Abstract
The sigma1 receptor is distinguished for its ability to bind various pharmacological agents including drugs of abuse such as cocaine and methamphetamine. Some endogenous ligands have been identified as putative sigma1 receptor regulators. High affinity ligands for the sigma1 receptor contain a nitrogen atom connected to long alkyl chains. We found that long alkyl chain primary amines including endogenous amines belonging to the sphingolipid family such as D-erythro-sphingosine and sphinganine bind with considerable affinity to the sigma1 receptor but not to the sigma2 receptor. The binding of D-erythro-sphingosine to the sigma1 receptor appears to be competitive in nature as assessed against the radioligand [3H]-(+)-pentazocine. Interestingly, the well studied sphingolipid mediator sphingosine-1 phosphate did not bind to the sigma1 or the sigma2 receptor. Sphingosine is converted to sphingosine-1 phosphate by a family of sphingosine kinases that regulate the relative levels of these two bioactive lipids in the cell. The selective binding of sphingosine but not sphingosine-1 phosphate to the sigma1 receptor suggests a mechanism for regulation of sigma1 receptor activity by the sphingosine kinase. We have successfully reconstituted this hypothetical model in HEK-293 cells overexpressing both the sigma1 receptor and sphingosine kinase-1. The data presented here strongly supports sphingosine as an endogenous modulator of the sigma1 receptor.
Index words: sigma1 receptor, sphingolipids, alkyl amines, ligand binding, endogenous ligand
1. Introduction
The sigma1 receptor is a unique intracellular binding site. The sigma1 receptor protein does not show significant similarity to any known mammalian proteins but resembles, with 30% identity, a C8-C7 sterol isomerase (ERG2) from yeast such as Saccharomyces cerevisiae, involved in ergosterol biosynthesis (Hanner et al., 1996). A sigma2 receptor has been identified based on ligand binding and photolabeling studies but is yet to be cloned (Hellewell et al., 1994; Vilner et al., 1995).
One distinguishing feature of the sigma1 receptor is its promiscuity in binding a wide range of different pharmacological agents although, how binding of these various compounds translates into function(s) through the sigma1 receptor is currently not clear (Hayashi and Su, 2004a). The sigma1 receptor has been demonstrated to regulate intracellular Ca2+ release from the endoplasmic reticulum (Hayashi and Su, 2001), through its interaction with the inositol phosphate 3 (IP3) receptor, which has been recently shown to occur via the C-terminal 122 amino acids of the sigma1 receptor (Wu and Bowen, 2008). Additionally, the sigma1 receptor has been implicated in modulation of K+, Cl− and Ca2+ ion channels (Aydar et al., 2002; Renaudo et al., 2007; Zhang and Cuevas, 2002), modulation of neurite sprouting in response to nerve growth factor (Takebayashi et al., 2002) and potentiation of brain-derived neurotrophic factor induced glutamate release (Yagasaki et al., 2006). Recently, the sigma1 receptor has been identified as a ligand regulated, Ca2+ sensitive chaperone at the mitochondrion-associated endoplasmic reticulum membrane in Chinese hamster ovary (CHO) cells (Hayashi and Su, 2007).
Sigma receptors have been reported to be associated with lipid containing microdomains. The sigma1 receptor was found to be present in cholesterol enriched, detergent insoluble lipid rafts of the endoplasmic reticulum (ER) in NG108 neuroblastoma cells where they were shown to be important in compartmentalization of endoplasmic reticulum synthesized lipids (Hayashi and Su, 2003a; 2003b). In the endoplasmic reticulum lipid droplets, the sigma1 receptor co-localized with caveolin-2, a cholesterol binding protein. In rat primary hippocampal cultures, sigma1 receptors were shown to form galactoceramide enriched lipid rafts and promote differentiation of oligodendrocytes (Hayashi and Su, 2004b). Additionally, the sigma1 receptor itself has been proposed to harbor cholesterol binding domains in its C-terminal region (Palmer et al., 2007). The sigma2 receptor has also been shown to localize to lipid raft fractions enriched in a raft marker flotillin 2 (Gebreselassie and Bowen, 2004). In MCF-7 and T47D breast tumor cells, sigma2 receptor ligands CB-184 and BD737 caused increases in ceramide and sphingomyelin levels (Crawford and Bowen, 2002).
We have previously purified the guinea pig sigma1 receptor from E. coli as a fusion protein with the E. coli maltose binding protein (Ramachandran et al., 2007). Using competition radioligand binding experiments with the pure sigma1 receptor we have found that long chain alkyl amines bind to the sigma1 receptor. This prompted us to investigate if endogenous sphingolipids that share the same basic structure (long alkyl chain amines) can interact with the sigma1 receptor. Here, we report that the long alkyl chain sphingolipid, sphingosine binds to the E.coli purified as well as to the membrane bound sigma1 receptor with high affinity but its phosphorylated counterpart sphingosine-1 phosphate which is endogenously generated by sphingosine kinase does not. High affinity sphingosine binding to the sigma1 receptor raises the possibility that sphingosine and methylated derivatives of sphingosine are potential endogenous regulators of the sigma1 receptor.
2. Materials and Methods
2.1 Materials
Dodecyl and stearyl amines were purchased from Acros Organics. [3H]-(+)-pentazocine and [3H]-DTG (1,3-Di-o-tolylguanidine) was purchased from Perkin Elmer Life Sciences, Wellesley, MA. Solvents and chemicals used in the synthesis were purchased from Sigma-Aldrich, St. Louis, MO. Sphingosine and all other sphingolipid analogues were purchased from Avanti Polar Lipids, Alabastar, AL and Cayman Chemicals, Ann Arbor, MI.
2.2 Overexpression and purification of the sigma1 receptor from E. coli
The guinea pig sigma1 receptor was purified as previously described (Ramachandran et al., 2007). Briefly, the pMal P2X plasmid (New England Biolabs) encoding the guinea pig sigma1 receptor on the C terminus of the E. coli maltose binding protein was used for the purification. The sigma1 receptor on its C terminus carried a HIS6 epitope tag. Protein was expressed in the E. coli strain BL21 DE3 (Novagen) with 0.7 mM IPTG. The E. coli pellet was sonicated and centrifuged at 100,000G for 1 hr to separate the particulate and soluble fractions. The particulate fraction was extracted with Triton X-100, centrifuged and the extract loaded onto an amylose resin (New England Biolabs, E-802). After washing the resin with buffer containing 1% Trition X-100, the MBP-sigma1 receptor fusion protein was eluted with buffer containing 10 mM maltose.
The MBP-sigma1 receptor fusion protein was cleaved with Factor Xa protease (Novagen) at room temperature for 24–48 h and the cleavage was monitored with SDS-polyacrylamide gel electrophoresis. The sigma1 receptor from the Factor Xa cleavage was purified with a HIS-Select HC Nickel affinity gel (Sigma-Aldrich, P6611) either in a batch or column format depending on the scale of the purification. The column was washed with buffer containing 0.5% Triton X-100 detergent. After washing to remove MBP the pure sigma1 receptor was eluted in buffer containing 250 mM imidazole.
To remove the undigested MBP-sigma1 receptor fusion protein that is carried over in the Ni2+ column purification, the eluate from the Ni2+ column was incubated with Anti-MBP antibody linked agarose (Vector laboratories, Burlingame, CA) at 4°C for 18–24 h. The beads were separated by centrifugation and the supernatant contained pure sigma1 receptor.
2.3 Preparation of guinea pig and rat liver membranes
Membranes were prepared as described previously (Kahoun and Ruoho, 1992). Liver tissue was homogenized (10 ml buffer/ g wet tissue) by 4 bursts of 10 s each using a brinkman polytron on setting 6 in ice cold sodium phosphate buffer (10 mM, pH 7.4) containing 0.32 M sucrose and a cocktail of protease inhibitors (20 µg/ml leupeptin, 5 µg/ml soybean trypsin inhibitor, 100 µM phenylmethylsulfonyl fluoride (PMSF), 100 µM benzamidine and 1 mM EDTA). The membrane suspension after homogenization was centrifuged for 10 min at 17000G and the supernatant was recentrifuged at 105000G for 1 h. The pellet was resuspended in homogenization buffer, snap frozen and stored at −80°C.
2.4 Ligand binding
Competition ligand binding to the pure sigma1 receptor or the guinea pig liver membranes was performed as described previously (Ramachandran et al., 2007). Binding was carried out in 50 mM Tris, pH 8.0 in a total volume 100 µl containing 50–100 ng of pure sigma1 receptor or 30 µg guinea pig liver membranes, 10 nM [3H]-(+)-pentazocine and various concentrations of inhibitor to be tested. Sigma2 receptor binding assays on rat liver membranes were performed as described (Matsumoto et al., 1995) with the radioligand [3H]-DTG and using 100 nM (+)-pentazocine to mask sigma1 sites. After incubation at 30°C for 1 h, the reaction was terminated by rapid filtration through glass fiber filters (Whatman GF/B), using a Brandel cell harvester (Brandel, Gaithersburg, MD). The glass fiber filters were previously soaked in 0.5% polyethyleneimine (PEI) for at least 1 h at room temperature. Filters were washed 4 times with 4 ml of ice-cold 50 mM Tris, pH 8.0. Radioactivity was quantified by liquid scintillation counting using a Packard model 1600CA scintillation counter. This method was suitable for both membranes and soluble sigma1 receptor, the latter due to the retention of soluble proteins on PEI coated filters based on charge interactions (Bruns et al., 1983; Ramachandran et al., 2007). Binding data were evaluated using GRAPHPAD PRISM (GRAPHPAD Software Inc., San Diego, CA, USA). For competition experiments Ki for ligands were calculated from the IC50 value using the Cheng Prussoff equation (Cheng and Prusoff, 1973), Ki = IC50 /(1 + [L]/Kd. The experimentally determined Kd value of [3H]-(+)-pentazocine was set as 10 nM. Stock solutions of sphingolipids were prepared in DMSO and diluted into the aqueous buffer for the binding assay. Final concentration of DMSO was adjusted to be no more than 2.0%.
2.5 Iodoazidococaine photolabeling
Radiochemical synthesis of the sigma1 receptor photolabel [125I]-Iodoazidococaine and photoaffinity labeling were performed as previously described (Kahoun and Ruoho, 1992). For photoaffinity labeling, E. coli purified sigma1 receptor (2 µg protein) was incubated in the presence and absence of 5 µM haloperidol in 60 mM Tris, pH 7.4 for 25 min at room temperature. [125I]-Iodoazidococaine was then added to a concentration of 1 nM and incubation continued for 15 min. Samples were irradiated for 6 s with a high pressure AH6-mercury lamp and the reaction was immediately quenched with sample buffer containing 250 mM β-mercaptoethanol. Photolabeled samples were separated by 12% SDS PAGE and the gel was placed on a PhophorImager (445 SI, Molecular dynamics) exposure cassette for at least 8 h after which the cassette was scanned to develop the autoradiogram.
2.6 Transient expression of sigma1 receptor and sphingosine kinase in HEK-293 cells
For sigma1 receptor expression in HEK-293 cells, the sigma1 receptor ORF in pcDNA 3.1(+) was used (Ramachandran et al., 2007). Mammalian expression vector pcDNA4/myc-His(A) harboring sphingosine kinase-1 was utilized for overexpression of C-terminally myc-His6 tagged kinase (Waters et al., 2006). DNA purification, cell culture and transient transfection were performed as previously described (Thiriot et al., 2002). Briefly HEK-293 cells from a confluent 150 mm culture dish were harvested with trypsin and transfected by electroporation with 25 µg of plasmid DNA using a Bio-Rad genepulser/capacitance extender unit. For co-transfection of SK1 and sigma1 receptor both plasmids were mixed together in a 1:1 w/w ratio. 48–72 h after transfection cells were harvested with trypsin and homogenized by passage through a 27-gauge needle in sphingosine kinase assay buffer A containing 50 mM Tris-HCl pH 7.4, 20% glycerol, 1mM β-mercaptoethanol, 1 mM EDTA, phosphatase inhibitors, 1 mM sodium orthovanadate, 15 mM sodium fluoride and protease inhibitors, 2 mM phenylmethylsulfonyl fluoride (PMSF), 10 µg/ml soybean trypsin inhibitor, pepstatin A and benzamidine. The homogenate was assayed for protein using BioRad protein assay method, aliquoted and frozen at −80°C.
2.7 Sphingosine kinase assay
Sphingosine kinase-1 activity was determined as previously described (Kohama et al., 1998) with some modifications. In a total volume of 190 µl of sphingosine kinase assay buffer A was included, 50 µg of HEK-293 cell homogenate containing phosphatase and protease inhibitors and 10 µl of 1 mM sphingosine (dissolved in 5% Triton X-100). The reaction was initiated by addition of 10 µl of γ[32P]-ATP (10 µCi, 2 mM) containing MgCl2 (10 mM) and incubated at 37°C for 20 min. Reactions were terminated with 20 µl of 1 N HCl and 0.8 ml of chloroform/methanol/HCl (100:200:1 v/v/v). After vigorous vortexing, 240 µl of chloroform and 240 µl of 2 M KCl were added and phases separated by centrifugation. The lipids were resolved by thin layer chromatography (TLC) on Silica Gel G60 with 1-butanol/ethanol/acetic acid/water (80:20:10:20, v/v). The TLC plate was dried and developed using a phosphorimager cassette. The sphingosine-1 phosphate spot confirmed by chromatography of commercially purchased authentic sphingosine-1 phosphate and visualization using ninhydrin spray.
2.8 Western blotting
Protein samples were first resolved by 12% SDS PAGE and then proteins were transferred onto polyvinyldifluoride (PVDF) membrane (Millipore, 0.45 µm) in CAPS buffer (10 mM 3-(Cyclohexylamino)-1-propanesulfonic acid (CAPS), pH 10.5, 0.5% w/v DTT and 15% v/v methanol) at 65 V for 1 h. The PVDF membrane was blocked with 5% non fat dry milk in PBST (PBS containing 0.05% Tween 20) for 1 h at room temperature. The membrane was then treated with primary antibody, anti-sigma1 receptor [rabbit polyclonal, (Ramachandran et al., 2007)] or anti-myc (Sigma-Aldrich, St. Louis, MO) antibody at room temperature for 1 h or overnight at 4°C. The blot was washed 3 times for 10 min each in buffer PBST (PBS containing 0.05% Tween 20) before incubating with horseradish peroxidase conjugated secondary antibody (1/20000-5000 X dilution) in PBST for 1 h at room temperature. Blots were developed with enhanced chemiluminescence (ECL) reagents from PIERCE.
3. Results
3.1 Alkylamines bind to the sigma1 receptor
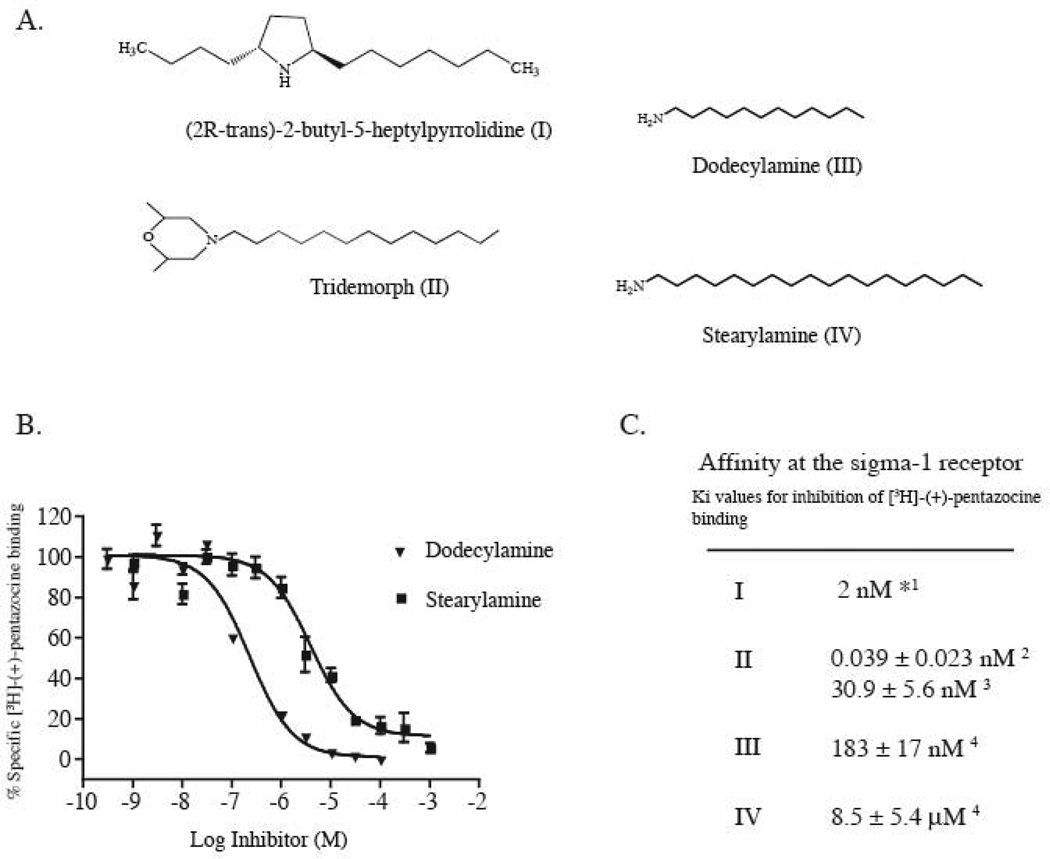
(2R-trans)-2-Butyl-5-heptylpyrrolidine (Fig. 1A, I), isolated from the culture broth of Streptomyces longispororuber has been shown to be a high affinity sigma1 receptor ligand (Kumagai et al., 2000) which inhibited [3H]-(+)-pentazocine binding with an IC50 of 2 nM (Fig. 1C) and also exhibited high affinity for the dopamine (D2) receptor (Kumagai et al., 2000). Another structurally similar ligand, tridemorph, (Fig. 1A, II), is an agricultural insecticide that inhibits the yeast sterol isomerase and has been reported to have a high affinity for the sigma1 receptor in guinea pig liver membranes as well as on the pure sigma1 receptor (Moebius et al., 1997; Ramachandran et al., 2007). Both of these compounds share a common long alkyl chain extending from a secondary or tertiary nitrogen atom.
Figure 1.
Long chain alkyl amines bind to the sigma1 receptor. A. Structures of sigma1 receptor ligands containing N-alkyl amines. Compound I was isolated from the culture broth of Streptomyces longisporuber (Kumagai et al., 2000). Compound II is a yeast sterol isomerase inhibitor (Moebius et al., 1997). B. Inhibition of [3H]-(+)-pentazocine binding to the purified sigma1 receptor by the long chain alkyl amines dodecylamine and stearylamine. C. Affinities of the indicated compounds at the sigma1 receptor. *IC50 value, 1as reported in (Kumagai et al., 2000). 2as reported in (Moebius et al., 1997). 3as reported in (Ramachandran et al., 2007), 4Ki values from inhibition curves shown in B. Data reported as mean ± standard error of the mean (S.E.M) (n=4).
Based on the structures of these compounds, alkyl amines such as dodecylamine and stearylamine (Fig. 1A) were tested for inhibition of [3H]-(+)-pentazocine binding to the E. coli purified sigma1 receptor. Fig 1B shows the inhibition curves against the sigma1 receptor radioligand [3H]-(+)-pentazocine. We found dodecylamine to inhibit [3H]-(+)-pentazocine binding to the pure sigma1 receptor with an Ki of 183 ± 17 nM and stearylamine showed a Ki of 8.5 ± 5.4 µM (Fig. 1C).
3.2 Endogenous sphingolipids bind to the sigma1 receptor
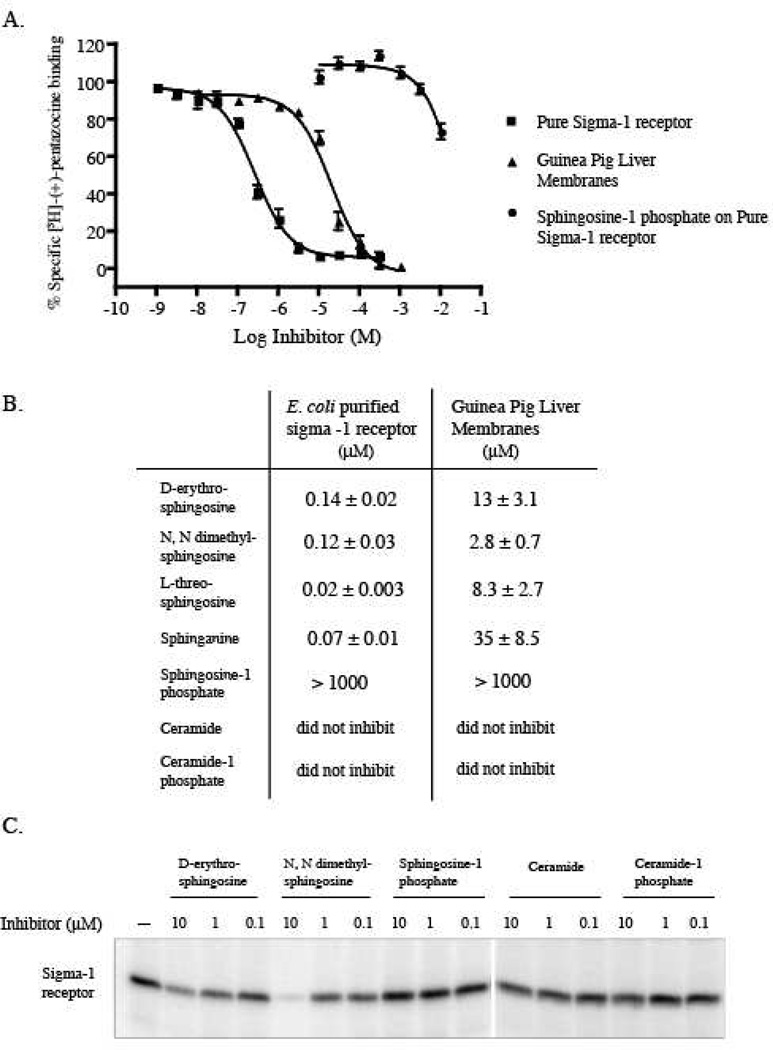
One or more endogenous ligands for the sigma1 receptor have not yet been identified. Since long alkyl chain amine compounds containing structures similar to tridemorph (Moebius et al., 1997) or the Streptomyces compound (Kumagai et al., 2000), bind to the sigma1 receptor, we examined the possibility that endogenous nitrogen containing lipids could also bind to the sigma1 receptor. D-erythro sphingosine, the major cellular sphingolipid base conforms to the structural motif of having a long alkyl chain with a positively charged nitrogen atom. D-erythro sphingosine and its derivatives are important endogenous lipid mediators variously involved in multiple signal transduction pathways (Hannun and Obeid, 2008). To determine if sphingosine and other similar compounds bind to the sigma1 receptor, sphingosine and its derivatives were tested for inhibition of [3H]-(+)-pentazocine binding to guinea pig liver membranes and the purified guinea pig sigma1 receptor (Fig. 2A).
Figure 2.
Interaction of sphingosine derivatives with the pure sigma1 receptor. A. Inhibition of [3H]-(+)-pentazocine binding by D-erythro-sphingosine in guinea pig liver membranes (▲) and to the pure sigma1 receptor (■) and by sphingosine-1 phosphate on the pure sigma1 receptor (●). B. Ki values of other sphingosine derivatives showing affinity at the sigma1 receptor. Data presented as mean ± SEM (n=3). C. Photoaffinity labeling of the pure sigma1 receptor using the sigma1 receptor photoprobe [125I]Iodoazidococaine (1 nM) (Chen et al., 2007) showing inhibition of photolabeling by 10 µM D-erythro-sphingsosine and N, N dimethylsphingosine. Sphingosine-1 phosphate and the acylated ceramides did not show significant inhibition of Iodoazidococaine photolabeling.
D-erythro sphingosine has two asymmetric chiral carbon atoms (2 and 3) and can exist in four possible streoisomers; (D-erythro (2S, 3R), D- threo (2R, 3R), L-erythro (2R, 3S) and L-threo (2S, 3S). We tested the D-erythro and L-threo stereoisomers of sphingosine, N, N dimethyl sphingosine and the C4 saturated analogue of D-erythro sphingosine (sphinganine) for ability to bind to the sigma1 receptor. Fig. 2A shows inhibition of [3H]-(+)-pentazocine binding by D-erythro-sphingosine on the purified protein as well as guinea pig liver membranes. All the sphingosine derivatives tested inhibited [3H]-(+)-pentazocine binding to the sigma1 receptor in both guinea pig liver membranes and the purified guinea pig sigma1 receptor (Fig. 2B). D-erythro-sphingosine, N, N dimethyl sphingosine, L-threo-sphingosine and sphinganine inhibited [3H]-(+)-pentazocine binding to the purified sigma1 receptor with Ki values of 0.14 ± 0.02, 0.12 ± 0.03, 0.02 ± 0.003 and 0.07 ± 0.01 µM respectively and to guinea pig liver membranes with Ki values of 13 ± 3.1, 2.9 ± 0.7, 8.3 ± 2.7 and 35 ± 8.5 µM respectively. For all four compounds that inhibited [3H]-(+)-pentazocine binding the Ki values for inhibition were lower by an order of magnitude on the pure sigma1 receptor as compared to guinea pig liver membranes. The apparent low affinity of these compounds for sigma1 receptors in guinea pig liver membranes is likely to be due to partitioning of the lipids into the membranes. Selectivity of binding was not observed between the D-erythro or L-threo stereoisomers of sphingosine however; the affinity of the L-threo derivative was better by an order of magnitude. Sphinganine also showed affinities comparable to sphingosine in both guinea pig liver membranes and the pure sigma1 receptor.
Sphingosine can be phosphorylated to sphingosine-1 phosphate by the enzyme sphingosine kinase (Spiegel and Milstien, 2007). We found that while sphingosine was able to inhibit [3H]-(+)-pentazocine binding to the sigma1 receptor, sphingosine-1 phosphate failed to do so (Fig. 2A). Moreover, the acylated derivatives of sphingosine, ceramide and ceramide-1 phosphate also failed to inhibit [3H]-(+)-pentazocine binding to the sigma1 receptor (Fig. 2B).
The sphingosine derivatives were also tested for their ability to inhibit photolabeling by the sigma1 receptor photoprobe [125I]-Iodoazidococaine on the pure sigma1 receptor (Fig. 2C). The compounds were used at 0.1 µM, 1 µM and 10 µM to inhibit [125I]-Iodoazidococaine photolabeling. The relative inhibition by the compounds tested was similar to the rank order of the Ki values for inhibition of [3H]-(+)-pentazocine binding. N, N dimethyl sphingosine was found marginally better than D-erythro sphingosine while sphingosine-1 phosphate, ceramide and ceramide-1 phosphate were not as effective in inhibiting [125I]-Iodoazidococaine photolabeling (Fig. 2C).
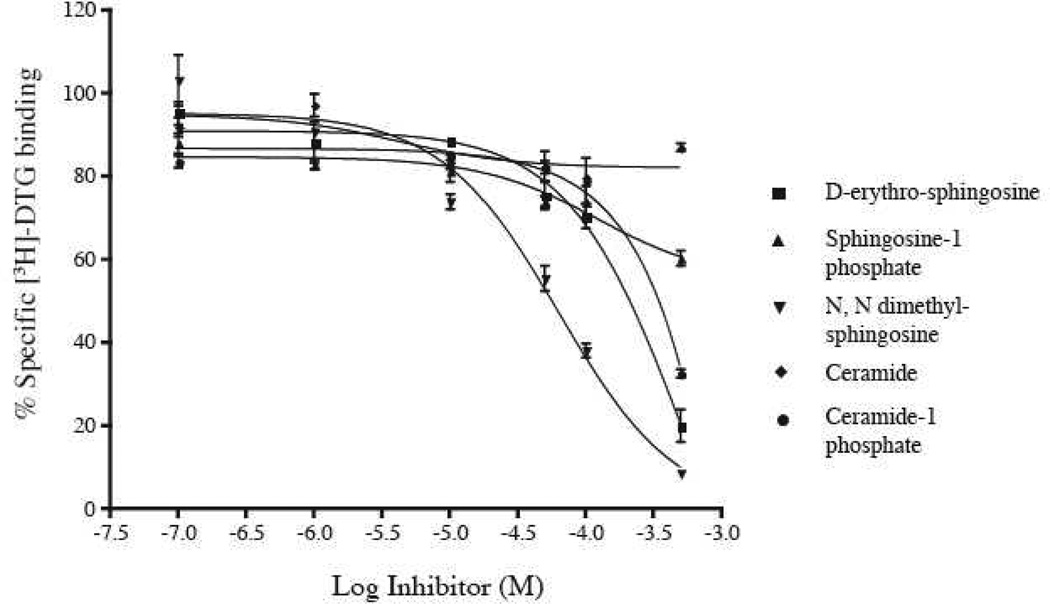
We also examined the binding affinity of the sphingosine derivatives for the sigma2 receptor in rat liver membranes using the radioligand [3H]-DTG (1,3-Di-o-tolylguanidine) (Fig. 3) in the presence of 100 nM (+)-pentazocine. The choice of rat liver membranes was due to the high density of sigma2 receptors in this tissue (Hellewell et al., 1994). None of the sphingolipids tested showed any appreciable affinity for the sigma2 receptor. Of the compounds tested N N dimethyl sphingosine showed a modest inhibition of [3H]-DTG binding with an IC50 of 62 µM.
Figure 3.
Inhibition of [3H]-DTG (20 nM) binding on rat liver membranes by the indicated sphingolipids. Binding was performed in the presence of 100 nM (+)-pentazocine to block sigma1 binding sites.
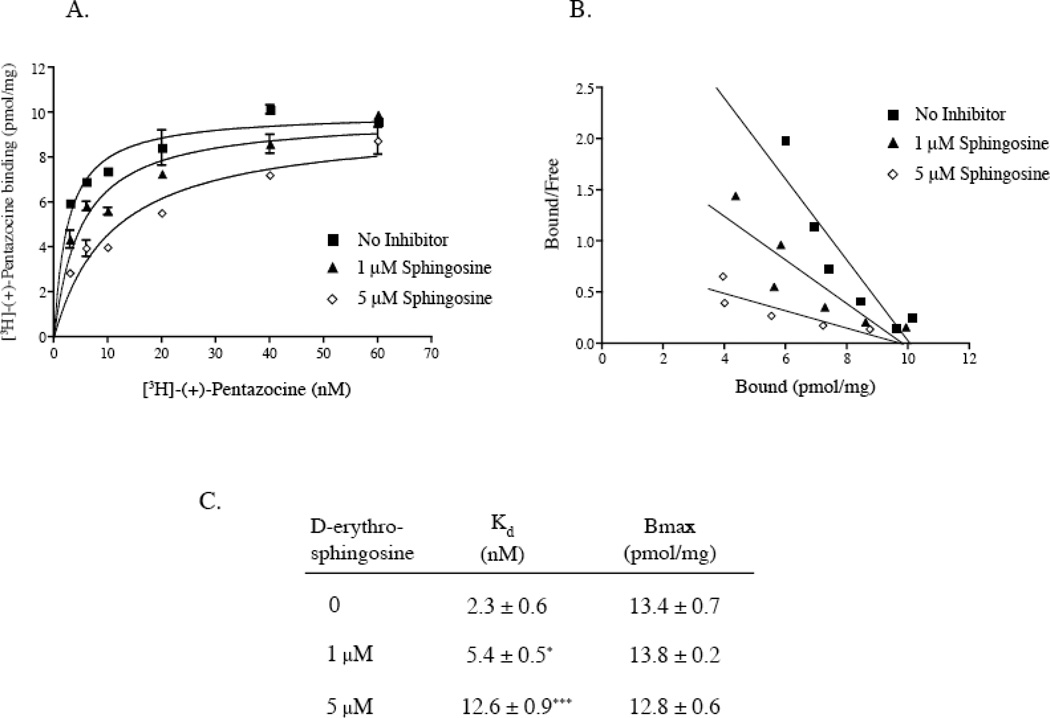
3.3 Characterization of D-erythro sphingosine interaction with the purified sigma1 receptor
To determine the mode of sphingosine inhibition of [3H]-(+)-pentazocine binding to the sigma receptor, saturation equilibrium binding of [3H]-(+)-pentazocine to guinea pig liver membranes (Fig. 4A) was performed followed by Scatchard transformation of the data (Fig. 4B). In the presence of both 1 µM and 5 µM D-erythro sphingosine there was a significant increase in the Kd of [3H]-(+)-pentazocine binding to the pure sigma1 receptor (Fig. 4C). The Kd value for [3H]-(+)-pentazocine increased significantly from 2.3 ± 0.6 nM with no added sphingosine to 5.4 ± 0.5 nM with 1 µM sphingosine and 12.6 ± 0.9 nM with 5 µM sphingosine. The Bmax of [3H]-(+)-pentazocine on guinea pig liver membranes was 13.4 ± 0.7 pM/mg which is in excellent correlation to values reported earlier (Hanner et al., 1996). However, in presence of 1 and 5 µM sphingosine the Bmax value for [3H]-(+)-pentazocine binding at was not significantly altered (Fig. 4C). A Scatchard transformation of the binding data confirmed these findings. The lines exhibited significantly different slopes indicating different Kd values but, intersected the X-axis at approximately the same Bmax value. These results suggests a simple competitive interaction of sphingosine against the radioligand [3H]-(+)-pentazocine.
Figure 4.
Sphingosine interaction with the sigma1 receptor. A. [3H]-(+)-pentazocine binding curves on guinea pig liver membranes in the presence of 1 µM and 5 µM sphingosine are shown. B. Scatchard transformation of the binding data in A. Data in A and B are from a single representative experiment. C. Kd and Bmax values of [3H]-(+)-pentazocine binding to the pure sigma1 receptor in the presence of 1 µM and 5 µM sphingosine. Data are presented as mean ± S.E.M (n=3). *P value < 0.05, (0.015), ***P value < 0.005, (0.0008).
3.4 Modulation of D-erythro sphingosine binding to the sigma1 receptor by the activity of sphingosine kinase-1
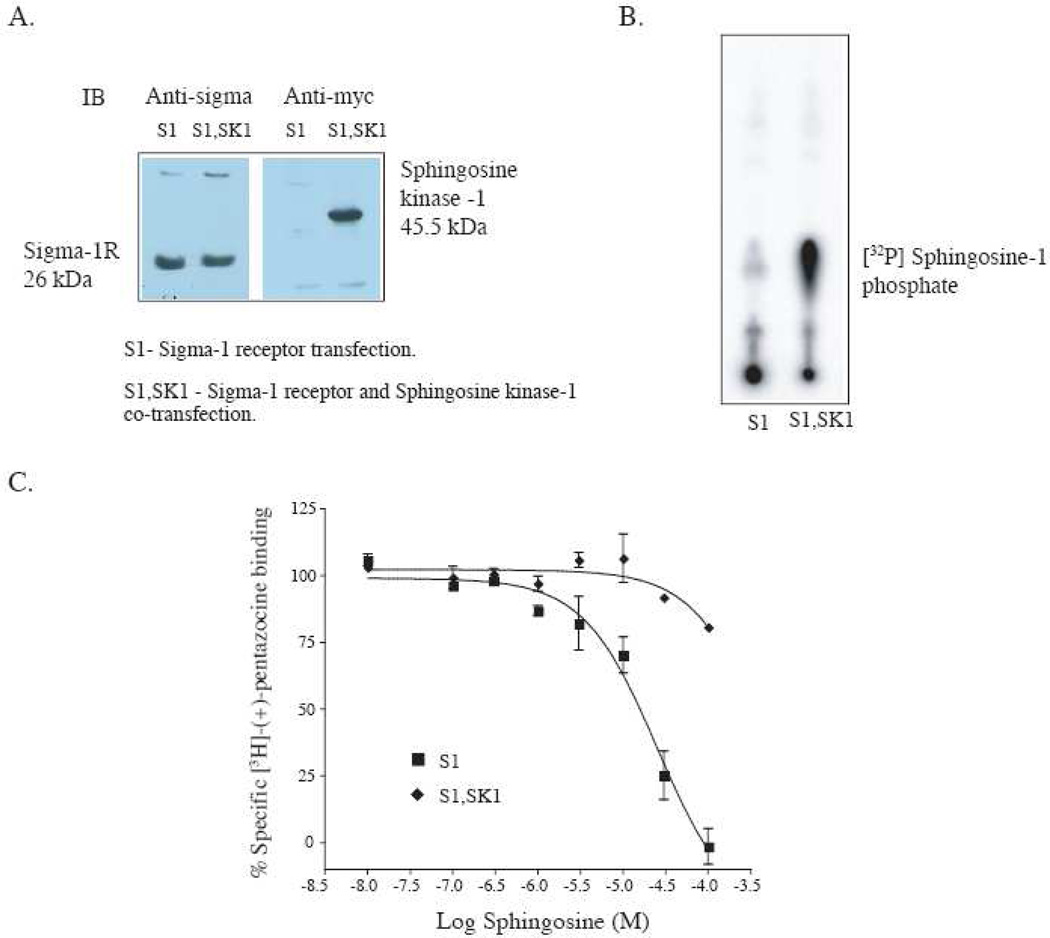
The sphingosine phosphorylating enzyme, sphingosine kinase-1, may modulate sphingosine binding to the sigma1 receptor by converting sphingosine to sphingosine-1 phosphate and thus regulate downstream sigma1 receptor signaling mechanisms. To test this hypothesis we reconstituted the system by transfection of the sigma1 receptor alone (Fig. 5A, S1) or co-transfection of sphingosine kinase 1 and sigma1 receptor (Fig. 5A, S1,SK1) into HEK-293 cells. Expression of both proteins was confirmed by western blotting (Fig. 5A) using a polyclonal antibody against the sigma1 receptor (Ramachandran et al., 2007) and an anti-myc antibody directed against a C-terminal myc tag on sphingosine kinase-1. The functional activity of the expressed sphingosine kinase was tested using an in vitro kinase assay with γ[32P]-ATP and exogenous D-erythro sphingosine. Homogenates from sphingosine kinase-1 transfected cells showed a robust phosphorylation of exogenously added D-erythro sphingosine indicating activity of the overexpressed kinase (Fig. 5B, S1,SK1). Very little endogenous kinase activity was seen in HEK-293 cells expressing sigma1 receptor alone (Fig. 5B, S1).
Figure 5.
Reconstitution of a sphingosine “switch” at the sigma1 receptor with transiently overexpressed sphingosine kinase-1 in HEK-293 cells. A. Western blot showing expression of the sigma1 receptor and sphingosine kinase-1 in HEK-293 cell lysate. The antibody used against the sigma1 receptor was a polyclonal antibody previously reported from our laboratory (Ramachandran et al., 2007). Sphingosine kinase-1 was detected via anti-myc antibody against the COOH terminal myc tag. B. Autoradiogram showing activity of the transiently expressed sphingosine kinase-1 in HEK-293 cells. The kinase assay was performed as described in Methods, section 2.7, using γ-[32P]-ATP, MgCl2, sphingosine and the homogenate from HEK-293 (S1 or S1,SK1) cells. The radiolabeled γ-[32P] sphingosine-1 phosphate migrated at the position of authentic non- radioactive sphingosine-1 phosphate C. Inhibition of [3H]-(+)-pentazocine binding by D-erythro-sphingosine in HEK-293 cells overexpressing the sigma1 receptor alone (S1) or the sigma1 receptor together with the sphingosine kinase (S1,SK1). Binding was performed under conditions conducive to sphingosine kinase-1 activity. The binding assay buffer included 2 mM ATP, 5 mM MgCl2, phosphatase inhibitors 1 mM Na3VO4 and 15 mM NaF as described in Methods, section 2.7
D-erythro sphingosine inhibition of [3H]-(+)-pentazocine binding in HEK-293 cells expressing sigma1 receptor alone was carried out in the presence and absence of sphingosine kinase-1. In these experiments binding was performed in the presence of 2 mM ATP and 5 mM MgCl2 to ensure activity of the sphingosine kinase. As shown in Fig. 5C (S1), D-erythro sphingosine inhibited [3H]-(+)-pentazocine binding in HEK-293 cells expressing sigma1 receptor alone with an Ki of 3.6 ± 0.6 µM. However in homogenates from HEK-293 cells expressing the sigma1 receptor and sphingosine kinase-1, D-erythro sphingosine did not show a significant inhibition of [3H]-(+)-pentazocine binding at concentrations as high as 100 µM (Fig. 5C, S1,SK1). The results indicate that in vitro phosphorylation of exogenous D-erythro sphingosine by sphingosine kinase-1 resulting in sphingosine-1 phosphate abrogated sphingosine inhibition of [3H]-(+)-pentazocine binding to the membrane bound sigma1 receptor.
4. Discussion
Studies aimed at better understanding the sigma1 receptor pharmacophore have indicated that certain high affinity sigma1 receptor ligands contain long alkyl chains and a basic amino group (Fontanilla et al., 2009; Glennon, 2005). Compounds not conforming to this basic structure such as endogenous steroids, progesterone and pregnenolone do interact with the sigma1 receptor however, their affinity for the sigma1 receptor is considerably lower since the pharmacophore requirements for high affinity binding are not fulfilled (Ablordeppey et al., 2000). The affinity of such steroids for the sigma1 receptor has also been explained due to their structural similarity to the steroidal substrates of the yeast (ERG2) and the mammalian (emopamil binding protein) sterol isomerase (Moebius et al., 1997). Laggner et al. (Laggner et al., 2005) have described pharmacophore models for high affinity binding to the sigma1 receptor, the yeast sterol isomerase (ERG2) and the mammalian emopamil binding protein (EBP). Their best scoring model for sigma1 receptor ligands was based on the compound, fenpropimorph, containing one positive ionizable center attached to hydrophobic groups (Laggner et al., 2005). We have previously utilized a fenpropimorph photolabel, [125I]-iodoazidofenpropimorph to probe the sigma1 receptor binding site (Pal et al., 2007). Other high affinity sigma1 receptor ligands include a natural compound isolated from the culture broth of Streptomyces longispororuber, (2R-trans)-2-Butyl-5-heptylpyrrolidine (Kumagai et al., 2000) (Fig. 1A, I) and the yeast sterol isomerase inhibitor, tridemorph (Fig. 1A, II) (Moebius et al., 1997; Ramachandran et al., 2007) both conforming to the presence of an ionizable nitrogen atom and long alkyl hydrophobic groups. We queried if the existing pharmacophore requirements could be further simplified by testing long chain alkyl amines for their ability to inhibit [3H]-(+)-pentazocine binding on the pure sigma1 receptor. Dodecylamine with 12 carbons showed higher affinity than stearylamine with 18 carbons (Fig. 1B, C), which implies that certain structural limitations occur in the sigma1 receptor binding site to accommodate long alkyl chains. Both of these compounds however, are structurally similar to the endogenously occurring long chain lipid, sphingosine.
The sigma1 receptor is implicated in lipid transport from the endoplasmic reticulum to the plasma membrane (Hayashi and Su, 2003b). It has been suggested that in the brain the sigma1 receptor localizes to galactosylceramide enriched lipid microdomains and plays a role in oligodendrocyte differentiation (Hayashi and Su, 2004b). In support of such links between the sigma receptors and sphingolipids, previous reports of high affinity long chain alkyl amine containing compound binding to the sigma1 receptor (Kumagai et al., 2000; Moebius et al., 1997), prompted us to evaluate direct D-erythro-sphingosine binding to the sigma1 receptor. Here, we report for the first time that the major sphingosine analogs D-erythro-sphingosine, N, N dimethyl sphingosine, sphinganine and L-threo-sphingosine bind to the sigma1 receptor with high affinity (Fig. 2). We observed sphingosine and analogs to inhibit [3H]-(+)-pentazocine binding both to the pure sigma1 receptor and guinea pig liver membrane preparations. There is an approximately 100-fold difference in the Ki values of these lipids for inhibition of [3H]-(+)-pentazocine binding between the pure sigma1 receptor and guinea pig liver membranes (Fig. 2A, B). This difference may be due to partitioning of the lipid into the membranes, which would reduce the effective free concentration at equilibrium. This suggests that the 13 ± 3.1 µM Ki value in guinea pig liver membranes does not reflect the true affinity of interaction between sphingosine and the sigma1 receptor and a more correct reflection of the interaction is the 0.14 ± 0.02 µM Ki value on the pure protein.
The synthetic L-threo sphingosine was found to be approximately 10 fold more potent at the pure sigma1 receptor as compared to the naturally occurring D-threo isomer. For binding to the sigma1 receptor in guinea pig liver membranes, however, the Ki values are similar. In a physiological system a lower affinity ligand is more suitable for regulation (i.e. in this case D-erythro vs. L-threo) since it provides a more facile regulation as compared to a high affinity ligand that would “trap” the receptor in a bound state. Indeed, it has been documented in many receptor systems that the endogenously occurring hormones or neurotransmitters have far lower affinity than their synthetic analogs. This is a reasonable explanation for the lower affinity of D-erythro-sphingosine as compared to its L-threo-isomer. The binding of D-erythro sphingosine, N, N dimethyl sphingosine and sphinganine is specific to the sigma1 receptor since these compounds demonstrated much lower binding affinity for sigma2 receptors as assessed by [3H]-DTG on rat liver membranes (Fig. 3).
The mechanism of sphingosine binding to the sigma1 receptor was studied using a saturation equilibrium [3H]-(+)-pentazocine binding in guinea pig liver membranes. Increasing concentrations of sphingosine (below its Kd of interaction) resulted in a significant change in the Kd of [3H]-(+)-pentazocine and an appreciable right shift of the [3H]-(+)-pentazocine binding curve. At these concentrations the Bmax values were constant indicating that inhibition by sphingosine was surmountable with increasing concentrations of the radioligand. A Scatchard transformation of the binding data (Fig. 4) confirmed these observations. These data support a model of competitive inhibition of [3H]-(+)-pentazocine binding by sphingosine (Fig. 4). The binding of sphingosine to the sigma1 receptor is specific for the nonphosphorylated analogue of sphingosine. Sphingosine-1 phosphate did not exhibit a measurable affinity for the sigma1 receptor (Fig. 2A). Sphingosine-1 phosphate is a novel extracellular mediator that signals through a family of G protein coupled receptors (Chun et al., 2002). Sphingosine-1 phosphate is formed from sphingosine by sphingosine kinase (Spiegel and Milstien, 2007) and is degraded by the enzymes sphingosine-1 phosphate lyase and sphingosine-1 phosphate phosphatases (Hannun and Obeid, 2008; Spiegel and Milstien, 2007). Sphingosine-1 phosphate enhances growth and survival in diverse cell types and its precursors ceramide and sphingosine are associated with growth arrest and cell death. Sphingosine is well known to cause a rapid release of Ca2+ from intracellular stores. Sphingosine, however, has no known specific receptor through which it mediates its cellular effects. Treatment of Jurkat T cell line with sphingosine leads to a rapid mobilization of intracellular Ca2+ with no effect on IP3 levels (Sakano et al., 1996). The rapid increase in intracellular Ca2+ has been attributed to an increase in diglyceride kinase activity and subsequently phosphatidic acid. Both sphingosine and N, N dimethyl sphingosine are potent inhibitors of protein kinase C and many downstream effects of sphingosine are attributed to this action (Igarashi et al., 1989).
The sphingolipid family of lipids plays essential roles both as structural cell membrane components and second messengers in cell signaling. In the resting state of cells, the balance between sphingosine-1 phosphate formation and degradation maintains the levels of the two signaling molecules, which in turn determines, in part, cell fate. This has been referred to as the sphingolipid rheostat. Several recent reviews are devoted to the biology and physiology of sphingolipid signaling (Hannun and Obeid, 2008; Spiegel and Milstien, 2003).
While sphingosine-1 phosphate generated by sphingosine kinase is transported outside the cell to act on its cognate sphingosine-1 phosphate GPCR, sphingosine interaction with the sigma1 receptor occurs presumably on the membrane at the sigma1 receptor binding site. The selective binding of sphingosine to the sigma1 receptor suggests a potential mechanism for regulation of sigma1 receptor activity in a physiological setting where phosphorylation of sphingosine by the kinase would release sphingosine bound to the sigma1 receptor. We have successfully reconstituted such a model in HEK-293 cells overexpressing both the sigma1 receptor and sphingosine kinase-1 (Fig. 5). Phosphorylation of sphingosine by an increase in sphingosine kinase-1 levels resulted in an abrogation of sphingosine inhibition of [3H]-(+)-pentazocine binding to the sigma1 receptor. This signaling pathway could function in both directions whereby phosphorylation of sphingosine by a kinase or removal of the phosphate by sphingosine phosphatase may function as a ‘switch’ to activate or inactivate receptor activity depending on whether sphingosine acts as an agonist or antagonist at the sigma1 receptor. The data reported in this paper indicate that a lipid-like molecule such as D-erythro sphingosine is a strong candidate to be an endogenous regulator of the sigma1 receptor.
Acknowledgments
This study was supported by the National Institutes of Health grant (NIH R01MH065503) to Arnold E. Ruoho.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ablordeppey SY, Fischer JB, Glennon RA. Is a nitrogen atom an important pharmacophoric element in sigma ligand binding? Bioorg. Med. Chem. 2000;8:2105–2111. doi: 10.1016/s0968-0896(00)00148-6. [DOI] [PubMed] [Google Scholar]
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Bruns RF, Lawson-Wendling K, Pugsley TA. A rapid filtration assay for soluble receptors using polyethylenimine-treated filters. Anal. Biochem. 1983;132:74–81. doi: 10.1016/0003-2697(83)90427-x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hajipour AR, Sievert MK, Arbabian M, Ruoho AE. Characterization of the cocaine binding site on the sigma-1 receptor. Biochemistry. 2007;46:3532–3542. doi: 10.1021/bi061727o. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, Pyne S, Tigyi G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- Crawford KW, Bowen WD. Sigma-2 receptor agonists activate a novel apoptotic pathway and potentiate antineoplastic drugs in breast tumor cell lines. Cancer Res. 2002;62:313–322. [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The Hallucinogen N,N-Dimethyltryptamine (DMT) is an Endogenous Sigma-1 Receptor Regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebreselassie D, Bowen WD. Sigma-2 receptors are specifically localized to lipid rafts in rat liver membranes. Eur. J. Pharmacol. 2004;493:19–28. doi: 10.1016/j.ejphar.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Glennon RA. Pharmacophore identification for sigma-1 (sigma1) receptor binding: application of the "deconstruction-reconstruction-elaboration" approach. Mini. Rev. Med. Chem. 2005;5:927–940. doi: 10.2174/138955705774329519. [DOI] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl. Acad. Sci. U S A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell. Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proc. Natl. Acad. Sci. U S A. 2001;98:491–496. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108-15 cells. J. Pharmacol. Exp. Ther. 2003a;306:726–733. doi: 10.1124/jpet.103.051292. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J. Pharmacol. Exp. Ther. 2003b;306:718–725. doi: 10.1124/jpet.103.051284. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS Drugs. 2004a;18:269–284. doi: 10.2165/00023210-200418050-00001. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proc. Natl. Acad. Sci. U S A. 2004b;101:14949–14954. doi: 10.1073/pnas.0402890101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 Receptor Chaperones at the ER- Mitochondrion Interface Regulate Ca(2+) Signaling and Cell Survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur. J. Pharmacol. 1994;268:9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Igarashi Y, Hakomori S, Toyokuni T, Dean B, Fujita S, Sugimoto M, Ogawa T, el-Ghendy K, Racker E. Effect of chemically well-defined sphingosine and its N-methyl derivatives on protein kinase C and src kinase activities. Biochemistry. 1989;28:6796–6800. doi: 10.1021/bi00443a002. [DOI] [PubMed] [Google Scholar]
- Kahoun JR, Ruoho AE. (125I)iodoazidococaine, a photoaffinity label for the haloperidol-sensitive sigma receptor. Proc. Natl. Acad. Sci. U S A. 1992;89:1393–1397. doi: 10.1073/pnas.89.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J. Biol. Chem. 1998;273:23722–23728. doi: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- Kumagai K, Shono K, Nakayama H, Ohno Y, Saji I. (2R-trans)-2-butyl-5-heptylpyrrolidine as a potent sigma receptor ligand produced by Streptomyces longispororuber. J. Antibiot. (Tokyo) 2000;53:467–473. doi: 10.7164/antibiotics.53.467. [DOI] [PubMed] [Google Scholar]
- Laggner C, Schieferer C, Fiechtner B, Poles G, Hoffmann RD, Glossmann H, Langer T, Moebius FF. Discovery of high-affinity ligands of sigma1 receptor, ERG2, and emopamil binding protein by pharmacophore modeling and virtual screening. J. Med. Chem. 2005;48:4754–4764. doi: 10.1021/jm049073+. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Bowen WD, Tom MA, Vo VN, Truong DD, De Costa BR. Characterization of two novel sigma receptor ligands: antidystonic effects in rats suggest sigma receptor antagonism. Eur. J. Pharmacol. 1995;280:301–310. doi: 10.1016/0014-2999(95)00208-3. [DOI] [PubMed] [Google Scholar]
- Moebius FF, Reiter RJ, Hanner M, Glossmann H. High affinity of sigma 1-binding sites for sterol isomerization inhibitors: evidence for a pharmacological relationship with the yeast sterol C8-C7 isomerase. Br. J. Pharmacol. 1997;121:1–6. doi: 10.1038/sj.bjp.0701079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A, Hajipour AR, Fontanilla D, Ramachandran S, Chu UB, Mavlyutov T, Ruoho AE. Identification of regions of the sigma-1 receptor ligand binding site using a novel photoprobe. Mol. Pharmacol. 2007;72:921–933. doi: 10.1124/mol.107.038307. [DOI] [PubMed] [Google Scholar]
- Palmer CP, Mahen R, Schnell E, Djamgoz MB, Aydar E. Sigma-1 receptors bind cholesterol and remodel lipid rafts in breast cancer cell lines. Cancer Res. 2007;67:11166–11175. doi: 10.1158/0008-5472.CAN-07-1771. [DOI] [PubMed] [Google Scholar]
- Ramachandran S, Lu H, Prabhu U, Ruoho AE. Purification and characterization of the guinea pig sigma-1 receptor functionally expressed in Escherichia coli. Protein. Expr. Purif. 2007;51:283–292. doi: 10.1016/j.pep.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudo A, L'Hoste S, Guizouarn H, Borgese F, Soriani O. Cancer cell cycle modulated by a functional coupling between sigma-1 receptors and Cl- channels. J. Biol. Chem. 2007;282:2259–2267. doi: 10.1074/jbc.M607915200. [DOI] [PubMed] [Google Scholar]
- Sakano S, Takemura H, Yamada K, Imoto K, Kaneko M, Ohshika H. Ca2+ mobilizing action of sphingosine in Jurkat human leukemia T cells. Evidence that sphingosine releases Ca2+ from inositol trisphosphate- and phosphatidic acid-sensitive intracellular stores through a mechanism independent of inositol trisphosphate. J. Biol. Chem. 1996;271:11148–11155. doi: 10.1074/jbc.271.19.11148. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell. Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J. Biol. Chem. 2007;282:2125–2129. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Hayashi T, Su TP. Nerve growth factor-induced neurite sprouting in PC12 cells involves sigma-1 receptors: implications for antidepressants. J. Pharmacol. Exp. Ther. 2002;303:1227–1237. doi: 10.1124/jpet.102.041970. [DOI] [PubMed] [Google Scholar]
- Thiriot DS, Sievert MK, Ruoho AE. Identification of human vesicle monoamine transporter (VMAT2) lumenal cysteines that form an intramolecular disulfide bond. Biochemistry. 2002;41:6346–6353. doi: 10.1021/bi015779j. [DOI] [PubMed] [Google Scholar]
- Vilner BJ, John CS, Bowen WD. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res. 1995;55:408–413. [PubMed] [Google Scholar]
- Waters CM, Long J, Gorshkova I, Fujiwara Y, Connell M, Belmonte KE, Tigyi G, Natarajan V, Pyne S, Pyne NJ. Cell migration activated by platelet-derived growth factor receptor is blocked by an inverse agonist of the sphingosine 1-phosphate receptor-1. Faseb J. 2006;20:509–511. doi: 10.1096/fj.05-4810fje. [DOI] [PubMed] [Google Scholar]
- Wu Z, Bowen WD. Role of sigma-1 receptor C-terminal segment in inositol 1,4,5-trisphosphate receptor activation: constitutive enhancement of calcium signaling in MCF-7 tumor cells. J. Biol. Chem. 2008;283:28198–28215. doi: 10.1074/jbc.M802099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagasaki Y, Numakawa T, Kumamaru E, Hayashi T, Su TP, Kunugi H. Chronic antidepressants potentiate via sigma-1 receptors the brain-derived neurotrophic factor-induced signaling for glutamate release. J. Biol. Chem. 2006;281:12941–12949. doi: 10.1074/jbc.M508157200. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cuevas J. Sigma receptors inhibit high-voltage-activated calcium channels in rat sympathetic and parasympathetic neurons. J. Neurophysiol. 2002;87:2867–2879. doi: 10.1152/jn.2002.87.6.2867. [DOI] [PubMed] [Google Scholar]