Abstract
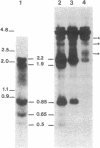
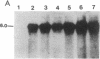
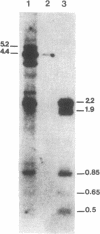
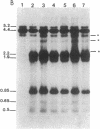
Synchronized chicken embryo fibroblasts, prepared by addition of serum to stationary cells arrested in Go, were exposed to the Prague strain of Rous sarcoma virus. At different times during the cell cycle, high molecular weight DNA was prepared from infected cells and examined for the presence of newly integrated viral DNA sequences. The results demonstrate that newly integrated viral sequences were first detected during S-phase DNA synthesis 9 hr after infection. The presence of colchicine prevented cellular division and delayed the appearance of progeny virus but it did not affect the appearance of viral specific DNA in the high molecular weight fraction of cellular DNA. Our results indicate that provirus integration, occurring during S-phase DNA synthesis, does not require cell division. Previous experiments have demonstrated that Rous sarcoma virus infection of chicken embryo fibroblasts requires cell division to initiate viral RNA synthesis and the production of progeny virus. The findings presented in this report support the hypothesis that division of the infected cells is required for an event that controls viral expression at the level of the integrated provirus.
Full text
PDF

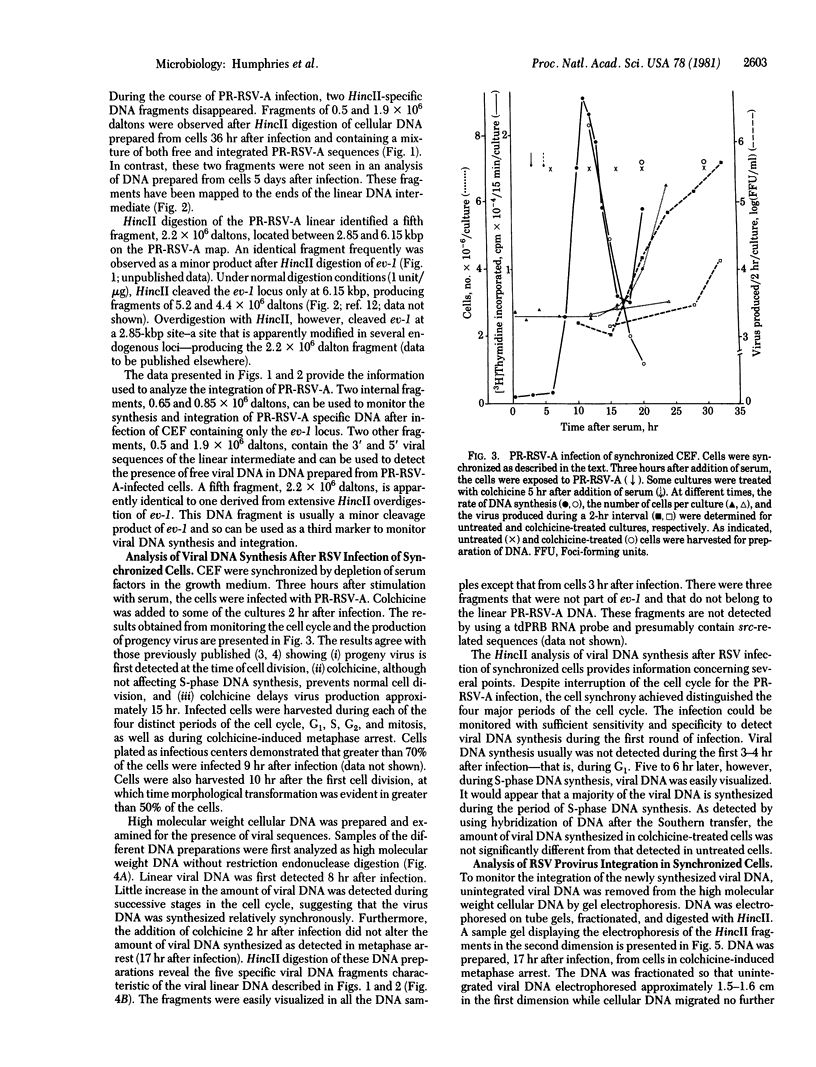

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrin S. M. Endogenous viral genes of the White Leghorn chicken: common site of residence and sites associated with specific phenotypes of viral gene expression. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5941–5945. doi: 10.1073/pnas.75.12.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battula N., Temin H. M. Sites of integration of infectious DNA of avian reticuloendotheliosis viruses in different avian cellular DNAs. Cell. 1978 Feb;13(2):387–398. doi: 10.1016/0092-8674(78)90207-6. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Temin H. M. Light inactivation of focus formation by chicken embryo fibroblasts infected with avian sarcoma virus in the presence of 5-bromodeoxyuridine. Nature. 1970 Nov 14;228(5272):622–624. doi: 10.1038/228622a0. [DOI] [PubMed] [Google Scholar]
- Ege T., Ringertz N. R. Preparation of microcells by enucleation of micronucleate cells. Exp Cell Res. 1974 Aug;87(2):378–382. doi: 10.1016/0014-4827(74)90494-7. [DOI] [PubMed] [Google Scholar]
- Fritsch E. F., Temin H. M. Inhibition of viral DNA synthesis in stationary chicken embryo fibroblasts infected with avian retroviruses. J Virol. 1977 Nov;24(2):461–469. doi: 10.1128/jvi.24.2.461-469.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Humphries E. H., Coffin J. M. Rate of virus-specific RNA synthesis in synchronized chicken embryo fibroblasts infected with avian leukosis virus. J Virol. 1976 Feb;17(2):393–401. doi: 10.1128/jvi.17.2.393-401.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries E. H., Glover C. Independent expression of avian sarcoma virus in doubly infected chicken embryo fibroblasts. J Virol. 1981 Feb;37(2):721–729. doi: 10.1128/jvi.37.2.721-729.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries E. H., Glover C., Weiss R. A., Arrand J. R. Differences between the endogenous and exogenous DNA sequences of Rous-associated virus-O. Cell. 1979 Nov;18(3):803–815. doi: 10.1016/0092-8674(79)90133-8. [DOI] [PubMed] [Google Scholar]
- Humphries E. H., Temin H. M. Cell cycle-dependent activation of rous sarcoma virus-infected stationary chicken cells: avian leukosis virus group-specific antigens and ribonucleic acid. J Virol. 1972 Jul;10(1):82–87. doi: 10.1128/jvi.10.1.82-87.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries E. H., Temin H. M. Requirement for cell division for initiation of transcription of Rous sarcoma virus RNA. J Virol. 1974 Sep;14(3):531–546. doi: 10.1128/jvi.14.3.531-546.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., O'Rear J. J., Temin H. M. DNA of noninfectious and infectious integrated spleen necrosis virus (SNV) is colinear with unintegrated SNV DNA and not grossly abnormal. Cell. 1979 Jan;16(1):51–61. doi: 10.1016/0092-8674(79)90187-9. [DOI] [PubMed] [Google Scholar]
- Murphy H. M. A new replication-defective variant of the Bryan high-titer strain Rous sarcoma virus. Virology. 1977 Apr;77(2):705–721. doi: 10.1016/0042-6822(77)90493-7. [DOI] [PubMed] [Google Scholar]
- Sabran J. L., Hsu T. W., Yeater C., Kaji A., Mason W. S., Taylor J. M. Analysis of integrated avian RNA tumor virus DNA in transformed chicken, duck and quail fibroblasts. J Virol. 1979 Jan;29(1):170–178. doi: 10.1128/jvi.29.1.170-178.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Padgett T., Heasley S., Simon G., Bishop J. M. Cellular functions are required for the synthesis and integration of avian sarcoma virus-specific DNA. Cell. 1977 Jun;11(2):307–319. doi: 10.1016/0092-8674(77)90047-2. [DOI] [PubMed] [Google Scholar]