Abstract
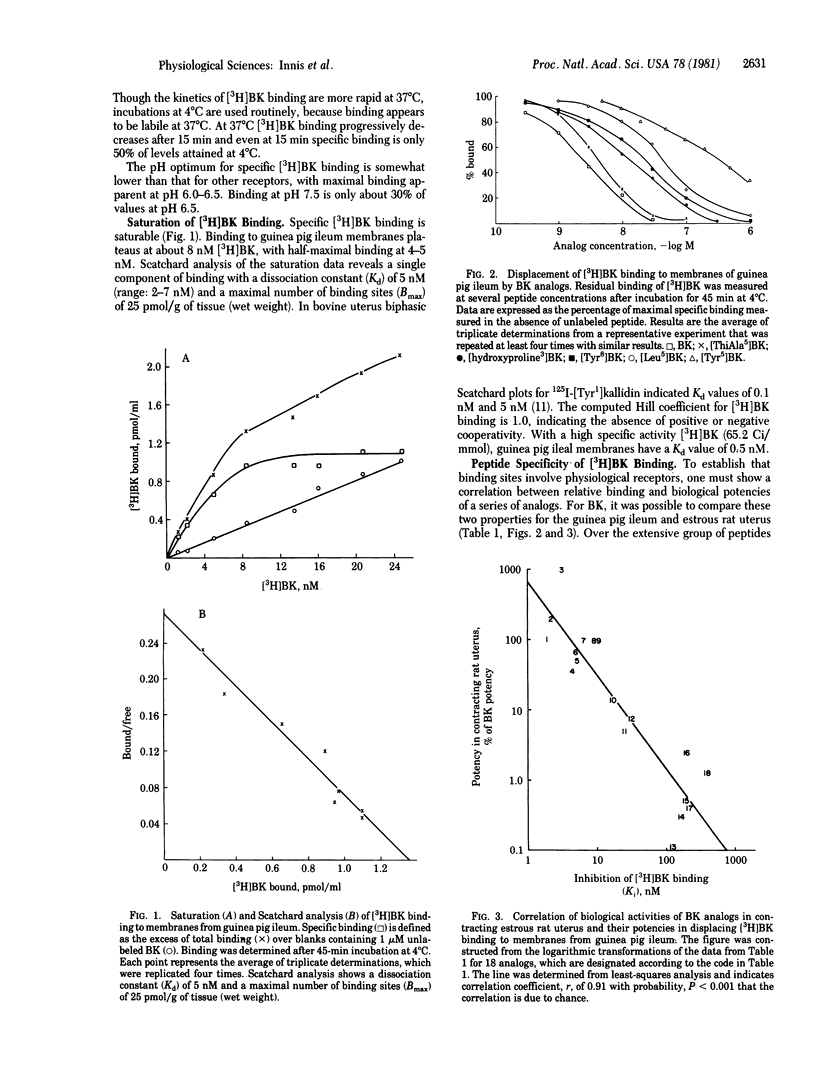
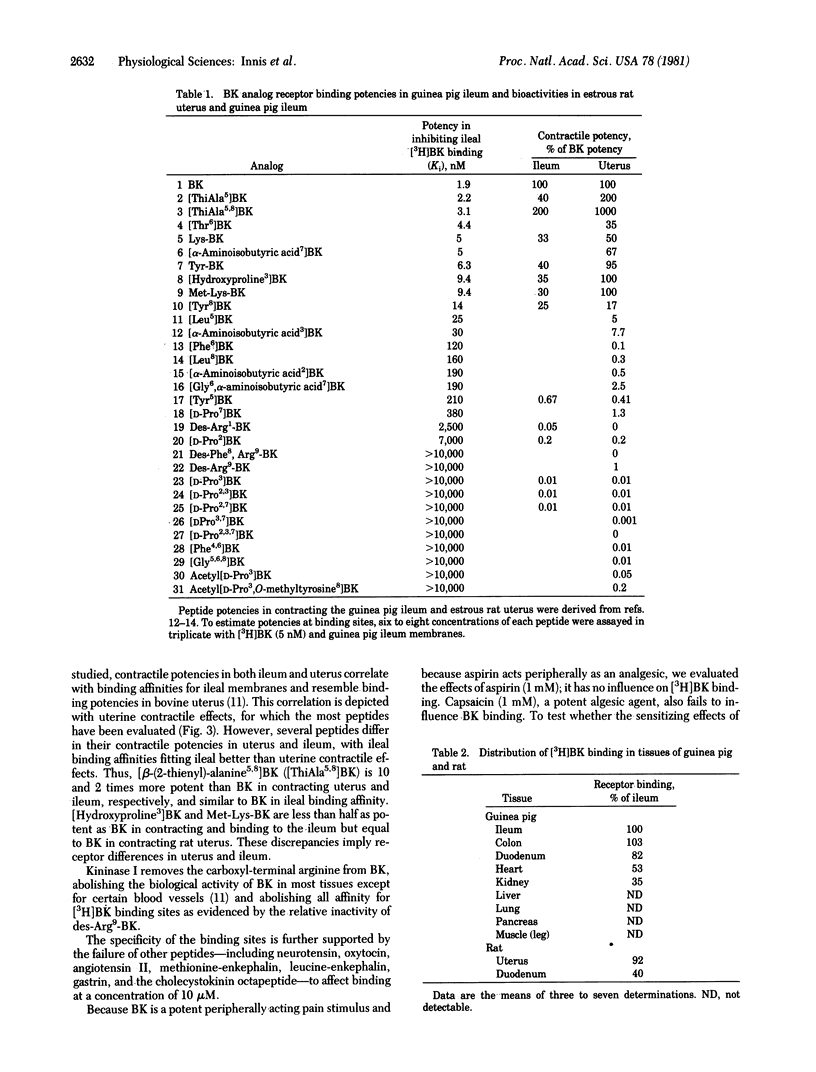
[3H]Bradykinin binds to membranes from a variety of mammalian tissues in a saturable fashion with a dissociation constant of about 5 nM. Highest levels of binding are detected in guinea pig ileum, colon, and duodenum and in the oestrus rat uterus. The relative potencies of bradykinin derivatives in competing for these binding sites in guinea pig ileum membranes correlate with their contractile potencies in the ileum better than with contractile effects in the uterus. Thus, receptor recognition sites may differ in ileum and uterus. Monovalent and divalent cations at physiological concentrations reduce [3H]bradykinin binding. Of the monovalent cations, medium lowers binding 50% at about 80 mM. Among the divalent cations, calcium lowers binding about 50% at 5 mM, suggesting a link to the calcium conductance channel.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belcher G. The effects of intra-arterial bradykinin, histamine, acetylcholine and prostaglandin E1 on nociceptive and non-nociceptive dorsal horn neurones of the cat. Eur J Pharmacol. 1979 Jul 1;56(4):385–395. doi: 10.1016/0014-2999(79)90270-x. [DOI] [PubMed] [Google Scholar]
- Chang R. S., Synder S. H. Histamine H1-receptor binding sites in guinea pig brain membranes: regulation of agonist interactions by guanine nucleotides and cations. J Neurochem. 1980 Apr;34(4):916–922. doi: 10.1111/j.1471-4159.1980.tb09666.x. [DOI] [PubMed] [Google Scholar]
- Corrêa F. M., Graeff F. G. On the mechanism of the hypertensive action of intraseptal bradykinin in the rat. Neuropharmacology. 1976 Nov;15(11):713–717. doi: 10.1016/0028-3908(76)90042-3. [DOI] [PubMed] [Google Scholar]
- Corrêa F. M., Innis R. B., Uhl G. R., Snyder S. H. Bradykinin-like immunoreactive neuronal systems localized histochemically in rat brain. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1489–1493. doi: 10.1073/pnas.76.3.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I., Usdin T. B., Snyder S. H. Dopamine receptor binding regulated by guanine nucleotides. Mol Pharmacol. 1979 Jul;16(1):69–76. [PubMed] [Google Scholar]
- Greenberg D. A., U'Prichard D. C., Sheehan P., Snyder S. H. alpha-Noradrenergic receptors in the brain: differential effects of sodium on binding of [3H]agonists and [3H]antagonists. Brain Res. 1978 Jan 27;140(2):378–384. doi: 10.1016/0006-8993(78)90472-9. [DOI] [PubMed] [Google Scholar]
- Innis R. B., Snyder S. H. Distinct cholecystokinin receptors in brain and pancreas. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6917–6921. doi: 10.1073/pnas.77.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan H., Lembeck F. Action of peptides and other algesic agents on paravascular pain receptors of the isolated perfused rabbit ear. Naunyn Schmiedebergs Arch Pharmacol. 1974;283(2):151–164. doi: 10.1007/BF00501142. [DOI] [PubMed] [Google Scholar]
- Mersey J. H., Williams G. H., Emanuel R., Dluhy R. G., Wong P. Y., Moore T. J. Plasma bradykinin levels and urinary kallikrein excretion in normal renin essential hypertension. J Clin Endocrinol Metab. 1979 Apr;48(4):642–647. doi: 10.1210/jcem-48-4-642. [DOI] [PubMed] [Google Scholar]
- Odya C. E., Goodfriend T. L., Peña C. Bradykinin receptor-like binding studied with iodinated analogues. Biochem Pharmacol. 1980 Feb;29(2):175–185. doi: 10.1016/0006-2952(80)90326-3. [DOI] [PubMed] [Google Scholar]
- Pasternak G. W., Snowman A. M., Snyder S. H. Selective enhancement of [3H]opiate agonist binding by divalent cations. Mol Pharmacol. 1975 Nov;11(6):735–744. [PubMed] [Google Scholar]
- ROCHA E SILVA M., ROSENTHAL S. R. Release of pharmacologically active substances from the rat skin in vivo following thermal injury. J Pharmacol Exp Ther. 1961 Apr;132:110–116. [PubMed] [Google Scholar]
- Regoli D., Barabé J., Park W. K. Receptors for bradykinin in rabbit aortae. Can J Physiol Pharmacol. 1977 Aug;55(4):855–867. doi: 10.1139/y77-115. [DOI] [PubMed] [Google Scholar]
- Ribeiro S. A., Corrado A. P., Graeff F. G. Antinociceptive action of intraventricular bradykinin. Neuropharmacology. 1971 Nov;10(6):725–731. doi: 10.1016/0028-3908(71)90087-6. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Goodman R. R. Multiple neurotransmitter receptors. J Neurochem. 1980 Jul;35(1):5–15. doi: 10.1111/j.1471-4159.1980.tb12483.x. [DOI] [PubMed] [Google Scholar]
- U'Prichard D. C., Snyder S. H. Interactions of divalent cations and guanine nucleotides at alpha 2-noradrenergic receptor binding sites in bovine brain mechanisms. J Neurochem. 1980 Feb;34(2):385–394. doi: 10.1111/j.1471-4159.1980.tb06608.x. [DOI] [PubMed] [Google Scholar]
- Vavrek R. J., Stewart J. M. Bradykinin analogs containing alpha-aminoisobutyric acid (Aib). Peptides. 1980 Fall;1(3):231–235. doi: 10.1016/0196-9781(80)90059-5. [DOI] [PubMed] [Google Scholar]



