Abstract
Purpose
This is a randomised controlled trial to examine whether intra-articular injection of tranexamic acid (TXA) decreases blood loss, as well as reducing leg swelling after total knee arthroplasty (TKA).
Methods
We performed 100 TKA in osteoarthritis patients. At closure, a total of 2,000 mg/20 ml TXA was injected into the knee joint through a closed suction drain (TXA group). For the control group, the same volume of physiological saline was injected. The pre-operative condition of the patients, post-operative haemoglobin (Hb) levels, discharge volumes from drain, D-dimer and needs for transfusion were compared between these two groups. Furthermore, leg diameters (thigh, suprapatellar portion and calf girth) were measured pre- and post-operatively to investigate whether TXA has an influence on leg swelling after surgery.
Results
The results revealed that post-operative decrease in Hb level was significantly reduced in the TXA group. Furthermore, knee joint swelling after operation was significantly suppressed in the TXA group compared to the control group.
Conclusions
The results revealed intra-articular administration of TXA decreased not only blood loss, but also knee joint swelling after TKA.
Introduction
Total knee arthroplasty (TKA) is an excellent surgical procedure for patients with painful arthritic knees. However, TKA has some potential unresolved problems, including blood loss, pain after operation and leg swelling. Resolution of these problems would increase patient satisfaction [1] and serve to raise the overall quality of TKA.
One of the main problems is the need for blood transfusion in some TKA patients. Although the incidence is low, serious complications involving allogeneic blood transfusions, such as viral infections and graft-versus-host disease, have been reported [2]. There are two current methods for avoiding allogeneic blood transfusions. One is by autologous blood transfusion, including pre-operative autologous donation, intra-operative or post-operative blood salvage [3–7]. The other is by reducing blood loss using techniques such as hypotensive anaesthesia [8], drain clamping [9–13], application of fibrin tissue adhesive [14, 15], compression bandage and cryotherapy [16]. When TKA is performed using a tourniquet, most blood loss occurs after surgery [17]. Recently, drain clamping has received increasing attention [9–13].
Tranexamic acid (TXA) is both an inhibitor of fibrinolysis and an activator of plasminogen [18] and is known to inhibit blood loss after TKA [19–21]. While there have been many reports outlining single or repeated intravenous administration of TXA in TKA patients [19, 20, 22], the ideal method of providing TXA is still controversial [22]. It is generally accepted that only a small percentage of the intravenously injected drug reaches the target location, and it is thought that the mechanism of TXA action is inhibition of tissue fibrinolysis and consequent stabilisation of clots by TXA entering the extravascular space and accumulating in tissues for up to 17 hours [23]. Thus a more efficient method of advanced application is desirable. As an alternative, a prospective randomised trial to test the hypothesis that intra-articular isolated injection of TXA combined with drain clamping was performed in this study. This is a method that has not been reported in the English literature, as far as we know. In addition, it is generally accepted that the control of swelling can improve wound healing, reduce pain and permit rapid rehabilitation of the knee after trauma or surgery [24]. Noble et al. have suggested that real improvements in the outcome of TKA must address the prevention of residual pain, stiffness and swelling [1]. We also consider that the reduction of post-operative leg swelling is desirable and has no disadvantages; however, until now there have been few studies investigating how to reduce leg swelling after TKA [25, 26]. Techniques such as thermal therapy and cryotherapy have been reported [25, 26]. In this study, we postulated that the reduction in blood loss achieved with TXA would also enable reduction of leg swelling after TKA. The other purpose of this study was to investigate the hypothesis that isolated intra-articular injection of TXA could reduce leg swelling after TKA.
Materials and methods
This was a prospective, randomised study. A total of 100 consecutive primary TKAs were performed in osteoarthritis (OA) patients (12 men and 88 women) between January 2008 and May 2009. Those with rheumatoid arthritis, revision TKA and simultaneous bilateral TKA were excluded. Patients were alternately assigned to one of two groups. In the first, drain clamping was performed after injection of TXA (2,000 mg/20 ml) into the knee joint (TXA group). In the second, drain clamping was performed after injection of saline (20 ml) (control group). The characteristics of the patients are shown in Table 1. The patients were comparable between the two groups. Surgical methods were consistent as described below: all knees were operated upon under spinal anaesthesia. All TKAs were unilateral using a medial parapatellar approach and a tourniquet. The tourniquet was not released before skin closure. For bony resection, an intramedullary alignment jig was used for the femur and an extramedullary device was used for the tibia. No patella was resurfaced. The posterior cruciate ligament (PCL) was resected in 86 TKAs and retained in 14. The types of implant used were: Advance (Wright Medical Technology Inc., Arlington, TN, USA) in 81 [posterior substituting (PS), 75; cruciate retaining (CR), six], LPS-Flex (Zimmer, Warsaw, IN, USA) in two, Scorpio NRG CR (Stryker Orthopedics, Mahwah, NJ, USA) in eight, Vanguard PS (Biomet, Warsaw, IN, USA) in eight and PFC Σ PS (DePuy, Warsaw, IN, USA) in one. Lateral retinacular releases were performed for seven TKAs in the control group and ten in the TXA group. Full cementation was performed in all TKAs. Immediately after wound closure, 20 ml TXA or saline was injected through a Port-VAC (Howmedica International S. de R.L., Limerick, Ireland) drain. Drain clamping methods were performed according to previously reported methods [12] as follows. Briefly, the tube was clamped and closed completely for 30 minutes; then the clamp was partially released until blood started to flow out. If the flow of blood ceased, the clamp was further opened until the blood flowed once again. Finally the clamp was fully opened. Pre-donation of autologous blood was not carried out for any patient and shed blood was not re-infused. In both groups, only one drain was placed deep to the fascia in the knee joint. In both groups, the drains were removed at 48 hours and the volume of fluid at one, three, six, 12, 24 and 48 hours was measured. Continuous passive motion began after removal of the drains. For the prevention of deep vein thrombosis, an arteriovenous impulse system (Novamedix, Andover, Hampshire, UK) was used in all patients for 24 hours after surgery. Furthermore, 10,000 IU heparin sodium (Ajinomoto, Tokyo, Japan) was administered intravenously for 24 hours, which was started soon after the operation. The pre-operative conditions, post-operative haemoglobin (Hb) levels, D-dimer, as an assessment for thromboembolic conditions, the rate of allogeneic blood transfusion and complications were recorded. The Hb levels were examined at day zero (three hours), one, seven (one week) and 14 (two weeks) after the operation. Furthermore, circumferential measurements of the leg at the superior patellar border (suprapatellar girth), ten cm above the border (thigh girth), and the maximum circumference of the calf (calf girth) were examined to investigate whether TXA influences leg swelling after surgery. The suprapatellar girth was recognised as the index of the intra-articular swelling. Measurements were performed pre-operation and one, two and four weeks after the operation. Measurements were performed twice each day in each patient by two authors blinded to clinical information.
Table 1.
Demographic data of the patients
| Groups | Age (years) | Gender | Height (cm) | Weight (kg) | BMI | Operated side | Operation time (min) |
|---|---|---|---|---|---|---|---|
| TXA group (n = 50) | 73.3 (5.0) | Male: 6; female: 44 | 148.3 (4.8) | 59.7 (9.0) | 26.7 (3.1) | R: 23; L: 27 | 106.5 (15.6) |
| Control group (n = 50) | 73.5 (6.1) | Male: 6; female: 44 | 150.6 (7.8) | 59.7 (10.1) | 27.4 (3.9) | R: 24; L: 26 | 105.0 (10.9) |
| p value | n.s. | n.s. | n.s. | n.s. | n.s. |
All data expressed as mean (SD) except for gender
TXA tranexamic acid, BMI body mass index
Data analysis
One-way analysis of variance (ANOVA) was used to analyse the difference in post-operative blood loss, and the changes in Hb levels and the circumferential measurements over time. All differences were considered to be significant when the p value was < 0.05. Results were analysed statistically using a statistical software package (Stat Mate III, ATMS Co., Ltd., Tokyo, Japan).
Results
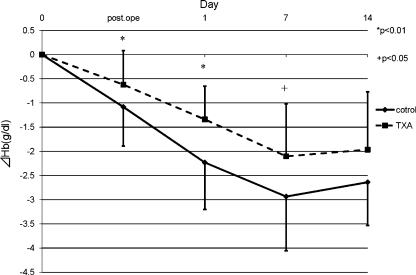
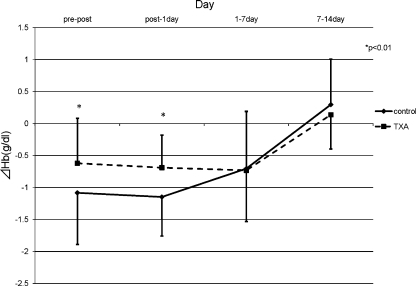
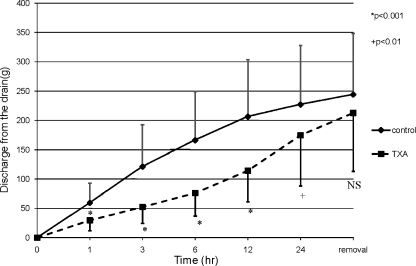
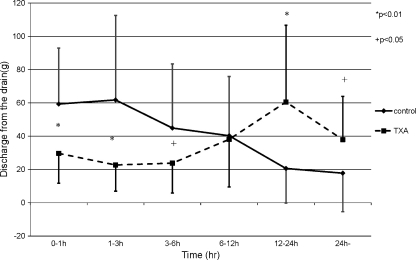
The pre-operative condition of the patients was comparable between the two groups (Table 2). In both groups, the Hb level decreased and had its minimum peak at one week post-operation and recovered gradually. The reductions in Hb levels in the TXA group were significantly smaller than the control group at both one day and one week post-operation (Fig. 1). The mean post-operative reduction in the Hb level for the two groups revealed that the significant suppression in Hb level reduction occurred in the TXA group by one day post-operation (p < 0.01) (Fig. 2). These results revealed that TXA could significantly reduce blood loss by one week post-operation, and the effect of TXA occurred soon after the operation. The results of the mean discharge volumes from the drain revealed that the volumes in the TXA group had significantly diminished compared to those in the control group by 24 hours post-operation. The volume in the TXA group increased thereafter, and finally, the total volumes were approximately the same among these two groups (Fig. 3). Furthermore, the results revealing the mean discharge volume increment from the drain showed that the drain volumes in the TXA group were remarkably diminished at between zero to one, one to three and three to six hours post-operation; however, the volume increment in the TXA group had significantly increased at between 12 and 24 hours, and thereafter in contrast (Fig. 4). These results revealed that TXA did not interfere with the effect of the drain.
Table 2.
Pre-operative assessment of the patients
| Groups | Hb (g/dl) | Ht (%) | Platelets (×101 μl) | Bleeding time (s) | APTT (s) | Prothrombin time (%) |
|---|---|---|---|---|---|---|
| TXA group | 12.5 (1.0) | 37.6 (2.6) | 21.1 (4.4) | 132.1 (56.9) | 28.6 (3.5) | 97.6 (16.3) |
| Control group | 12.6 (1.0) | 37.6 (2.9) | 21.5 (4.0) | 126.4 (52.3) | 28.2 (2.8) | 99.8 (13.3) |
| p value | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
All data expressed as mean (SD)
Hb haemoglobin, Ht haematocrit, APTT activated partial thromboplastin time
Fig. 1.
The mean post-operative reduction in the Hb level for the two groups. In both groups, the Hb level had its minimum peak at 1 week post-operation and recovered gradually. The reduction in Hb levels was significantly decreased in the TXA group compared to the control group by 2 weeks post-operation (*p < 0.01; +p < 0.05) (solid line control group, broken line TXA group)
Fig. 2.
The mean post-operative reduction in the Hb level at the different time points. A significantly decreased reduction in Hb level was seen from pre-operation to 1 day post-operation (*p < 0.01) (solid line control group, broken line TXA group)
Fig. 3.
The mean discharge volumes from the drain. The discharge volumes from the drain in the TXA group had significantly diminished compared to the control group by 24 h post-operation. The volume in the TXA group increased thereafter, and finally, the total volumes of the drain fluid were approximately the same among these two groups (*p < 0.001; +p < 0.01) (solid line control group, broken line TXA group)
Fig. 4.
The mean discharge volume increment from the drain. The drain volumes in the TXA group were remarkably diminished at between 0–1, 1–3 and 3–6 h post-operation; however, the volume increment in the TXA group had significantly increased at between 12 and 24 h, and thereafter in contrast (*p < 0.01; +p < 0.05) (solid line control group, broken line TXA group)
The results of thigh girth measurements showed that the post-operative increments were also the same between the two groups after the operation (Table 3). The results of the suprapatellar girth measurements representing the circumference at the superior patellar border revealed that the increments were significantly reduced in the TXA group compared to the control group at one week post-operation (p < 0.01, Table 3). The results of calf girth measurements showing the widest part of the calf revealed that the increments were significantly reduced in the TXA group compared to the control group at two weeks post-operation (p < 0.05, Table 3); however, the increments were almost the same between the two groups at one week post-operation.
Table 3.
Mean (SD) increments in circumferences of the leg over time for the two groups (cm)
| Groups | Pre-operation (cm) | Pre-operative increment | ||
|---|---|---|---|---|
| 1 week | 2 weeks | 4 weeks | ||
| Thigh girtha | ||||
| TXA group | 41.4 (5.4) | 1.1 (2.0) | −0.7 (2.1) | −1.8 (2.1) |
| Control group | 40.8 (3.8) | 1.4 (1.7) | −0.6 (1.9) | −1.6 (2.2) |
| p value | n.s. | n.s. | n.s. | n.s. |
| Suprapatellar girthb | ||||
| TXA group | 38.3 (3.6) | 1.6 (1.2) | 0.7 (1.0) | 0.1 (1.1) |
| Control group | 37.5 (3.0) | 2.5 (1.2) | 1.1 (1.1) | −0.1 (1.3) |
| p value | n.s. | <0.01* | n.s. | n.s. |
| Calf girthc | ||||
| TXA group | 32.8 (3.1) | 0.5 (1.6) | −1.1 (1.4) | −1.2 (1.3) |
| Control group | 32.9 (3.2) | 0.9 (2.0) | −0.3 (1.6) | −1.0 (1.1) |
| p value | n.s. | n.s. | <0.05* | n.s. |
aThigh girth: circumference at the thigh, 10 cm proximal portion from the top of the patella. Post-operative increment (cm): increment of the circumference compared with that of the pre-operation. A statistically significant difference was recognised when the p value was under 0.05
bSuprapatellar girth: circumference at the superior patellar border. Post-operative increment (cm): increment of the circumference compared with that of the pre-operation. *Statistically significant difference between the two groups: p < 0.05
cCalf girth: the maximum circumference of the calf. Post-operative increment (cm): increment of the circumference compared with that of the pre-operation. *Statistically significant difference between the two groups: p < 0.05
Allogeneic blood transfusion was required for one patient (2%) in the control group. The mean D-dimer levels one week post-operation were 10.6 (4.7–19.7) in the TXA group and 11.2 (5.1–23.5) in the control group, respectively. The levels were similar in these two groups. There were no specific complications and no revision arthroplasty in either group at an average of 21.5 (12–27) months after the operations.
Discussion
One of the important findings in this study is that the intra-articular administration of TXA can effectively diminish the reduction of Hb levels by one week post-operation, and these results were consistent with other studies investigating the effect of intravenous administration of TXA in TKA patients [19, 20, 22]. As Ralley et al. recently proposed that many of the dosing schedules reported were not ideally suited for routine application [22], this intra-articular administration of TXA, which was applied through the drain at the end of the operation, is simple, easy and suitable for clinical application.
In a meta-analysis, Cid et al. reported that the reduction in the risk of receiving a blood transfusion was independent of the total dose of TXA given [27]. They mentioned that both a low-dose (15–35 mg/kg) and a high-dose (135–150 mg/kg) protocol resulted in similar transfusion rates [27]. The total amount of 2,000 mg TXA used in this study was not a high-dose protocol, but not such a low dose compared with other reports. Therefore, we might be able to reduce the total amount of TXA applied into the joint and this needs further investigation.
Good et al. reported that the administration of TXA intravenously decreased external blood loss but not hidden blood loss after TKA [20]. Their report also revealed that TXA reduces drainage volumes by about 50% [20]. In contrast to their report, the results in this study revealed that the discharge volume from the drain between the two groups was also the same. Furthermore, the results also revealed that TXA could reduce the suprapatellar swelling at one week post-operation. These results indicated that the method in this study had the benefit of reducing the joint swelling by diminishing the hidden blood loss in the joint, since the same drainage effect by drain was obtained in both groups.
Regarding the results of calf girth measurements, calf swelling was reduced only at two weeks, not at one week post-operation. Additionally, the results of the thigh girth measurements showed that the circumference was equally decreased, at least by four weeks post-operation in both groups. In general, post-operative leg swelling is influenced by multiple factors, such as venous thromboembolic events (VTE), soft tissue inflammation and renal dysfunction. As a result, whether this method could reduce the leg swelling was not confirmed in this study.
There have been many reports investigating whether intravenously administered TXA increases VTE [19, 20, 22]. Kagoma et al. reviewed the previously published randomised trials and reported that TXA did not increase the risk of VTE [28]. In this study, there were no symptomatic VTE in either group and the results of D-dimer measurements revealed no significant difference between the two groups. However, the methods of detecting clinically asymptomatic VTE, such as ultrasound and venography, were not performed and the true ratio of this incidence is unknown in this study. Further studies are needed to investigate whether the intra-articular administration of TXA elevated the risk of VTE.
There were some limitations in this study. Firstly, the correlation between the leg swelling and the patients’ pain and discomfort was not examined and this is essential in further investigations. As the subjective assessment of pain is difficult, a self-assessment questionnaire or a visual analogue scale would be useful. In addition, the correlation to clinical results remained unknown. Furthermore, investigations comparing the different methods, such as intravenous injection of TXA, would reveal a more detailed effectiveness of TXA.
In summary, this study showed that the intra-articular administration of TXA reduced knee joint swelling as well as blood loss after TKA. We consider the methods employed in this study to be effective, efficient and reproducible for the reduction of blood loss and knee joint swelling, which has the potential to reduce patient discomfort after TKA.
Acknowledgement
We thank Ms. Janina Tubby for English rewriting.
References
- 1.Noble PC, Conditt MA, Cook KF, Mathis KB. The John Insall Award: Patient expectations affect satisfaction with total knee arthroplasty. Clin Orthop Relat Res. 2006;452:35–43. doi: 10.1097/01.blo.0000238825.63648.1e. [DOI] [PubMed] [Google Scholar]
- 2.Fiebig E. Safety of the blood supply. Clin Orthop Relat Res. 1998;357:6–18. doi: 10.1097/00003086-199812000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Groh GI, Buchert PK, Allen WC. A comparison of transfusion requirements after total knee arthroplasty using the Solcotrans autotransfusion system. J Arthroplasty. 1990;5:281–285. doi: 10.1016/S0883-5403(08)80084-8. [DOI] [PubMed] [Google Scholar]
- 4.Gannon DM, Lombardi AV, Jr, Mallory TH, Vaughn BK, Finney CR, Niemcryk S. An evaluation of the efficacy of postoperative blood salvage after total joint arthroplasty. A prospective randomized trial. J Arthroplasty. 1991;6:109–114. doi: 10.1016/S0883-5403(11)80004-5. [DOI] [PubMed] [Google Scholar]
- 5.Clements DH, Sculco TP, Burke SW, Mayer K, Levine DB. Salvage and reinfusion of postoperative sanguineous wound drainage. A preliminary report. J Bone Joint Surg Am. 1992;74:646–651. [PubMed] [Google Scholar]
- 6.Sinha A, Sinha M, Burgert S. Reinfusion of drained blood as an alternative to homologous blood transfusion after total knee replacement. Int Orthop. 2001;25:257–259. doi: 10.1007/s002640100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda K, Nozawa M, Katsube S, Maezawa K, Kurosawa H. Reinfusion of unwashed salvaged blood after total knee arthroplasty in patients with rheumatoid arthritis. Int Orthop. 2009;33:1615–1618. doi: 10.1007/s00264-008-0661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juelsgaard P, Larsen UT, Sørensen JV, Madsen F, Søballe K. Hypotensive epidural anesthesia in total knee replacement without tourniquet: reduced blood loss and transfusion. Reg Anesth Pain Med. 2001;26:105–110. doi: 10.1053/rapm.2001.21094. [DOI] [PubMed] [Google Scholar]
- 9.Ryu J, Sakamoto A, Honda T, Saito S. The postoperative drain-clamping method for hemostasis in total knee arthroplasty. Reducing postoperative bleeding in total knee arthroplasty. Bull Hosp Jt Dis. 1997;56:251–254. [PubMed] [Google Scholar]
- 10.Yamada K, Imaizumi T, Uemura M, Takada N, Kim Y. Comparison between 1-hour and 24-hour drain clamping using diluted epinephrine solution after total knee arthroplasty. J Arthroplasty. 2001;16:458–462. doi: 10.1054/arth.2001.23620. [DOI] [PubMed] [Google Scholar]
- 11.Kiely N, Hockings M, Gambhir A. Does temporary clamping of drains following knee arthroplasty reduce blood loss? A randomised controlled trial. Knee. 2001;8:325–327. doi: 10.1016/S0968-0160(01)00095-3. [DOI] [PubMed] [Google Scholar]
- 12.Tsumara N, Yoshiya S, Chin T, Shiba R, Kohso K, Doita M. A prospective comparison of clamping the drain or post-operative salvage of blood in reducing blood loss after total knee arthroplasty. J Bone Joint Surg Br. 2006;88:49–53. doi: 10.1302/0301-620X.88B1.16653. [DOI] [PubMed] [Google Scholar]
- 13.Stucinskas J, Tarasevicius S, Cebatorius A, Robertsson O, Smailys A, Wingstrand H. Conventional drainage versus four hour clamping drainage after total knee arthroplasty in severe osteoarthritis: a prospective, randomised trial. Int Orthop. 2009;33:1275–1278. doi: 10.1007/s00264-008-0662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang GJ, Hungerford DS, Savory CG, Rosenberg AG, Mont MA, Burks SG, Mayers SL, Spotnitz WD. Use of fibrin sealant to reduce bloody drainage and hemoglobin loss after total knee arthroplasty: a brief note on a randomized prospective trial. J Bone Joint Surg Am. 2001;83-A:1503–1505. doi: 10.2106/00004623-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Levy O, Martinowitz U, Oran A, Tauber C, Horoszowski H. The use of fibrin tissue adhesive to reduce blood loss and the need for blood transfusion after total knee arthroplasty. A prospective, randomized, multicenter study. J Bone Joint Surg Am. 1999;81:1580–1588. doi: 10.2106/00004623-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Gibbons CE, Solan MC, Ricketts DM, Patterson M. Cryotherapy compared with Robert Jones bandage after total knee replacement: a prospective randomized trial. Int Orthop. 2001;25:250–252. doi: 10.1007/s002640100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li B, Wen Y, Wu H, Qian Q, Lin X, Zhao H. The effect of tourniquet use on hidden blood loss in total knee arthroplasty. Int Orthop. 2009;33:1263–1268. doi: 10.1007/s00264-008-0647-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Astedt B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol Suppl. 1987;137:22–25. doi: 10.3109/00365528709089756. [DOI] [PubMed] [Google Scholar]
- 19.Benoni G, Carlsson A, Petersson C, Fredin H. Does tranexamic acid reduce blood loss in knee arthroplasty? Am J Knee Surg. 1995;8:88–92. [PubMed] [Google Scholar]
- 20.Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90:596–599. doi: 10.1093/bja/aeg111. [DOI] [PubMed] [Google Scholar]
- 21.Akizuki S, Yasukawa Y, Takizawa T. A new method of hemostasis for cementless total knee arthroplasty. Bull Hosp Jt Dis. 1997;56:222–224. [PubMed] [Google Scholar]
- 22.Ralley FE, Berta D, Binns V, Howard J, Naudie DD. One intraoperative dose of tranexamic acid for patients having primary hip or knee arthroplasty. Clin Orthop Relat Res. 2010;468:1905–1911. doi: 10.1007/s11999-009-1217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mannucci PM. Hemostatic drugs. N Engl J Med. 1998;339:245–253. doi: 10.1056/NEJM199807233390407. [DOI] [PubMed] [Google Scholar]
- 24.McMaster WC, Liddle S. Cryotherapy influence on posttraumatic limb edema. Clin Orthop Relat Res. 1980;150:283–287. [PubMed] [Google Scholar]
- 25.Healy WL, Seidman J, Pfeifer BA, Brown DG. Cold compressive dressing after total knee arthroplasty. Clin Orthop Relat Res. 1994;299:143–146. [PubMed] [Google Scholar]
- 26.Hecht PJ, Bachmann S, Booth RE, Jr, Rothman RH. Effects of thermal therapy on rehabilitation after total knee arthroplasty. A prospective randomized study. Clin Orthop Relat Res. 1983;178:198–201. [PubMed] [Google Scholar]
- 27.Cid J, Lozano M. Tranexamic acid reduces allogeneic red cell transfusions in patients undergoing total knee arthroplasty: results of a meta-analysis of randomized controlled trials. Transfusion. 2005;45:1302–1307. doi: 10.1111/j.1537-2995.2005.00204.x. [DOI] [PubMed] [Google Scholar]
- 28.Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009;123:687–696. doi: 10.1016/j.thromres.2008.09.015. [DOI] [PubMed] [Google Scholar]