Abstract
This analysis assessed the effect of lenalidomide on progression-free survival (PFS). Patients with relapsed or refractory multiple myeloma (RRMM) who received lenalidomide plus dexamethasone in the MM-009 and MM-010 trials were pooled and those who had not progressed and were still receiving lenalidomide at 12 months were included. The median follow-up of surviving patients was 48 months. Of 353 patients who received lenalidomide plus dexamethasone, 116 (33%) had not progressed. Overall, 52 patients (45%) had no dose reductions, 25 (22%) had dose reductions ⩾12 months and 39 (34%) had dose reductions before 12 months. Patients who had dose reductions ⩾12 months had a significantly longer median PFS than those who had reductions before 12 months (P=0.007) or no dose reductions (P=0.039) (not reached vs 28.0 vs 36.8 months, respectively). In a multivariate Cox regression model, dose reduction ⩾12 months was an independent predictor of improved PFS (hazard ratio, 0.47; 95% confidence interval, 0.23–0.98) after adjusting for patient characteristics. The data suggest that to achieve maximum PFS benefit, patients with RRMM should be treated for ⩾12 months with full-dose lenalidomide plus dexamethasone. Thereafter, patients may benefit from lower-dose continued therapy; prospective studies are needed to confirm these findings.
Keywords: dexamethasone, lenalidomide, multiple myeloma, progression-free survival, refractory, relapsed
Introduction
Despite recent improvements in survival outcomes,1, 2 multiple myeloma (MM) remains an incurable disease associated with uncontrolled plasma cell growth, immunodeficiency and high rates of relapse. Lenalidomide is an oral ImiDs® immunomodulatory compound with a dual mechanism of action. It has a tumoricidal effect that leads directly to tumor cell death and an immunomodulatory effect that keeps the tumor in remission.3 The combination of these effects provides both immediate and sustained myeloma control when lenalidomide is used long term. Currently, lenalidomide is indicated in combination with dexamethasone for the treatment of patients with relapsed or refractory (RR) MM who have received at least one previous therapy. Approval was based primarily on the results of two pivotal, phase III, randomized, double-blind, placebo-controlled trials conducted mainly in North America (MM-009)4 and Europe (MM-010).5 Compared with dexamethasone alone, lenalidomide plus dexamethasone significantly prolonged time to progression and overall survival, and had an acceptable safety profile.4, 5, 6
Exploratory sub-analyses of data from the MM-009 and MM-010 trials indicate that response rates appear to improve with continued treatment with lenalidomide plus dexamethasone,7 and that continued treatment after achievement of best response is associated with prolonged survival compared with earlier discontinuation.8 These findings demonstrate that continuing lenalidomide-based therapy is essential for maximum therapeutic benefit, although the optimal dose and schedule of long-term lenalidomide-based continuous therapy in this setting is unknown. Furthermore, certain adverse events associated with lenalidomide, such as neutropenia and thrombocytopenia, often warrant dose reductions or delays, which may hinder the delivery of long-term therapy. We therefore performed an exploratory analysis using data from the MM-009 and MM-010 trials to determine whether reducing the dose of lenalidomide and the timing of the dose reduction influences progression-free survival (PFS).
Patients and methods
Data from patients who received lenalidomide plus dexamethasone in the MM-009 (up to 23 July 2008) and MM-010 (up to 2 March 2008) trials were included in this analysis. These trials were approved by the relevant institutional review boards and ethics committees, and all patients gave written informed consent. To control for selection bias, only patients who had not progressed and were still receiving lenalidomide at 12 months were included. These patients were categorized as having dose reductions or no dose reductions, and those requiring dose reductions were further classified as having dose reductions after ⩾12 months or within 12 months of starting therapy. The median follow-up of surviving patients included in this analysis was 48 months.
All patients began lenalidomide therapy at 25 mg/day for 21 days of each 28-day cycle. All patients received the same initial dose of oral dexamethasone (40 mg on days 1–4, 9–12 and 17–20 of each 28-day cycle; after the fourth cycle, dexamethasone was administered on days 1–4 only). As previously described,4, 5 protocol-sanctioned dose reductions of lenalidomide and dexamethasone were allowed for adverse events.
A series of landmark analyses were performed, including only patients still on study at 6, 9 and 12 months in order to determine the impact of lenalidomide dose reductions on PFS at the different time points. PFS was evaluated using Kaplan–Meier analyses, and the log-rank test was used for comparing the groups. At 6 and 9 months, median PFS for patients with a dose reduction after the specified time was better than for the other two groups, but not significantly so. However, at 12 months, there was a statistically significant difference in PFS between groups. Therefore, 12 months was used in this study.
Patient characteristics and laboratory values at baseline and 12 months were included in a univariate Cox regression analysis of PFS. Dose reduction (‘<12 months', ‘⩾12 months' or ‘no dose reduction') was coded as two dummy variables. All covariates with P<0.25 in the univariate analysis were included in a multivariate analysis. All possible regression models were run and the ‘best' subset of covariates was selected. To identify baseline covariates that predicted dose reduction after ⩾12 months, a logistic regression analysis was performed using ‘dose reduction ⩾12 months' as the dependent variable.
Results
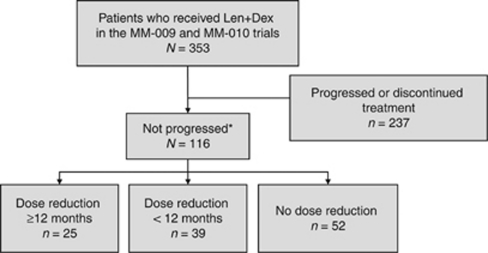
A total of 353 patients received lenalidomide plus dexamethasone in the MM-009 (N=177) and MM-010 (N=176) trials. Of these, 116 (32.9%) had not progressed and were still receiving study treatment at 12 months and, therefore, were included in the current analysis (60 patients from MM-009 and 56 patients from MM-010). The remaining 237 patients had documented disease progression or discontinued study treatment within the first 12 months of therapy. Of the 116 evaluable patients, 52 patients (45%) had no dose reductions, 39 patients (34%) had lenalidomide dose reductions before 12 months and 25 patients (22%) had lenalidomide dose reductions after ⩾12 months. Patient disposition is summarized in Figure 1.
Figure 1.
Flow diagram demonstrating the disposition and lenalidomide dose reductions of 353 patients included in the study. Len+Dex, lenalidomide plus dexamethasone. *Patients still receiving lenalidomide at 12 months.
Patient demographics and clinical characteristics are shown in Table 1. Baseline characteristics were comparable between the three groups; however, among patients with early or late dose reduction, a higher proportion had an Eastern Cooperative Oncology Group (ECOG) performance status score of ⩾1 (56% and 76%, respectively), compared with patients who had no dose reduction (38%). In addition, the burden of disease (measured by a β2-microglobulin level of ⩾2.5 mg/l) was higher in patients with dose reductions before 12 months (77%) compared with patients who had dose reductions after ⩾12 months (60%) or no dose reduction (48%).
Table 1. Demographic and clinical characteristics.
|
Dose reduction |
|||
|---|---|---|---|
| ⩾12 Months (n=25) | <12 Months (n=39) | No dose reduction (n=52) | |
| Age, years | |||
| Median | 64 | 65 | 59.5 |
| Range | 45–81 | 46–81 | 33–81 |
| Sex, n (%) | |||
| Male | 15 (60.0) | 24 (61.5) | 29 (55.8) |
| Female | 10 (40.0) | 15 (38.5) | 23 (44.2) |
| Time since diagnosis, years | |||
| Median | 3.4 | 3.2 | 2.9 |
| Range | 0.4–10.4 | 0.8–14.6 | 0.5–13.6 |
| Durie–Salmon stage, n (%) | |||
| I | 3 (12.0) | 2 (5.1) | 2 (3.8) |
| II | 9 (36.0) | 12 (30.8) | 16 (30.8) |
| III | 13 (52.0) | 25 (64.1) | 34 (65.4) |
| ECOG performance status, n (%) | |||
| 0 | 5 (20.0) | 15 (38.5) | 29 (55.8) |
| 1 | 13 (52.0) | 16 (41.0) | 19 (36.5) |
| 2 | 6 (24.0) | 6 (15.4) | 1 (1.9) |
| Missing | 1 (4.0) | 2 (5.1) | 3 (5.8) |
| Previous therapy, n (%) | |||
| 1 previous therapy | 10 (40.0) | 15 (38.5) | 20 (38.5) |
| ⩾2 previous therapies | 12 (48.0) | 22 (56.4) | 25 (48.1) |
| Type of therapy, n (%) | |||
| Thalidomide | 7 (28.0) | 16 (41.0) | 14 (26.9) |
| Bortezomib | 2 (8.0) | 5 (12.8) | 3 (5.8) |
| Stem-cell transplantation | 12 (48.0) | 21 (53.8) | 32 (61.5) |
| β2-Microglobulin level, n (%) | |||
| <2.5 mg/l | 10 (40.0) | 9 (23.1) | 27 (51.9) |
| ⩾2.5 mg/l | 15 (60.0) | 30 (76.9) | 25 (48.1) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Response and outcomes
Response rates were similarly high in all three patient subgroups: a partial response or better was achieved in all patients with a dose reduction and 49 of the 52 patients (94%) without a dose reduction. The rate of complete response was higher in patients with dose reductions after ⩾12 months (52%), compared with those with early (31%) or no (40%) dose reductions. Median PFS for each patient subgroup is shown in Table 2 and Figure 2. Patients who had dose reductions after ⩾12 months had significantly longer PFS (not reached (NR)) than those who had dose reductions before 12 months of starting therapy (28.0 months, P=0.007) and those without dose reductions (36.8 months, P=0.039) (Table 2). Median overall survival was NR in any of the three subgroups. Consistent with the PFS findings, median overall survival was significantly longer in patients who had dose reductions after ⩾12 months (NR; 95% confidence interval (CI), NR–NR) compared with those who had dose reductions before 12 months (NR; 95% CI, 38.5–NR; P=0.022) and those who had no dose reductions (NR; 95% CI, 47.1–NR; P=0.103).
Table 2. Landmark analyses performed at 6, 9 and 12 months to determine the impact of lenalidomide dose on PFS.
|
Median PFS, months (95% CI) |
|||
|---|---|---|---|
| Dose reduction at or after time point | Dose reduction before time point | No dose reduction | |
| 6 months | n=50 | n=40 | n=79 |
| 28.6 (20.5–46.9) | 24.7 (16.6–36.7) | 19.8 (25.1–29.0) | |
| P=0.388 | P=0.069 | ||
| 9 months | n=39 | n=37 | n=63 |
| 44.4 (21.9–NR) | 24.7 (17.0–36.7) | 26.2 (19.7–39.6) | |
| P=0.256 | P=0.128 | ||
| 12 months | n=25 | n=39 | n=52 |
| NR (35.7–NR) | 28.0 (17.5–36.7) | 36.8 (22.1–NR) | |
| P=0.007 | P=0.039 | ||
Abbreviations: CI, confidence interval; NR, not reached; PFS, progression-free survival.
P-values are vs dose reduction after time point (log-rank test).
Figure 2.
PFS (months) of MM patients according to lenalidomide dose reduction group, <12 months (n=39), ⩾12 months (n=25) or no dose reduction (n=52). Median PFS was significantly longer in patients who had dose reductions ⩾12 months as compared with those who had dose reductions <12 months (P=0.007) and those without dose reductions (P=0.039).
Predictors of PFS in Cox regression analysis
Covariates considered for analysis are shown in Supplementary Table S1. Univariate analysis indicated that dose reductions after ⩾12 months were associated with longer PFS compared with the other two subgroups, and dose reductions before 12 months of starting therapy were associated with shorter PFS compared with the other two subgroups. Other relevant factors identified in the univariate analysis are listed in Table 3. In a multivariate analysis, lenalidomide dose reductions after ⩾12 months remained an independent predictor of PFS (hazard ratio, 0.47; 95% CI, 0.23–0.98). Other independent predictors of PFS were serum albumin, the percentage of neutrophils and M-protein, all at 12 months (Table 3).
Table 3. Cox regression analysis of PFS.
| Covariate |
HR (95% CI) |
|
|---|---|---|
| Univariate modela (n=116) | Multivariate model (n=101) | |
| Dose reduced after ⩾12 months (yes vs no) | 0.42 (0.21–0.86)b | 0.47 (0.23–0.98) |
| Dose reduced before 12 months (yes vs no) | 1.73 (1.03–2.91)b | |
| Age, years | 1.02 (1.00–1.05) | |
| ISS disease stage at baseline (stage 1, 2, 3) | 1.45 (0.92–2.28) | |
| Bone marrow cellularity, % | 1.01 (1.00–1.02) | |
| Bone marrow cellularity (<33% vs ⩾33%) | 1.43 (0.86–2.36) | |
| Serum albumin at 12 months, g/dl | 0.53 (0.25–1.26) | 0.29 (0.11–0.75) |
| Calcium at baseline (⩽10% vs >10%) | 0.56 (0.30–1.07) | |
| Calcium at 12 months (⩽10% vs >10%) | 2.38 (0.73–7.73) | |
| Neutrophils at 12 months, % | 1.02 (1.00–1.04) | 1.02 (1.00–1.04) |
| Neutrophils (absolute) at 12 months, × 103/l | 1.06 (0.84–1.34) | |
| M-protein at 12 months, g/l | 1.07 (1.00–1.14)b | 1.07 (1.00–1.14) |
| ECOG score at baseline (⩾1 vs 0) | 1.00 (0.60–1.67) | |
| Chi-square(df) | Chi-square(4)=16.60 | |
| P=0.003 | ||
| AIC=423.56 | ||
Abbreviations: AIC, Akaike's information criterion; CI, confidence interval; df, degrees of freedom; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; ISS, International Staging System.
All listed univariate covariates had a P-value of <0.25 and were considered in the multivariate analysis.
P<0.05 for the univariate model.
Proportion of dose received and days treated
As expected, the proportion of full-dose lenalidomide received (25 mg/day for 21 days of each 28-day cycle) was lower in the groups that required dose reductions, compared with those who did not require dose reductions (Table 4). Nevertheless, the proportion of days treated was similar among the three subgroups, indicating that patients in all three subgroups required dose interruptions at some point during treatment. The median dose of dexamethasone was similar in all three subgroups; however, differences in the proportion of the target dexamethasone dose received were noted between the groups, especially after the second year of treatment (Table 4). Both subgroups of patients with dose reductions received 46–56% of the target dexamethasone dose during the third and fourth years of treatment, compared with 86–88% for patients with no dose reductions.
Table 4. Proportion of full-dose lenalidomide received, days treated and median dexamethasone dose, according to dose reduction.
|
Dose reduction |
|||
|---|---|---|---|
| ⩾12 Months (n=25) | <12 Months (n=39) | No dose reduction (n=52) | |
| Median proportion of lenalidomide dose received, % (25%, 75%)a | 72.2 (63.4, 84.0) | 50.5 (34.5, 63.8) | 90.4 (83.5, 98.4) |
| Median proportion of days treated, % (25%, 75%)b | 88.7 (79.2, 96.4) | 89.1 (82.2, 93.6) | 90.4 (83.5, 98.4) |
| Median dexamethasone dose, mg (25%, 75%)c | 40.0 (30.3, 40.0) | 40.0 (37.2, 40.0) | 36.3 (29.4, 40.0) |
| Proportion of dexamethasone dose received, %d | |||
| Year 1 (target 9.2 mg/day) | 87 (n=25) | 88 (n=39) | 96 (n=52) |
| Year 2 (target 5.7 mg/day) | 109 (n=25) | 114 (n=39) | 91 (n=52) |
| Year 3 (target 5.7 mg/day) | 56 (n=22) | 49 (n=19) | 86 (n=33) |
| Year 4 (target 5.7 mg/day) | 53 (n=15) | 46 (n=11) | 88 (n=23) |
Total target dose was 25 mg/day.
Total target days of treatment was 21 days per 28-day cycle.
Reflects dose on days of treatment. Per protocol patients received 40 mg/day dexamethasone for days 1–4, 9–12 and 17–20 for four cycles, and then 40 mg/day for days 1–4 for subsequent cycles.
Median target dose is calculated as total dose received/days on study.
Neutropenia and thrombocytopenia
As expected, neutropenia and thrombocytopenia were more common in patients who had dose reductions than in those who had no dose reductions (Table 5). Among those who required dose reductions, the incidence of grade 3 or 4 neutropenia was 64% in those who had dose reductions after ⩾12 months and 67% in those who had dose reductions before 12 months of starting therapy; the incidence was 39% in those with no dose reductions (Table 5). Of note, the incidence of thrombocytopenia was higher in patients who had dose reductions before 12 months of therapy (31%), compared with those who had dose reductions after ⩾12 months or no dose reductions (8% and 6%, respectively). The proportion of patients without any grade ⩾3 events of neutropenia or thrombocytopenia was greater in the group that had no dose reductions (58%) than in those with dose reductions (36% and 23% for those with late and early dose reductions, respectively). Among patients who developed grade 3 or 4 neutropenia, 44–50% received granulocyte colony-stimulating factor in each dose reduction group (Table 5). Notably, granulocyte colony-stimulating factor was more readily administered for neutropenia in patients who had no dose reductions (59% of events), than in patients who had dose reductions before 12 months (42% of events) or dose reductions after ⩾12 months (28% of events) (data not shown). Neutropenia or infection was the cause for dose reductions in 18 of 25 patients (72%) who had dose reductions after ⩾12 months. The remaining seven patients had dose reductions for other adverse events, including asthenia, edema and neuropathy.
Table 5. Incidence of grade 3 or 4 neutropenia, febrile neutropenia and thrombocytopenia.
| Grade 3 or 4 event, n (%) |
Dose reduction |
||
|---|---|---|---|
| ⩾12 Months (n=25) | <12 Months (n=39) | No dose reduction (n=52) | |
| Neutropeniaa | 16 (64) | 26 (67) | 20 (39) |
| G-CSF | 7 (44) | 12 (46) | 10 (50) |
| No G-CSF | 9 (56) | 14 (54) | 10 (50) |
| Febrile neutropeniab | 1 (4) | 3 (8) | 1 (2) |
| Thrombocytopenia | 2 (8) | 12 (31) | 3 (6) |
| No events | 9 (36) | 9 (23) | 30 (58) |
Abbreviation: G-CSF, granulocyte colony-stimulating factor.
Neutropenia included the terms ‘neutropenia', ‘febrile neutropenia' and ‘neutrophil count decreased'.
Febrile neutropenia included the terms ‘febrile neutropenia' and ‘neutropenic sepsis'.
Predictor of dose reductions after 12 months in logistic regression analysis: performance status
Logistic regression identified ECOG score at baseline (⩾1 vs 0) as the strongest predictor of dose reductions after ⩾12 months (odds ratio, 0.25; 95% CI, 0.09–0.73). Of the 110 evaluable patients with ECOG scores available, 61 patients (56%) had an ECOG score of ⩾1. Overall, 79% of patients who had lenalidomide dose reductions after ⩾12 months, 59% of patients with dose reductions before 12 months and 41% of patients with no dose reductions had ECOG scores of ⩾1. Patients with an ECOG score of ⩾1 were three times more likely to have a dose reduction after ⩾12 months than those who had an ECOG score of 0 (31 vs 10% P<0.01).
Discussion
This subanalysis of data from the MM-009 and MM-010 trials showed that, among patients who required lenalidomide dose reductions, those who had dose reductions after ⩾12 months of full-dose lenalidomide (25 mg/day for 21 days of each 28-day cycle) had a significantly higher median PFS and overall survival than those who had earlier dose reductions. These data underscore the importance of continuing full-dose lenalidomide therapy for at least 12 months in patients who are able to tolerate the treatment. Patients who had dose reductions after ⩾12 months also had a significantly higher median PFS than those who had never had dose reductions. Therefore, the prolonged PFS in patients who received full-dose lenalidomide for ⩾12 months before dose reductions cannot be attributed to a selection bias of generally healthier patients. In fact, this group had the highest proportion of patients with an ECOG score of ⩾1, which is associated with a worse prognosis. Furthermore, the relationship between dose reductions after ⩾12 months and PFS was independent of other factors measuring disease severity, such as β2-microglobulin or International Staging System disease stage, as found in the Cox regression analysis. Of note, it is unlikely that the imbalance between the groups in β2-microglobulin levels confounded the results because there was no association between β2-microglobulin levels and PFS in the univariate analysis.
As expected, neutropenia and thrombocytopenia were more frequently observed in patients who had dose reductions. Careful monitoring and management of myelosuppression with growth factors during the first 12 months of treatment may help to avoid severe cases of neutropenia or thrombocytopenia, which necessitate early dose reductions or interruptions. The inferior PFS observed in patients who had dose reductions within 12 months of starting therapy underscores the importance of adverse-event management and delivering full doses of lenalidomide therapy for ⩾12 months. The present data also suggest that after 12 months, the lenalidomide dose can be reduced for adverse events without compromising efficacy.
This analysis has several limitations. It is exploratory in nature and is based on a small number of patients in each subgroup. After adjusting for all measured covariates at baseline and at 12 months, patients with dose reductions after ⩾12 months still had significantly longer PFS. However, because decisions to reduce the dose were based on investigators' assessment of individual clinical status, and not as defined by the protocol, it is possible that there are patient clinical parameters not included in this analysis that could potentially explain the longer PFS among patients with dose reductions after ⩾12 months.
This analysis of pooled data from the MM-009 and MM-010 trials is consistent with evidence that continued therapy with lenalidomide plus dexamethasone is associated with clinical benefit. Harousseau et al.7 reported that half of the patients initially classified as having a partial response to lenalidomide plus dexamethasone eventually had a complete response or near-complete response after receiving additional cycles of therapy. In the present analysis, the rate of complete response was higher in patients with dose reductions after ⩾12 months (52%) than in patients with dose reductions before 12 months (31%) or no dose reductions (40%), suggesting deepening responses with continued treatment. San Miguel et al.8 demonstrated that continuing treatment after achievement of best response significantly prolonged survival compared with earlier discontinuation. Together, these findings confirm the importance of continuing treatment with lenalidomide plus dexamethasone in patients with RRMM.
The cellular effects of lenalidomide suggest that chronic use may be beneficial.9 Lenalidomide exerts both direct tumoricidal effects that lead to tumor cell death and immunomodulatory effects that may help to keep tumors in remission. These occur via the direct inhibition of myeloma cell growth and induction of apoptosis;10, 11, 12 immunomodulation, including inhibition of myeloid cells and co-stimulation of lymphoid cells;13, 14, 15, 16, 17 and inhibition of angiogenesis.18, 19, 20, 21 Lenalidomide also modulates several cytokines, including inhibition of myeloid cell production of tumor necrosis factor-α, a growth and survival factor for MM cells.13, 22 Moreover, lenalidomide also stimulates T cells as well as natural killer cells, which may help to develop innate anticancer immunity.15, 16, 17, 23, 24
It has been suggested that, although the tumoricidal effects of lenalidomide in combination with dexamethasone may be responsible for the initial response to therapy, the immunomodulatory effects of lenalidomide may have an important role in maintaining long-term disease control.12 On the basis of in vitro pharmacology, the immunomodulatory effects of lenalidomide are more potent than its direct tumoricidal effects, and therefore these effects may be predominant at lower doses. The maximum plasma concentration for lenalidomide following a 25 mg dose in patients with normal renal function is 2.19 μ (568 ng/ml).25 Proliferation of 4 of 10 myeloma cell lines is inhibited by lenalidomide with a half maximal inhibitory concentration (IC50) of ⩽2.1 μ.12 In comparison, lenalidomide shows immunomodulatory properties at concentrations of <40 n (<10.4 ng/ml), reaching maximal enhancement at 1 μ (259 ng/ml).12 The present findings combined with these in vitro observations suggest that, following at least 12 months of treatment, lenalidomide's immunomodulatory properties, exerted even at lower doses, may be sufficient for adequate control of the residual tumor in RRMM. Further studies are needed to confirm this hypothesis.
Although lenalidomide acts synergistically with dexamethasone to inhibit myeloma cell proliferation in vitro, its immunomodulatory activity is antagonized by dexamethasone.12, 26, 27 In this analysis, the median dexamethasone dose was comparable in all three subgroups (40, 40 and 36.3 mg in the ⩾12 months, <12 months or no reduction groups, respectively). This reflects actual dose received on days in which there was no dose interruption divided by the number of actual treatment days. After 2 years of treatment, patients with lenalidomide dose reductions (⩾12 months and <12 months) also received a lower proportion of the target dexamethasone dose compared with those without lenalidomide dose reductions. The enhanced efficacy and improved tolerability achieved when administering full-dose lenalidomide with reduced-dose dexamethasone, defined as ⩽20 mg/day for just 4 days during the first 4 cycles or ⩽20 mg/day in subsequent cycles, was demonstrated by San Miguel et al.28 in another subset analysis from MM-009 and MM-010 trials. In a recent ECOG study (E4A03) conducted in patients with newly diagnosed MM, a regimen of lenalidomide plus low-dose dexamethasone was more clinically effective than the standard regimen.29 One explanation for the results is the improved tolerability of the low-dose dexamethasone regimen, which resulted in lower incidence of serious adverse events, including thromboembolism.29 Another explanation could be that lower doses of the immunosuppressant dexamethasone led to less antagonism of the immunomodulatory effects of lenalidomide, which in turn resulted in better disease control, as discussed above.12, 26, 27 This is supported by a number of studies that investigated steroid-sparing approaches and that demonstrated the efficacy and tolerability of single-agent lenalidomide in patients with MM.30, 31, 32 Furthermore, emerging lenalidomide maintenance phase III studies in the frontline setting suggest that maintenance therapy with single-agent lenalidomide (10–15 mg/day, for 21 of 28 days or continuously) significantly prolongs PFS and duration of response, reducing the risk of disease progression by up to 61%.33, 34, 35 In the absence of maintenance studies in the relapsed setting, consensus guidelines for treatment of patients with RRMM recommend long-term continued treatment with lenalidomide plus dexamethasone with dose modifications for adverse events for both agents if required.36
Recently, cases of second primary malignancies (SPMs) have been observed in newly diagnosed patients with myeloma treated with lenalidomide maintenance after high-dose chemotherapy. In a post hoc analysis of pooled MM-009 and MM-010 data, the incidence rates of SPM were assessed in comparison with background cancer rates based on cancer registry data (SEER database) to better characterize the significance of these observations.37, 38 The incidence of SPMs was low during double-blind treatment and no acute myeloid leukemia or B-cell malignancies were observed. Importantly, the observed incidence rates of solid-tumor SPMs were not different from the incidence rates observed in the general population.38 Considering a survival benefit was observed during long-term follow-up of the MM-009 and MM-010 trials, despite a significant number of patients in the placebo and dexamethasone arm crossing over to receive lenalidomide-based therapy, the low number and type of SPMs observed did not change the benefit–risk profile for lenalidomide in RRMM patients.38
In this study, PFS benefit was seen in patients with lenalidomide dose reductions after ⩾12 months; and a reduction in dexamethasone dose was observed after the second year. Altogether, the present findings can be explained by the mechanism of action of lenalidomide; full-dose lenalidomide in combination with dexamethasone seems to be directly tumoricidal whereas lenalidomide, even at reduced doses, in combination with lower-dose dexamethasone may provide immunomodulatory effects.
A better understanding of the immune effects of lenalidomide in the patient may help to determine an appropriate dose and schedule for optimal biologic effect during maintenance therapy. Additional studies on the immunomodulatory properties of continuous lenalidomide therapy are in progress.
Acknowledgments
This paper describes a pooled analysis of the Celgene-sponsored MM-009 and MM-010 clinical trials. The authors received editorial support from Excerpta Medica in the preparation of this paper, funded by Celgene Corporation. The authors were fully responsible for content and editorial decisions for this paper. This work was first presented at the 51st American Society of Hematology Annual Meeting and Exposition, New Orleans, LA, USA, 5–8 December 2009.
Professor Dimopoulos has acted as a consultant/advisory role for Celgene Corporation and received honoraria from Celgene Corporation. Drs Hussein and Swern are employed by Celgene Corporation. Dr Weber has received research funding and other remuneration from Celgene Corporation.
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanan-Khan AA, Cheson BD. Lenalidomide for the treatment of B-cell malignancies. J Clin Oncol. 2008;26:1544–1552. doi: 10.1200/JCO.2007.14.5367. [DOI] [PubMed] [Google Scholar]
- Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Chen C, Spencer A, Niesvizky R, Attal M, Stadtmauer EA, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23:2147–2152. doi: 10.1038/leu.2009.147. [DOI] [PubMed] [Google Scholar]
- Harousseau JL, Dimopoulos MA, Wang M, Corso A, Chen C, Attal M, et al. Better quality of response to lenalidomide plus dexamethasone is associated with improved clinical outcomes in patients with relapsed or refractory multiple myeloma. Haematologica. 2010;95:1738–1744. doi: 10.3324/haematol.2009.015917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Miguel JF, Dimopoulos MA, Stadtmauer EA, Rajkumar SV, Siegel D, Bravo ML, et al. Effects of lenalidomide and dexamethasone treatment duration on survival in patients with relapsed or refractory multiple myeloma treated with lenalidomide and dexamethasone. Clin Lymphoma Myeloma Leuk. 2011;11:38–43. doi: 10.3816/CLML.2010.n.120. [DOI] [PubMed] [Google Scholar]
- Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4:314–322. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai YT, et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–2950. [PubMed] [Google Scholar]
- Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, et al. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99:4525–4530. doi: 10.1182/blood.v99.12.4525. [DOI] [PubMed] [Google Scholar]
- Gandhi AK, Kang J, Capone L, Parton A, Wu L, Zhang LH, et al. Dexamethasone synergizes with lenalidomide to inhibit multiple myeloma tumor growth, but reduces lenalidomide-induced immunomodulation of T and NK cell function. Curr Cancer Drug Targets. 2010;10:155–167. doi: 10.2174/156800910791054239. [DOI] [PubMed] [Google Scholar]
- Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163:380–386. [PubMed] [Google Scholar]
- Haslett PA, Hanekom WA, Muller G, Kaplan G. Thalidomide and a thalidomide analogue drug costimulate virus-specific CD8+ T cells in vitro. J Infect Dis. 2003;187:946–955. doi: 10.1086/368126. [DOI] [PubMed] [Google Scholar]
- Marriott JB, Clarke IA, Dredge K, Muller G, Stirling D, Dalgleish AG. Thalidomide and its analogues have distinct and opposing effects on TNF-alpha and TNFR2 during co-stimulation of both CD4+ and CD8+ T cells. Clin Exp Immunol. 2002;130:75–84. doi: 10.1046/j.1365-2249.2002.01954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Raje N, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical implications. Br J Haematol. 2005;128:192–203. doi: 10.1111/j.1365-2141.2004.05286.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Payvandi F, Wu L, Zhang LH, Hariri RJ, Man HW, et al. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc Res. 2009;77:78–86. doi: 10.1016/j.mvr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Dredge K, Horsfall R, Robinson SP, Zhang LH, Lu L, Tang Y, et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005;69:56–63. doi: 10.1016/j.mvr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Lentzsch S, LeBlanc R, Podar K, Davies F, Lin B, Hideshima T, et al. Immunomodulatory analogs of thalidomide inhibit growth of Hs Sultan cells and angiogenesis in vivo. Leukemia. 2003;17:41–44. doi: 10.1038/sj.leu.2402745. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Schlossman RL, Weller E, Hideshima T, Mitsiades C, Davies F, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100:3063–3067. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- Bartlett JB, Michael A, Clarke IA, Dredge K, Nicholson S, Kristeleit H, et al. Phase I study to determine the safety, tolerability and immunostimulatory activity of thalidomide analogue CC-5013 in patients with metastatic malignant melanoma and other advanced cancers. Br J Cancer. 2004;90:955–961. doi: 10.1038/sj.bjc.6601579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YT, Li XF, Catley L, Coffey R, Breitkreutz I, Bae J, et al. Immunomodulatory drug lenalidomide (CC-5013, IMiD3) augments anti-CD40 SGN-40-induced cytotoxicity in human multiple myeloma: clinical implications. Cancer Res. 2005;65:11712–11720. doi: 10.1158/0008-5472.CAN-05-1657. [DOI] [PubMed] [Google Scholar]
- Chang DH, Liu N, Klimek V, Hassoun H, Mazumder A, Nimer SD, et al. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Blood. 2006;108:618–621. doi: 10.1182/blood-2005-10-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Lau H, Kong L, Kumar G, Zeldis JB, Knight R, et al. Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J Clin Pharmacol. 2007;47:1466–1475. doi: 10.1177/0091270007309563. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS. How ‘immunomodulatory' are IMIDS. Blood. 2011;117:1440–1441. doi: 10.1182/blood-2010-11-317156. [DOI] [PubMed] [Google Scholar]
- Hsu AK, Quach H, Tai T, Prince M, Harrison SJ, Trapani JA, et al. The immunostimulatory effect of lenalidomide on NK-cell function is profoundly inhibited by concurrent dexamethasone therapy. Blood. 2011;117:1605–1613. doi: 10.1182/blood-2010-04-278432. [DOI] [PubMed] [Google Scholar]
- San Miguel JF, Dimopoulos M, Weber D, Olesnyckyj M, Yu Z, Zeldis J, et al. Dexamethasone dose adjustments seem to result in better efficacy and improved tolerability in patients with relapsed/refractory multiple myeloma who are treated with lenalidomide/dexamethasone (MM009/010 sub-analysis) Blood 2007110(abstract 2712). [Google Scholar]
- Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Blood E, Mitsiades CS, Jagannath S, Zeldenrust SR, Alsina M, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P, Jagannath S, Hussein M, Berenson J, Singhal S, Irwin D, et al. Safety and efficacy of single-agent lenalidomide in patients with relapsed and refractory multiple myeloma. Blood. 2009;114:772–778. doi: 10.1182/blood-2008-12-196238. [DOI] [PubMed] [Google Scholar]
- Baz R, Patel M, Finley-Oliver E, Lebovic D, Hussein MA, Miller KC, et al. Single agent lenalidomide in newly diagnosed multiple myeloma: a retrospective analysis. Leukemia Lymphoma. 2010;51:1015–1019. doi: 10.3109/10428191003721342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy PL, Owzar K, Anderson KC, Hofmeister CC, Hurd DD, Hassoun H, et al. Phase III intergroup study of lenalidomide versus placebo maintenance therapy following single autologous hematopoietic stem cell transplantation (AHSCT) for multiple myeloma: CALGB 100104 Blood 2010116(abstract 37). [Google Scholar]
- Attal M, Lauwers-Cancès V, Marit G, Caillot D, Facon T, Hulin C, et al. Maintenance treatment with lenalidomide after transplantation for MYELOMA: final analysis of the IFM 2005-02 Blood 2010116(abstract 310). [Google Scholar]
- Palumbo A, Delforge M, Catalano J, Hajek R, Kropff M, Petrucci MT, et al. A phase 3 study evaluating the efficacy and safety of lenalidomide combined with melphalan and prednisone in patients ≥65 years with newly diagnosed multiple myeloma (NDMM): continuous use of lenalidomide vs fixed-duration regimens Blood 2010116(abstract 622). [Google Scholar]
- Dimopoulos MA, Palumbo A, Attal M, Beksaç M, Davies FE, Delforge M, et al. Optimizing the use of lenalidomide in relapsed or refractory multiple myeloma: consensus statement. Leukemia. 2011;25:749–760. doi: 10.1038/leu.2011.3. [DOI] [PubMed] [Google Scholar]
- Howlander N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2008 National Cancer Institute: Bethesda, MD; . http://seer.cancer.gov/csr/1975_2008/ , based on November 2010 SEER data submission, posted to SEER website,2011 [Google Scholar]
- Dimopoulos M, Weber D, Richardson P, Orlowski R, Niesvizky R, Attal M, et al. Lenalidomide and dexamethasone (Len+Dex) treatment in relapsed/refractory multiple myeloma (RRMM) patients does not increase the risk of second primary malignancies (SPM): analysis of MM-009/010 Haematologica 201196S2abstract 1009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.