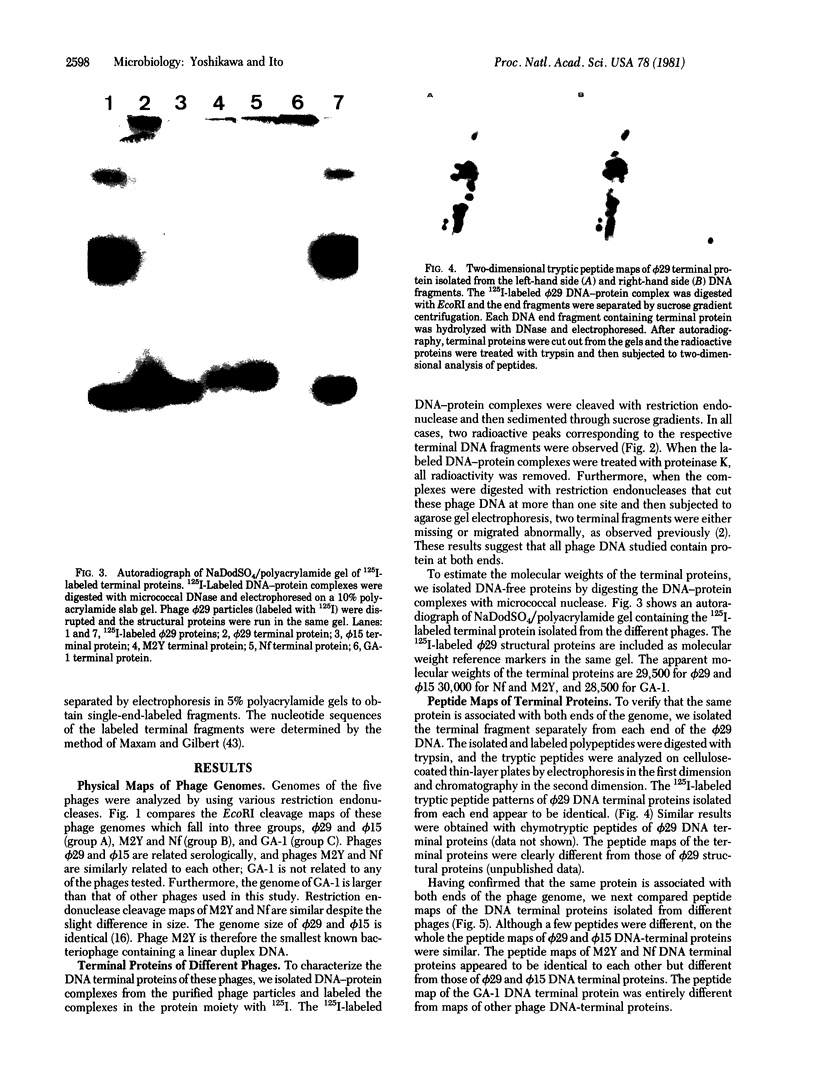
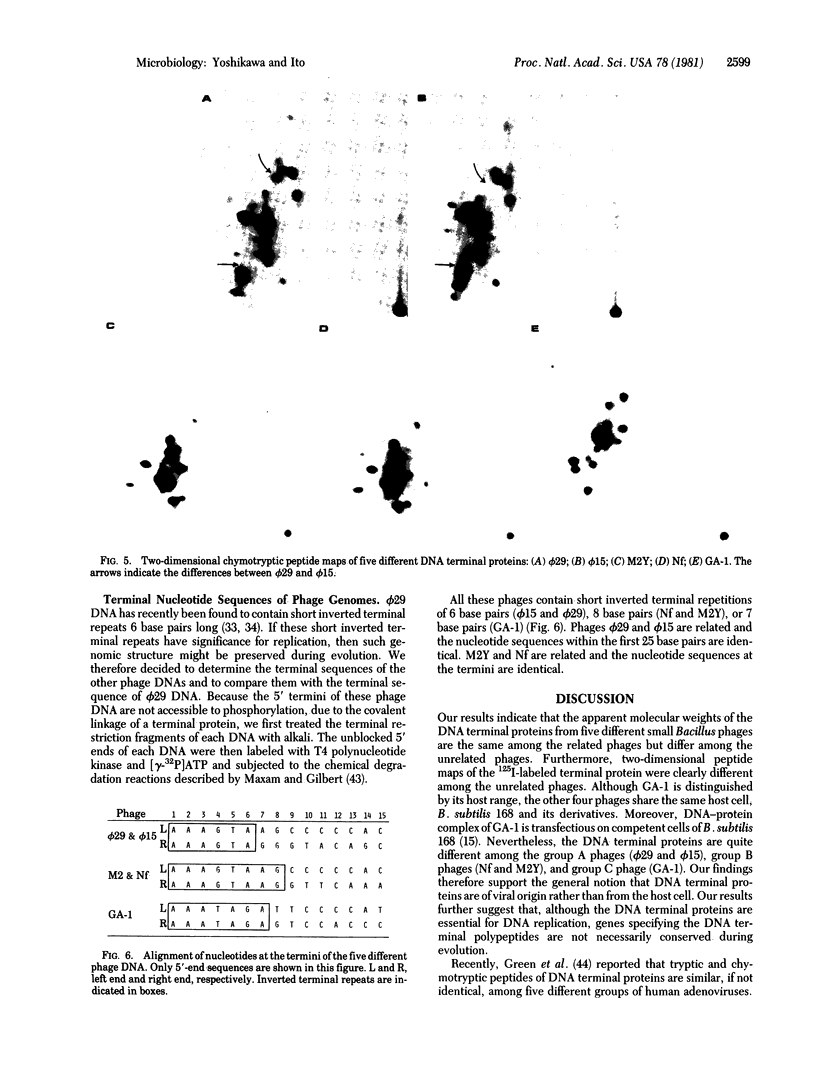

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. L., Hickman D. D., Reilly B. E. Structure of Bacillus subtilis bacteriophage phi 29 and the length of phi 29 deoxyribonucleic acid. J Bacteriol. 1966 May;91(5):2081–2089. doi: 10.1128/jb.91.5.2081-2089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. L., Reilly B. E. Analysis of bacteriophage phi 29 gene function: protein synthesis in suppressor-sensitive mutant infection of Bacillus subtilis. J Virol. 1974 Jan;13(1):211–221. doi: 10.1128/jvi.13.1.211-221.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrand J. R., Roberts R. J. The nucleotide sequences at the termini of adenovirus-2 DNA. J Mol Biol. 1979 Mar 15;128(4):577–594. doi: 10.1016/0022-2836(79)90294-8. [DOI] [PubMed] [Google Scholar]
- Arwert F., Venema G. Protease-sensitive transfection of Bacillus subtilis with bacteriophage GA-1 DNA: a probable case of heterologous transfection. J Virol. 1974 Mar;13(3):584–589. doi: 10.1128/jvi.13.3.584-589.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D. G., Helinski D. R. Relaxation complexes of plasmid DNA and protein. I. Strand-specific association of protein and DNA in the relaxed complexes of plasmids ColE1 and ColE2. J Biol Chem. 1975 Nov 25;250(22):8785–8789. [PubMed] [Google Scholar]
- Carrascosa J. L., Camacho A., Moreno F., Jiménez F., Mellado R. P., Viñuela E., Salas M. Bacillus subtilis phage phi29. Characterization of gene products and functions. Eur J Biochem. 1976 Jul 1;66(2):229–241. doi: 10.1111/j.1432-1033.1976.tb10512.x. [DOI] [PubMed] [Google Scholar]
- David G. S. Solid state lactoperoxidase: a highly stable enzyme for simple, gentle iodination of proteins. Biochem Biophys Res Commun. 1972 Jul 25;48(2):464–471. doi: 10.1016/s0006-291x(72)80074-3. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Griffith J., Kornberg A. phiX174 cistron A protein is a multifunctional enzyme in DNA replication. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3198–3202. doi: 10.1073/pnas.74.8.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Escarmís C., Salas M. Nucleotide sequence at the termini of the DNA of Bacillus subtilis phage phi 29. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1446–1450. doi: 10.1073/pnas.78.3.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanegan J. B., Petterson R. F., Ambros V., Hewlett N. J., Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5'-terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci U S A. 1977 Mar;74(3):961–965. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Brackmann K., Wold W. S., Cartas M., Thornton H., Elder J. H. Conserved primary sequences of the DNA terminal proteins of five different human adenovirus groups. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4380–4384. doi: 10.1073/pnas.76.9.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen E. W., Reilly B. E., Tosi M. E., Anderson D. L. Analysis of gene function of bacteriophage phi 29 of Bacillus subtilis: identification of cistrons essential for viral assembly. J Virol. 1976 Aug;19(2):501–517. doi: 10.1128/jvi.19.2.501-517.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding N. E., Ito J. DNA Replication of bacteriophage phi29: isolation of a DNA-protein complex from Bacillus subtilis cells infected with wild-type and with a suppressor-sensitive mutant. Virology. 1976 Sep;73(2):389–401. doi: 10.1016/0042-6822(76)90400-1. [DOI] [PubMed] [Google Scholar]
- Harding N. E., Ito J. DNA replication of bacteriophage phi 29: characterization of the intermediates and location of the termini of replication. Virology. 1980 Jul 30;104(2):323–338. doi: 10.1016/0042-6822(80)90337-2. [DOI] [PubMed] [Google Scholar]
- Harding N. E., Ito J., David G. S. Identification of the protein firmly bound to the ends of bacteriophage phi 29 DNA. Virology. 1978 Feb;84(2):279–292. doi: 10.1016/0042-6822(78)90248-9. [DOI] [PubMed] [Google Scholar]
- Hirokawa H. Transfecting deoxyribonucleic acid of Bacillus bacteriophage phi 29 that is protease sensitive. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1555–1559. doi: 10.1073/pnas.69.6.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inciarte M. R., Salas M., Sogo J. M. Structure of replicating DNA molecules of Bacillus subtilis bacteriophage phi 29. J Virol. 1980 Apr;34(1):187–199. doi: 10.1128/jvi.34.1.187-199.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J. Bacteriophage phi29 terminal protein: its association with the 5' termini of the phi29 genome. J Virol. 1978 Dec;28(3):895–904. doi: 10.1128/jvi.28.3.895-904.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Harding N. E., Saigo K. phi29 DNA-protein complex and the structure of replicating DNA molecules. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):525–536. doi: 10.1101/sqb.1979.043.01.058. [DOI] [PubMed] [Google Scholar]
- Ito J., Kawamura F., Yanofsky S. Analysis of phi 29 and phi 15 genomes by bacterial restriction endonucleases, EcoR1 and Hpal. Virology. 1976 Mar;70(1):37–51. doi: 10.1016/0042-6822(76)90234-8. [DOI] [PubMed] [Google Scholar]
- Ito J., Meinke W., Hathaway G., Spizizen J. Studies on Bacillus subtilis bacteriophage phi 15. Virology. 1973 Nov;56(1):110–122. doi: 10.1016/0042-6822(73)90291-2. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Wu M. Structure of a nicked DNA-protein complex isolated from simian virus 40: covalent attachment of the protein to DNA and nick specificity. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1945–1949. doi: 10.1073/pnas.73.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura F., Ito J. Studies of Bacillus subtilis phage M2: a physical map of the M2 genome. Virology. 1977 Dec;83(2):233–245. doi: 10.1016/0042-6822(77)90168-4. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Thomas C. A., Jr An intermediate in the replication of bacteriophage T7 DNA molecules. J Mol Biol. 1969 Sep 28;44(3):459–475. doi: 10.1016/0022-2836(69)90373-8. [DOI] [PubMed] [Google Scholar]
- Lee Y. F., Nomoto A., Detjen B. M., Wimmer E. A protein covalently linked to poliovirus genome RNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):59–63. doi: 10.1073/pnas.74.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mellado R. P., Peñalva M. A., Inciarte M. R., Salas M. The protein covalently linked to the 5' termini of the DNA of Bacillus subtilis phage phi 29 is involved in the initiation of DNA replication. Virology. 1980 Jul 15;104(1):84–96. doi: 10.1016/0042-6822(80)90367-0. [DOI] [PubMed] [Google Scholar]
- Ortin J., Viñuela E., Salas M., Vasquez C. DNA-protein complex in circular DNA from phage phi-29. Nat New Biol. 1971 Dec 29;234(52):275–277. doi: 10.1038/newbio234275a0. [DOI] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Salas M., Mellado R. P., Viñuela E. Characterization of a protein covalently linked to the 5' termini of the DNA of Bacillus subtilis phage phi29. J Mol Biol. 1978 Feb 25;119(2):269–291. doi: 10.1016/0022-2836(78)90438-2. [DOI] [PubMed] [Google Scholar]
- Sangar D. V., Rowlands D. J., Harris T. J., Brown F. Protein covalently linked to foot-and-mouth disease virus RNA. Nature. 1977 Aug 18;268(5621):648–650. doi: 10.1038/268648a0. [DOI] [PubMed] [Google Scholar]
- Shinagawa M., Padmanabhan R. Nucleotide sequence at the inverted terminal repetition of adenovirus type 2 DNA. Biochem Biophys Res Commun. 1979 Apr 13;87(3):671–678. doi: 10.1016/0006-291x(79)92011-4. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Inciarte M. R., Corral J., Viñuela E., Salas M. RNA polymerase binding sites and transcription map of the DNA of Bacillus subtilis phage phi29. J Mol Biol. 1979 Feb 5;127(4):411–436. doi: 10.1016/0022-2836(79)90230-4. [DOI] [PubMed] [Google Scholar]
- Steenbergh P. H., Maat J., van Ormondt H., Sussenbach J. S. The nucleotide sequence at the termini of adenovirus type 5 DNA. Nucleic Acids Res. 1977 Dec;4(12):4371–4389. doi: 10.1093/nar/4.12.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera A., Salas M., Viñuela E. Temperature-sensitive mutants affected in DNA synthesis in phage phi29 of Bacillus subtilis. Eur J Biochem. 1972 Dec 4;31(2):367–371. doi: 10.1111/j.1432-1033.1972.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Thomas C. A., Jr, Saigo K., McLeod E., Ito J. The separation of DNA segments attached to proteins. Anal Biochem. 1979 Feb;93(1):158–166. [PubMed] [Google Scholar]
- Winnacker E. L. Adenovirus DNA: structure and function of a novel replicon. Cell. 1978 Aug;14(4):761–773. doi: 10.1016/0092-8674(78)90332-x. [DOI] [PubMed] [Google Scholar]
- YOUNG E. T., 2nd, SINSHEIMER R. L. NOVEL INTRA-CELLULAR FORMS OF LAMBDA DNA. J Mol Biol. 1964 Dec;10:562–564. doi: 10.1016/s0022-2836(64)80080-2. [DOI] [PubMed] [Google Scholar]
- Yanofsky S., Kawamura F., Ito J. Thermolabile transfecting DNA from temperature-sensitive mutant of phage phi29. Nature. 1976 Jan 1;259(5538):60–63. doi: 10.1038/259060a0. [DOI] [PubMed] [Google Scholar]
- Yehle C. O. Genome-linked protein associated with the 5' termini of bacteriophage phi29 DNA. J Virol. 1978 Sep;27(3):776–783. doi: 10.1128/jvi.27.3.776-783.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H., Friedmann T., Ito J. Nucleotide sequences at the termini of phi 29 DNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1336–1340. doi: 10.1073/pnas.78.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]