Abstract
Event-related potentials offer evidence for face distinctive neural activity that peaks at about 170 ms following the onset of face stimuli (the N170 effect). We investigated the role of the perceptual mechanism reflected by the N170 effect by comparing the adaptation of the N170 amplitude when target faces were preceded either by identical face images or by different faces relative to when they were preceded by objects. In two experiments, we demonstrate that the N170 is equally adapted by repetition of the same or different faces. Thus, our findings show that the N170 is sensitive to the category rather than the identity of a face. This outcome supports the hypothesis that the N170 effect reflects the activity of a perceptual mechanism which discriminates faces from objects and streams face stimuli to dedicated circuits, specialized in encoding and decoding information about the face.
Keywords: Neural adaptation, N170, Face perception, Face categorization, Face identification
The modulation of the human ERP response by presentation of faces or face components over and above any other stimulus peaking at N170 ms post-stimulus (the N170 effect) has become one of the hallmarks of face processing in the visual system (Bentin et al. 1996). Yet, nearly 15 years since the initial descriptions of this effect, there are still arguments about the particular perceptual mechanism it reflects. One view is that the N170 effect reflects the detection of physiognomic information in the visual field, which streams the input to face-specific structural encoding processes (Bentin et al. 1999; Sagiv and Bentin 2001; Zion-Golumbic and Bentin 2007). Importantly, according to this “streaming” view, the N170 does not reflect the process of structural encoding per se. That is, it is not involved in the formation of individual face representations containing diagnostic features which allow the putative Face Recognition Units (Bruce and Young 1986) to determine the familiarity of the face. This view is a revision of the original position suggested by Bentin et al. (1996) and is based on more recent findings showing that: (a) The N170 is insensitive to face familiarity (e.g., Bentin and Deouell 2000; Eimer 2000a; Jemel et al. 2003; Schweinberger et al. 2002b; for a similar pattern see also Rossion et al. 1999 albeit the term N170 was not used in that paper; similar results were found in MEG: see Ewbank et al. 2008); (b) A robust N170 is elicited by schematic faces (like “smiley” faces) whose physiognomic value is determined entirely by canonic global organization (Sagiv and Bentin 2001; Bentin et al. 2006) and are typically not discriminated individually; (c) A robust N170 is elicited by individual face components (Bentin et al. 1996; Itier et al. 2007) even if they are presented in isolation and scrambled, thus destroying the global structure of a face and preventing configural computations, which are a major aspect of the face individuation process (Zion-Golumbic and Bentin 2007).
A different view is that the N170 does reflect the face-specific configural structural encoding process leading to face individuation (Jacques and Rossion 2006) albeit it is not related to the activation of semantic knowledge that is typically linked to a familiar face (Eimer 2000b). Importantly, according to this view, the N170 effect is process—rather than stimulus—specific. The sensitivity of the N170 to configural processes (used for face individuation) derived from studies showing that (a) the N170 peak is delayed when faces are inverted, thus impeding recognition (e.g., Bentin et al. 1996; Jacques et al. 2007; Rossion and Gauthier 2002); (b) albeit significantly reduced, modulation of the N170 was found in response to objects of expertise which were not faces, such as birds and dogs (Tanaka and Curran 2001); (c) Simultaneous presentations of a face and another object, which the subject is an expert in recognizing, reduces the N170, suggesting competition over similar processing resources (Rossion et al. 2004).
Since both views are supported by different sets of data, a resolution of this controversy is presently difficult. To shed more light on the perceptual mechanism reflected by the N170 effect, we need an experimental manipulation that would lead to different results depending on whether this effect is associated with face individuation or with face detection at the categorical level only. Recent studies suggested that the adaptation of the N170 when faces are repeated might be used for such a purpose.
Previous studies have shown that the neural response following the presentation of repeated stimuli is attenuated, and this attenuation may occur at multiple temporal and spatial scales. As noted by Grill-Spector et al. (2006), this stimulus-specific reduction in neuronal firing has been variously referred to as mnemonic filtering (Miller et al. 1993), repetition suppression (Desimone 1996), decremented responses (Brown and Xiang 1998), neural priming (Maccotta and Buckner 2004; Grill-Spector et al. 2006), or adaptation (Sobotka and Ringo 1994; Ringo 1996; Grill-Spector and Malach 2001), which is the term we will use here. Adaptation is being extensively exploited to investigate the response characteristics of neurons using non-invasive measures such as fMRI and EEG. It is surmised that adaptation should manifest in neurons that are excited by the second of two successive stimuli if the neurons are insensitive to differences that might exist between the two stimuli (Krekelberg et al. 2006). Neurons that show such adaptation can be described as ‘generalizing’ across such differences.
Within this context, since the neurons responsible for the N170 effect are sensitive to faces more than to non-faces, a smaller N170 effect should be elicited by a face following a face than by a face following a non-face stimulus. In other words, we should expect category-specific adaptation. However, the question remains open regarding face identity. By the account suggesting that the N170 effect is involved in individuation, neurons should be sensitive to individual differences, and thus, the N170 effect should be larger (less adapted) in response to a face preceded by a different face than in response to a face preceded by the same face. In contrast, according to the ‘streaming’ account, individual differences are immaterial at this stage, and thus, the two responses should be similar, reflecting similar levels of adaptation.
Surprisingly, only a few studies investigated category-specific adaptation of the N170 potential, as observed when a face is preceded by another face as opposed to a non-face object (i.e., basic-level category adaptation), and none explored directly the possible modulation of the N170 effect (that is, the difference between faces and objects) as a result of adaptation. All these studies revealed adaptation of the N170 elicited by a face preceded by another face, and some showed similar adaptation effects for non-face stimuli. For example, using a gender categorization task, Kovacs et al. (2006) observed an attenuation of the N170 when faces were shown after participants had been previously exposed to an adaptor face in comparison with when they were previously exposed to an oval image containing a spatial-frequency scrambled version of the same stimulus. This effect occurred despite the fact that the identity of the two faces was different. However, a similar N170 adaptation effect was found in this study for hands, suggesting that the N170 effect may not have been modulated. Moreover, in a more recent study, Kloth et al. (2010) report that the N170 elicited by faces in a gender classification task was adapted when preceded by a face adaptor but not when preceded by a gender-biasing human-voice adaptor. Consequently, they conclude that the N170 is adapted by faces as a category but not by subordinate information. In another face adaptation study, Maurer et al. (2008) found adaptation effects when they compared the N170 elicited by faces preceded by other faces to that of faces preceded by words. Note, however, that since different words as well as different faces were presented in both sequences, a possible account for this categorical difference is that while faces form a distinguished basic-level category, words are considered, at most, a supraordinate category. Hence, this study does not speak directly to the question of category-specific adaptation of the N170. Interestingly, there was no adaptation of the N170 amplitude elicited by repetition of words suggesting that the N170 effect was also adapted. A categorical face adaptation effect was found in an MEG study (Harris and Nakayama 2007). Presenting pairs of house–face or face–face stimulus-pairs, these authors demonstrated an adaptation of the face distinctive M170 when the S1–S2 stimulus onset asynchrony (SOA) was shorter than ~650 ms, with higher adaptation in the face–face than in the house–face condition even though different faces were presented at S1 and S2. However, since the adaptation of houses (following houses) was not assessed, this study does not reveal whether the M170 effect was or was not modulated by adaptation.
The studies reviewed above support a categorical adaptation effect which is, indeed, predicted by both accounts for the N170 effect. We turn now to N170 adaptation studies, in which the effect of identical face repetition was compared to that of categorical (non-identical) face repetition. Finding larger adaptation for identical faces as opposed to repetition of non-identical faces (categorical repetition) should support the possibility that the N170 effect reflects face individuation processes. Unfortunately, the outcomes of these experiments have thus far been inconclusive. Some studies found attenuation of N170 in response to the presentation of the same face (as opposed to different faces) in succession (Caharel et al. 2009; Campanella et al. 2000; Itier and Taylor 2002, 2004; Martens et al. 2006; Heisz et al. 2006; Jacques et al. 2007; Jacques and Rossion 2006). In all these studies, however, the effect was very small, and other studies had not found this effect to occur at all (Schweinberger et al. 1995, 2002a, b). Furthermore, an MEG study showed that whereas the M170 elicited by identical face repetitions is reduced relative to that elicited by faces preceded by different faces, this occurs only if the two faces are presented from the same viewpoint. This pattern suggests that the adaptation effect is viewpoint dependent (Ewbank et al. 2008). It extends a previous EEG study from the same group also showing viewpoint dependence for the adaptation of the N170 (Ewbank and Andrews 2006) and is in sharp contrast to a recent study which reported viewpoint independence of the N170 adaptation effect (Caharel et al. 2009). On the other hand, comparing the M170 elicited by an upright target face with that elicited by the adapting face (upright and inverted alike) Harris and Nakayama (2008) found that across the orientation of the adapting faces, the adaptation of the M170 was larger when the adaptor and target faces were of the same person as opposed to different persons.
As the above review shows, current N170 adaptation data are still indecisive. Whereas the categorical adaptation effect seems to be convincing, it is not clear whether this effect is different for faces and for other stimulus categories, and therefore, it is not clear whether face adaptation reduces the N170 effect. Furthermore, whereas some studies suggest that adaptation is larger when the adaptor and the test face are identical, other studies did not find such effects. Moreover, conflicting data about viewpoint dependence of the identical-face-adaptation effect does not permit an unequivocal dismissal of the possibility that the larger adaptation induced by identity relative to categorical repetition is explained by low-level vision factors. Finally, a complete assessment of the characteristics of the face adaptation effects on the N170 would benefit from direct comparisons between the effects of categorical and identical face adaptation on the N170. The present study provides a step toward achieving this goal.
The present design enabled us to directly compare the effects of categorical and identical face adaptation within-subjects. In two experiments, the N170 adaptation induced by repetition of one or two identical faces was compared with that induced by repetitions of different faces. Importantly, both effects were measured relative to a baseline where the N170 was elicited by faces preceded by two painted Easter eggs, hence, not adapted. Higher N170 adaptation in the identical relative to the different faces condition would support the hypothesis that neurons that contribute to this potential are sensitive to perceptual differences between individual faces. By contrast, similar adaptation in the two conditions relative to the baseline N170 would support the hypothesis that these neurons generalize across individual identities and, therefore, are not involved in the individuation process. In addition, we examined whether high-level visual adaptation is peculiar to faces by comparing the N170 elicited by eggs preceded by eggs (either identical or different) with the N170 elicited by eggs preceded by faces. Similar patterns of adaptation for eggs and faces should not reduce the N170 effect, indicating that the perceptual mechanism which is adapted is not selective for faces. Such an outcome would raise caveats about using adaptation designs in studies of face processing.
Experiment 1
Methods
Participants
The participants were 19 undergraduate students from the Hebrew University of Jerusalem (mean age = 21.2, 11 female) who participated for payment or credit toward a course requirement. Of these, 3 subjects were not considered in our analysis, since they did not show a clear N170 effect for faces. They all had normal or corrected-to-normal vision. The participants signed an informed consent as required by the Hebrew University committee for ethics in human experiments.
Stimuli
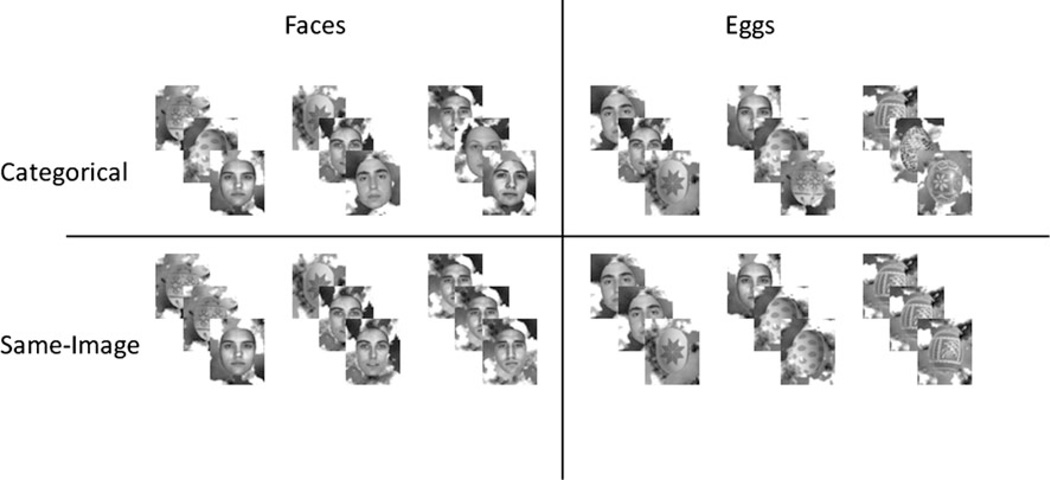
The stimuli used were 177 grayscale photographic images of faces and 177 grayscale images of Easter eggs, presented over a random pattern of black and white “clouds” background 1 (Fig. 1). Stimuli were 5.27 × 6.33 cm and occupied a visual angle of 5.17 × 4.31° around fixation.
Fig. 1.
Experimental Design. Faces (left panels) and eggs (right panels) were preceded by two, one, or no repetitions of other faces/eggs. These repetitions were either of different stimuli (“categorical” adaptation: top row) or of the same stimulus (“same-image” adaptation: bottom row)
Task and design
ERPs were recorded in four successive experimental blocks of 240 trials each. Each trial consisted of three successive images of either faces or eggs, followed by a question mark. The participants were instructed to remember the sequence of stimuli presented and, when cued by the question mark, to respond with three button presses according to the sequence of the stimuli that were presented in that trial, with one button indicating “face” and another “egg”. For instance, if three faces were shown in succession, the participant was instructed to press the “face” button three times consecutively. This task was designed so that attention would be equally allocated to all three stimuli in a sequence. Participants received auditory feedback contingent on accuracy in each trial.
Six different triad sequences were presented with equal probability: I. Face–Face–Face; II. Egg–Face–Face; III. Egg–Egg–Face; IV. Egg–Egg–Egg; V. Face–Egg–Egg; VI. Face–Face–Egg. There were two Adaptation-type conditions (Fig. 1). In the categorical adaptation condition, all stimuli within and across trials were different. In the same-image adaptation condition, repeated stimuli within a trial were identical, but there were no repetitions across trials. The Adaptation-type conditions were blocked, with two blocks per conditions. The order of the blocks followed an ABBA design, with half the subjects starting with categorical adaptation and half with the same-image adaptation condition.
Procedure
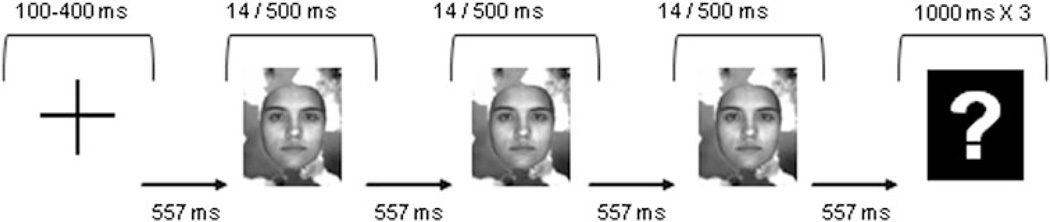
The experiment was conducted in a Faradically isolated and sound-attenuated chamber. After the electrode cap was mounted, the subjects performed one practice block, preceding all four experimental blocks. The practice presented all 6 triad sequences (1 trial per each of the 6 sequences), and the subjects were encouraged to repeat the practice block until they felt comfortable with the task and performed at a reasonable accuracy (a hit rate of at least 67%). The practice block consisted either of the categorical or the same-image condition, counterbalanced by subject). In the experimental blocks, the trial sequence was randomized. Each trial began with a fixation cross presented for 100–400 ms, followed by the three images that were presented for 14 ms each2 (one refresh cycle of the monitor run at 70 Hz), and an inter-stimulus interval (ISI) of 557 ms. An interval of 557 ms also separated the offset of the third stimulus from the onset of a question mark, at which time subjects were required to report the preceding sequence by three consecutive button presses, with a time limit of 1 s for each press in the response sequence (Fig. 2). A new trial began immediately following the response.
Fig. 2.
Experimental procedures. After a short fixation, three successive images appeared, followed by a question mark. The task was to indicate via button presses which three images appeared, which could have been either faces or eggs. The exposure time for each image was 14 ms in Experiment 1 and 500 ms in Experiment 2
Importantly, since the exposure time was very short (~14 ms), one could question whether participants were able to distinguish between identical repetition and repetition of different faces. Although a previous study from our laboratory demonstrated that participants are able to identify previously learned faces at an exposure time similar to the one used in the present study (R. Albeck-Elimelech 2004, unpublished doctoral dissertation), we investigated the validity of this caveat in a control experiment. Twelve undergraduate students who did not participate (mean age = 27.75, 6 female) in the main experiment were presented with a random mixture of 24 pairs of identical faces and 24 pairs of different faces and instructed to distinguish between the two conditions. Using the same time course and presentation procedures as in the main experiment and allowing only one second to respond, we found that the participants were well above chance in this task (overall correct responses = 75%, SD = 6.9%; d’ = 1.64, SD = 0.65).
Recording procedures and ERP analysis
The EEG was recorded continuously using a Biosemi Active 2 system (http://www.biosemi.com) via 64 Ag–AgCl pin-type active electrodes mounted on an elastic cap according to the extended 10–20 system (American Encephalographic Society 1994) and from 2 additional electrodes placed at the right and left mastoids and one placed on the tip of the nose. Eye movements, as well as blinks, were monitored using two pairs of electrodes, one pair attached to the left and right external canthi and the other to the infraorbital and supraorbital regions of the right eye. Both EEG and EOG were sampled at 256 Hz. A 67-Hz low-pass filter was used during recording to avoid aliasing of high frequencies. All electrodes were referenced during recording to a common-mode signal (CMS) electrode between POz and PO3 and were subsequently re-referenced digitally. The digitized EEG was saved and processed off-line.
The EEG was re-referenced off-line to the tip of the nose and digitally filtered using a band-pass of 0.8–17 Hz (24 dB/octave, zero-phase Butterworth filter). Artifacts were removed by excluding from the data analysis epochs of 200 ms equally placed before and after events where the EEG or the EOG exceeded ± 100 µV. Artifact-free EEG was segmented separately for each of the 12 trial types starting 100 ms before and ending 400 ms after the onset of the third stimulus in each triad. ERPs were obtained by averaging these segments. The minimum number of segments per condition in any participant was 41 with the exclusion of one condition in one participant which included only 21 trials. Exclusion of this subject from the analysis did not change the pattern of results.
Based on previous studies and observing a classical distribution of the N170 component, the N170 amplitude was determined as the most negative amplitude peak occurring between 140 and 220 ms from stimulus onset at the P9 and P10 sites.3 The baseline was adjusted by subtracting the mean amplitude of the 400 ms prestimulus period of the first stimulus in each series, for each ERP from all the data points in the epoch.
The N170 amplitudes elicited by the third stimulus in each triad were initially analyzed by a within-subject ANOVA with repeated measures. The factors were Stimulus type (face, egg), Adaptation type (categorical, same-image), Repetition level (none, one, two), and Hemisphere (left, right). Further, we calculated a measure of the N170 effect separately in each condition by calculating the ratio (face − egg)/(face + egg).4 This ratio weights the face–egg difference by the absolute N170 amplitudes in each participant. It is distributed from −1 to 1 with positive values indicating higher amplitudes for faces than for eggs. In order to assess how adaptation influences this measure, we tested the effects of Adaptation-type, Repetition-level and Hemisphere factors by within-subjects ANOVA.
Lastly, although we focused on the N170, we also analyzed the N250r component, which has been previously shown to be affected by adaptation of both familiar and unfamiliar faces (Schweinberger et al. 1995). The dependent variable was the mean amplitude between 230 and 330 ms. For this analysis, we collapsed across repetition levels. Hence, the independent factors were Stimulus (face, eggs), Repetition (same-image, category, unrepeated), and Hemisphere.
Results
Performance
The participants were very accurate at the experimental task, performing at a rate of 92.81% (standard error = 0.88%) in the same-image-adaptation condition, and at a rate of 92.88% (standard error = 1.17%) in the categorical adaptation condition.
ERP results
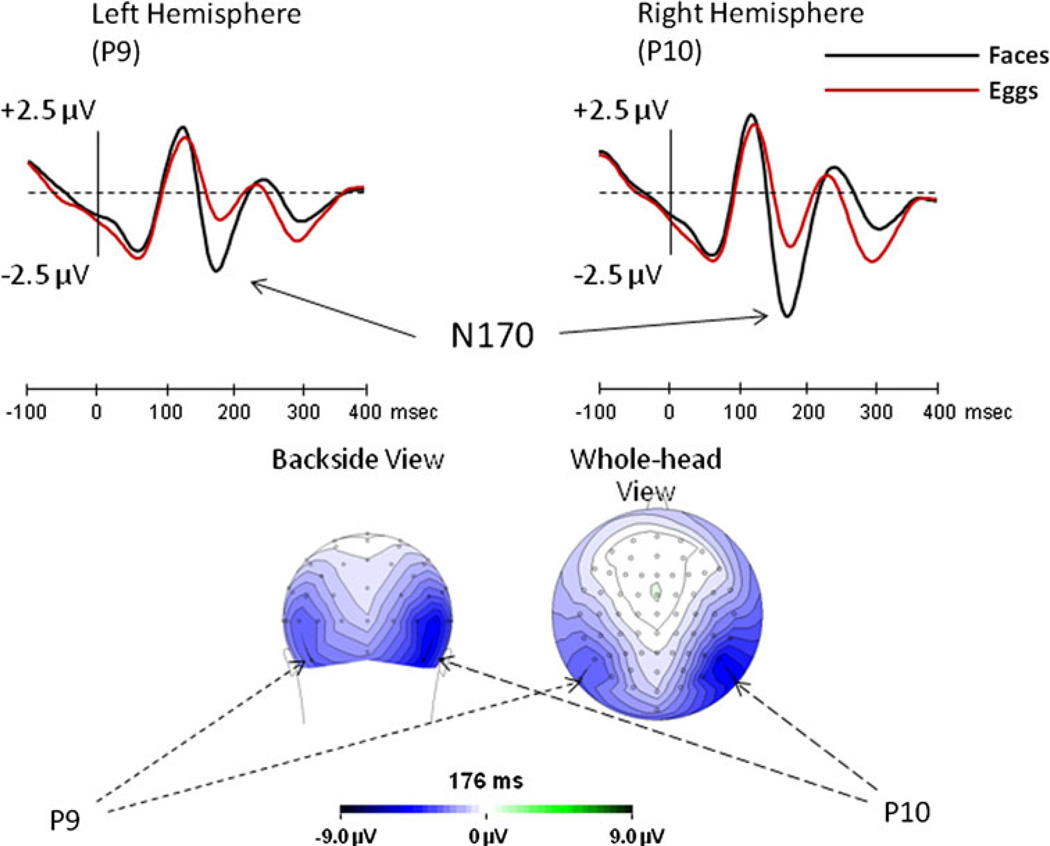
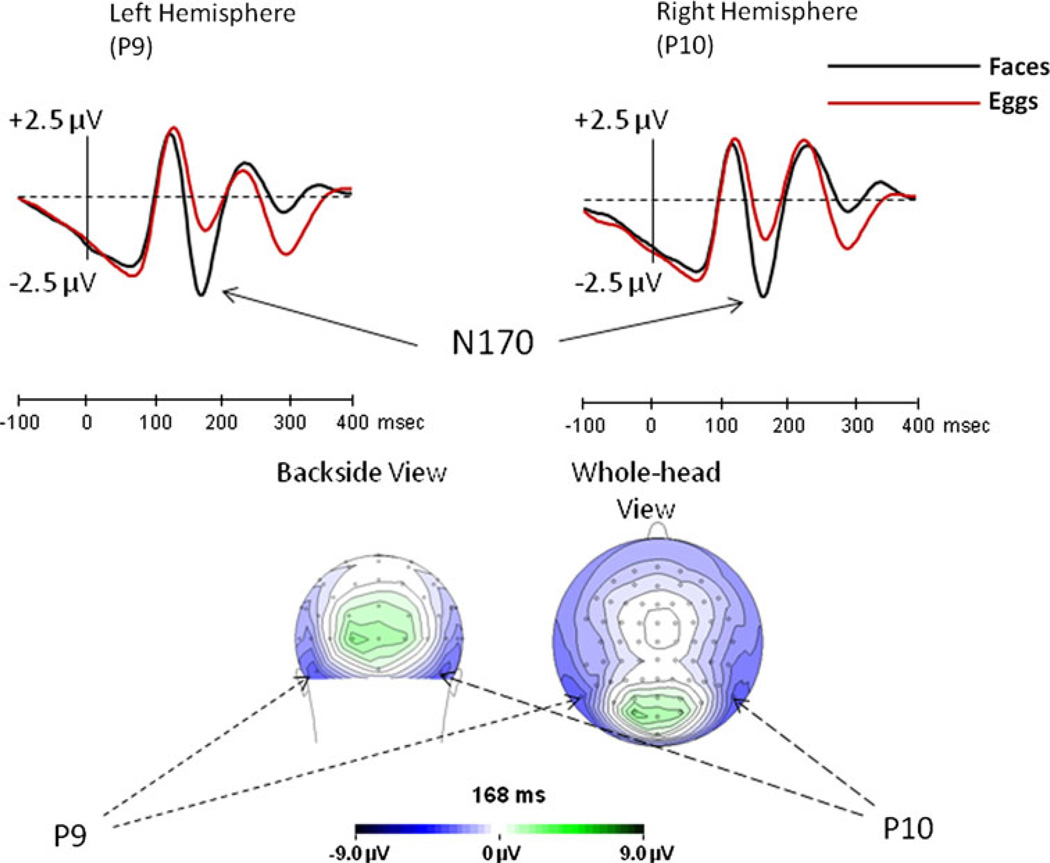
Given the very high accuracy, we did not exclude trials from the ERP analysis. Demonstrating once again the special tuning of the N170 to faces, ANOVA of Stimulus type (face, egg), Adaptation type (categorical, same-image), Repetition level (none, one, two), and Hemisphere (left, right) showed that across all conditions the N170 elicited by faces was significantly larger [F(1,15) = 37.2, MSe = 15.3, p < 0.001] than that elicited by eggs (Fig. 3). The potentials were larger at the right than at the left hemisphere [F(1,15) = 20.6, MSe = 9.2, p < 0.001] but since the Hemisphere effect did not interact with any other factor, it will not be addressed further. There was no Hemisphere effect on N170 latency.
Fig. 3.
Experiment 1: the overall effect of faces (black line) and eggs (red line), collapsed over all experimental conditions, along with the N170 scalp distribution for faces
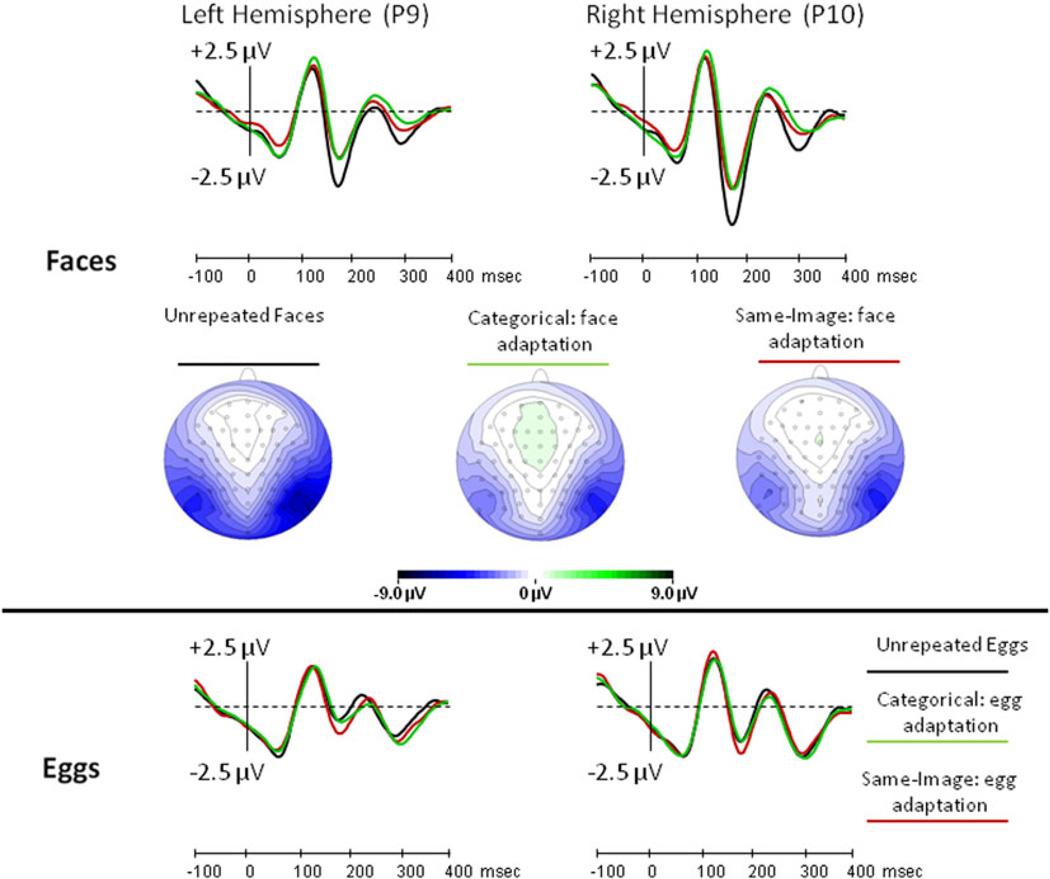
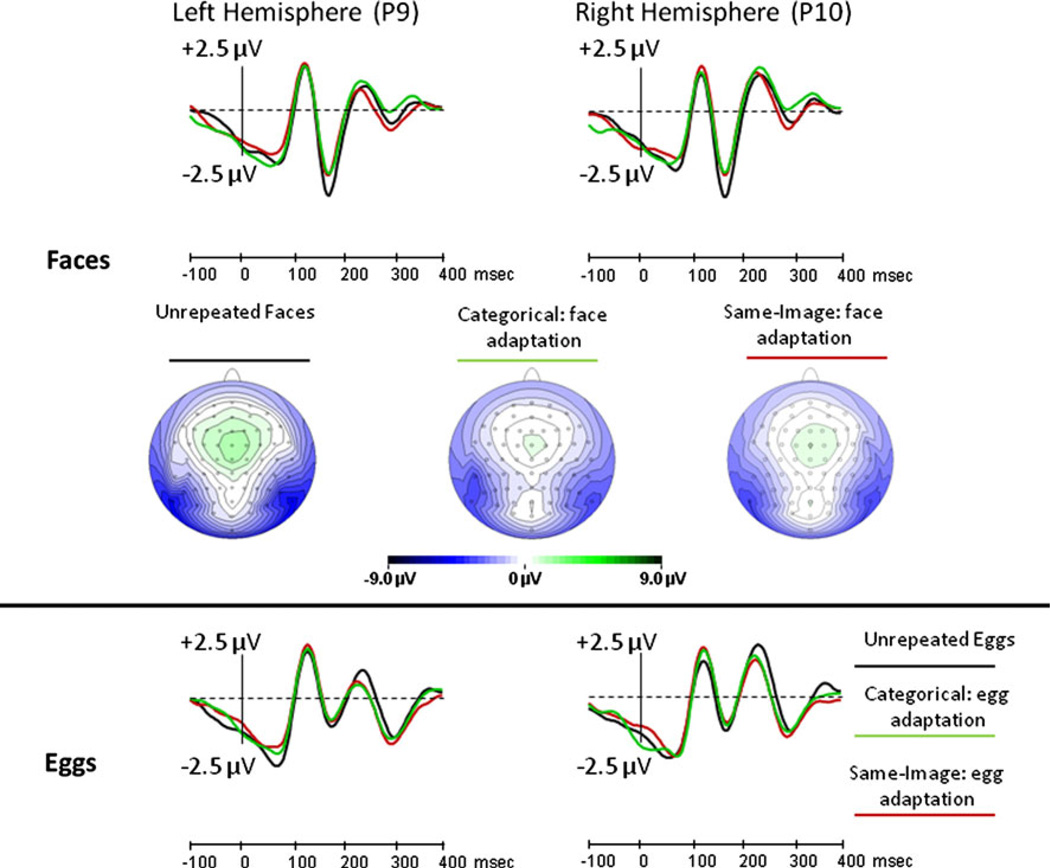
A main effect of Repetition level on the N170 amplitude [F(2,30) = 8.2, MSe = 3.6, p < 0.01] demonstrated adaptation, but this effect was modulated by an interaction with Stimulus type [F(2,30)5 = 19.6, MSe = 2.3, p < 0.001]. Importantly, there was no main effect of Adaptation type [F(1,15) = 1.6, MSe = 2.7, p > 0.2], and no first-order or second-order interactions between Adaptation type and any other factor [for all these interactions, p > 0.1]. The N170 elicited in the same-image-adaptation condition peaked somewhat faster than that which was elicited in the category adaptation condition [F(1,15) = 15.5, MSe = 88.6, p < 0.05], but this difference was very small (3 ms), at the threshold of the temporal resolution (~3.91 ms) at 256 Hz. In any case, this latency effect did not interact with either Stimulus type or Repetition level. Therefore, we conclude that in the present study, same-image adaptation was equal to categorical adaptation in all aspects (Fig. 4 and Supplementary Figure 1).
Fig. 4.
Experiment 1: the effect of categorical (green line) and same-image (red line) adaptations relative to no adaptation (black line), for faces (top row) and eggs (bottom row). The scalp distribution for the three face adaptation conditions is displayed
The interaction between the Repetition level and Stimulus type on the N170 amplitude was further investigated by ANOVAs administrated separately for faces and eggs. These analyses demonstrated a significant adaptation effect for faces [F(2,30) = 32.6, MSe = 2.0, p < 0.001], but no adaptation for eggs [F(2,30) = 2.6, MSe = 3.9, p = 0.09]. The Repetition level factor did not interact with Adaptation type either for faces or for eggs (both F’s < 1.0). Post hoc pairwise contrasts showed that the N170 amplitude was larger when the third face was preceded by eggs (−5.93 µV) than when it was preceded by either one face (−4.04 µV) or two faces (−4.44 µV), with no difference between the latter two conditions (Supplementary Table 1; for all significant pairwise comparisons, p < 0.05, Bonferroni corrected).
As expected, the normalized N170 effect was positive in all conditions confirming a robust face preference (Supplementary Table 2). In addition, like the N170 amplitude, the N170 effect was reduced by repetition, suggesting adaptation.
ANOVA showed a significant effect of Repetition level [F(2,30) = 13.3, MSe = 0.02, p < 0.001] on the normalized N170 effect, showing that the N170 effect was larger for faces following eggs than for faces following faces (p < 0.01) with no significant difference between faces preceded by one or two faces (p > 0.5). Consistent with the differential effects found for faces and eggs above, this demonstrates that the normalized difference between faces and eggs was adapted by repetition.
There were no other significant main effects or interactions (all p values > 0.2). That is, like for the absolute N170 amplitude, the adaptation of the N170 effect was not increased when identical face images were shown relative to when different faces were shown.
The N250r analysis yielded a significant Stimulus × Repetition interaction [F(2,30) = 7.7, MSe = 0.63, p < 0.01]. Separate ANOVAs for faces and eggs showed a significant effect of Repetition for faces [F(2,30) = 5.2, MSe = 1.2, p < 0.05] but not for eggs [F(2,30) < 1.0]. Pairwise comparisons revealed that the N250r elicited by unrepeated faces was more negative than for repeated faces and no difference between same-image and categorical repetition.
Discussion
The present results join previous studies in demonstrating a significant adaptation of the N170’s absolute amplitude when faces are repeated in succession (Jacques and Rossion 2006). This adaptation was assessed relative to a baseline condition in which faces followed painted eggs. Painted eggs were preferred as baseline as they share with faces the oval shape, and like faces, have internal detail and depth cues (unlike simple ovals that were previously used). In addition, we found similar adaptation of the N170 effect, that is, the normalized difference between the response to faces and to eggs. These effects were found despite the very brief stimulus exposure and rather long ISIs. However, in contrast to some previous studies, we did not find a difference in the adaptation induced by identical or different faces. It is also worth noting that the adaptation effect was limited to faces: The N170 elicited by eggs was not adapted either in the categorical or in the same-image repetition conditions. Finally, the size of the adaptation was similar for one and for two repetitions.
Importantly, although our control behavioral experiment (see Sect. Methods) showed that participants are capable of distinguishing between facial identities under our experimental design, their performance was not perfect. Moreover, 14 ms presentation durations differ from those used in previous studies that did find identity effects, leaving open the concern that the lack of identity effect stems from insufficient presentation times. Therefore, we replicated the procedures of this experiment with a new group of participants using 500 ms stimulus exposure durations.
Experiment 2
Methods
The experiment was identical to Experiment 1 except that each stimulus was exposed for 500 ms instead of ~14 ms, while maintaining the ISI as in Experiment 1 (~557 ms). Thus, the SOA in this experiment was ~1,057 ms. Sixteen naive participants were tested in this experiment (mean age = 20.94, 13 female).
Performance
Subjects were accurate in 93.01% (standard error = 1.24%) of the trials in the same-image-adaptation condition and at a rate of 94.27% (standard error = 1.11%) in the categorical adaptation condition. The difference between the performances in the two conditions was not significant (p > 0.2).
ERP results
As in Experiment 1, all trials were included in the ERP analysis. The ANOVA of Stimulus type (face, egg), Adaptation type (categorical, same-image), Repetition level (none, one, two), and Hemisphere (left, right) showed that across all conditions the N170 elicited by faces was significantly larger [F(1,15) = 93.1, MSe = 8.0, p < 0.001] and peaked earlier [F(1,15) = 9.0, MSe = 208.2, p < 0.01] than that elicited by eggs (Fig. 5). There was a significant interaction between Stimulus type and Adaptation type on the latency of the N170 [F(2,30) = 6.5, MSe = 50.4, p < 0.05], suggesting a larger peak-latency difference between faces and eggs in the same-image than in the categorical repetition condition. There was no effect of Hemisphere, and no interaction between Hemisphere and Adaptation type (Fig. 6).
Fig. 5.
Experiment 2: the overall effect of faces (black line) and eggs (red line), collapsed over all experimental conditions, along with the N170 scalp distribution for faces
Fig. 6.
Experiment 2: The effect of categorical (green line) and same-image (red line) adaptations relative to no adaptation (black line), for faces (top row) and eggs (bottom row). The scalp distribution for the three face adaptation conditions is displayed
As in Experiment 1, we found a main effect of Repetition level on the N170 amplitude [F(2,30) = 7.6, MSe = 3.6, p < 0.01] and an interaction of Repetition level with Stimulus type [F(2,30) = 6.4, MSe = 3.4, p < 0.01]. Again, there was no main effect of Adaptation type [F(1,15) < 1.0], and no first-order or second-order interactions between Adaptation type and any other factor on the amplitude of the N170 (p > 0.05 for all interactions).
The interaction between the Repetition level and Stimulus type on the N170 amplitude was further investigated by ANOVAs administrated separately for faces and eggs. These analyses demonstrated a significant effect of repetition for faces [F(2,30) = 11.3, MSe = 3.8, p < 0.01], but not for eggs [F(2,30) = 2.0, MSe = 3.3, p > 0.15], again replicating the results of Experiment 1. Post hoc pairwise contrasts (Bonferroni corrected) showed that the N170 amplitude was larger when the third face was preceded by eggs (−5.9 µV) than when it was preceded by either one face (−4.33 µV) or two faces (−4.72 µV), with no difference between the latter two conditions (for all significant pairwise comparison p > 0.05).
The N250r analysis showed only a tendency for a repetition effect [F(2,30) = 2.6, MSe = 2.1, p = 0.09], and no Stimulus × Repetition interaction [F(2,30) = 1.9, MSe = 2.2, p = 0.16].
Hence, the longer exposure time did not change the outcome: Despite a longer SOA, face repetition still resulted in significant N170 adaptation, and this effect was the same for repetitions of identical and different faces.
General discussion
The outcome of the present study does not lend any support to the conjecture that the N170 response reflects the activity of neurons that are sensitive to (and by extension directly contribute to the processing of) face identities. Within the context of the models presented in the introduction, these data support the view that the N170 effect reflects the activity of a perceptual mechanism that detects faces in the visual field but is not directly involved in face individuation. Assuming that face recognition entails a particular perceptual process tuned to extract exemplar-specific diagnostic features (e.g., the computation of individual texture and coloration cues and spatial relations between face components), early detection of stimuli that potentially require this process is necessary to stream only relevant stimuli via this type of structural encoding (or trigger it only when necessary; Bentin et al. 1999). The detection should be based on visual characteristics of faces as a category, such as their specific (and universal) global shape (first-order relations) or specific components, among which the eyes are most conspicuous. Congruently, the N170 effect is robust when visual stimuli contain such characteristics, even if additional structural encoding computations are not possible (e.g., if natural face components are scrambled) or not applied (e.g., when uniform schematic faces are presented). In contrast, the N170 effect is not elicited by schematic drawings if the inner components are scrambled so that the stimulus contains neither the global structure of the face nor the visual characteristics of inner components, unless the observers are primed to activate the face detection mechanism (Bentin and Golland 2002; see also Bentin et al. 2002). Interestingly, a similar conclusion has been reached in a recent study using different analytic procedures (Kahn et al. 2010). This study suggests that specific face identity computations are manifested by ERPs later than the P1/N170 complex.
Whereas the present data support the face detection rather than the structural encoding account for the N170 effect, we should address the discrepancy between these data and previous studies which supported the opposite view. First, as reviewed in the introduction, there are a few studies reporting modulation of N170 amplitude by face familiarity (e.g., Caharel et al. 2002; Caharel et al. 2006; Marzi and Viggiano 2007). However, these findings were inconsistent. For example, whereas Caharel and her colleagues found larger N170 amplitudes for familiar than unfamiliar faces, the opposite pattern was reported by Marzi and Viggiano (2007). Furthermore, only a few familiar faces were used in Caharel and colleagues’ studies, which were repeated many times, and familiarity effects were observed only for faces of people that were personally important to the participants but not when familiarity was determined by fame (Caharel et al. 2005). Hence, it is conceivable that the enhanced N170 in response to familiar faces reflected factors associated with attention or emotions rather than simple face processing.
Stronger evidence for the structural encoding view, which is directly relevant to the present study, came from studies of adaptation in which, in contrast to our present findings, adaptation following identical repetition was larger than following categorical repetition (Caharel et al. 2009; Jacques et al. 2007; Jacques and Rossion 2006). This contradictory evidence is intriguing and must be accounted for. There are several ways in which such studies differed from ours. One difference is that whereas our present task directed the participants’ attention to the categorical level of the stimulus, with the exception of Jacques and Rossion (2006), all previous studies in which identical face repetition yielded higher adaptation than the repetition of different faces used tasks that directed the participants’ attention to the identity of the face. Whereas it is possible that this difference may explain the contradictory results, we note that several studies showed that the N170 is robust to task manipulations (e.g. Carmel and Bentin 2002; Stahl et al. 2010). An important way in which the study by Jacques and Rossion (2006) differs from our own is that in their study there was no ISI, resulting in continuous stimulation of the retina. Hence, it is possible that the level of adaptation in Rossion and colleagues’ studies occurred much lower in the visual hierarchy than in ours, reflecting the effect of perceptual identity rather than face identity. The low-level adaptation may modulate the amplitude of the N170 downstream. This interpretation is supported by studies showing that retinal position invariance affects the adaptation of the N170 when the adaptation time is long (~5,000 ms) but not when it is shorter (~500 ms; Kovacs et al. 2007; Kovacs et al. 2005). We notice, however, that this explanation may not hold for Caharel et al.’s study (2009) in which identical faces induced stronger adaptation despite presenting the adaptor and the test face in different orientations. However, as mentioned in the introduction, other studies did not find adaptation of the N170 using different views of the same face (e.g., Ewbank and Andrews 2006).
In conclusion, taking the present and previous evidence together, we believe that the strong position taken by Jacques and Rossion (2006) about the association of the N170 to face individuation is premature. The detection and streaming hypothesis is viable, and at this time, it is at least as strongly supported by evidence as its alternative.
Supplementary Material
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00221-011-2546-x) contains supplementary material, which is available to authorized users.
As with the presentation duration, this background was chosen because this study was done in preparation for a masking experiment, for which such backgrounds are beneficial.
Very short presentation times were chosen because this study was done in preparation for a masking experiment, in which short presentation times were crucial.
Similar analysis including the additional sites P7/8, P07/8, yielded a similar pattern of adaptation (Supplementary Figure 1). For the sake of clarity, we present here only the P9/P10 analysis. The full analysis is available from the corresponding author upon request.
Since in some participants the amplitudes elicited by eggs were positive and in order to avoid ratios larger than 1 (which would be meaningless), we elevated the baseline by subtracting the maximum positive amplitude observed across eggs and faces and participants from all individual ERPs.
The Mauchly’s test of sphericity was insignificant throughout; therefore, the degrees of freedom were not corrected.
Contributor Information
Ido Amihai, Email: idoamihai@yahoo.com, Department of Neurobiology, The Hebrew University of Jerusalem, Jerusalem 91905, Israel.
Leon Y. Deouell, Email: msleon@mscc.huji.ac.il, Department of Psychology, The Hebrew University of Jerusalem, 91905 Jerusalem, Israel; Interdisciplinary Center for Neural Computation, The Hebrew University of Jerusalem, 91905 Jerusalem, Israel.
Shlomo Bentin, Email: Shlomo.Bentin@huji.ac.il, Department of Psychology, The Hebrew University of Jerusalem, 91905 Jerusalem, Israel; Interdisciplinary Center for Neural Computation, The Hebrew University of Jerusalem, 91905 Jerusalem, Israel.
References
- Albeck-Elimelech R. Unpublished doctoral dissertation. Hebrew University of Jerusalem; 2004. Attentional bias and cognitive strategy in unilateral neglect: face perception as a model. [Google Scholar]
- American Electroencephalographic Society. Guidelines for standard electrode position nomenclature. J Clin Neurophysiol. 1994;11:111–113. [PubMed] [Google Scholar]
- Bentin S, Deouell LY. Structural encoding and identification in face processing: ERP evidence for separate mechanisms. Cognitive Neuropsychology. 2000;17(1–3):35–54. doi: 10.1080/026432900380472. [DOI] [PubMed] [Google Scholar]
- Bentin S, Golland Y. Meaningful processing of meaningless stimuli: the influence of perceptual experience on early visual processing of faces. Cognition. 2002;86(1):B1–B14. doi: 10.1016/s0010-0277(02)00124-5. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cogn Neurosci. 1996;8(6):551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Deouell LY, Soroker N. Selective visual streaming in face recognition: evidence from developmental prosopagnosia. Neuroreport. 1999;10(4):823–827. doi: 10.1097/00001756-199903170-00029. [DOI] [PubMed] [Google Scholar]
- Bentin S, Sagiv N, Mecklinger A, Friederici A, von Cramon YD. Priming visual face-processing mechanisms: electrophysiological evidence. Psychol Sci. 2002;13(2):190–193. doi: 10.1111/1467-9280.00435. [DOI] [PubMed] [Google Scholar]
- Bentin S, Golland Y, Flevaris A, Robertson LC, Moscovitch M. Processing the trees and the forest during initial stages of face perception: Electrophysiological evidence. J Cogn Neurosci. 2006;18(8):1406–1421. doi: 10.1162/jocn.2006.18.8.1406. [DOI] [PubMed] [Google Scholar]
- Brown MW, Xiang JZ. Recognition memory: neuronal substrates of the judgement of prior occurrence. Prog Neurobiol. 1998;55(2):149–189. doi: 10.1016/s0301-0082(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Bruce V, Young A. Understanding face recognition. Br J Psychol. 1986;77:305–327. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Caharel S, Poiroux S, Bernard C, Thibaut F, Lalonde R, Rebai M. ERPs associated with familiarity and degree of familiarity during face recognition. Int J Neurosci. 2002;112(12):1499–1512. doi: 10.1080/00207450290158368. [DOI] [PubMed] [Google Scholar]
- Caharel S, Courtay N, Bernard C, Lalonde R, Rebai M. Familiarity and emotional expression influence an early stage of face processing: an electrophysiological study. Brain Cogn. 2005;59(1):96–100. doi: 10.1016/j.bandc.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Caharel S, Fiori N, Bernard C, Lalonde R, Rebai M. The effects of inversion and eye displacements of familiar and unknown faces on early and late-stage ERPs. Int J Psychophysiol. 2006;62(1):141–151. doi: 10.1016/j.ijpsycho.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Caharel S, d’Arripe O, Ramon M, Jacques C, Rossion B. Early adaptation to repeated unfamiliar faces across viewpoint changes in the right hemisphere: evidence from the N170 ERP component. Neuropsychologia. 2009;47(3):639–643. doi: 10.1016/j.neuropsychologia.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Campanella S, Hanoteau C, Depy D, Rossion B, Bruyer R, Crommelinck M, Guerit JM. Right N170 modulation in a face discrimination task: an account for categorical perception of familiar faces. Psychophysiology. 2000;37(6):796–806. [PubMed] [Google Scholar]
- Carmel D, Bentin S. Domain specificity versus expertise: factors influencing distinct processing of faces. Cognition. 2002;83(1):1–29. doi: 10.1016/s0010-0277(01)00162-7. [DOI] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci U S A. 1996;93(24):13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M. Event-related brain potentials distinguish processing stages involved in face perception and recognition. Clin Neurophysiol. 2000a;111(4):694–705. doi: 10.1016/s1388-2457(99)00285-0. [DOI] [PubMed] [Google Scholar]
- Eimer M. The face-specific N170 component reflects late stages in the structural encoding of faces. Neuroreport. 2000b;11(10):2319–2324. doi: 10.1097/00001756-200007140-00050. [DOI] [PubMed] [Google Scholar]
- Ewbank MP, Andrews TJ. Size-invariant, but viewpoint-specific adaptation of the N170 potential to faces. Proceedings of the12th annual meeting of the Organization for Human Brain Mapping; Florence. 2006. p. S113. [Google Scholar]
- Ewbank MP, Smith WAP, Hancock ER, Andrews TJ. The M170 reflects a viewpoint-dependent representation for both familiar and unfamiliar faces. Cereb Cortex. 2008;18(2):364–370. doi: 10.1093/cercor/bhm060. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. FMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol (Amst) 2001;107(1–3):293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10(1):14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Harris A, Nakayama K. Rapid face-selective adaptation of an early extrastriate component in MEG. Cereb Cortex. 2007;17(1):63–70. doi: 10.1093/cercor/bhj124. [DOI] [PubMed] [Google Scholar]
- Harris A, Nakayama K. Rapid adaptation of the M170 response: importance of face parts. Cereb Cortex. 2008;18(2):467–476. doi: 10.1093/cercor/bhm078. [DOI] [PubMed] [Google Scholar]
- Heisz JJ, Watter S, Shedden JM. Progressive N170 habituation to unattended repeated faces. Vision Res. 2006;46(1–2):47–56. doi: 10.1016/j.visres.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Taylor MJ. Inversion and contrast polarity reversal affect both encoding and recognition processes of unfamiliar faces: a repetition study using ERPs. Neuroimage. 2002;15(2):353–372. doi: 10.1006/nimg.2001.0982. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Taylor MJ. Effects of repetition learning on upright, inverted and contrast-reversed face processing using ERPs. Neuroimage. 2004;21(4):1518–1532. doi: 10.1016/j.neuroimage.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Alain C, Sedore K, McIntosh AR. Early face processing specificity: it’s in the eyes! J Cogn Neurosci. 2007;19:1815–1826. doi: 10.1162/jocn.2007.19.11.1815. [DOI] [PubMed] [Google Scholar]
- Jacques C, Rossion B. The speed of individual face categorization. Psychol Sci. 2006;17(6):485–492. doi: 10.1111/j.1467-9280.2006.01733.x. [DOI] [PubMed] [Google Scholar]
- Jacques C, d’Arripe O, Rossion B. The time course of the inversion effect during individual face discrimination. J Vis. 2007;7(8) doi: 10.1167/7.8.3. [DOI] [PubMed] [Google Scholar]
- Jemel B, Pisani M, Calabria M, Crommelinck M, Bruyer R. Is the N170 for faces cognitively penetrable? Evidence from repetition priming of mooney faces of familiar and unfamiliar persons. Cogn Brain Res. 2003;17(2):431–446. doi: 10.1016/s0926-6410(03)00145-9. [DOI] [PubMed] [Google Scholar]
- Kahn DA, Harris AM, Wolk DA, Aguirre GK. Temporally distinct neural coding of perceptual similarity and prototype bias. J Vis. 2010;10(10):12, 1–12. doi: 10.1167/10.10.12. http://www.journalofvision.org/content/10/10/12. [DOI] [PubMed] [Google Scholar]
- Kloth N, Schweinberger SR, Kovacs G. Neural correlates of generic versus gender-specific face adaptation. J Cogn Neurosci. 2010;22(10):2345–2356. doi: 10.1162/jocn.2009.21329. [DOI] [PubMed] [Google Scholar]
- Kovacs G, Zimmer M, Harza I, Antal A, Vidnyanszky Z. Position-specificity of facial adaptation. Neuroreport. 2005;16(17):1945–1949. doi: 10.1097/01.wnr.0000187635.76127.bc. [DOI] [PubMed] [Google Scholar]
- Kovacs G, Zimmer M, Banko E, Harza I, Antal A, Vidnyanszky Z. Electrophysiological correlates of visual adaptation to faces and body parts in humans. Cereb Cortex. 2006;16(5):742–753. doi: 10.1093/cercor/bhj020. [DOI] [PubMed] [Google Scholar]
- Kovacs G, Zimmer M, Harza I, Vidnyanszky Z. Adaptation duration affects the spatial selectivity of facial aftereffects. Vision Res. 2007;47(25):3141–3149. doi: 10.1016/j.visres.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Krekelberg B, Boynton GM, van Wezel RJA. Adaptation: from single cells to bold signals. Trends Neurosci. 2006;29(5):250–256. doi: 10.1016/j.tins.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Maccotta L, Buckner RL. Evidence for neural effects of repetition that directly correlate with behavioral priming. J Cogn Neurosci. 2004;16(9):1625–1632. doi: 10.1162/0898929042568451. [DOI] [PubMed] [Google Scholar]
- Martens U, Schweinberger SR, Kiefer M, Burton AM. Masked and unmasked electrophysiological repetition effects of famous faces. Brain Res. 2006;1109:146–157. doi: 10.1016/j.brainres.2006.06.066. [DOI] [PubMed] [Google Scholar]
- Marzi T, Viggiano MP. Interplay between familiarity and orientation in face processing: an ERP study. Int J Psychophysiol. 2007;65:182–192. doi: 10.1016/j.ijpsycho.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Maurer U, Rossion B, McCandliss BD. Category specificity in early perception: face and word N170 responses differ in both lateralization and habituation properties. Front Hum Neurosci. 2008;2 doi: 10.3389/neuro.09.018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term-memory task. J Neurosci. 1993;13(4):1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringo JL. Stimulus specific adaptation in inferior temporal and medial temporal cortex of the monkey. Behav Brain Res. 1996;76(1–2):191–197. doi: 10.1016/0166-4328(95)00197-2. [DOI] [PubMed] [Google Scholar]
- Rossion B, Gauthier I. How does the brain process upright and inverted faces? Behav Cogn Neurosci Rev. 2002;1(1):63–75. doi: 10.1177/1534582302001001004. [DOI] [PubMed] [Google Scholar]
- Rossion B, Campanella S, Gomez CM, Delinte A, Debatisse D, Liard L, Dubois S, Bruyer R, Crommelinck M, Guerit JM. Task modulation of brain activity related to familiar and unfamiliar face processing: an ERP study. Clin Neurophysiol. 1999;110(3):449–462. doi: 10.1016/s1388-2457(98)00037-6. [DOI] [PubMed] [Google Scholar]
- Rossion B, Kung CC, Tarr MJ. Visual expertise with nonface objects leads to competition with the early perceptual processing of faces in the human occipitotemporal cortex. Proc Natl Acad Sci USA. 2004;101(40):14521–14526. doi: 10.1073/pnas.0405613101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv N, Bentin S. Structural encoding of human and schematic faces: holistic and part-based processes. J Cogn Neurosci. 2001;13(7):937–951. doi: 10.1162/089892901753165854. [DOI] [PubMed] [Google Scholar]
- Schweinberger SR, Pfutze EM, Sommer W. Repetition priming and associative priming of face recognition: evidence from event-related potentials. J Exp Psychol Learn. 1995;21(3):722–736. [Google Scholar]
- Schweinberger SR, Pickering EC, Burton AM, Kaufmann JM. Human brain potential correlates of repetition priming in face and name recognition. Neuropsychologia. 2002a;40(12):2057–2073. doi: 10.1016/s0028-3932(02)00050-7. [DOI] [PubMed] [Google Scholar]
- Schweinberger SR, Pickering EC, Jentzsch I, Burton AM, Kaufmann JM. Event-related brain potential evidence for a response of inferior temporal cortex to familiar face repetitions. Cogn Brain Res. 2002b;14(3):398–409. doi: 10.1016/s0926-6410(02)00142-8. [DOI] [PubMed] [Google Scholar]
- Sobotka S, Ringo JL. Stimulus-specific adaptation in excited but not in inhibited cells in inferotemporal cortex of macaque. Brain Res. 1994;646(1):95–99. doi: 10.1016/0006-8993(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Stahl J, Wiese H, Schweinberger SR. Learning task affects ERP-correlates of the own-race bias, but not recognition memory performance. Neuropsychologia. 2010;48(7):2027–2040. doi: 10.1016/j.neuropsychologia.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Curran T. A neural basis for expert object recognition. Psychol Sci. 2001;12(1):43–47. doi: 10.1111/1467-9280.00308. [DOI] [PubMed] [Google Scholar]
- Zion-Golumbic E, Bentin S. Dissociated neural mechanisms for face detection and configural encoding: evidence from N170 and induced gamma-band oscillation effects. Cereb Cortex. 2007;17(8):1741–1749. doi: 10.1093/cercor/bhl100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.