Abstract
The laboratory diagnosis of Clostridium difficile infection (CDI) continues to be challenging. Recent guidelines from professional societies in the United States note that enzyme immunoassays for toxins A and B do not have adequate sensitivity to be used alone for detecting CDI, yet the optimal method for diagnosing this infection remains unclear. Nucleic acid amplification tests (NAATs) that target chromosomal toxin genes (usually the toxin B gene, tcdB) show high sensitivity and specificity, provide rapid results, and are amenable to both batch and on-demand testing, but these tests were not universally recommended for routine use in the recent guidelines. Rather, two-step algorithms that use glutamate dehydrogenase (GDH) assays to screen for C. difficile in stool specimens, followed by either direct cytotoxin testing or culture to identify toxin-producing C. difficile isolates, were recommended in one guideline and either GDH algorithms or NAATs were recommended in another guideline. Unfortunately, neither culture nor direct cytotoxin testing is widely available. In addition, this two-step approach requires 48 to 92 hours to complete, which may delay the initiation of therapy and critical infection control measures. Recent studies also show the sensitivity of several GDH assays to be <90%. This review considers the role of NAATs for diagnosing CDI and explores their potential advantages over two-step algorithms, including shorter time to results, while providing comparable, if not superior, accuracy.
The laboratory diagnosis of Clostridium difficile infection (CDI) is, according to the latest clinical practice guidelines issued by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA), in a state of flux.1 The most widely used tests in clinical microbiology laboratories for detection of CDI [ie, enzyme immunoassays (EIAs) for toxins A and B] are no longer considered to have adequate sensitivity to be used as stand-alone tests for CDI.1,2 A comprehensive survey of the literature, conducted by Crobach and colleagues3 for the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), showed that the mean sensitivity of well-type EIAs for toxins A and B was 66%, whereas the mean sensitivity for membrane-type EIAs for toxins A and B was only 52%, when compared with toxigenic culture as the reference method. Nucleic acid amplification tests (NAATs), which typically show both high sensitivity and specificity for detection of CDI, may ultimately be the best tests, according to recent commentaries and practice guidelines from the American Society for Microbiology (ASM, http://www.asm.org/images/pdf/Clinical/clostridiumdifficile9-21.pdf, last accessed April 25, 2011)4–6; however, at publication of the SHEA-IDSA guidelines, there was insufficient data in the literature on which to base a recommendation for their use. The mean sensitivity of PCR in the ESCMID survey was 86%, whereas the mean specificity was 97%.3 In lieu of using EIA tests or PCR assays as stand-alone tests, the SHEA-IDSA guidelines recommended a two-step algorithm using glutamate dehydrogenase (GDH) as a screening test, followed by either a cell culture cytotoxin neutralization (CCCN) assay or bacterial culture coupled with a toxin assay on the purified organism (ie, toxigenic culture) for confirmation.1 The recommendations did not support the use of an EIA test for confirmation of GDH-positive samples because of a lack of sensitivity of the EIA tests. This is consistent with multiple reports,7–10 all of which note that EIA confirmation of GDH-positive samples is too insensitive for routine use, a conclusion also consistent with the ASM guidelines. The SHEA-IDSA recommendations and the guidelines from ASM are in sharp contrast to those of ESCMID, which require a combination of two positive test results (EIA, GDH, and/or PCR) for diagnosis of CDI but accept any negative test result, including a single EIA toxin A/B test, as an indication that the diarrheal disease is not caused by C. difficile. This ESCMID guideline is based on the high negative predictive values of EIAs, even though the sensitivities of the assays can be <50%,3 a statistical phenomenon common to poorly performing tests when used in a low-prevalence disease setting. This phenomenon may also result in unacceptable positive predictive values. Given these conflicting recommendations, the optimal methods for diagnosis of CDI are worthy of closer examination.
Organism Description
The organism now known as C. difficile was described by Hall and O'Toole in 1935,11 but it was not until 1978 that Bartlett and colleagues12 identified C. difficile as the causative agent of antibiotic-associated pseudomembranous colitis. C. difficile is a Gram-positive, anaerobic, spore-forming rod. It is found in humans, a variety of animals, and the environment.13,14 Two toxins, designated A and B, encoded by the chromosomal genes tcdA and tcdB, respectively, are part of a pathogenicity locus (PaLoc) that is typically present in those strains of C. difficile that cause disease. The toxins are regulated by two additional genes, tcdC and tcdR; a holinlike protein is also encoded by tcdE.15 According to an elegant series of experiments by Lyras et al,16 toxin B is the most critical determinant of pathogenicity for human infections. Although this has been recently challenged by Kuehne et al,17 studies by Leav and colleagues18 support the critical role of toxin B in infection, noting that patients with low levels of antibody specific for toxin B, but not for toxin A, are more likely to have recurrent disease. An additional toxin called the binary toxin, which is present only in a few strains of C. difficile, is encoded by two genes designated cdtA and cdtB, located on the bacterial chromosome outside of PaLoc.19 The role of binary toxin in pathogenesis remains controversial.
The organism's spores are resistant to heat and desiccation and can remain viable in the hospital environment for weeks.20 Spore production, which varies among strains of C. difficile,21 enhances the ability of the organism to spread among hospitalized patients.22 Some strains of C. difficile appear to have enhanced capability for spreading and causing outbreaks. These include the J strain described in 199923 and the 027/NAP1/BI strain independently described in North America and Europe24,25 in 2005 and 2006, respectively.
Laboratory Methods
A variety of laboratory methods can be used for diagnosis of CDI. A sample of their reported performance characteristics is summarized in Table 1 and reviewed later.
Table 1.
Product Performance Data for Selected Tests for Laboratory Diagnosis of CDI When Compared with the Results of CCCN and TC
| Type and name of test | Sensitivity |
Specificity |
Predictive value of positive result |
Predictive value of negative result |
||||
|---|---|---|---|---|---|---|---|---|
| CCCN | TC | CCCN | TC | CCCN | TC | CCCN | TC | |
| CCCN2,26,27 | ||||||||
| Laboratory-developed cytotoxin | 76 | 100 | 100 | 97 | ||||
| TechLab (Wampole) | 64–67 | 99 | 93 | 94 | ||||
| EIA for toxins2,7,28–30 | ||||||||
| Meridian AB | 96–99 | 48–58 | 94–97 | 95–98 | 51–88 | 69–88 | 99 | 87–92 |
| TechLab AB | 60–96 | 87–99 | 96 | 92 | ||||
| Remel Xpect | 96 | 48 | 99 | 84 | 95 | 46 | 99 | 85 |
| Lateral flow for toxins7,28,29,31 | ||||||||
| TechLab | 43–80 | 99 | 94 | 97 | 76 | 97 | ||
| Meridian Immunocard | 96 | 48 | 99 | 99 | 95 | 91 | 99 | 87 |
| GDH2,6,7,28,31,32 | ||||||||
| Marion latex (no longer available) | 68 | 95 | 59 | 96 | ||||
| Biocite Triage | 32–80 | 100 | 100 | 84–95 | ||||
| TechLab | 83–94 | 97 | 83–88 | 97–98 | ||||
| NAATs26,27,33–37 | ||||||||
| Gen-Probe ProGastro (PCR) | 85–92 | 77 | 95–96 | 98 | 69 | 94 | 98 | 96 |
| BD GeneOhm (PCR) | 84–96 | 84–94 | 95 | 98 | 65–70 | 84–90 | 99 | 97–99 |
| Cepheid Xpert (PCR) | 94–96 | 96–97 | 80–84 | 98–99 | ||||
| Meridian Illumigene (LAMP) | 92–98 | 98–99 | 92 | 99 | ||||
CCCN and TC were the reference methods.
TechLab, Blacksburg, VA; Meridian Biosciences, Inc., Cincinnati, OH; Remel, Lenexa, KS; Biocite, Inc, San Diego, CA; Gen-Probe, San Diego, CA; BD GenOhm, La Jolla, CA; Cepheid, Sunnyvale, CA.
CCCN, cell culture cytotoxin neutralization; TC, toxigenic culture.
Culture on Agar Media
Although culture methods for propagation of anaerobic organisms used to be common in many clinical microbiology laboratories in the 1970s and 1980s, few laboratories, at least in the United States, continue to use culture-based methods for C. difficile detection.38 According to a recent survey conducted by the Association of Practitioners of Infection Control, most testing for identification of C. difficile in clinical samples is by non–culture-based methods.38 Culture methods for C. difficile are considered sensitive but not specific for diagnosis because nontoxigenic strains of C. difficile, which are not considered to be pathogenic, can be recovered from stool samples of both symptomatic and asymptomatic patients. Nevertheless, because of its high sensitivity, culture, together with the identification of toxin production from pure cultures of organisms (referred to as toxigenic culture), has replaced the CCCN assay as the reference method for CDI diagnosis in most studies.1,3 However, some investigators3,6 still hold that the identification of toxin in stool, and not the detection of the organism or the gene that encodes the critical toxin, should be considered the reference method for diagnostic studies.
Enriching for Spores
One of the challenges of recovering bacterial agents of diarrheal disease is the competing flora present in stool samples. However, the resiliency of C. difficile spores provides a means of enriching for C. difficile, by treating the stool sample with either ethanol or heat before culture on solid agar.39 These shock methods reduce the other stool flora present and, when combined with chemicals that stimulate growth of vegetative forms, such as taurocholate, work well to enhance the sensitivity of the culture method.
Selective Media
One of the most widely used selective agar media for recovery of C. difficile from stool is pre-reduced cycloserine-cefoxitin-fructose agar,40 which may be supplemented with taurocholate to enhance spore germination.41 This medium often is used in conjunction with an enrichment broth, such as cycloserine-cefoxitin-mannitol broth with taurocholate, lysozyme, and cysteine, to enhance recovery of C. difficile. Selective media typically are incubated at 35°C for 48 hours before examination for C. difficile colonies, which have a ground-glass appearance and smell of para-cresol (similar to a horse barn). The presence of large, Gram-positive, obligately anaerobic rods on the agar medium, which are susceptible to 5 μg of vancomycin, is presumptive evidence of C. difficile. Gas-liquid chromatography, demonstrating the presence of isocaproic, isovaleric, and isobutyric acids as end products of glucose fermentation, can be used for species confirmation.42 Direct plating of stool on selective agar media, which is common in many laboratories that perform anaerobic cultures for C. difficile, is 18% to 20% less sensitive than using broth enrichment to enhance recovery of organisms in culture; however, broth enrichment adds at least 24 hours to the turnaround time of testing, which is already slow.33
CCCN Test
For many years, the CCCN test was considered the gold standard for diagnosis of CDI because this was a direct indication of the presence of a toxin (ie, toxin B) in a clinical sample.1,2,5 Strains of C. difficile that do not produce toxins are common and considered nonpathogenic, which is why culture methods alone are not sufficient for diagnosis of CDI. Both older published results32 and the conclusions of newer studies2,5 suggest that the CCCN test lacks adequate sensitivity for detection of toxin-producing strains, partially because of the degradation of the toxin over time.43 Eastwood et al2 compared the results of EIA, CCCN, and toxigenic culture for detection of C. difficile from a series of stool samples. Compared with culture, CCCN was only 66.7% sensitive. A more recent study5 reiterated that a commercial CCCN assay was only 67.0% sensitive when compared with toxigenic culture.
Antigen Detection Methods
EIA Methods
EIA methods for toxins A and B have been among the most widely used diagnostic tests for diagnosing CDI over the last two decades because they are rapid, simple to perform, and relatively inexpensive.2 Although toxin B is the determinant of pathogenicity,16 testing for both toxins can potentially add sensitivity to an assay, because of the differential lability of toxins in feces.43 Most of the EIA assays were compared with CCCN during initial evaluations, which made the test appear to have adequate sensitivity for routine laboratory use.2 However, more recent studies,28,44 in which toxigenic culture with broth enrichment was used as the reference method, indicate that many EIA assays have sensitivities no better than 60%.
GDH Assays
GDH is a cell-associated enzyme antigen (protein) found on most isolates of C. difficile and occasionally on the surface of other Clostridium species. It is relatively stable in the feces and, because of its apparent ubiquity on isolates of C. difficile, has been proposed as a sensitive but nonspecific screening test for C. difficile in stool samples.1,45 Because GDH assays are purported to be highly sensitive but not specific for toxin-producing C. difficile isolates, this assay is usually used to screen stool specimens as part of a two- or three-step algorithm. Data from recent studies46,47 supported the use of a two-step approach, although another study7 raised concerns about using EIA tests for toxins A and B as confirmatory tests for GDH-positive samples because of the low sensitivity of the toxin EIAs. The SHEA-IDSA guidelines recommend screening liquid stools using a GDH EIA test and confirming positive results with either CCCN or toxigenic culture.1 However, this approach often requires several days to complete, and neither assay is commonly available in clinical laboratories. The recent comprehensive study of C. difficile detection methods,2 which reported the sensitivity of a commonly used GDH assay as 87.6% when compared with toxigenic culture, is consistent with concerns of falsely GDH-negative samples raised by the report of Larson et al.48 Novak-Weekley et al34 reported that initial GDH screening failed to identify approximately 15% of samples containing toxigenic C. difficile isolates. In addition, the mean sensitivity of membrane-type GDH assays in the ESCMID survey was only 60% when compared with toxigenic culture,3 suggesting that GDH screening may not be as highly sensitive as previously assumed.13 A recent meta-analysis of GDH tests by Shetty and colleagues45 reported that, when compared with the results of toxigenic culture, the sensitivity of GDH assays ranged from a low of 79.2% to 98% and varied with the prevalence of CDI. This, along with the data on GDH sensitivity reported by Peterson and Robicsek,5 calls into question the utility of a two-step approach. Indeed, a recent point-counterpoint article6 on C. difficile laboratory methods cited the sensitivity of GDH assays as an unresolved issue. A recent report by Tenover and colleagues33 suggests that some of the variability of the sensitivity of GDH assays reported in the literature may be because of reduced sensitivity of GDH assays for detecting PCR ribotypes other than type 027.
Why Are There Disparities in the Published Reports of Test Performance for Antigen-Based Assays?
What accounts for the stark performance differences between immunoassays, particularly the GDH and toxin A and B EIAs, and other direct detection methods reported in the literature? One explanation could be degradation of toxin proteins in transport or during storage before batch testing.43 Toxin A and, especially, toxin B are subject to time-dependent degradation due to proteolysis and pH effects.32 Although these proteins are generally stable in stool at 4°C, this is not the ambient temperature of the gastrointestinal tract, within which degradation is probably a continuous process. Specimens may sit for several minutes to hours at room temperature in a bedpan before being placed in a transport container and sent to the laboratory for processing. Thus, ample opportunities exist for toxin degradation before the specimen reaches the laboratory. Another possible explanation for the variation in test sensitivity is the sequestration of toxin proteins via naturally occurring polymers in the gut. Toxin A binds carbohydrate components, and both toxins A and B are bound by anionic polymers.49 Toxin sequestration by natural dietary sources of anionic carbohydrate polymers, such as carrageenans, within the gastrointestinal tract could make the toxins less available for detection with antibody-based assays.50 Sucralfate may also bind toxin B, reducing its detection by antigen-based assays.51
A third explanation for the disparity in results among antigen detection assays is related to the genetic diversity of the target proteins, particularly the antigenic variation of the toxins, which may decrease the sensitivity of antibody-based assays, depending on the strain mix present in a hospital or a community. For example, a recent study33 of 350 human isolates of toxigenic C. difficile from North America reported that, although 18 different PCR ribotypes and seven different pulsed-field gel electrophoresis patterns were detected, approximately 30% of the isolates demonstrated unique undesignated ribotyping patterns and 46% were novel undesignated pulsed-field gel electrophoresis patterns, indicating a high degree of genetic diversity among the isolates. Of those organisms that were typable, a disparity in performance of both toxins A and B and GDH antigen tests compared with PCR was observed. Although GDH assays showed equivalent sensitivity to the PCR test for ribotype 027 strains when compared with the results of toxigenic culture, the sensitivity of GDH screening assays for non-027 strains decreased to 69.4% compared with toxigenic culture.33 The sensitivities of toxins A and B EIA test results were significantly lower for ribotypes 002 (15.4%), 027 (78.4%), and 106 (18.8%) compared with sensitivities of 100%, 100%, and 75% for the PCR assay, respectively (P < 0.0001, P < 0.0001, and P < 0.0005, respectively).33 This suggests that variations in the protein sequences, particularly within tcdB, directly affect the sensitivity of the antigen-based assays, whereas the PCR tests, which target conserved DNA regions, are less affected by sequence variation. To investigate this further, the amino acid sequences of toxin B from 16 isolates of C. difficile (based on DNA sequences available in GenBank) were aligned. The amino acid sequence heterogeneity at each residue position is indicated in the red regions in Figure 1 (higher peaks indicate greater sequence diversity). The antigenicity of the sequences, which was calculated using the algorithm of Kolaskar and Tongaonkar,52 is indicated by the black lines (higher peaks, either above or below the midline indicate greater antigenicity). Many of the regions of high antigenicity (black lines) also show highly variable amino acid sequences (red regions), suggesting the possibility of strain-to-strain variation in antigenicity. Thus, antibodies raised against a limited number of common-type strains of C. difficile may have difficulty detecting the full range of strain types of C. difficile that cause disease in humans, as indicated in the North American study.33 On the other hand, nucleic acid–based assays have primarily targeted sequences in the conserved regions of tcdB, such as those labeled with blue bars above the graph (where the amino acid sequence variability shown in red is low). These gene sequences appear to be conserved across the various strain types, making the impact on the sensitivity of PCR-based diagnostic tests low.
Figure 1.
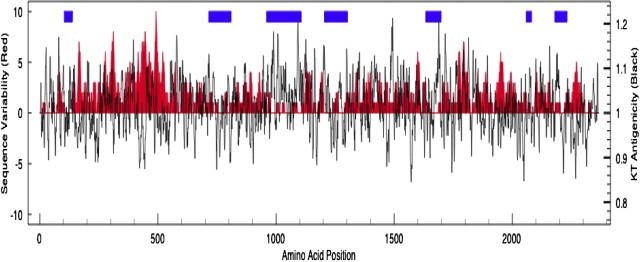
Amino acid heterogeneity (red) and predicted antigenicity of toxin B protein (black lines) based on an alignment of amino acid sequences of toxin B from 16 C. difficile isolates. The red-shaded plot is the variability of the amino acid sequences of toxin B at each amino acid position; great amino acid diversity is indicated by higher peaks. The black line indicates the predicted antigenicity of the protein based on the algorithm of Kolaskar and Tongaonkar (KT)52 (high peaks indicate high antigenicity). The blue bars indicate regions of DNA sequence conservation among the 16 isolates. The 16 isolates include strains of PCR ribotypes 001, 017, 027, and 078 and pulsed-field gel electrophoresis types NAP1, NAP7, and NAP8.
Nucleic Acid Amplification Methods
A comparison of the number of steps and length of time required to perform the four US Food and Drug Administration (FDA)–cleared nucleic acid amplification assays for laboratory diagnosis of CDI, based on their respective package inserts, is shown in Figure 2. The tests require anywhere from 45 minutes to 3 hours to perform and vary in complexity from 3 to 12 steps. Those assays that have multiple manual steps are more prone to crossover contamination than those assays in which extraction and amplification are performed in sealed tubes.
Figure 2.
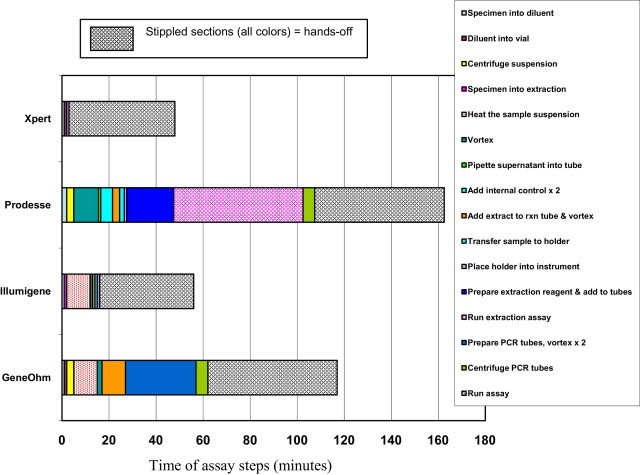
Steps involved in four FDA-cleared nucleic acid amplification assays for the detection of CDI. Data were extracted from package inserts and published literature.34,36,53,54 rxn, reaction.
PCR Assays
The use of a PCR assay to detect tcdB of C. difficile was first reported by Gumerlock et al55 in 1993. Since then, a variety of PCR assays targeting either tcdB or the tcdC regulator gene have been described.28,34,56 All three FDA-cleared commercial PCR-based assays for C. difficile use tcdB as their major target. Evaluations of several commercial PCR assays26,34,35 have appeared in the literature, and several investigators8–10 have proposed using PCR assays to confirm GDH screening tests in lieu of using EIA assays because of the low sensitivity of the EIA component of the assays. The SHEA-IDSA guidelines suggest that PCR may be the most sensitive and specific diagnostic method for CDI detection, but additional studies in which toxigenic culture is used as the reference method are needed to validate this approach, particularly because PCR assays tend to be more expensive than antigen-based methods.1 In their investigation comparing EIA, GDH, and PCR-based methods, Larson et al48 retested GDH-negative stool samples by PCR and identified four additional positive samples among their cohort of patients. All four patients had subsequent stool samples that were positive by both PCR and toxigenic culture, and a review of the patients' medical records revealed that three of four patients already had been treated for CDI.48 Thus, retesting of GDH-negative samples by PCR lowered the sensitivity of the GDH algorithm from 97.1% to 83.8%, and the negative predictive value also decreased from 99.7% to 97.9%.
The report of Larson and colleagues48 addresses the key controversy of using PCR methods for detection of CDI, which is whether the increased sensitivity and speed of direct PCR testing of liquid stool samples is worth the added expense of the method when compared with EIA testing. In their cost analysis, Larson et al48 concluded that the increased cost of either direct PCR testing or the three-step algorithm (GDH testing, followed by EIA confirmation of positive samples, followed by PCR testing of GDH-positive EIA-negative samples) was justified by the earlier detection of CDI cases, which would prevent additional cases of nosocomial CDI and shorten the length of stay for patients with CDI. Babady et al, 57 in their recent study of a commercial PCR assay, also concluded that using PCR as a stand-alone test for CDI was cost-effective after considering both turnaround time and labor costs. Using PCR for tcdB as a stand-alone test for CDI detection also is advocated strongly in a recent point-counterpoint editorial by Fang6, whereas Wilcox and colleagues6 argue in the same article that the best approach is still unknown but probably requires at least two methods used in either parallel or series. The uncertainty, as presented by Wilcox and colleagues6 in the same article, is that CCCN, which they feel is the best indicator of disease, clearly lacks sensitivity compared with toxigenic culture and, thus, cannot be used as a stand-alone test. The issue of whether PCR assays identify C. difficile isolates that harbor tcdB genes that are not expressed is often raised. Although there are no published data to address this issue directly, the problem can be mitigated by testing only liquid stool samples from those patients who are suspected of having CDI, as noted in the package inserts of each of the products that are FDA cleared in the United States. It is conceivable that some patients may be colonized with toxigenic C. difficile strains but have symptoms based on another diarrheal pathogen; however, such instances are likely rare in most institutions.
LAMP Assays
Commercial loop-mediated amplification (LAMP) assays are isothermal nucleic acid amplification reactions that do not require expensive instrumentation to perform. The first LAMP assay for C. difficile was described by Kato et al58 in 2005 and targeted tcdB. More recently, a commercial LAMP assay that targets the tcdA gene (toxin A) of C. difficile has been described. Norén et al36 reported sensitivity and specificity values of 98% and 98%, respectively, for 272 samples using toxigenic culture as the gold standard. However, this report also notes that targeting tcdA may be suboptimal because of the “importance of toxin B in virulence and the existence of toxin A-negative strains.”36 Although some C. difficile strains, such as toxinotypes VIII and X, are reported as toxin A negative (but toxin B positive), vestigial tcdA sequences still present in such isolates apparently are sufficient to provide a positive amplification signal.59 A more recent study37 of 472 stool samples reported sensitivity and specificity results of 91.8% and 99.1%, respectively, although the researchers did not specifically report testing known toxin A–negative isolates. Data on detection of other C. difficile strain types, such as toxinotypes XIV and XX19, which are toxin A negative and have more substantial deletions of tcdA, are lacking. One commercial LAMP assay has been cleared by the FDA for testing symptomatic children between 1 and 2 years of age. The three FDA-cleared PCR assays are not specifically cleared for use in samples from children less than 2 years of age. Since infants are frequently colonized shortly after birth with toxigenic C. difficile strains that they may still harbor asymptomatically until 2 years of age, testing samples from children between the ages of 1 and 2 years continues to be controversial.
Potential Limitations of Direct Detection Methods
Tests for C. difficile, whether culture, antigen, or nucleic acid based, perform two important, but slightly divergent, functions: first, they are used to diagnose patients with clinical presentations consistent with CDI so that appropriate therapy can be initiated; and second, they identify the human reservoirs of C. difficile that require timely infection control measures to prevent hospital spread. For the latter purpose, CCCN-based testing is probably inadequate because it will miss up to 30% of positive specimens, and EIA typically will miss, on average, 50% of cases; in a worst-case scenario, EIA may miss up to 85% (if ribotype 002 was the predominant hospital strain) of potentially infectious cases in need of contact isolation precautions.33 However, direct detection methods, whether based on culture or NAAT, run the potential risk of overestimating the etiological role of C. difficile in nosocomial diarrheal disease, especially in patients with extended hospitalization who may develop diarrhea because of any number of other infectious or noninfectious causes. Clearly, as described by Larson et al,48 patients who test negative by toxin assays (either A and B or GDH) but who are positive by both PCR and culture can benefit from therapy, but additional studies are needed to clarify optimal management of such patients.
Effect of Various Testing Algorithms on Isolation of Patients with CDI
A review of various testing algorithms and their impact on the isolation of patients with CDI is presented in Table 2. This model assumes testing of 1000 patients in a hospital with 10% prevalence of CDI (ie, 100 true-positive patients and 900 true-negative patients). The average cost per test reflects reagents only and not the cost of capital equipment or labor. Sensitivity and specificity values are based on published literature2,28,34,53,62 and represent midrange values. For the model, the sensitivity of the GDH portion of the GDH-EIA testing algorithm was set at 87%,2,34,45 so the sensitivity of the testing algorithms that reflex to NAATs, CCCN, or toxigenic culture testing for GDH-positive/EIA-negative specimens cannot exceed this value, because specimens that are negative by GDH will be excluded from further testing. GDH and EIA testing may be either in parallel (ie, together in the same test) or sequential, if a stand-alone GDH assay is used, followed by an independent EIA toxin A/B test.2,34 The model assumes that 32 specimens will be GDH positive and EIA negative and, thus, available for reflex testing. The sensitivities of the reflex tests are as follows: NAATs, 95%26,33,34,56,57; toxigenic culture, 99%42; and cytotoxin testing, 70%.2,5,37 The isolation protocols are based on data from studies by Lee et al63 and Peterson et al.64 The model assumes that only one laboratory test is performed each day (on the first shift), so reflex test results for NAATs would be available the following day (because one protocol requires 3 hours to complete), toxigenic culture results in 4 days, and CCCN (direct cytotoxin testing) results in 2 days. The length of patient stay in the health care facility is set at 5 days. Thus, toxigenic culture results are typically available 1 day before the patient is discharged in this model, because they require 4 days to complete. Patients with negative test results are not placed in isolation if test results are available the same day (ie, for GDH/EIA testing alone and NAAT alone). For the three reflex tests, the model assumes that patients will be placed in isolation if the GDH component is positive and not removed from isolation until negative results are received for the reflex test; however, patients will not be treated for CDI until a final positive laboratory test result is available. This is based on infection control protocols in use at several US hospitals.5,20
Table 2.
The Effect of Various Testing Algorithms on Isolation of Patients with CDI
| Testing approach | Average cost/test ($) | Sensitivity of test/algorithm (%)⁎ | No. of patients positive for CDI missed | Specificity of test/algorithm (%)⁎ | No. of false-positive test results | Patients in isolation with CDI | Patients in isolation without CDI | Patients with CDI not in isolation |
|---|---|---|---|---|---|---|---|---|
| GDH or EIA alone | 18.00 | 55 | 45 | 94 | 54 | Days 1–5, 55 | Days 1–5, 54 | Days 1–5, 45 |
| Reflex to NAAT for GDH+ and EIA- | 19.12 | 85 | 15 | 93.9 | 55 | Day 1, 55; days 2–5, 85 | Day 1, 54; days 2–5, 55 | Day 1, 45; days 2–5, 15 |
| Reflex to toxigenic culture for GDH+ and EIA- | 18.51 | 86 | 14 | 93.9 | 55 | Days 1–4, 55; day 5, 86 | Days 1–4, 54; day 5, 55 | Days 1–4, 45; day 5, 14 |
| Reflex to direct cytotoxin for GDH+ and EIA- | 18.32 | 77 | 23 | 93.9 | 55 | Days 1–2, 55; days 3–5, 77 | Days 1–2, 54; days 3–5, 55 | Days 1–2, 45; days 3–5, 23 |
| NAAT alone | 35.00 | 95 | 5 | 96 | 36 | Days 1–5, 95 | Days 1–5, 36 | Days 1–5, 5 |
Assumptions: 1000 patients tested with each test or algorithm; 10% prevalence (ie, 100 true-positive patients and 900 true-negative patients); GDH-positive and EIA-negative samples = 32, which will be retested using one of the reflex methods; testing on first shift of each day; one test type is performed per day; no pre-emptive isolation if test results are reported on same day as ordered (ie, <8-hour turnaround time); pre-emptive isolation for CDI orders includes time for other tests; isolation continues until the day the test result is negative; assumes a 5-day length of stay.
Sensitivity and specificity values are based on published literature.2–5,7–10,26–30,32–37,44–48,53–56,58–61
The model illustrates several points. First, if GDH/EIA testing results are used without further testing, there are almost as many patients in contact isolation who have CDI (true positives, 55 patients) as who do not have CDI (false positives, 54 patients). In addition, 45 patients with CDI are not in isolation (false negatives) and will continue to spread disease on the hospital wards. In contrast, GDH/EIA algorithms that reflex to PCR, toxigenic culture, or CCCN testing increase the sensitivity of detecting CDI cases over using GDH/EIA alone, although the false-positive results remain high (55 patients), as do the costs of unnecessarily isolating these patients. This is consistent with the results of several reports.7–10 Although CCCN is recommended in the SHEA-IDSA guidelines,1 this approach is less desirable as a reflex test because of its reduced sensitivity compared with NAATs and toxigenic culture and, from a more practical standpoint, because of its general lack of availability in most hospital laboratories. Although reflex testing using toxigenic culture produces statistically equivalent sensitivity and specificity to NAAT reflex testing, the delay in finalizing results would likely have a negative impact on both infection control costs and, potentially, on the initiation of therapy for patients with CDI. In contrast, NAATs identify 95% of the CDI-positive patients, while only indicating that 36 patients without CDI should be placed in isolation. More important, only five CDI-positive patients are missed and have the opportunity to continue to spread disease. Thus, NAATs as stand-alone methods or as a reflex test for GDH-positive/EIA-negative specimens have advantages in time to detection and overall sensitivity over the SHEA-IDSA–recommended algorithms and are consistent with the ASM recommendations. However, there are few data available on the cost-effectiveness of this approach; such studies would be of value. From a regulatory standpoint, using only the GDH portion of the combined assay and not reporting the EIA portion of the test may require validation in a separate study (ie, apart from verification studies for combined GDH/EIA results) by the laboratory before implementation, because this may be considered off-label use.60
Summary
Timely and accurate laboratory diagnosis of CDI is important for both patient management and limitation of the nosocomial spread of C. difficile in health care settings.1,3,6 The clinical practice guidelines from SHEA-IDSA provided an interim recommendation to screen liquid or semiformed stool samples with a GDH assay and confirm positive results with CCCN or toxigenic culture.1 The latter confirmatory procedures are not available in most laboratories and, in the case of CCCN confirmation, may result in false-negative results. The ASM guidelines, on the other hand, support the use of NAATs as stand-alone methods for the detection of CDI. The inherent delays in the time to results for the two-step algorithm (48 to 92 hours for a positive case) and the associated costs of unnecessary isolation and delayed therapy argue for faster direct methods, such as those based on nucleic acid amplification. Although more expensive than antigen-based methods, there are significant opportunities to reduce multiple test orders because of the high negative predictive values of NAATs.61 Recent data from several published studies5,48,57 on both the utility and cost-effectiveness of NAATs suggest that the molecular amplification methods are worth consideration.
Acknowledgment
We thank Robert Jones for his assistance in preparing the amino acid sequence analysis and figure.
Footnotes
Supported by BD GeneOhm, Cepheid, and Roche (L.R.P.).
F.C.T., E.J.B., and D.H.P. are employees and shareholders of Cepheid.
References
- 1.Cohen S.H., Gerding D.N., Johnson S., Kelly C.P., Loo V.G., McDonald L.C., Pepin J., Wilcox M.H. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 2.Eastwood K., Else P., Charlett A., Wilcox M. Comparison of nine commercially available Clostridium difficile toxin detection assays, a real-time PCR assay for C. difficile tcdB, and a glutamate dehydrogenase detection assay to cytotoxin testing and cytotoxigenic culture methods. J Clin Microbiol. 2009;47:3211–3217. doi: 10.1128/JCM.01082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crobach M.J., Dekkers O.M., Wilcox M.H., Kuijper E.J. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): data review and recommendations for diagnosing Clostridium difficile-infection (CDI) Clin Microbiol Infect. 2009;15:1053–1066. doi: 10.1111/j.1469-0691.2009.03098.x. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt M.L., Gilligan P.H. Clostridium difficile testing algorithms: what is practical and feasible? Anaerobe. 2009;15:270–273. doi: 10.1016/j.anaerobe.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Peterson L.R., Robicsek A. Does my patient have Clostridium difficile infection? Ann Intern Med. 2009;151:176–179. doi: 10.7326/0003-4819-151-3-200908040-00005. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox M.H., Planche T., Fang F.C., Gilligan P. Point-counterpoint: what is the current role of algorithmic approaches for diagnosis of Clostridium difficile infection? J Clin Microbiol. 2010;48:4347–4353. doi: 10.1128/JCM.02028-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilligan P.H. Is a two-step glutamate dehydrogenase antigen-cytotoxicity neutralization assay algorithm superior to the premier toxin A and B enzyme immunoassay for laboratory detection of Clostridium difficile? J Clin Microbiol. 2008;46:1523–1525. doi: 10.1128/JCM.02100-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swindells J., Brenwald N., Reading N., Oppenheim B. Evaluation of diagnostic tests for Clostridium difficile infection. J Clin Microbiol. 2010;48:606–608. doi: 10.1128/JCM.01579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doing K.M., Hintz M.S., Keefe C., Horne S., LeVasseur S., Kulikowski M.L. Reevaluation of the Premier Clostridium difficile toxin A and B immunoassay with comparison to glutamate dehydrogenase common antigen testing evaluating Bartels cytotoxin and Prodesse ProGastro Cd polymerase chain reaction as confirmatory procedures. Diagn Microbiol Infect Dis. 2010;66:129–134. doi: 10.1016/j.diagmicrobio.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Quinn C.D., Sefers S.E., Babiker W., He Y., Alcabasa R., Stratton C.W., Carroll K.C., Tang Y.W. C. Diff Quik Chek Complete enzyme immunoassay provides a reliable first-line method for detection of Clostridium difficile in stool specimens. J Clin Microbiol. 2010;48:603–605. doi: 10.1128/JCM.01614-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall I.C., O'Toole E. Intestinal flora in new-born infants with a description of a new pathogenic anaerobe: Bacillus difficilis. Am J Dis Child. 1935;49:390–402. [Google Scholar]
- 12.Bartlett J.G., Chang T.W., Gurwith M., Gorbach S.L., Onderdonk A.B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298:531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- 13.Lyerly D.M., Krivan H.C., Wilkins T.D. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988;1:1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samore M.H., Venkataraman L., DeGirolami P.C., Arbeit R.D., Karchmer A.W. Clinical and molecular epidemiology of sporadic and clustered cases of nosocomial Clostridium difficile diarrhea. Am J Med. 1996;100:32–40. doi: 10.1016/s0002-9343(96)90008-x. [DOI] [PubMed] [Google Scholar]
- 15.Rupnik M., Dupuy B., Fairweather N.F., Gerding D.N., Johnson S., Just I., Lyerly D.M., Popoff M.R., Rood J.I., Sonenshein A.L., Thelestam M., Wren B.W., Wilkins T.D., von Eichel-Streiber C. Revised nomenclature of Clostridium difficile toxins and associated genes. J Med Microbiol. 2005;54:113–117. doi: 10.1099/jmm.0.45810-0. [DOI] [PubMed] [Google Scholar]
- 16.Lyras D., O'Connor J.R., Howarth P.M., Sambol S.P., Carter G.P., Phumoonna T., Poon R., Adams V., Vedantam G., Johnson S., Gerding D.N., Rood J.I. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176–1179. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuehne S.A., Cartman S.T., Heap J.T., Kelly M.L., Cockayne A., Minton N.P. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467:711–713. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 18.Leav B.A., Blair B., Leney M., Knauber M., Reilly C., Lowy I., Gerding D.N., Kelly C.P., Katchar K., Baxter R., Ambrosino D., Molrine D. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI) Vaccine. 2010;28:965–969. doi: 10.1016/j.vaccine.2009.10.144. [DOI] [PubMed] [Google Scholar]
- 19.Rupnik M. Heterogeneity of large clostridial toxins: importance of Clostridium difficile toxinotypes. FEMS Microbiol Rev. 2008;32:541–555. doi: 10.1111/j.1574-6976.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- 20.Dubberke E.R., Gerding D.N., Classen D., Arias K.M., Podgorny K., Anderson D.J., Burstin H., Calfee D.P., Coffin S.E., Fraser V., Griffin F.A., Gross P., Kaye K.S., Klompas M., Lo E., Marschall J., Mermel L.A., Nicolle L., Pegues D.A., Perl T.M., Saint S., Salgado C.D., Weinstein R.A., Wise R., Yokoe D.S. Strategies to prevent Clostridium difficile infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S81–S92. doi: 10.1086/591065. [DOI] [PubMed] [Google Scholar]
- 21.Akerlund T., Persson I., Unemo M., Norén T., Svenungsson B., Wullt M., Burman L.G. Increased sporulation rate of epidemic Clostridium difficile Type 027/NAP1. J Clin Microbiol. 2008;46:1530–1533. doi: 10.1128/JCM.01964-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McFarland L.V. What's lurking under the bed?: persistence and predominance of particular Clostridium difficile strains in a hospital and the potential role of environmental contamination. Infect Control Hosp Epidemiol. 2002;23:639–640. doi: 10.1086/501986. [DOI] [PubMed] [Google Scholar]
- 23.Johnson S., Samore M.H., Farrow K.A., Killgore G.E., Tenover F.C., Lyras D., Rood J.I., DeGirolami P., Baltch A.L., Rafferty M.E., Pear S.M., Gerding D.N. Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N Engl J Med. 1999;341:1645–1651. doi: 10.1056/NEJM199911253412203. [DOI] [PubMed] [Google Scholar]
- 24.Kuijper E.J., Coignard B., Tull P. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect. 2006;12(Suppl 6):2–18. doi: 10.1111/j.1469-0691.2006.01580.x. [DOI] [PubMed] [Google Scholar]
- 25.McDonald L.C., Killgore G.E., Thompson A., Owens R.C., Jr, Kazakova S.V., Sambol S.P., Johnson S., Gerding D.N. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 26.Stamper P.D., Alcabasa R., Aird D., Babiker W., Wehrlin J., Ikpeama I., Carroll K.C. Comparison of a commercial real-time PCR assay for tcdB detection to a cell culture cytotoxicity assay and toxigenic culture for direct detection of toxin-producing Clostridium difficile in clinical samples. J Clin Microbiol. 2009;47:373–378. doi: 10.1128/JCM.01613-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbut F., Braun M., Burghoffer B., Lalande V., Eckert C. Rapid detection of toxigenic strains of Clostridium difficile in diarrheal stools by real-time PCR. J Clin Microbiol. 2009;47:1276–1277. doi: 10.1128/JCM.00309-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sloan L.M., Duresko B.J., Gustafson D.R., Rosenblatt J.E. Comparison of real-time PCR for detection of the tcdC gene with four toxin immunoassays and culture in diagnosis of Clostridium difficile infection. J Clin Microbiol. 2008;46:1996–2001. doi: 10.1128/JCM.00032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musher D.M., Manhas A., Jain P., Nuila F., Waqar A., Logan N., Marino B., Graviss E.A. Detection of Clostridium difficile toxin: comparison of enzyme immunoassay results with results obtained by cytotoxicity assay. J Clin Microbiol. 2007;45:2737–2739. doi: 10.1128/JCM.00686-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Berg R.J., Vaessen N., Endtz H.P., Schulin T., van der Vorm E.R., Kuijper E.J. Evaluation of real-time PCR and conventional diagnostic methods for the detection of Clostridium difficile-associated diarrhoea in a prospective multicentre study. J Med Microbiol. 2007;56:36–42. doi: 10.1099/jmm.0.46680-0. [DOI] [PubMed] [Google Scholar]
- 31.Reyes R.C., John M.A., Ayotte D.L., Covacich A., Milburn S., Hussain Z. Performance of TechLab C. DIFF QUIK CHEK and TechLab C: DIFFICILE TOX A/B II for the detection of Clostridium difficile in stool samples. Diagn Microbiol Infect Dis. 2007;59:33–37. doi: 10.1016/j.diagmicrobio.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Peterson L.R., Olson M.M., Shanholtzer C.J., Gerding D.N. Results of a prospective, 18-month clinical evaluation of culture, cytotoxin testing, and culturette brand (CDT) latex testing in the diagnosis of Clostridium difficile-associated diarrhea. Diagn Microbiol Infect Dis. 1988;10:85–91. doi: 10.1016/0732-8893(88)90045-4. [DOI] [PubMed] [Google Scholar]
- 33.Tenover F.C., Novak-Weekley S., Woods C.W., Peterson L.R., Davis T., Schreckenberger P., Fang F.C., Dascal A., Gerding D.N., Nomura J.H., Goering R.V., Akerlund T., Weissfeld A.S., Baron E.J., Wong E., Marlowe E.M., Whitmore J., Persing D.H. Impact of strain types on detection of toxigenic Clostridium difficile: comparison of molecular diagnostic and enzyme immunoassay approaches. J Clin Microbiol. 2010;48:3719–3724. doi: 10.1128/JCM.00427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novak-Weekley S.M., Marlowe E.M., Miller J.M., Cumpio J., Nomura J.H., Vance P.H., Weissfeld A. Clostridium difficile testing in the clinical laboratory using multiple testing algorithms. J Clin Microbiol. 2010;48:889–893. doi: 10.1128/JCM.01801-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamper P.D., Babiker W., Alcabasa R., Aird D., Wehrlin J., Ikpeama I., Gluck L., Carroll K.C. Evaluation of a new commercial TaqMan PCR assay for direct detection of the Clostridium difficile toxin B gene in clinical stool specimens. J Clin Microbiol. 2009;47:3846–3850. doi: 10.1128/JCM.01490-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norén T., Alriksson I., Andersson J., Akerlund T., Unemo M. Rapid and sensitive loop-mediated isothermal amplification test for Clostridium difficile detection challenges cytotoxin B cell test and culture as gold standard. J Clin Microbiol. 2011;49:710–711. doi: 10.1128/JCM.01824-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lalande V., Barrault L., Wadel S., Eckert C., Petit J.C., Barbut F. Evaluation of a loop-mediated isothermal amplification assay for diagnosis of Clostridium difficile infections. J Clin Microbiol. 2011;49:2714–2716. doi: 10.1128/JCM.01835-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarvis W.R., Schlosser J., Jarvis A.A., Chinn R.Y. National point prevalence of Clostridium difficile in US health care facility inpatients, 2008. Am J Infect Control. 2009;37:263–270. doi: 10.1016/j.ajic.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Koransky, Allen S.D., Dowell V.R., Jr Use of ethanol for selective isolation of sporeforming microorganisms. Appl Environ Microbiol. 1978;35:762–765. doi: 10.1128/aem.35.4.762-765.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.George W.L., Sutter V.L., Citron D., Finegold S.M. Selective and differential medium for isolation of Clostridium difficile. J Clin Microbiol. 1979;9:214–219. doi: 10.1128/jcm.9.2.214-219.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson K.H., Kennedy M.J., Fekety F.R. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J Clin Microbiol. 1982;15:443–446. doi: 10.1128/jcm.15.3.443-446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson E.A., Summanen P., Finegold S.M. Clostridium. In: Murray P.R., Baron E.J., Jorgensen J.H., Landry M.L., Pfaller M.A., editors. Manual of Clinical Microbiology. 9th Edition. ASM Press; Washington: 2007. pp. 889–910. [Google Scholar]
- 43.Freeman J., Wilcox M. The effects of storage conditions on viability of Clostridium difficile vegetative cells and spores and toxin activity in human faeces. J Clin Pathol. 2003;56:126–128. doi: 10.1136/jcp.56.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alcala L., Sanchez-Cambronero L., Catalan M.P., Sanchez-Somolinos M., Pelaez M.T., Marin M., Bouza E. Comparison of three commercial methods for rapid detection of Clostridium difficile toxins A and B from fecal specimens. J Clin Microbiol. 2008;46:3833–3835. doi: 10.1128/JCM.01060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shetty N., Wren M.W., Coen P.G. The role of glutamate dehydrogenase for the detection of Clostridium difficile in faecal samples: a meta-analysis. J Hosp Infect. 2011;77:1–6. doi: 10.1016/j.jhin.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 46.Reller M.E., Lema C.A., Perl T.M., Cai M., Ross T.L., Speck K.A., Carroll K.C. Yield of stool culture with isolate toxin testing versus a two-step algorithm including stool toxin testing for detection of toxigenic Clostridium difficile. J Clin Microbiol. 2007;45:3601–3605. doi: 10.1128/JCM.01305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ticehurst J.R., Aird D.Z., Dam L.M., Borek A.P., Hargrove J.T., Carroll K.C. Effective detection of toxigenic Clostridium difficile by a two-step algorithm including tests for antigen and cytotoxin. J Clin Microbiol. 2006;44:1145–1149. doi: 10.1128/JCM.44.3.1145-1149.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larson A.M., Fung A.M., Fang F.C. Evaluation of tcdB real-time PCR in a three-step diagnostic algorithm for detection of toxigenic Clostridium difficile. J Clin Microbiol. 2010;48:124–130. doi: 10.1128/JCM.00734-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurtz C.B., Cannon E.P., Brezzani A., Pitruzzello M., Dinardo C., Rinard E., Acheson D.W., Fitzpatrick R., Kelly P., Shackett K., Papoulis A.T., Goddard P.J., Barker R.H., Jr, Palace G.P., Klinger J.D. GT160-246, a toxin binding polymer for treatment of Clostridium difficile colitis. Antimicrob Agents Chemother. 2001;45:2340–2347. doi: 10.1128/AAC.45.8.2340-2347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo J.-H., Skinner G.W., Harcum W.W., Barnum P.E. Pharmaceutical applications of naturally occurring water soluble polymers. Pharm Sci Technol Today. 1998;1:254–261. [Google Scholar]
- 51.Jensen G.L., Bross J.E., Bourbeau P.P., Naumovitz D.W., Streater M., Gianferante L.E. Risk factors for Clostridium difficile stool cytotoxin b among critically ill patients: role of sucralfate. J Infect Dis. 1994;170:227–230. doi: 10.1093/infdis/170.1.227. [DOI] [PubMed] [Google Scholar]
- 52.Kolaskar A.S., Tongaonkar P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990;276:172–174. doi: 10.1016/0014-5793(90)80535-q. [DOI] [PubMed] [Google Scholar]
- 53.Karre T., Sloan L., Patel R., Mandrekar J., Rosenblatt J. Comparison of two commercial molecular assays to a laboratory developed molecular assay for diagnosis of Clostridium difficile infection. J Clin Microbiol. 2011;49:725–727. doi: 10.1128/JCM.01028-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kvach E.J., Ferguson D., Riska P.F., Landry M.L. Comparison of BD GeneOhm Cdiff real-time PCR assay with a two-step algorithm and a toxin A/B enzyme-linked immunosorbent assay for diagnosis of toxigenic Clostridium difficile infection. J Clin Microbiol. 2009;48:109–114. doi: 10.1128/JCM.01630-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gumerlock P.H., Tang Y.J., Weiss J.B., Silva J., Jr Specific detection of toxigenic strains of Clostridium difficile in stool specimens. J Clin Microbiol. 1993;31:507–511. doi: 10.1128/jcm.31.3.507-511.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang H., Weintraub A., Fang H., Nord C.E. Comparison of a commercial multiplex real-time PCR to the cell cytotoxicity neutralization assay for diagnosis of Clostridium difficile infections. J Clin Microbiol. 2009;47:3729–3731. doi: 10.1128/JCM.01280-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Babady N.E., Stile J., Ruggiero P., Khosa P., Huang D., Shuptar S., Kamboj M., Kiehn T.E. Evaluation of the Cepheid Xpert Clostridium difficile epi assay for diagnosis C. difficile infection and typing of the NAP1 strain at a cancer hospital. J Clin Microbiol. 2010;48:4519–4524. doi: 10.1128/JCM.01648-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kato H., Yokoyama T., Arakawa Y. Rapid and simple method for detecting the toxin B gene of Clostridium difficile in stool specimens by loop-mediated isothermal amplification. J Clin Microbiol. 2005;43:6108–6112. doi: 10.1128/JCM.43.12.6108-6112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Couturier B., She R.C. 2010. The Illumigene C. difficile Assay Detects Both A+B+ and A-B+ Toxin-Producing Strains of Clostridium difficile: (Abstract ID55, 16th Annual Meeting of the Association for Molecular Pathology) p. 34. Edited by the Association for Molecular Pathology, Bethesda, MD. [Google Scholar]
- 60.Clinical and Laboratory Standards Institute . Molecular Diagnostic Methods for Infectious Diseases, MM3-A2 Approved Guideline. ed 2. 2006. p. 25. Edited by the Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 61.Luo R.F., Banaei N. Is repeat PCR needed for diagnosis of Clostridium difficile infection? J Clin Microbiol. 2010;48:3738–3741. doi: 10.1128/JCM.00722-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharp S.E., Ruden L.O., Pohl J.C., Hatcher P.A., Jayne L.M., Ivie W.M. Evaluation of the C. Diff Quik Chek Complete Assay, a new glutamate dehydrogenase and A/B toxin combination lateral flow assay for use in rapid, simple diagnosis of Clostridium difficile disease. J Clin Microbiol. 2010;48:2082–2086. doi: 10.1128/JCM.00129-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee T.L., Hacek D.M., Stroupe K.T., Collins S.M., Peterson L.R. Three surveillance strategies for vancomycin-resistant enterococci in hospitalized patients: detection of colonization efficiency and a cost-effectiveness model. Infect Control Hosp Epidemiol. 2005;26:39–46. doi: 10.1086/502485. [DOI] [PubMed] [Google Scholar]
- 64.Peterson L.R., Hacek D.M., Robicsek A. 5 Million Lives Campaign: Case study: an MRSA intervention at Evanston Northwestern Healthcare. Jt Comm J Qual Patient Saf. 2007;33:732–738. doi: 10.1016/s1553-7250(07)33088-2. [DOI] [PubMed] [Google Scholar]


