Abstract
Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) is widely used for rapid and reliable identification of bacteria and yeast grown on agar plates. Moreover, MALDI-TOF MS also holds promise for bacterial identification from blood culture (BC) broths in hospital laboratories. The most important technical step for the identification of bacteria from positive BCs by MALDI-TOF MS is sample preparation to remove blood cells and host proteins. We present a method for novel, rapid sample preparation using differential lysis of blood cells. We demonstrate the efficacy and ease of use of this sample preparation and subsequent MALDI-TOF MS identification, applying it to a total of 500 aerobic and anaerobic BCs reported to be positive by a Bactec 9240 system. In 86.5% of all BCs, the microorganism species were correctly identified. Moreover, in 18/27 mixed cultures at least one isolate was correctly identified. A novel method that adjusts the score value for MALDI-TOF MS results is proposed, further improving the proportion of correctly identified samples. The results of the present study show that the MALDI-TOF MS-based method allows rapid (<20 minutes) bacterial identification directly from positive BCs and with high accuracy.
Bloodstream infections and infectious endocarditis remain associated with high morbidity and mortality, even though diagnosis and treatment have greatly improved.1 Rapid identification of the causative microorganism and its associated resistance pattern is a prerequisite for specific antibiotic therapy, which reduces mortality for bloodstream infections. Blood cultures (BCs) are still the gold standard for diagnosis of bloodstream infections, despite the time delay to positive reading of BCs [(time to positivity (TTP)], and despite frequent negative findings in patients treated with antibiotics.2 During the last decade, culture media have been intensively improved to enhance the cultivation of bacteria and yeasts, as well as to reduce TTP. Because further improvements in TTP are difficult to achieve, the focus is shifting to time for identification of microorganisms from positive BCs cultures.
Biochemical differentiation of bacteria and yeasts directly from positive BCs requires at least 6 to 8 hours,3 and it is difficult to separate and correctly identify mixed cultures. Molecular methods such as microarray analysis (eg, the Prove-it sepsis assay; Mobidiag, Helsinki, Finland) and fluorescence in situ hybridization [eg, peptide nucleic acid- fluorescence in situ hybridization (PNA-FISH); AdvanDx, Woburn, MA] are costly, and are restricted to a preselected panel of bacteria and yeasts.4–6 Other molecular methods include real-time PCR and, more recently, broad-spectrum PCR coupled with mass spectrometry to identify the amplification products.7 A complex workflow and the need for expensive instruments have limited the availability of these molecular methods in routine laboratories.
A few years ago, matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was introduced as a rapid and reliable method for bacterial identification from plate cultures,8,9 based on protein profiles characteristic for each organism. This approach has been used to identify a broad spectrum of organisms, including Gram-positive and Gram-negative bacteria, mycobacteria, yeasts, and molds.10–12 The rapid and reproducible MALDI-TOF MS technology occasions only minimal consumable costs and has already replaced biochemical differentiation in several microbiological routine laboratories. More recently, MALDI-TOF MS has been successfully used as a rapid method for the direct identification of bacteria and yeasts from positive BC bottles.13–18 The host proteins and blood cells have to be substantially removed to reveal species-specific protein spectra of bacteria and yeasts. Thus, the sample preparation of BCs for MALDI-TOF MS proves to be a key step for the successful identification.
In previous studies, sample preparation protocols relying on differential centrifugation were applied. Under these protocols, collection tubes with separator gels are used to separate bacteria and yeasts from blood cells.18 Others have applied centrifugation and/or lysis of blood cells by adding sterile water, trifluoroacetic acid, or formic acid.13,16 In the present study, we established and applied an easy-to-perform sample preparation protocol (MALDI Sepsityper; Bruker Daltonik, Bremen, Germany) in combination with a commercially available mass spectrometry typing system (MALDI Biotyper; Bruker Daltonik) for the rapid and reliable identification of bacteria from positive BC bottles. Using the new protocol, the time for sample preparation could be reduced from up to 30 minutes to less than 10 minutes. We evaluated this novel sample preparation method in a prospective survey of 500 positive BC bottles over a 3-month period (Figure 1).
Figure 1.
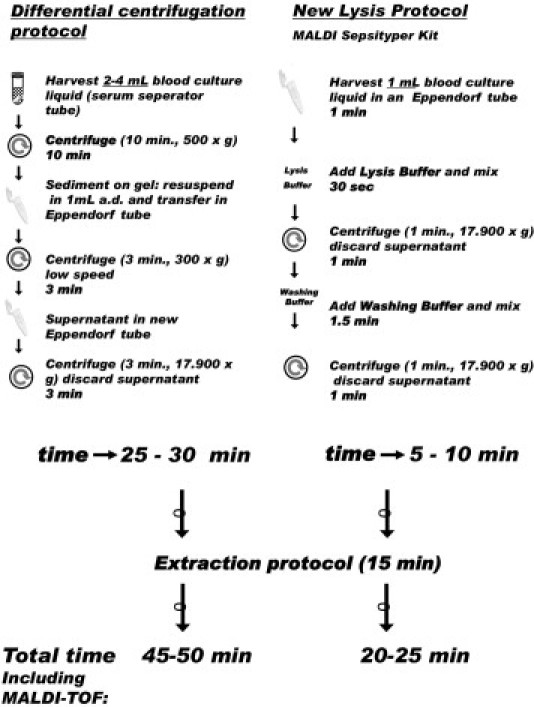
Workflow for the differential centrifugation, a commonly used sample preparation technique for MALDI-TOF MS of positive blood cultures, and a new lysis protocol, MALDI Sepsityper.
Materials and Methods
Blood Culture Procedure
A total of 500 positive BCs were consecutively collected in a 3-month period and analyzed. All BCs were processed using a Bactec 9240 blood culture system (Becton Dickinson, Heidelberg, Germany) and Standard 10 Aerobic/F and 10 Anaerobic/F blood culture media (Becton Dickinson). Once placed in the incubator, inoculated bottles appeared positive between 6 and 24 hours. In parallel with direct identification by MALDI-TOF MS, all of the BCs were subcultured on agar plates, and identification of subcultured isolates was performed.
Preparation of Bacteria for MALDI-TOF Analysis
Sample preparation of the positive BCs was performed using the MALDI Sepsityper kit (Bruker Daltonik) according to the manufacturer's instructions (Figure 1). Technicians of the routine laboratory staff performed the MALDI-TOF MS measurements for the present study. All of them are well trained with the MALDI-TOF MS system and have 2 years of experience using the instrument and the software. In brief, culture fluids (1 mL) drawn from positive BC bottles were transferred to a 1.5-mL reaction tube (Eppendorf, Hamburg, Germany). After addition of 200 μL of the lysis buffer, the sample was mixed using a vortex shaker for 10 s and then centrifuged for 1 minute at 17,900 × g using an Eppendorf 5417 centrifuge (Eppendorf, Germany). The supernatant was discarded and the pellet was resuspended in 1 mL of washing buffer. After a second centrifugation step (1 minute at 17,900 × g), the supernatant was discarded and the pellet was suspended in 300 μL of distilled water.
Extraction of Bacteria for MALDI-TOF MS Analysis
After addition of 900 μL ethanol, the sample was centrifuged at 17,900 × g for 2 minutes. Supernatant was discarded, and residual ethanol was removed after repeated centrifugation. The pellet was suspended in 30 μL of 70% formic acid. Acetonitrile (30 μL) was added, and the sample was mixed using a vortex shaker and briefly centrifuged.
Matrix Preparation and Spotting the MALDI-TOF Plate
The MALDI-TOF α-cyano-4-hydroxycinnamic acid matrix was prepared daily as a saturated solution in 50% acetonitrile and 2.5% trifluoroacetic acid (TFA). Subsequently, 1 μL of the extract of the sample to be analyzed was spotted onto a steel target plate (Bruker Daltonik) and allowed to dry. Next, 1 μL of matrix solution was added and air dried. The target plate was then placed into the MALDI-TOF MS apparatus.
Calibration and Spectral Measurement
Samples were evaluated using a Microflex LT mass spectrometer (Bruker Daltonik) in linear positive-ion mode across the m/z range of 2 to 20 kDa. Each spot was measured by using 240 laser shots at 60 Hz in groups of 40 shots per sampling area of the spot. Spectra were analyzed by using MALDI Biotyper software (version 3.0) (MALDI Biotyper Library version 3.1.1.0; Bruker Daltonik), which includes a proprietary algorithm for spectral pattern matching that yields a logarithmic score from 0 to 3. For identification of bacterial colonies on agar plates, the MALDI Biotyper manufacturer recommends that score values be divided into three intervals; a log(score) of ≥2.0 is mainly considered to be the threshold for a match at the species level, a score of 1.7 to 2.0 indicates a close relationship (at least at the genus level), and a score of <1.7 indicates a non-reliable identification.18,19 The applicability of these criteria for the direct identification of BC isolates was evaluated as part of the present study.
All direct identifications of positive BCs by MALDI-TOF MS analysis were compared with those obtained from single-colony cultures on agar plates. These cultures were then analyzed by MALDI-TOF MS, as well as by standard biochemical identifications, which included oxidase, catalase, a commercial identification strip system (API; bioMérieux, Nürtingen, Germany), and the Phoenix automated system (Becton Dickinson, Heidelberg, Germany). In the case of noncongruence between MALDI and biochemical typing and mass spectrometry fingerprinting, full-length 16S rRNA gene sequencing was performed and the results were compared with annotated sequences from the NCBI GenBank database. For this, we performed PCR using the universal prokaryotic primers fD1 (5′-AGAGTTTGATCCTGGCTCAG-3′) and 800r (5′-CTACCAGGGTATCTAATCC-3′), as described previously, to amplify a 796-bp segment corresponding to a part of the 16S rRNA gene (corresponding to Escherichia coli positions 8 to 803; CP000948.1).20 The nucleotide sequences of both strands of the amplified DNA fragment were determined at Eurofins MWG Operon (Ebersberg, Germany) using an ABI Prism 1.1 BigDye sequencing kit and an ABI 3730 DNA analyzer (Applied Biosystems, Foster City, CA). Nucleotide sequence analysis was performed using Lasergene software version 8.0 (DNASTAR, Madison, WI). The results were compared against the GenBank database (http://www.ncbi.nlm.nih.gov) using BLAST algorithms and against the Ribosomal Database Project (RPD-II; Michigan State University, East Lansing, MI). A 98.5% sequence similarity was chosen as the cutoff value for species identification.
Results
In three consecutive months, 500 positive blood vials were analyzed, of which 483 were monomicrobial. (Table 1). Among these 483 monomicrobial BCs, we identified 98 as Gram-negative (GN) and 358 as Grampositive (GP), as well as 17 fungi, representing 30 genera and 53 species or groups. We first applied the score values proposed by the manufacturer for identification of bacterial colonies on agar plates by MALDI-TOF MS. Because we were not satisfied with the results obtained, we applied a modified interpretation rule for BCs, as previously described for the differential centrifugation method.11 This adapted rule takes into account score values and the identity of consecutively proposed identifications (Table 1). We compared results for the two different rule sets by retrospective analysis of the data. We were able to demonstrate that the cutoff score values could be lowered to ≥1.5 and still provide accurate identification. In the case of a score value between ≥1.5 and <2, we could obtain correct species identifications if the first three proposed results were identical. Thus, applying the modified interpretation rule for BCs, we were able to correctly identify 86.5% of the BCs, compared with 60.7% if using the original manufacturer's protocol. Within all groups, no identifications diverged from those obtained from subcultures. The improvement of the identification rate using the new score interpretation rule differed across groups. For Gram-negative bacteria, the identification rate rose from 85.7% to 89.8%. The improvement of identification rates was even more pronounced for Gram-positive bacteria and yeasts, with a shift from 54.5% to 86.3% and 47% to 70.6%, respectively (Table 1). Gram-positive bacteria and yeasts are generally more difficult to identify by MALDI-TOF MS. In most cases, the identification scores obtained were generally lower than those obtained with Enterobacteriaceae, as has been reported from studies using MALDI-TOF for direct identification of bacteria and yeasts in positive BCs. These lower scores may be due in part to the more compact cell walls in Gram-positive bacteria and yeasts, compared with Gram-negative bacteria, or to the different growth behaviors, which can lead to differing amounts of BC proteins within isolated bacterial clusters.
Table 1.
Identification of Bacteria and Yeasts from Monomicrobial Positive Blood Cultures by Means of MALDI-TOF MS and a Novel Sample Preparation Protocol Using (A) Manufacturer's and (B) Adapted Interpretation Thresholds
| Identification | Isolates [no. (%)] | Threshold A [no. (%)] |
Threshold B [no. (%)] |
||
|---|---|---|---|---|---|
| Identified | Not identified | Identified | Not identified | ||
| Gram-negative | 98 (20.7) | 84 (85.7) | 14 (14.3) | 88 (89.8) | 10 (10.2) |
| Enterobacteriaceae | 3 | ||||
| Citrobacter koseri | 3 | 3 | 8 | ||
| Enterobacter cloacae | 8 | 8 | 43 | 3 | |
| Escherichia coli | 46 | 42 | 4 | 1 | |
| Klebsiella oxytoca | 1 | 1 | 7 | ||
| Klebsiella pneumoniae | 7 | 7 | 1 | ||
| Morganella morganii | 1 | 1 | 1 | ||
| Proteus mirabilis | 1 | 1 | 3 | ||
| Salmonella enterica | 3 | 3 | 2 | ||
| Serratia marcescens | 2 | 2 | |||
| Nonfermenting bacteria | |||||
| Acinetobacter baumannii | 1 | 1 | 1 | ||
| Burkholderia multivorans | 1 | 1 | 1 | ||
| Pseudomonas aeruginosa | 15 | 15 | 15 | ||
| Stenotrophomonas maltophilia | 2 | 2 | 2 | ||
| Haematobacter massiliensis | 1 | 1 | 2 | 1 | |
| Others | |||||
| Aggregatibacter aphrophilus | 3 | 3 | 3 | ||
| Bacteroides fragilis | 2 | 2 | 1 | 1 | |
| Fusobacterium necrophorum | 1 | 1 | 1 | ||
| Gram-positive | 358 (75.7) | 195 (54.5) | 163 (45.5) | 309 (86.3) | 49 (13.7) |
| Staphylococcaceae | |||||
| Staphylococcus aureus | 52 | 48 | 4 | 50 | 2 |
| Staphylococcus capitis | 11 | 8 | 3 | 10 | 1 |
| Staphylococcus caprae | 1 | 1 | 1 | ||
| Staphylococcus epidermidis | 178 | 77 | 101 | 162 | 16 |
| Staphylococcus haemolyticus | 21 | 15 | 6 | 18 | 3 |
| Staphylococcus hominis | 13 | 8 | 5 | 10 | 3 |
| Staphylococcus pettenkoferi | 1 | 1 | 1 | ||
| Staphylococcus saccharolyticus | 1 | 1 | 1 | ||
| Staphylococcus warneri | 5 | 3 | 2 | 5 | |
| Streptococcaceae | |||||
| Enterococcus faecalis | 21 | 13 | 8 | 20 | 1 |
| Enterococcus faecium | 18 | 15 | 3 | 17 | 1 |
| Streptococcus anginosus | 1 | 1 | 1 | ||
| Streptococcus gordonii | 2 | 2 | 2 | ||
| Streptococcus mitis | 4 | 4 | 4 | ||
| Streptococcus oralis | 2 | 2 | 2 | ||
| Streptococcus pneumoniae | 6 | 1 | 5⁎ | 1 | 5⁎ |
| Streptococcus salivarius | 1 | 1 | 1 | ||
| Others | |||||
| Micrococcus luteus | 2 | 2 | 1 | 1 | |
| Rothia mucilaginosa | 1 | 1 | 1 | ||
| Paenibacillus amylolyticus | 1 | 1 | 1 | ||
| Brevibacillus borstelensis | 1 | 1 | 1 | ||
| Bacillus cereus | 1 | 1 | 1 | ||
| Bacillus licheniformis | 1 | 1 | 1 | ||
| Corynebacterium amycolatum | 1 | 1 | 1 | ||
| Corynebacterium coyleae | 2 | 2 | 2 | ||
| Corynebacterium glucuronolyticum | 1 | 1 | 1 | ||
| Corynebacterium jeikeium | 1 | 1 | 1 | ||
| Corynebacterium pseudodiphtheriticum | 1 | 1 | 1 | ||
| Listeria monocytogenes | 1 | 1 | 1 | ||
| Brevibacterium paucivorans | 1 | 1 | 1 | ||
| Propionibacterium acnes | 5 | 1 | 4 | 2 | 3 |
| Fungi | 17 (3.6) | 8 (47) | 9 (52.9) | 12 (70.6) | 5 (29.4) |
| Candida albicans | 5 | 4 | 1 | 5 | |
| Candida glabrata | 7 | 2 | 5 | 5 | 2 |
| Candida guilliermondii | 1 | 1 | 1 | ||
| Candida parapsilosis | 2 | 1 | 1 | 1 | 1 |
| Geotrichum capitatum | 2 | 2 | 2 | ||
These Streptococcus mitis isolates were misidentified as Str. pneumoniae. The necessity to differentiate Str. mitis and Str. pneumoniae by a further test is indicated by the manufacturer of the MALDI Biotyper system (Bruker Daltonic, Bremen, Germany).
In 89.8% of Gram-negative bacteria and 86.3% of Gram-positive bacteria, identification was correct at the species level (Table 1). Enterobacteriaceae constituted 15.2% of all species found, Staphylococcaceae constituted 59.9%, Streptococcaceae and Enterococcaceae together constituted 11.6%, nonfermenting Gram-negative rod bacteria constituted 4.2%, and fungi constituted 3.6%. In 25 of the 27 polymicrobial samples, at least one of the species was correctly identified (92.6%). Five isolates remained misidentified as Streptococcus pneumoniae, all belonging to Streptococcus mitis. Yeasts from positive BCs were less accurately identified by MALDI-TOF MS. In 17 BCs positive for Candida and Geotrichum species (5 C. albicans, 7 C. glabrata, 1 C. guilliermondii, 2 C. parapsilosis, and 2 G. capitatum), 12 microorganisms were identified at the species level (70.6%). This identification rate of Candida and Geotrichum species from positive BCs is superior to that of a previous study using a differential centrifugation protocol.14 In that study, 18 BCs positive for Candida species were investigated; no microorganisms were identified at the species level, and only 1 of the 18 (5.6%) was detected at the genus level.14
Discussion
MALDI-TOF MS is well suited for identification of bacteria and yeasts from positive BC bottles. Compared with the biochemical method of identification for bacteria and yeasts from positive BCs, the use of a MALDI-TOF-based technique saves between 6 and 12 hours for Enterobacteriaceae and up to 48 hours for anaerobic bacteria. The novel sample preparation proposed here is rapid and accurate. It requires only two centrifugation steps, with volumes of 1 mL, and all centrifugation steps are performed in a single tube in the same centrifuge. This approach reduces hands-on preparation time from the 25 to 30 minutes reported from previous studies13–18 down to 5 to 10 minutes, which is compatible with the workflow of a routine microbiology laboratory (Figure 1). The workflow of the novel sample preparation method is more convenient, saves the time of laboratory personnel, and enables the use of MALDI-TOF MS in daily BC culture diagnostics. Established and published methods for preparing positive BC samples for MALDI-TOF MS apply differential centrifugation with up to five different centrifugation steps. Other protocols of differential centrifugation suggest the use of serum separator tubes with a gel at the bottom of the tube to separate bacteria and yeasts from blood cells.15,17 Published sample preparation protocols relying on the lysis of blood cells either use up to four centrifugation steps or work with sample volumes as large as up to 40 mL.13,16,17 We believe the new protocol is more suitable for the use in routine laboratory diagnostics. Moreover, the majority of the published protocols provide MALDI-TOF MS results with a correct identification rate of <80%. Here we describe for the first time a convenient and easy-to-use sample preparation method that leads to MALDI-TOF MS identification rates of >85%, including organisms difficult to analyze, such as Gram-positive bacteria and yeasts.
Compared with the costs of sample preparation by differential centrifugation using serum separation tubes ($2 to $3, USD), the Sepsityper kit is about twice the price. Using the MALDI-TOF MS for identification of bacteria and yeasts, a comparison of the measured mass spectrum with entries of the database is given as a ranking list. The best matching database entry, which reveals the highest score value of comparison, represents the identified species. Up to 10 hits are given in the ranking list of a single measurement. As observed earlier by others,17 and also in the present study, MALDI Biotyper sometimes proposes correct identifications with scores of <1.9. After comparing the results of the direct identification of positive BCs with those obtained from subcultured isolates, we decided to take a lower score value into account so long as the first, second, and third best match from the ranking list refer to a single species. Thus, adapted thresholds of MALDI Biotyper identification score values as low as 1.5 could successfully be implemented.
By applying the adapted thresholds we obtained 86.5% correct identification out of the 500 positive BCs used. Because the estimate of percentage correct identification using the new rule is obtained using the same data from which the rule was derived, the estimate is statistically biased and may be significantly higher than would be obtained if an independent validation set were used for testing the new rule. However, no false identification on the species level was observed, with the exception of inaccurate identification of five Str. mitis isolates as Str. pneumoniae. The misidentification of these two closely related species is a known drawback of MALDI-TOF MS-based methods, one that has been described previously for BC identification and that can be explained by the very close phylogenetic relationship of the two species.17,18,21 Of note, Str. mitis isolates have been misidentified as Str. pneumoniae, but not vice versa. This is probably a result of the database design, which does not allow Str. pneumoniae isolates to be missed. Thus, additional tests such as a bile solubility test directly on the BC broth or antigen tests should be applied to all those organisms identified as being Str. pneumoniae by MALDI-TOF MS.17 This is also advised by the manufacturer of the MALDI Biotyper system.
Further studies are needed to prove universal validity of our proposed method for adaptation of score value thresholds. One may argue that the correct identification rate of 86.5% is too low for clinical use. We propose that the technique of species identification directly from positive BCs using MALDI-TOF MS described here should be envisioned as the first step in a two-step system. The first step is early identification directly from the positive BC bottle. The second step, on the next day, is identification of the residual 13.5% of (subcultured) samples that have not been characterized in the first step. Thus, samples that have failed the first step are not lost, but are processed as before, with the result that exact and correct species identification is achieved for 86.5% of the bacteria and yeasts in less than 30 minutes after the BC bottle has been reported to be positive. This is up to 2 days earlier than with conventional protocols. Thus, important information for patient treatment is available much earlier, which makes the technique suitable for routine diagnostics. A limitation of the present study may be the bias of a restricted spectrum of species identified (the included data set samples were obtained solely from a university hospital).
In summary, the use of the novel sample preparation for MALDI-TOF MS to identify blood culture isolates directly from positive broth cultures is accurate and rapid. It provides a decreased turnaround time for identification of bacteria isolated from blood cultures, making this technology suitable for routine diagnostics. Thus, this novel sample preparation technique combines a rapid and reliable identification of septicemia-causing bacteria and yeasts with easy performance and low additional costs. This technique can allow for more rapid identification of infecting microorganisms and treatment options in infectious disease, thereby preventing inappropriate treatment of patients and unnecessary use of broad-spectrum antibiotics.
Acknowledgment
We thank Lukas Schneider for critical reading of the manuscript.
Footnotes
Supported by a grant from the Bavarian Research Foundation (Bayerische Forschungsstiftung; Forschungsverbund FORPROTECT “Infektionsprotektion durch neue Diagnostik Diagnostikverfahren instead of Diagnostik-verfahren verfahren und Therapieansätze” to S.S.). Bruker Daltonik GmbH (Bremen, Germany) supplied reagents and technical support to the Max von Pettenkofer-Institute, Ludwig-Maximilians-University, Munich, Germany.
References
- 1.Paolucci M., Landini M.P., Sambri V. Conventional and molecular techniques for the early diagnosis of bacteraemia. Int J Antimicrob Agents. 2010;36(Suppl 2):S6–S16. doi: 10.1016/j.ijantimicag.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Peralta G., Roiz M.P., Sánchez M.B., Garrido J.C., Ceballos B., Rodríguez-Lera M.J., Mateos F., De Benito I. Time-to-positivity in patients with Escherichia coli bacteraemia. Clin Microbiol Infect. 2007;13:1077–1082. doi: 10.1111/j.1469-0691.2007.01817.x. [DOI] [PubMed] [Google Scholar]
- 3.Funke G., Funke-Kissling P. Use of the BD PHOENIX Automated Microbiology System for direct identification and susceptibility testing of gram-negative rods from positive blood cultures in a three-phase trial. J Clin Microbiol. 2004;42:1466–1470. doi: 10.1128/JCM.42.4.1466-1470.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan M., Marlowe E., Della-Latta P., Salimnia H., Novak-Weekley S., Wu F., Crystal B.S. Multicenter evaluation of a new shortened peptide nucleic acid fluorescence in situ hybridization procedure for species identification of select Gram-negative bacilli from blood cultures. J Clin Microbiol. 2010;48:2268–2270. doi: 10.1128/JCM.00166-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tissari P., Zumla A., Tarkka E., Mero S., Savolainen L., Vaara M., Aittakorpi A., Laakso S., Lindfors M., Piiparinen H., Mäki M., Carder C., Huggett J., Gant V. Accurate and rapid identification of bacterial species from positive blood cultures with a DNA-based microarray platform: an observational study. Lancet. 2010;375:224–230. doi: 10.1016/S0140-6736(09)61569-5. [DOI] [PubMed] [Google Scholar]
- 6.Forrest G.N., Roghmann M.C., Toombs L.S., Johnson J.K., Weekes E., Lincalis D.P., Venezia R.A. Peptide nucleic acid fluorescent in situ hybridization for hospital-acquired enterococcal bacteremia: delivering earlier effective antimicrobial therapy. Antimicrob Agents Chemother. 2008;52:3558–3563. doi: 10.1128/AAC.00283-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ecker D.J., Sampath R., Li H., Massire C., Matthews H.E., Toleno D., Hall T.A., Blyn L.B., Eshoo M.W., Ranken R., Hofstadler S.A., Tang Y.W. New technology for rapid molecular diagnosis of bloodstream infections. Expert Rev Mol Diagn. 2010;10:399–415. doi: 10.1586/erm.10.24. [DOI] [PubMed] [Google Scholar]
- 8.Fenselau C., Demirev P.A. Characterization of intact microorganisms by MALDI mass spectrometry. Mass Spectrom Rev. 2001;20:157–171. doi: 10.1002/mas.10004. [DOI] [PubMed] [Google Scholar]
- 9.Seng P., Drancourt M., Gouriet F., La Scola B., Fournier P.E., Rolain J.M., Raoult D. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis. 2009;49:543–551. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- 10.Haag A.M., Taylor S.N., Johnston K.H., Cole R.B. Rapid identification and speciation of Haemophilus bacteria by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Mass Spectrom. 1998;33:750–756. doi: 10.1002/(SICI)1096-9888(199808)33:8<750::AID-JMS680>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Hettick J.M., Kashon M.L., Simpson J.P., Siegel P.D., Mazurek G.H., Weissman D.N. Proteomic profiling of intact mycobacteria by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal Chem. 2004;76:5769–5776. doi: 10.1021/ac049410m. [DOI] [PubMed] [Google Scholar]
- 12.Amiri-Eliasi B., Fenselau C. Characterization of protein biomarkers desorbed by MALDI from whole fungal cells. Anal Chem. 2001;73:5228–5231. doi: 10.1021/ac010651t. [DOI] [PubMed] [Google Scholar]
- 13.La Scola B., Raoult D. Direct identification of bacteria in positive blood culture bottles by matrix-assisted laser desorption ionisation time-of-flight mass spectrometry. PLoS One. 2009;4:e8041. doi: 10.1371/journal.pone.0008041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira L., Sánchez-Juanes F., Porras-Guerra I., García-García M.I., García-Sánchez J.E., González-Buitrago J.M., Muñoz-Bellido J.L. Microorganisms direct identification from blood culture by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Clin Microbiol Infect. 2011;17:546–551. doi: 10.1111/j.1469-0691.2010.03257.x. [DOI] [PubMed] [Google Scholar]
- 15.Christner M., Rohde H., Wolters M., Sobottka I., Wegscheider K., Aepfelbacher M. Rapid identification of bacteria from positive blood culture bottles by use of matrix-assisted laser desorption-ionization time of flight mass spectrometry fingerprinting. J Clin Microbiol. 2010;48:1584–1591. doi: 10.1128/JCM.01831-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prod'hom G., Bizzini A., Durussel C., Bille J., Greub G. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for direct bacterial identification from positive blood culture pellets. J Clin Microbiol. 2010;48:1481–1483. doi: 10.1128/JCM.01780-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevenson L.G., Drake S.K., Murray P.R. Rapid identification of bacteria in positive blood culture broths by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2010;48:444–447. doi: 10.1128/JCM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moussaoui W., Jaulhac B., Hoffmann A.M., Ludes B., Kostrzewa M., Riegel P., Prévost G. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry identifies 90% of bacteria directly from blood culture vials. Clin Microbiol Infect. 2010;16:1631–1638. doi: 10.1111/j.1469-0691.2010.03356.x. [DOI] [PubMed] [Google Scholar]
- 19.Mellmann A., Bimet F., Bizet C., Borovskaya A.D., Drake R.R., Eigner U., Fahr A.M., He Y., Ilina E.N., Kostrzewa M., Maier T., Mancinelli L., Moussaoui W., Prévost G., Putignani L., Seachord C.L., Tang Y.W., Harmsen D. High interlaboratory reproducibility of matrix-assisted laser desorption ionization-time of flight mass spectrometry-based species identification of nonfermenting bacteria. J Clin Microbiol. 2009;47:3732–3734. doi: 10.1128/JCM.00921-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al M.M., Armougom F., Scheld W.M., Dufour H., Roche P.H., Drancourt M., Raoult D. The expansion of the microbiological spectrum of brain abscesses with use of multiple 16S ribosomal DNA sequencing. Clin Infect Dis. 2009;48:1169–1178. doi: 10.1086/597578. [DOI] [PubMed] [Google Scholar]
- 21.Ferroni A., Suarez S., Beretti J.L., Dauphin B., Bille E., Meyer J., Bougnoux M.E., Alanio A., Berche P., Nassif X. Real-time identification of bacteria and Candida species in positive blood culture broths by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2010;48:1542–1548. doi: 10.1128/JCM.02485-09. [DOI] [PMC free article] [PubMed] [Google Scholar]


