Abstract
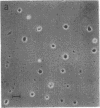
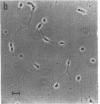
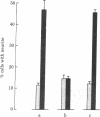
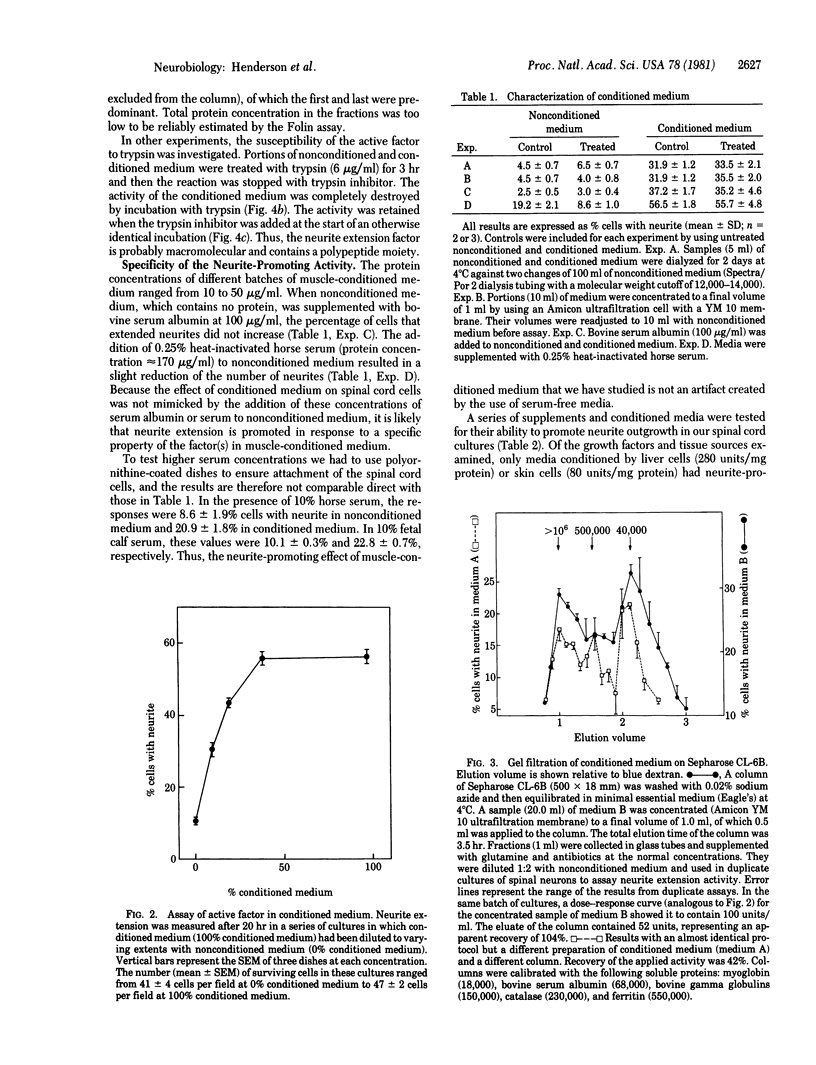
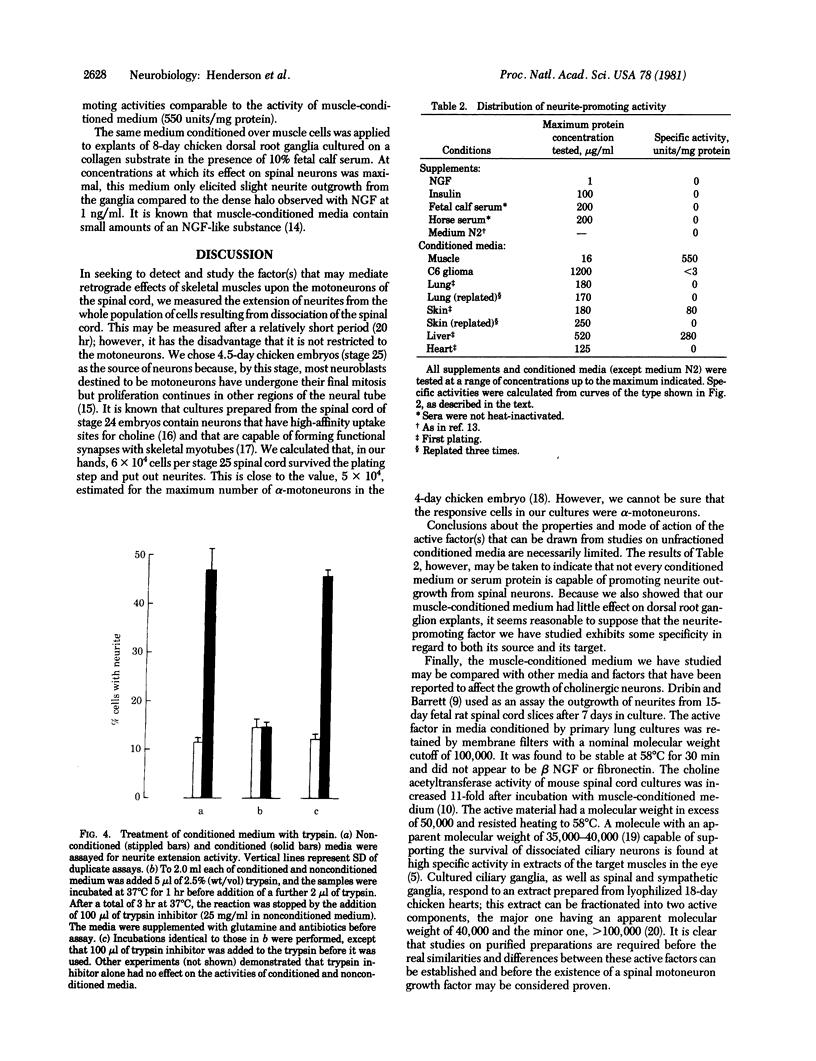
The effect of media conditioned by muscle cells on the development in vitro of chicken spinal neurons was studied. Neural tube cells of 4.5-day chicken embryos were dissociated after trypsinization and cultured in serum-free minimum essential medium conditioned for 4 days over cultures of fused chicken myotubes. After 20 hr in conditioned medium (protein concentration, 10--50 microgram/ml), about 50% of surviving cells had extended neurites, whereas in cultures in nonconditioned medium this value was about 10%. The active factor(s) in conditioned medium is macromolecular and its activity was completely destroyed by incubation with trypsin. Concentrated samples of conditioned medium were analyzed by gel filtration on columns of Sepharose CL-6B. The activity was recovered in peaks with apparent molecular weights of 40,000 and 500,000 and at the exclusion volume of the column. Media conditioned neurite-promoting activity but at lower levels. No activity was detected in Nerve Growth Factor, insulin, fetal calf serum, or horse serum or in media conditioned by chicken lung, chicken heart, or C6 glioma cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., Landa K. B., Manthorpe M., Varon S. Cholinergic neuronotrophic factors: intraocular distribution of trophic activity for ciliary neurons. Science. 1979 Jun 29;204(4400):1434–1436. doi: 10.1126/science.451576. [DOI] [PubMed] [Google Scholar]
- Barald K. F., Berg D. K. Autoradiographic labeling of spinal cord neurons with high affinity choline uptake in cell culture. Dev Biol. 1979 Sep;72(1):1–14. doi: 10.1016/0012-1606(79)90093-9. [DOI] [PubMed] [Google Scholar]
- Barald K. F., Berg D. K. High affinity choline uptake by spinal cord neurons in dissociated cell culture. Dev Biol. 1978 Jul;65(1):90–99. doi: 10.1016/0012-1606(78)90182-3. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Lai K., Nurcombe V. Identification of embryonic motoneurons in vitro: their survival is dependent on skeletal muscle. Brain Res. 1980 May 26;190(2):537–542. doi: 10.1016/0006-8993(80)90295-4. [DOI] [PubMed] [Google Scholar]
- Berg D. K., Fischbach G. D. Enrichment of spinal cord cell cultures with motoneurons. J Cell Biol. 1978 Apr;77(1):83–98. doi: 10.1083/jcb.77.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes N., Burt D. R., Goldberg A. M., Bierkamper G. G. The influence of muscle-conditioned medium on cholinergic maturation in spinal cord cell cultures. Brain Res. 1980 Mar 31;186(2):474–479. doi: 10.1016/0006-8993(80)90994-4. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976 Dec 23;264(5588):705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- Dribin L. B., Barrett J. N. Conditioned medium enhances neuritic outgrowth from rat spinal cord explants. Dev Biol. 1980 Jan;74(1):184–195. doi: 10.1016/0012-1606(80)90060-3. [DOI] [PubMed] [Google Scholar]
- Ebendal T., Belew M., Jacobson C. O., Porath J. Neurite outgrowth elicited by embryonic chick heart: partial purification of the active factor. Neurosci Lett. 1979 Sep;14(1):91–95. doi: 10.1016/0304-3940(79)95350-3. [DOI] [PubMed] [Google Scholar]
- Giller E. L., Jr, Neale J. H., Bullock P. N., Schrier B. K., Nelson P. G. Choline acetyltransferase activity of spinal cord cell cultures increased by co-culture with muscle and by muscle-conditioned medium. J Cell Biol. 1977 Jul;74(1):16–29. doi: 10.1083/jcb.74.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMBURGER V. Regression versus peripheral control of differentiation in motor hypoplasia. Am J Anat. 1958 May;102(3):365–409. doi: 10.1002/aja.1001020303. [DOI] [PubMed] [Google Scholar]
- Hollyday M., Hamburger V. Reduction of the naturally occurring motor neuron loss by enlargement of the periphery. J Comp Neurol. 1976 Dec 1;170(3):311–320. doi: 10.1002/cne.901700304. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Manthorpe M., Skaper S., Adler R., Landa K., Varon S. Cholinergic neuronotrophic factors: fractionation properties of an extract from selected chick embryonic eye tissues. J Neurochem. 1980 Jan;34(1):69–75. doi: 10.1111/j.1471-4159.1980.tb04622.x. [DOI] [PubMed] [Google Scholar]
- Murphy R. A., Singer R. H., Saide J. D., Pantazis N. J., Blanchard M. H., Byron K. S., Arnason B. G., Young M. Synthesis and secretion of a high molecular weight form of nerve growth factor by skeletal muscle cells in culture. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4496–4500. doi: 10.1073/pnas.74.10.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon S. S., Bunge R. P. Trophic mechanisms in the peripheral nervous system. Annu Rev Neurosci. 1978;1:327–361. doi: 10.1146/annurev.ne.01.030178.001551. [DOI] [PubMed] [Google Scholar]