Abstract
Axonal injury is the major correlate of permanent disability in neurodegenerative diseases such as multiple sclerosis (MS), especially in secondary-progressive MS following relapsing-remitting disease course. Proteolytic enzyme, calpain, is a potential candidate for causing axonal injury. Most current treatment options only target the inflammatory component of MS. Previous work using calpain inhibitor CYLA in our laboratory showed significant reduction in clinical sign, demyelination and tissue calpain content in acute experimental autoimmune encephalomyelitis (EAE). Here we evaluated markers of axonal injury (amyloid precursor protein, Nav1.6 channels), neuronal calpain content and the effect of CYLA on axonal protection using histological methods in chronic EAE [myelin oligodendrocyte glycoprotein (MOG) – induced disease model of MS].
Intraperitoneal application of CYLA (2mg/mouse/day) significantly reduced the clinical signs, tissue calpain content, demyelination and inflammatory infiltration of EAE. Similarly, markers for axonal injury were barely detectable in the treated mice. Thus, this novel drug, which markedly suppresses the disease course, axonal injury and its progression, is a candidate for the treatment of a neurodegenerative disease such as multiple sclerosis.
Introduction
Multiple sclerosis, a chronic disabling disease of young adults consists of an inflammatory and neurodegenerative phase (Steinman, 2001). Demyelination associated with inflammation with relative sparing of axons is considered the pathologic hallmark of MS (Martin and McFarland, 1995). Multiple studies using MRI (Barnes et al., 1991; Bruck et al., 1997) and histopathology (Ferguson et al., 1997; Trapp et al., 1998), have emphasized the role of axonal injury in addition to the well-known demyelination and inflammation (Trapp et al., 1999a; Wujek et al., 2002; Petzold et al., 2005).
Experimental autoimmune encephalomyelitis, an animal model of MS, has been extensively used to study the pathogenesis of MS (Mokhtarian et al., 1984; Mokhtarian and Swoveland, 1987; Ofosu-Appiah et al., 1994; Mokhtarian et al., 1996; Mokhtarian et al., 1999) as well as treatment options (Mokhtarian et al., 1996; Gilgun-Sherki et al., 2003a; Gilgun-Sherki et al., 2003b; Bechtold et al., 2004; Hassen et al., 2006). Inflammation that leads to the production of nitric oxide (NO), tumor necrosis factor (TNF)-alpha and proteases, including calpain, contributes to the destruction of myelin and eventually causes injury to the axons (Banati et al., 1993; Lannes-Vieira et al., 1994; Gehrmann et al., 1995; Benveniste, 1997).
Demyelination leads to impairment or loss of axonal conduction (Craner et al., 2004a; Waxman et al., 2004). Improvements of MS symptoms are, in part, the result of increased expression of Na+ channels. Increased expression and redistribution of these Na+ channels leads to temporary restoration of axonal conduction. This process causes increased influx of noxious Ca2+ that ultimately activates multiple enzyme cascades including axonal calpain (Craner et al., 2004a; Waxman et al., 2004; Stys et al., 1992; Craner et al., 2004b). The abnormal and prolonged activation of axonal calpain has been proposed as a major component in the pathophysiology of axonal injury in MS and EAE (Stys, 2005; Hendriks et al., 2005) that ultimately leads to neurodegenaration and subsequently to permanent disability. In fact, in chronic forms of MS (Barnes et al., 1991; Bruck et al., 1997; Wujek et al., 2002; Bjartmar et al., 2002; De Stefano et al., 1998) and EAE (Wujek et al., 2002) the severity of the disease and degree of permanent disability corresponds more to the extent of axonal damage than myelin damage (Petzold et al., 2005; Bjartmar et al., 2003; Trapp et al., 1999b).
Amyloid precursor protein (APP) is an early and sensitive marker of axonal injury. It is a membrane–spanning glycoprotein that is produced in the neurons and axonally transported via fast anterograd axonal transport (Hendriks et al., 2005; Koo et al., 1990). This transport is mediated by the direct binding of APP to the kinesin light chain, a microtubule motor protein (Koo et al., 1990; Sisodia et al., 1993) (Ferguson et al., 1997; Craner et al., 2004a; Waxman et al., 2004). In MS tissue APP accumulates in the axon to a degree that can be detected using histological methods (Ferguson et al., 1997; Trapp et al., 1998).
Current treatments options target the inflammatory component of the MS and little attention has been given to the treatment options of the neurodegenerative component of the disease (Steinman, 2001; Rizvi and Agius, 2004). A drug capable of crossing the BBB and inhibiting calpain has a potential as a therapeutic agent in the chronic forms of MS, especially secondary-progressive MS following relapsing-remitting MS (RR-MS) that results from repeated exacerbations leading to the accumulation of axonal injury (Stys, 2005; Bjartmar et al., 2003; Stys, 2004).
Taurine (2-aminoethanesulfonic acid) is a β-amino acid that is transported through cell membranes via a Na+-dependent transport system to pass through cell membranes (Huxtable, 1996). To date, two distinct Na+-dependent high-affinity taurine transporters have been cloned. The brain synthesizes only limited amounts of taurine and thus significant amounts must be transported into regions of the brain that require it (Kishi et al., 1988). Cysteic acid (α-amino-β-sulfo-propionic acid) shares structural similarities with taurine. It is a competitive inhibitor of taurine transport and utilizes the same transport mechanisms (Koyama et al., 1990; Lahdesmaki and Oja, 1973). Cysteic acid contains a carboxyl group in addition to the sulfonic acid and amino groups. This provides another functional group, not required for transport, to which other compounds such as the calpain inhibitor leucyl-argininal can be attached. CYLA is synthesized by attaching cysteic acid to the leucyl-argininal of leupeptin and enables CYLA to use taurine transporters to cross the blood-brain barrier (BBB). The striking characteristics of CYLA to cross the BBB, permeate cell membranes, making it available to the central nervous system (CNS) are essential for enhancing the uptake of the drug in the CNS and for reducing calpain activity. The effect of CYLA in suppressing inflammation and demyelination in acute EAE was reported in our previous work (Hassen et al., 2006).
Given the role of calpain in axonal injury and neurodegeneration, we hypothesize that CYLA with its calpain inhibition property and ability to cross the BBB can be used as a therapeutic agent in suppressing or reducing axonal damage in EAE, an animal model of MS and other neurodegenerative diseases.
2. Materials and methods
2.1. Animals, preparation of the peptide antigen and induction of EAE
The experimental methods used were similar to those described in our earlier publication (Hassen et al., 2006). EAE was induced by injecting each mouse subcutaneously (s.c.) in each flank with a 100μl of an emulsion containing incomplete Freund’s adjuvant (IFA) supplemented with 500μg heat inactivated mycobacterium tuberculosis (Sigma St. Louis, MO, ) and 300μg MOG 35–55. Pertussis toxin (100ng) (Sigma, St. Louis, MO) was administered intraperitoneally (i.p) at the time of immunization on day 0 and 48h later (Hassen et al., 2006).
C57BL/6J mice were separated into two large groups (control and experimental). Each large group had three subgroups. The control subgroups consisted of naïve mice (naïve control: n=4), mice immunized with only complete Freund’s adjuvant (CFA control; n=6) and naïve mice treated with only CYLA (CYLA control; n=4). The experimental subgroups consisted of MOG/CFA immunized groups (EAE; n=6), EAE treated with CYLA (n=6) starting from the onset of the clinical signs [day 12 post immunization (pi)] and EAE treated with CYLA (n=6) starting before the onset of the clinical signs (day 7pi). EAE mice were assigned randomly to the different subgroups after induction of EAE.
2.2. Clinical evaluation
All mice were evaluated on a daily basis by two independent observers for the extent of neurological deficit up to the termination of the experiment on day 31pi. Clinical severity was assessed as previously described (Hassen et al., 2006). Histological evaluation was completed following the termination of the experiment on day 31pi.
2.3. Preparation and application of CYLA
CYLA was prepared for the experiment as described previously (Hassen et al., 2006). A total of 2mg (1mg injected twice a day in 200μl Dulbecco’s phosphate-buffered saline (D-PBS) was administered i.p. for mouse in one group starting from day 7pi, prior to the onset of the clinical signs and in another group starting from day 12pi, after the onset of clinical signs.
2.4. Tissue collection
The experiment was terminated on day 31pi and mice were anesthetized with Metofane (Schering-Plough Animal Health, Union, NJ). They were then perfused transcardially with paraformaldehyde. Finally, spinal cord tissue was fixed in 4% Paraformaldehyde for 4–6h, transferred to sucrose gradients and subsequently embedded in Shandon M-1 embedding matrix for frozen sectioning (Thermo Electron Corporation, Pittsburgh, PA), and mounted on glass slides.
2.5. Bielschowsky’s silver impregnation method
Qualitative assessment of axonal integrity was made using a silver impregnation method on 6–10μm lumbar spinal cord sections. Frozen spinal cord slides were placed the slides in pre-warmed (40 °C) 10% silver nitrate solution and stained for 15 minutes, turning the sections light brown. Slides were then immersed the in distilled water and washed 3 times for one minute. Concentrated ammonium hydroxide was added drop by drop to the silver nitrate solution, until the precipitate was just clear. The slides incubated in the ammonium silver solution in 40 °C oven for 30 min until sections became dark brown. Next, the sections were developed for 1 min in a working solution (8 drops of developer stock solution, 8 drops of concentrated ammonium hydroxide and 50 ml of distilled water). The slides were then dipped for 1 min in 1% ammonium hydroxide solution to stop the silver reaction. After washing the slides with distilled water 3 times the slides were placed in 5% sodium thiosulfate solution for 5 min, followed by rinsing three times in distilled water, dehydrated and cleared through 95% ethyl alcohol, 100% alcohol and xylene. Finally, slides were mounted, coverslipped and images acquired using Olympus BX50 microscope with Olympus DP11 digital camera.
Nine consecutive slides from each group were assessed and every third slide from each group was randomly chosen for analysis. The mean of those selected slides from the individual groups were compared with the means of the other groups. The final results were represented in a bar graph. Semi-quantitative analysis of axonal damage was performed as described with minor modification (Einstein et al., 2006).
2.6. Immunofluorescent (IF) labeling and confocal microscopy
Frozen sections (50μm) were prepared from paraformaldehyde-fixed spinal cord tissues for immunofluorescent labeling as previously described (Hassen et al., 2006). The sections were blocked for one hour with 10% goat serum and incubated for 1h with the following primary antibodies (1:100): anti-calpain (kindly provided by Dr. Banik, Medical University of South Carolina, Charleston, SC), anti-NeuN (Abcam Inc, Cambridge, MA), anti-APP (Chemicon, Temecula, CA) and anti-Nav1.6 (Alomone, Jerusalem, Israel). The following secondary antibodies (1:200) were applied for 30min: Alexa586-conjugated goat anti-rat secondary antibodies for Nav1.6 (Caltag, Burlingame, CA), Alexa647-conjugated donkey anti-rabbit antibody for calpain (Molecular probes, Eugene, Oregon), Alexa488-conjugated goat anti-mouse for APP and NeuN (BD PharMingen, San Diego, CA). Finally, sections were washed, mounted with Vectashield media (Vector, Burlingame, CA) and visualized with a BioRad MRC-1042 laser scanning confocal system with Olympus IX-70 microscope.
Nine consecutive slides from each group were assessed and every third slide from each group was used for pixel analysis. The mean of the selected slides from the individual groups were compared with the means of the other groups. The final result was represented as a bar graph.
Pixels were quantified as total number of pixels above background using ImageJ image analysis software from NIH and the data is graphically represented as mean ± S.E.M.
2.7. Statistical Analysis
The following statistical tests were used to compare differences among the groups. One-way ANOVA followed by Tukey post hoc-test was used for the IF studies and silver staining analysis.
The Kruskal-Wallis test followed by Wilcoxon two-sample test was used to compare the clinical scores in control groups, EAE and CYLA-treated groups.
3. Results
3.1. Neurological deficit
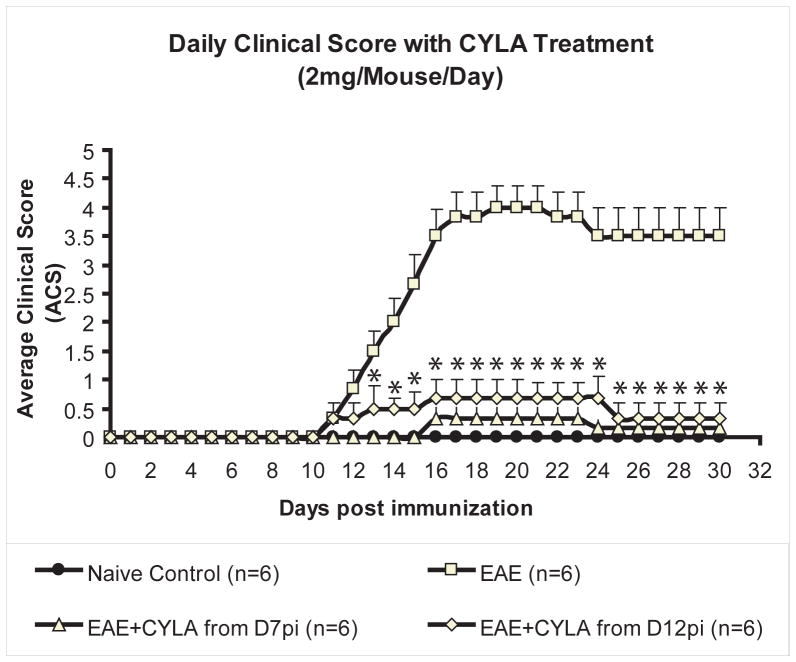
All mice were observed for 30 days post immunization. In general, early neurological signs of EAE such as floppy tail and hind limb weakness appeared on day 12pi. Over time the mice exhibited hind limb paralysis and some weakness in the fore limbs between days 14 and 16pi. Neurological deficits increased progressively in all EAE mice by days 18 and 19pi and peaked by day 20pi. After day 20pi, all EAE mice more or less maintained their clinical scores for the remaining observation time. Treatment with CYLA starting from day 7pi and day 12pi arrested the progression of the disease. Analysis of the average clinical score (ACS) of the groups, using Wilcoxon two-sample test, showed a statistically significant difference with treatment of 2mg/mouse/day (p<0.001; Fig.1).
Figure 1.
Changes in daily ACS of all groups over time during the course of treatment with 2mg CYLA following induction of EAE. EAE was induced in C57/Bl6 mice with MOG/CFA emulsion. CYLA, a total of 2mg/mouse/day (1mg per injection was applied i.p. twice daily). Early neurological signs such as floppy tail and hind limb weakness appeared in general about day 12pi. Over the course of time mice exhibited hind limb paralysis and some weakness in the fore limbs between days 14 and16pi. Their neurological deficit increased progressively in all EAE mice and reached its peak by day 20pi. After day 20pi until day 30pi EAE mice more or less maintained their ACS. Treatment with a total of 2mg CYLA per mouse per day starting from day 12pi led to suppression of the clinical signs to a significant level. Treatment with a total of 2mg CYLA per mouse per day starting from day 7pi caused a delayed onset and significant improvement of the clinical signs, slowing disease progression. No control groups (naïve, CFA and CYLA) exhibited neurological deficits. The experiment was terminated on day 31pi. Each graph presents daily ACS ± S.E.M. The difference between treatments and naïve control was considered significant at *P<0.05.
3.2. Axonal integrity
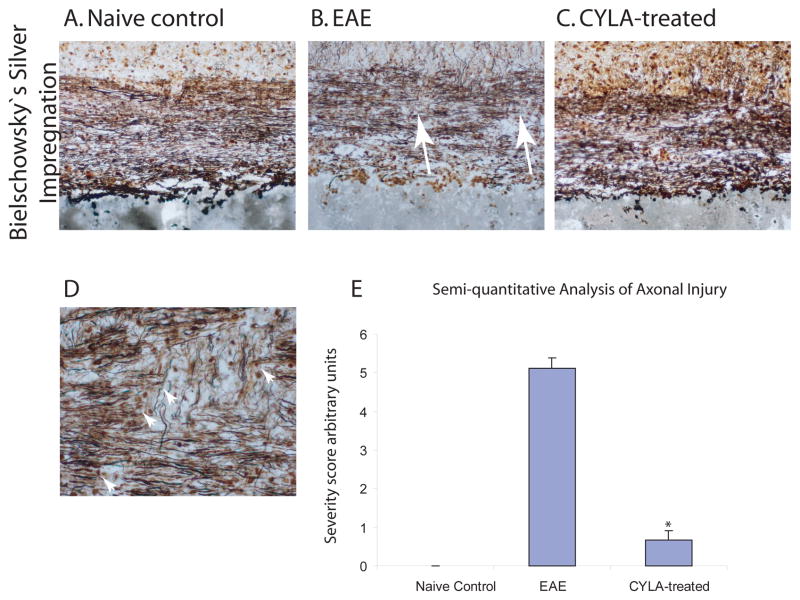
Qualitative analysis for axonal injury was done using Bielshowesky’s silver staining method. The naïve control groups showed a linear staining pattern of continuous axonal fibers without interruptions. In the spinal cord tissue of EAE mice the silver staining demonstrated extensive axonal injury with interruptions of the axons at areas corresponding to the lesions as well as extensive inflammation and demyelination (Fig. 2B). The staining pattern for the CYLA-treated mice exhibited preserved axonal morphology (Fig. 2C), similar to that observed in the control group (Fig. 2A). The staining patter among all control groups was similar. No difference was observed between the treatment groups (day 7pi and day 12pi).
Figure 2.
Qualitative assessment of axonal integrity of the lumbar spinal cord using silver impregnation method for all groups. EAE was induced in C57Bl/6J mice with MOG/CFA emulsion. CYLA, a total of 2mg/mouse/day (1mg per injection was applied i.p. twice daily). Silver staining was performed on 6–10μm cryosections. The spinal cord sections of all control groups [naïve (n=4), CFA (n=6) and CYLA (n=4)] showed normal axonal morphology (A). Extensive axonal damage (arrows) was highlighted in the spinal cords of chronic EAE mice (B). Preserved axonal morphology was observed after CYLA treatment (C). 20X magnification.
Representative section from EAE mice with signs of axonal injury such as axonal bulbs, spheroids and ovoids (arrow heads, D, 40X magnification). The staining pattern among all control groups was similar. No difference was observed between the treatment groups. Nine consecutive slides from each group were assessed and every third slide from each group was chosen randomly for analysis. The mean of the selected slides from the individual groups were compared with the means of the other groups. The final result was represented as a bar graph (E). The difference between treatments and control was considered significant at *P<0.05.
3.3. Distribution of APP
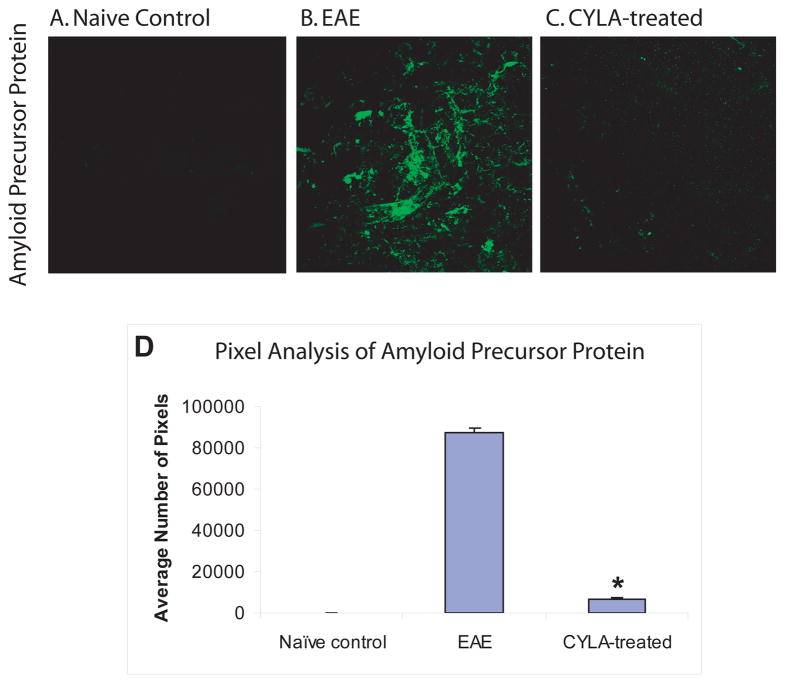
Additional qualitative assessment of axonal injury was measured using IF labeling for APP. No APP immunopositivity was visible in any of the control groups (Fig. 3A). Accumulation of extensive APP was apparent in spinal cord sections from untreated mice (Fig. 3B), corresponding to the extensive axonal injury within the spinal cords of this group. APP was barely detectable in the CYLA-treated group (Fig. 3C). Treatment with CYLA significantly reduced the accumulation of APP (Fig. 3D). The labeling pattern in all control groups was similar. In addition, no difference was observed between the treatment groups (day 7pi and day 12pi).
Figure 3.
Evaluation of axonal injury using APP in control, EAE and CYLA-treated groups. EAE was induced in C57Bl/6J mice with MOG/CFA emulsion. CYLA, a total of 2mg/mouse/day (1mg per injection was applied i.p. twice daily). IF labeling was performed on 50μm cryosections. Disruption of axonal transport leads to accumulation of APP within the injured axons. The spinal cord sections of all control groups [naïve (n=4), CFA (n=6) and CYLA (n=4)] showed no APP labeling (A). Extensive accumulation of APP was visible in spinal cord of chronic EAE (B). APP immunoreactivity in sections of CYLA-treated mice (day 7pi group) was similar to the control group (C). Similar result as in day 7pi groups was observed in day 12pi groups.
The quantitative data is graphically represented through pixel analysis using image analyzing software ImageJ from NIH. Pixels were quantified as total number of pixels above background. Nine consecutive slides from each group were assessed and every third slide from each group was chosen randomly for pixel analysis. The mean of the selected slides from the individual groups were compared with the means of the other groups. The final result was represented as a bar graph. The difference between treatments and control was considered significant at *P<0.05 (D). 120X magnification with oil.
3.4. Distribution of Nav 1.6 channels
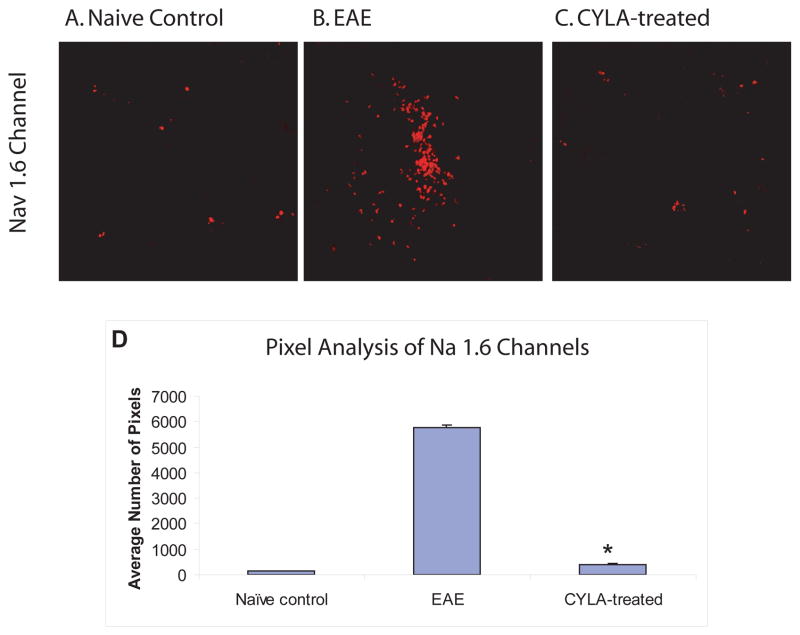
Labeling for Nav 1.6 channels demonstrated increased expression and abnormal distribution of Nav1.6 channels (clustered at areas of axonal injury) in untreated EAE mice (Fig. 4B) compared to the normal scattered distribution of these subtypes of Nav 1.6 channels in the CYLA-treated groups (Fig. 4C) and control groups (Fig. 4A). Treatment with CYLA significantly reduced the expression of Nav1.6 channels (Fig. 4D). The labeling pattern observed in all control groups was similar. No difference was observed between the treatment groups (day 7pi and day 12pi).
Figure 4.
Differences in Nav1.6 expression in the axons of lumbar spinal cord from control, EAE and CYLA-treated groups. EAE was induced in C57Bl/6J mice with MOG/CFA emulsion. CYLA, a total of 2mg/mouse/day (1mg per injection was applied i.p. twice daily). IF labeling was performed on 50μm cryosections. The spinal cord sections of all control groups [naïve (n=4), CFA (n=6) and CYLA (n=4)] showed normal scattered distribution of Nav1.6 channels corresponding to the areas of the node of Ranvier (A). Increased and clustered Nav1.6 channels in areas of demyelination in EAE mice spinal cord were observed. Similar expression of Nav1.6 channels in spinal cords of treated mice as in control group (D). The results from day 7pi and day 12pi groups were similar.
The quantitative data is graphically represented through pixel analysis using image analyzing software ImageJ from NIH. Pixels were quantified as total number of pixels above background. Nine consecutive slides from each group were assessed and every third slide from each group was chosen randomly for pixel analysis. The mean of the selected slides from the individual groups were compared with the means of the other groups. The difference between treatments and control was considered significant at *P<0.05 (D). 120X magnification with oil.
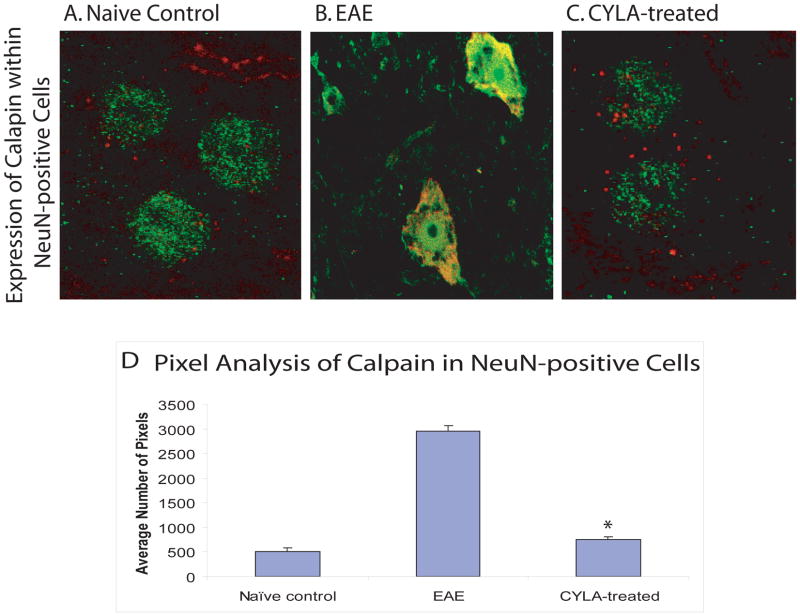
3.5. Distribution of calpain with NeuN-positive cells
Changes in calpain content within NeuN-positive cells (neurons) of the spinal cord sections were evaluated using IF labeling with anti-calpain antibody and NeuN (neuronal marker). Normal labeling for calpain was observed within NeuN-positive cells in the naive groups (Fig. 5A). By contrast, increased calpain staining within NeuN positive cells was observed in the untreated groups (Fig. 5B). Treatment with CYLA significantly decreased the calpain content within NeuN positive cells (Fig. 5C–D), corresponding to the lower ACS. Labeling among all control groups was similar. No difference was observed between the treatment groups (day 7pi and day 12pi).
Figure 5.
Fluorescent double-labeling using anti-calpain and anti-NeuN antibodies to identify the distribution of calpain within the neurons in chronic EAE. EAE was induced in C57Bl/6J mice with MOG/CFA emulsion. CYLA, a total of 2mg/mouse/day (1mg per injection was applied i.p. twice daily). IF with NeuN (neuronal marker) and calpain was performed on 50μm cryosections. Normal calpain distribution was visible as red dots within NeuN-positive neuron of all control groups [naïve (n=4), CFA (n=6) and CYLA (n=4)] (A). Increased calpain expression was observed within NeuN-positive neurons of EAE mice (B). The calpain distribution within NeuN-positive spinal cord neurons of CYLA-treated mice (day 7pi) was similar to control groups (C). The results of day 7pi and day 12pi groups were similar.
The quantitative data is graphically represented through pixel analysis using image analyzing software ImageJ from NIH. Pixels were quantified as total number of pixels above background. Nine consecutive slides from each group were assessed and every third slide from each group was chosen randomly for pixel analysis. The mean of the selected slides from the individual groups were compared with the means of the other groups. The difference between treatments and control was considered significant at *P<0.05 (D). 120X magnification with oil.
Discussion
Mice injected with MOG/CFA exhibit a complex immunopathological cascade that results in activation of macrophages and microglia with production and activation of calpain, an important factor in the pathogenesis of EAE (Hassen et al., 2006; Mokhtarian, 1988). In our previous publication (Hassen et al., 2006), we showed that CYLA reduced the inflammation, demyelination and clinical signs of acute EAE in a dose-and time-dependent manner. In this report, we evaluated the role of CYLA in preventing or ameliorating axonal injury in chronic EAE.
Treatment groups received 2mg CYLA per day divided into two doses of 1mg twice a day beginning on day 7pi in one group and day 12pi in another group and the experiment was terminated on day 31pi. CYLA treatment caused a significant decrease in neurological deficits as evidenced by the lower ACS as well as suppression of the progression of the disease, regardless of the time point CYLA treatment was started. The neurological deficits were very minimal and the disease progression was also suppressed in both therapy regimens (whether begun on day 7pi or on day 12pi).
Histological evaluation of the spinal cord tissues from CYLA-treated animals revealed reduced demyelination and inflammatory reaction when compared with the untreated group similar to that reported in our previous publication (Hassen et al., 2006).
Treatment with CYLA prevented axonal injury as evidenced by the preserved axonal morphology seen with the silver impregnation staining and barely-detectable immunopositivity for APP when compared to the untreated groups. The silver stain revealed extensive axonal injury in the non-treated group. Axonal morphology was preserved in the CYLA-treated. Anti-APP immunolabeling showed extensive accumulation of APP in the spinal cord sections of non-treated mice groups when compared with control and CYLA-treated mice.
The IF labeling with anti-calpain and anti-NeuN antibodies highlighted the increased content of calpain within NeuN-positive neurons. This may indicate that neuronal calpain, in addition to calpain from inflammatory cells, contributes to the axonal injury in the chronic form of EAE. Our results support the hypothesis that a calpain inhibitor capable of crossing the BBB and inhibiting calpain within the CNS, especially in the chronic stages of the disease, may be crucial in the therapeutic strategies of a neurodegenerative disease such as MS. It has been reported that during active myelin formation, some of the myelin components are synthesized in the cell body of the neurons and are transported to enter the myelin sheath (Haley and Ledeen, 1979). Our result also showed that minimal reactivity to APP was present in the acute phase of the disease (data not shown). This finding suggests that early axonal injury may contribute to the impaired remyelination in chronic EAE due to the lack of myelin components necessary for myelin formation. Thus, targeting mechanisms that lead to axonal injury, even in the acute phase of the disease, could prevent further deterioration and progression of the disease. In addition, calpain is involved in the late phase of the pathologic process allowing a window for therapeutic intervention that spans early to late time points in the disease process.
The exact mechanism of axonal injury is not fully understood. Calpain is only one factor believed to play a role in the pathophysiology of axonal injury, especially in the chronic phase of MS and EAE (Stys, 2005; Stys, 2004). Demyelination leads to the impairment or loss of conduction of electrical signal. Over time, the neurons attempt to compensate for the impaired conduction by expressing additional sodium channels (Nav1.6 channels) on the axons (Waxman et al., 2004; Stys, 2005; Kornek et al., 2001). The increased influx of sodium through these channels into the axons is the main drive for the Na+/Ca2+− exchanger (NCX), leading to the increased Ca2+-influx into the axons, which activates a number of enzyme cascades including calpain (Stys et al., 1992; Stys, 2005). It has been shown that inhibition of sodium channel using flecainide and phenytoin reduces axonal injury in EAE, further implicating sodium channels in axonal pathology (Bechtold et al., 2004; Lo et al., 2002).
Our IF labeling with anti-Nav1.6 antibody highlighted the accumulation of sodium channels clusters corresponding to areas of demyelination as compared to scattered immunostaining in control and CYLA-treated groups reflecting normal Nav1.6 distribution at the nodes of Ranvier (Craner et al., 2004a; Waxman et al., 2004; Craner et al., 2004b). Preliminary results from double immunolabeling showed co-localization of APP and Nav1.6 in tissues of untreated groups (data not shown). Similar results were observed in tissues of MS patients (Craner et al., 2004a).
Calpain degrades a variety of neuronal proteins such as spectrin, neurofilaments and myelin proteins (Deshpande et al., 1995; Banik et al., 1984; Siman et al., 1984; Saido et al., 1992). The western blot analysis from our previous paper showed increased degradation of α-fodrin in the chronic EAE compared to reduced degradation in the treated group (Hassen et al., 2006). The degree of injury to the neuronal scaffolding proteins correlated with the permanent disability in MS patients especially those who enter the secondary-progressive MS (SP-MS) course (Wujek et al., 2002; Lassmann, 2003; Diaz-Sanchez et al., 2006). It is generally believed that damage to the axons accumulates behind closed BBB from repeated relapses (Benveniste, 1997), leading to progressive damage to the axons with subsequent permanent disability over time.
In summary, treatment with CYLA is shown to be effective in reducing the severity of inflammation, demyelination and axonal injury as evidenced by the preserved axonal morphology in the silver staining and barely detectable APP accumulation and preserved myelin sheath and axons after treatment with CYLA in the chronic phase of EAE. CYLA has a potential for the treatment of MS especially RR-MS to prevent the worsening to SP-MS. It could be used as an agent for supplemental therapy during the remission period. Further trials with models of RR-EAE are necessary in order to study the effect of CYLA on relapses and prevention of secondary progression.
The advantage of calpain inhibitors when compared to the majority of current MS therapaeutic agents that predominantly target the inflammatory component of the disease is that calpain inhibitors target both inflammatory components as shown by our previous paper and the neurodegenerative component as reported here.
It has been reported that calpain is involved in the pathophysiology of a number of other human diseases (Ray and Banik, 2003; Hayes et al., 1998). An agent that can reach the CNS and inhibit calpain may be useful for the treatment of amyotrophic lateral sclerosis (ALS)(Ray and Banik, 2003), Alzheimer’s disease(Nixon, 1989; Nixon, 2000; Saito et al., 1993), Huntington’s disease (Gafni and Ellerby, 2002; Gafni et al., 2004), traumatic brain injury(TBI)(Ray et al., 2003) and cerebral stroke (Bartus et al., 1994; Kampfl et al., 1996).
Acknowledgments
Assistance from the following individuals was very much appreciated: Riccardo Bianchi, Barbara Concepcion, Hemavathy Kirugaval, Susanna Popp, William Oxberry, Lynette Sheffield, Mark Stewart and Jeremy Weedon. This work was performed in partial fulfillment of the requirements for a PhD degree of Getaw Worku Hassen, at SUNY Downstate at Brooklyn, School of Graduate Studies. This work was partially supported by Ceptor Corp., NY, New York.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993;7:111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- Banik NL, Hogan EL, Whetstine LJ, Balentine JD. Changes in myelin and axonal proteins in CaCl2-induced myelopathy in rat spinal cord. Cent Nerv Syst Trauma. 1984;1:131–137. doi: 10.1089/cns.1984.1.131. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Hayward NJ, Elliott PJ, Sawyer SD, Baker KL, Dean RL, Akiyama A, Straub JA, Harbeson SL, Li Z, et al. Calpain inhibitor AK295 protects neurons from focal brain ischemia. Effects of postocclusion intra-arterial administration. Stroke. 1994;25:2265–2270. doi: 10.1161/01.str.25.11.2265. [DOI] [PubMed] [Google Scholar]
- Benveniste EN. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med. 1997;75:165–173. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Battistuta J, Terada N, Dupree E, Trapp BD. N-acetylaspartate is an axon-specific marker of mature white matter in vivo: a biochemical and immunohistochemical study on the rat optic nerve. Ann Neurol. 2002;51:51–58. doi: 10.1002/ana.10052. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Wujek JR, Trapp BD. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci. 2003;206:165–171. doi: 10.1016/s0022-510x(02)00069-2. [DOI] [PubMed] [Google Scholar]
- Bruck W, Bitsch A, Kolenda H, Bruck Y, Stiefel M, Lassmann H. 1997 doi: 10.1002/ana.410420515. [DOI] [PubMed] [Google Scholar]
- Craner MJ, Newcombe J, Black JA, Hartle C, Cuzner ML, Waxman SG. Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger. Proc Natl Acad Sci U S A. 2004a;101:8168–8173. doi: 10.1073/pnas.0402765101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craner MJ, Hains BC, Lo AC, Black JA, Waxman SG. Co-localization of sodium channel Nav1.6 and the sodium-calcium exchanger at sites of axonal injury in the spinal cord in EAE. Brain. 2004b;127:294–303. doi: 10.1093/brain/awh032. [DOI] [PubMed] [Google Scholar]
- Deshpande RV, Goust JM, Hogan EL, Banik NL. Calpain secreted by activated human lymphoid cells degrades myelin. J Neurosci Res. 1995;42:259–265. doi: 10.1002/jnr.490420214. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Matthews PM, Fu L, Narayanan S, Stanley J, Francis GS, Antel JP, Arnold DL. Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain. 1998;121 ( Pt 8):1469–1477. doi: 10.1093/brain/121.8.1469. [DOI] [PubMed] [Google Scholar]
- Diaz-Sanchez M, Williams K, Deluca GC, Esiri MM. Protein co-expression with axonal injury in multiple sclerosis plaques. Acta Neuropathol (Berl) 2006;111:289–299. doi: 10.1007/s00401-006-0045-0. [DOI] [PubMed] [Google Scholar]
- Einstein O, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Polyzoidou E, Lavon I, Milonas I, Karussis D, Abramsky O, Ben-Hur T. Transplanted neural precursor cells reduce brain inflammation to attenuate chronic experimental autoimmune encephalomyelitis. Exp Neurol. 2006;198:275–284. doi: 10.1016/j.expneurol.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120 ( Pt 3):393–399. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- Gafni J, Ellerby LM. Calpain activation in Huntington’s disease. J Neurosci. 2002;22:4842–4849. doi: 10.1523/JNEUROSCI.22-12-04842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni J, Hermel E, Young JE, Wellington CL, Hayden MR, Ellerby LM. Inhibition of calpain cleavage of huntingtin reduces toxicity: accumulation of calpain/caspase fragments in the nucleus. J Biol Chem. 2004;279:20211–20220. doi: 10.1074/jbc.M401267200. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Panet H, Holdengreber V, Mosberg-Galili R, Offen D. Axonal damage is reduced following glatiramer acetate treatment in C57/bl mice with chronic-induced experimental autoimmune encephalomyelitis. Neurosci Res. 2003a;47:201–207. doi: 10.1016/s0168-0102(03)00217-7. [DOI] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Panet H, Melamed E, Offen D. Riluzole suppresses experimental autoimmune encephalomyelitis: implications for the treatment of multiple sclerosis. Brain Res. 2003b;989:196–204. doi: 10.1016/s0006-8993(03)03343-2. [DOI] [PubMed] [Google Scholar]
- Haley JE, Ledeen RW. Incorporation of axonally transported substances into myelin lipids. J Neurochem. 1979;32:735–742. doi: 10.1111/j.1471-4159.1979.tb04556.x. [DOI] [PubMed] [Google Scholar]
- Hayes RL, Wang KK, Kampfl A, Posmantur RM, Newcomb JK, Clifton GL. Potential contribution of proteases to neuronal damage. Drug News Perspect. 1998;11:215–222. [PubMed] [Google Scholar]
- Hassen GW, Feliberti J, Kesner L, Stracher A, Mokhtarian F. A novel calpain inhibitor for the treatment of acute experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;180:135–146. doi: 10.1016/j.jneuroim.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Hendriks JJ, Teunissen CE, de Vries HE, Dijkstra CD. Macrophages and neurodegeneration. Brain Res Brain Res Rev. 2005;48:185–195. doi: 10.1016/j.brainresrev.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Huxtable RJ. Taurine. Past, present, and future. Adv Exp Med Biol. 1996;403:641–650. [PubMed] [Google Scholar]
- Kampfl A, Posmantur R, Nixon R, Grynspan F, Zhao X, Liu SJ, Newcomb JK, Clifton GL, Hayes RL. mu-calpain activation and calpain-mediated cytoskeletal proteolysis following traumatic brain injury. J Neurochem. 1996;67:1575–1583. doi: 10.1046/j.1471-4159.1996.67041575.x. [DOI] [PubMed] [Google Scholar]
- Kishi M, Ohkuma S, Kimori M, Kuriyama K. Characteristics of taurine transport system and its developmental pattern in mouse cerebral cortical neurons in primary culture. Biochim Biophys Acta. 1988;939:615–623. doi: 10.1016/0005-2736(88)90109-5. [DOI] [PubMed] [Google Scholar]
- Koo EH, Sisodia SS, Archer DR, Martin LJ, Weidemann A, Beyreuther K, Fischer P, Masters CL, Price DL. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990;87:1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornek B, Storch MK, Bauer J, Djamshidian A, Weissert R, Wallstroem E, Stefferl A, Zimprich F, Olsson T, Linington C, Schmidbauer M, Lassmann H. Distribution of a calcium channel subunit in dystrophic axons in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain. 2001;124:1114–1124. doi: 10.1093/brain/124.6.1114. [DOI] [PubMed] [Google Scholar]
- Kornek B, Lassmann H. Neuropathology of multiple sclerosis-new concepts. Brain Res Bull. 2003;61:321–326. doi: 10.1016/s0361-9230(03)00095-9. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Baba A, Iwata H. Characteristics of Cl(−)-dependent L-[35S]cysteic acid transport into rat brain synaptic membrane vesicles. Neurochem Res. 1990;15:1153–1158. doi: 10.1007/BF01208574. [DOI] [PubMed] [Google Scholar]
- Lahdesmaki P, Oja SS. On the mechanism of taurine transport at brain cell membranes. J Neurochem. 1973;20:1411–1417. doi: 10.1111/j.1471-4159.1973.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Lannes-Vieira J, Gehrmann J, Kreutzberg GW, Wekerle H. The inflammatory lesion of T cell line transferred experimental autoimmune encephalomyelitis of the Lewis rat: distinct nature of parenchymal and perivascular infiltrates. Acta Neuropathol (Berl) 1994;87:435–442. doi: 10.1007/BF00294169. [DOI] [PubMed] [Google Scholar]
- Lo AC, Black JA, Waxman SG. Neuroprotection of axons with phenytoin in experimental allergic encephalomyelitis. Neuroreport. 2002;13:1909–1912. doi: 10.1097/00001756-200210280-00015. [DOI] [PubMed] [Google Scholar]
- Martin R, McFarland HF. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit Rev Clin Lab Sci. 1995;32:121–182. doi: 10.3109/10408369509084683. [DOI] [PubMed] [Google Scholar]
- Mokhtarian F, McFarlin DE, Raine CS. Adoptive transfer of myelin basic protein-sensitized T cells produces chronic relapsing demyelinating disease in mice. Nature. 1984;309:356–358. doi: 10.1038/309356a0. [DOI] [PubMed] [Google Scholar]
- Mokhtarian F. Role of Ia antigen in the induction of adoptively transferred acute and chronic relapsing demyelinating disease in mice. Clin Immunol Immunopathol. 1988;49:308–317. doi: 10.1016/0090-1229(88)90121-3. [DOI] [PubMed] [Google Scholar]
- Mokhtarian F, Shirazian T, Batuman O, Shi Y. The effects of oral myelin basic protein and dexamethasone treatment on experimental autoimmune encephalomyelitis. Ann N Y Acad Sci. 1996;778:414–417. doi: 10.1111/j.1749-6632.1996.tb21160.x. [DOI] [PubMed] [Google Scholar]
- Mokhtarian F, Zhang Z, Shi Y, Gonzales E, Sobel RA. Molecular mimicry between a viral peptide and a myelin oligodendrocyte glycoprotein peptide induces autoimmune demyelinating disease in mice. J Neuroimmunol. 1999;95:43–54. doi: 10.1016/s0165-5728(98)00254-9. [DOI] [PubMed] [Google Scholar]
- Nixon RA. Calcium-activated neutral proteinases as regulators of cellular function. Implications for Alzheimer’s disease pathogenesis. Ann N Y Acad Sci. 1989;568:198–208. doi: 10.1111/j.1749-6632.1989.tb12509.x. [DOI] [PubMed] [Google Scholar]
- Nixon RA. A “protease activation cascade” in the pathogenesis of Alzheimer’s disease. Ann N Y Acad Sci. 2000;924:117–131. [PubMed] [Google Scholar]
- Ofosu-Appiah W, Mokhtarian F, Shirazian D, Grob D. Production of anti-acetylcholine receptor-alpha antibody in vitro by peripheral blood lymphocytes of patients with myasthenia gravis: role of immunoregulatory T cells and monocytes. J Lab Clin Med. 1994;124:231–241. [PubMed] [Google Scholar]
- Petzold A, Eikelenboom MJ, Keir G, Grant D, Lazeron RH, Polman CH, Uitdehaag BM, Thompson EJ, Giovannoni G. Axonal damage accumulates in the progressive phase of multiple sclerosis: three year follow up study. J Neurol Neurosurg Psychiatry. 2005;76:206–211. doi: 10.1136/jnnp.2004.043315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SK, Banik NL. Calpain and its involvement in the pathophysiology of CNS injuries and diseases: therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2003;2:173–189. doi: 10.2174/1568007033482887. [DOI] [PubMed] [Google Scholar]
- Rizvi SA, Agius MA. Current approved options for treating patients with multiple sclerosis. Neurology. 2004;63:S8–14. doi: 10.1212/wnl.63.12_suppl_6.s8. [DOI] [PubMed] [Google Scholar]
- Saido TC, Nagao S, Shiramine M, Tsukaguchi M, Sorimachi H, Murofushi H, Tsuchiya T, Ito H, Suzuki K. Autolytic transition of mu-calpain upon activation as resolved by antibodies distinguishing between the pre- and post-autolysis forms. J Biochem (Tokyo) 1992;111:81–86. doi: 10.1093/oxfordjournals.jbchem.a123723. [DOI] [PubMed] [Google Scholar]
- Saito K, Elce JS, Hamos JE, Nixon RA. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci U S A. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaecher K, Rocchini A, Dinkins J, Matzelle DD, Banik NL. Calpain expression and infiltration of activated T cells in experimental allergic encephalomyelitis over time: increased calpain activity begins with onset of disease. J Neuroimmunol. 2002;129:1–9. doi: 10.1016/s0165-5728(02)00142-x. [DOI] [PubMed] [Google Scholar]
- Shields DC, Banik NL. Upregulation of calpain activity and expression in experimental allergic encephalomyelitis: a putative role for calpain in demyelination. Brain Res. 1998;794:68–74. doi: 10.1016/s0006-8993(98)00193-0. [DOI] [PubMed] [Google Scholar]
- Shields DC, Schaecher KE, Saido TC, Banik NL. A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc Natl Acad Sci U S A. 1999;96:11486–11491. doi: 10.1073/pnas.96.20.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R, Baudry M, Lynch G. Brain fodrin: substrate for calpain I, an endogenous calcium-activated protease. Proc Natl Acad Sci U S A. 1984;81:3572–3576. doi: 10.1073/pnas.81.11.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia SS, Koo EH, Hoffman PN, Perry G, Price DL. Identification and transport of full-length amyloid precursor proteins in rat peripheral nervous system. J Neurosci. 1993;13:3136–3142. doi: 10.1523/JNEUROSCI.13-07-03136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- Stys PK, Waxman SG, Ransom BR. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na(+)-Ca2+ exchanger. J Neurosci. 1992;12:430–439. doi: 10.1523/JNEUROSCI.12-02-00430.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys PK. General mechanisms of axonal damage and its prevention. J Neurol Sci. 2005;233:3–13. doi: 10.1016/j.jns.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Stys PK. White matter injury mechanisms. Curr Mol Med. 2004;4:113–130. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Ransohoff R, Rudick R. Axonal pathology in multiple sclerosis:relationship to neurologic disability. Curr Opin Neurol. 1999a;12:295–302. doi: 10.1097/00019052-199906000-00008. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Bo L, Mork S, Chang A. Pathogenesis of tissue injury in MS lesions. J Neuroimmunol. 1999b;98:49–56. doi: 10.1016/s0165-5728(99)00081-8. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Craner MJ, Black JA. Na+ channel expression along axons in multiple sclerosis and its models. Trends Pharmacol Sci. 2004;25:584–591. doi: 10.1016/j.tips.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Wujek JR, Bjartmar C, Richer E, Ransohoff RM, Yu M, Tuohy VK, Trapp BD. Axon loss in the spinal cord determines permanent neurological disability in an animal model of multiple sclerosis. J Neuropathol Exp Neurol. 2002;61:23–32. doi: 10.1093/jnen/61.1.23. [DOI] [PubMed] [Google Scholar]