Abstract
Serum levels of inflammatory cytokines, for example, tumor necrosis factor alpha (TNFα), interleukin-6 (IL-6), and IL-1 beta (IL-1β), are elevated in subjects with major depressive disorder (MDD). The reason why this occurs is unclear. Elevated levels of inflammatory cytokines could be a result of brain dysfunction in MDD. It is also possible that inflammatory cytokines contribute to depressive symptoms in MDD. If the first assumption is correct, one would expect levels to normalize with resolution of the depressive episode after treatment. Several studies have measured changes in cytokine levels during antidepressant treatment; however, the results vary. The purpose of this study was to pool all available data on changes in serum levels of TNFα, IL-6, and IL-1β during antidepressant treatment to determine whether these levels change. Studies were included if they used an approved pharmacological treatment for depression, patients had a diagnosis of MDD, and serum levels of TNFα, IL-6, and/or IL-1β were measured before and after treatment. Twenty-two studies fulfilled these criteria. Meta-analysis of these studies showed that, overall, while pharmacological antidepressant treatment reduced depressive symptoms, it did not reduce serum levels of TNFα. On the other hand, antidepressant treatment did reduce levels of IL-1β and possibly those of IL-6. Stratified subgroup analysis by class of antidepressant indicated that serotonin reuptake inhibitors may reduce levels of IL-6 and TNFα. Other antidepressants, while efficacious for depressive symptoms, did not appear to reduce cytokine levels. These results argue against the notion that resolution of a depressive episode is associated with normalization of levels of circulating inflammatory cytokines; however, the results are consistent with the possibility that inflammatory cytokines contribute to depressive symptoms and that antidepressants block the effects of inflammatory cytokines on the brain.
Keywords: depression, inflammation, TNF, IL-6, IL-1, antidepressant
INTRODUCTION
Major depressive disorder (MDD) is a prevalent and debilitating disorder the pathogenesis of which is incompletely understood. MDD, in the absence of medical illnesses, is associated with increased levels of the inflammatory cytokines tumor necrosis factor alpha (TNFα), interleukin-6 (IL-6), and IL-1 beta (IL-1β) (Howren et al, 2009; Dowlati et al, 2010). There are several potential reasons why elevated levels of inflammatory cytokines are associated with MDD: (1) there may be common etiologies that lead to both MDD and elevated levels of these inflammatory cytokines, without a causal relation between the two phenomena; (2) MDD may cause brain alterations that impair the brain's ability to modulate the immune system via hypothalamic-pituitary-adrenal axis activity and autonomic outflow; and (3) systemic triggers (eg, exposure to pathogens), genetic differences in the immune system, or differences in the immune system's exposure to commensal microorganisms, may lead to increased production of inflammatory cytokines, which in turn contribute to depressive symptoms (Miller et al, 2009; Raison et al, 2010). Supporting (3) are studies showing that acute and chronic immune stimuli, which increase serum levels of inflammatory cytokines, can elicit depressive symptoms in humans (Reichenberg et al, 2001; Wright et al, 2005; Capuron et al, 2009; Eisenberger et al, 2009; DellaGioia and Hannestad, 2010), and studies showing that anti-inflammatory and anti-TNFα drugs can ameliorate depressive symptoms (Muller et al, 2006; Tyring et al, 2006). If (2) above is true, that is, elevated cytokine levels are a consequence of depression, then the treatment of depression with successful resolution of depressive symptoms would be expected to normalize levels. If, on the other hand, (3) above is true, that is, elevated levels of TNFα and IL-6 is a result of processes inherent to the immune system, then antidepressant treatment may not normalize levels, unless they have a direct effect on innate immune cells. Some studies suggest such a direct effect on the immune system, for example, in rodents both sub-chronic (Yirmiya et al, 2001) and acute (Roumestan et al, 2007) administration of antidepressants has anti-inflammatory effects, while some studies in humans did not find this effect (Hannestad et al, 2011). Although multiple studies have measured the effect of antidepressant treatment on circulating cytokine levels in patients with MDD, the results are not consistent. The goal of this meta-analysis was to determine whether pharmacological treatment of MDD is associated with changes in circulating levels of inflammatory cytokines, specifically TNFα, IL-6, and IL-1β; these cytokines were chosen because they are elevated in depression (Howren et al, 2009; Dowlati et al, 2010).
MATERIALS AND METHODS
Search Strategy
PubMed, PsychINFO, EMBASE, and the Cochrane Library were searched by two reviewers (JH and ND) for relevant trials using the following search strategies: (tumor necrosis factor OR interleukin) AND (antidepressant OR serotonin reuptake inhibitor OR tricyclic). The Cochrane Library was also searched using the broader terms ‘interleukin AND depressive' and ‘tumor necrosis factor AND depressive'. The references of all papers included in this meta-analysis as well as select review papers on this topic were searched for citations of further relevant published and unpublished research. Studies were limited to those that assessed human subjects. There were no language limitations on included studies.
Criteria for Inclusion of Studies in this Review
Studies were included in this meta-analysis if they examined the effect of a pharmacological treatment for depression (ie, an antidepressant) on serum levels of TNFα, IL-6, and/or IL-1β. Studies were only included if the pharmacological treatment used was a medication of demonstrated efficacy and approved by FDA for the treatment of MDD. Studies examining the effect of antidepressant treatment on other cytokines or that assessed cytokine production in vitro or ex vivo were not included. Studies of the effect of non-pharmacological treatments for depression (eg, electroconvulsive therapy, psychotherapy, sleep deprivation, herbal remedies, and so on) were not included. Studies were only included if the human subjects had a diagnosis of MDD and were medically healthy adults.
Meta-Analytic Methods
The primary outcome measure was change in mean serum level of TNFα, IL-6, or IL-1β over the course of a period of treatment with an antidepressant medication. As different cytokine assays have different sensitivity, comparisons were only made within each study. Some studies presented data in graph form only. Authors were contacted and asked to provide the actual values; when this information was not provided by the author, the mean change in cytokine serum level, and SD were estimated from measuring the published graphs with a ruler.
Standardized mean difference (SMD) was chosen as the summary statistic for meta-analysis and pooled using the generic inverse variance method in RevMan 5. A random-effects model was chosen for meta-analysis because random-effects models are preferred when there is significant heterogeneity between trials. Publication bias was assessed by plotting the effect size against sample size for each trial (funnel plot). Heterogeneity in changes in cytokine levels was assessed visually from the forest plot of SMD of individual studies. Statistical estimates of heterogeneity were assessed using the I2 heterogeneity statistic in RevMan 5. In addition, a sensitivity analysis was performed to examine our decision to use a random-effect model rather than a fixed-effects model for meta-analysis.
Studies were stratified based on class of antidepressant used (specific serotonin reuptake inhibitor, SSRI; serotonin and norepinephrine reuptake inhibitor, SNRI; and tricyclic antidepressants, TCA). Studies that used >2 different antidepressant classes and that did not give cytokine data for each antidepressant class used, were classified as ‘Miscellaneous'. For TNFα, stratification included only SSRI and Miscellaneous, because there was only one study each which used only a TCA or only an SNRI. For IL-6, stratification included SSRI, TCA, and Miscellaneous, but not SNRI since there was only one study, which used only an SNRI. For IL-1β, no stratification was performed because 5 out of 6 studies used SSRIs. We used the test for subgroup differences in RevMan 5 to determine whether subgroups reduced overall heterogeneity. For all statistical analysis we used a significance threshold of p<0.05.
RESULTS
Selection of Studies
Twenty-two studies, comprising 603 subjects, fulfilled the inclusion criteria (Maes et al, 1995, 1997; Sluzewska et al, 1995; Frommberger et al, 1997; Hinze-Selch et al, 2000; Kubera et al, 2000; Kagaya et al, 2001; Mikova et al, 2001; Kraus et al, 2002; Tuglu et al, 2003; Basterzi et al, 2005; Himmerich et al, 2006; Leo et al, 2006; Sutcigil et al, 2007; Eller et al, 2008; Hernandez et al, 2008; Piletz et al, 2009; Song et al, 2009; Yoshimura et al, 2009; Chen et al, 2010; Jazayeri et al, 2010; Fornaro et al, 2011). Details about these studies are presented in Table 1. Eight studies used more than one class of antidepressant and were classified as miscellaneous; the other studies were classified based on the use of SSRIs, SNRIs, and TCAs. Most (18 out of 22) studies assessed depression severity with the Hamilton Depression Rating Scale (HDRS); only two used the Montgomery-sberg Depression Rating Scale. Baseline depression severity was very similar across studies. The weighted mean HDRS score at baseline was 25.5±1.0 in the 18 studies that used this scale. The degree of improvement (as a percent reduction from the baseline severity score) was also very homogeneous. In the 18 studies that provided this information, the weighted mean reduction in depression severity was 52±3%. As a result of such homogeneity in baseline severity and degree of improvement, no meta-regression by baseline severity or degree of improvement was performed. With regards to the cytokine assays used, 19 out of 22 studies used enzyme-linked immunosorbent assays (ELISA) to measure serum levels of cytokines; however, there was a wide range of manufacturers (Table 1).
Table 1. Studies Included in the Meta-Analysis.
| Study | N | Cytokine(s) assessed | Antidepressant class | Rating scale/baseline severity/percent reduction | Assay type/manufacturer |
|---|---|---|---|---|---|
| Basterzi et al (2005) | 23 | IL-6 | SSRI | HDRS/21/49% | ELISA/CytImmune Sciences |
| Chen et al (2010) | 43 | TNF, IL-6 | Misc. | HDRS/31/47% | ELISA/Diaclone (now Gen-Probe) |
| Eller et al (2008) | 100 | TNF | SSRI | MADRS/29/68% | Chemiluminescence/Immulite |
| Frommberger et al (1997) | 10 | IL-6 | TCA | MADRS/34/65% | Bioassay |
| Fornaro et al (2011) | 16 | IL-6 | SNRI | HDRS/21/56% | ELISA/Bender MedSystems |
| Hernandez et al (2008) | 31 | IL-1 | SSRI | HDRS/20/50% | ELISA/DuoSet (R&D Systems) |
| Himmerich et al (2006) | 67 | TNF | Misc. | HDRS/27/66% | ELISA/BioSource |
| Hinze-Selch et al (2000) | 22 | TNF | SSRI or TCA | ND | ELISA/Medgenix |
| Jazayeri et al (2010) | 14 | IL-6, IL-1 | SSRI | HDRS/29/64% | ELISA/Bender MedSystems |
| Kagaya et al (2001) | 12 | TNF, IL-6, IL-1 | TCA | HDRS/23/49% | ELISA/BioSource |
| Kraus et al (2002) | 20 | TNF | SNRI | ND | ELISA/Medgenix |
| Kubera et al (2000) | 9 | IL-6 | ND | HDRS/22/76% | ELISA/Eurogenetics |
| Leo et al (2006) | 20 | TNF, IL-6, IL-1 | SSRI | HDRS/24/39% | ELISA/Quantikine (R&D Systems) |
| Maes et al (1995) | 17 | IL-6 | SSRI or TCA | HDRS/24/40% | ELISA/Eurogenetics |
| Maes et al (1997) | 25 | IL-6 | Misc. | HDRS/25/47% | ELISA/Eurogenetics |
| Mikova et al (2001) | 23 | TNF, IL-6 | SSRI and TCA | HDRS/>18/ND 9 resp, 5 non resp | ELISA/Eurogenetics |
| Piletz et al (2009) | 12 | TNF, IL-1 | SNRI | HDRS/26/58% | ELISA/Quantikine |
| Sluzewska et al (1995) | 9 | IL-6 | SSRI | HDRS/23/ND | ELISA/ND |
| Song et al (2009) | 30 | TNF, IL-1 | SSRI | HDRS/22/50% | ELISA/GeneMay |
| Sutcigil et al (2007) | 23 | TNF | SSRI | HDRS/∼26/∼50% | ELISA/Bender MedSystems |
| Tuglu et al (2003) | 26 | TNF | SSRI | HDRS/27/67% | Chemiluminescence/Immulite |
| Yoshimura et al (2009) | 51 | TNF, IL-6 | SSRI and SNRI | HDRS/22/68% | ELISA/Quantikine |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; HDRS, Hamilton Depression Rating Scale; IL-1, interleukin-1; IL-6, interleukin-6; MADRS, Montgomery-sberg Depression Rating Scale; ND, no data; SNRI, serotonin norepinephrine reuptake inhibitor; SSRI, serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
List of studies included in this meta-analysis. The table shows the number of subjects in each study, the cytokines measured, the antidepressant treatment used (by class), the depression severity measure, the baseline depression severity score, the percent decrease in depression severity, the type of cytokine assay used, and the manufacturer of the assay.
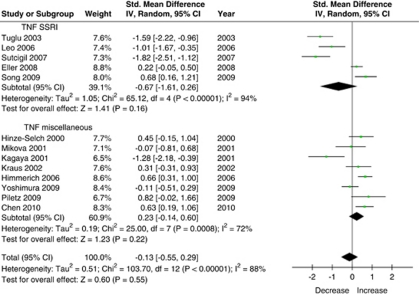
Tumor Necrosis Factor Alpha
A total of 13 studies (n=438 subjects) that measured serum levels of TNFα were included in this meta-analysis (Figure 1). Using the random-effects model, antidepressant treatment had no effect on serum levels of TNFα (SMD=−0.13 (95% CI: −0.55, 0.29), Z=0.6, p=0.55). This did not change when the fixed-effects model was used (SMD=0.09 (95% CI: −0.05, 0.23), Z=1.3, p=0.19). There was substantial heterogeneity between studies (τ2=0.5; χ2=103.7, df=12, p<0.00001, I2=88%), and the asymmetry in the funnel plot was indicative of possible publication bias. Stratified subgroup analysis showed that there was a significant difference by medication class (χ2=13.6, df=1, p=0.0002), because studies that used SSRI treatment showed greater reduction in TNFα levels. Using the random-effects model, SSRI treatment (n=199 subjects) did not have an effect on TNFα levels (SMD=−0.67 (95% CI: −1.61, 0.26), Z=1.4, p=0.16); however, using the fixed-effects model there was a trend effect of SSRI treatment on TNFα levels (SMD=−0.20 (95% CI: −0.40, 0.01), Z=1.9, p=0.06). There was substantial heterogeneity between SSRI studies (τ2=1.1; χ2=65.1, df=4, p<0.00001, I2=94%).
Figure 1.
Effect of antidepressant treatment on TNF serum levels.
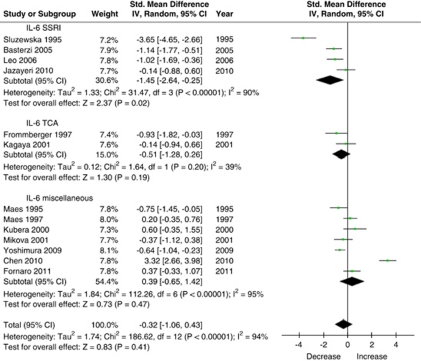
Interleukin-6
A total of 13 studies (n=274 subjects) measured the effect of antidepressant treatment on IL-6 levels and were included in this meta-analysis (Figure 2). Using the random-effects model, antidepressant treatment had no effect on IL-6 levels (SMD=−0.32 (95% CI: −1.06, 0.43), Z=0.8, p=0.41); however, using the fixed-effects model there was a significant, albeit very small, effect (SMD=−0.24 (95% CI: −0.43, −0.05), Z=2.5, p=0.01). Similarly, excluding two outliers (Sluzewska et al, 1995; Chen et al, 2010) rendered the overall results statistically significant, albeit with a very small effect (SMD=−0.38 (95% CI: −0.72, −0.05), Z=2.3, p=0.02). There was substantial heterogeneity between studies (τ2=1.7; χ2=186.6, df=12, p<0.00001, I2=94%). Stratified subgroup analysis showed that there was a significant difference by medication class (χ2=51.4, df=2, p<0.00001) because studies that used SSRI treatment showed greater reduction in IL-6 levels than studies using other antidepressants. Using the random-effects model, there was a significant effect of SSRI treatment (n=79 subjects) on IL-6 levels (SMD=−1.45 (95% CI: −2.64, −0.25), Z=2.4, p=0.02). Excluding one SSRI outlier (Sluzewska et al, 1995) did not change this (SMD=−0.80 (95% CI: −1.38, −0.21), Z=2.7, p<0.008). There was significant heterogeneity between the SSRI studies (τ2=1.3; χ2=31.5, df=3, p<0.00001, I2=90%). Using the random-effects model, there was no effect of TCA treatment (n=24 subjects) on IL-6 levels (SMD=−0.51 (95% CI: −1.28, 0.26), Z=1.3, p=0.2).
Figure 2.
Effect of antidepressant treatment on IL-6 serum levels.
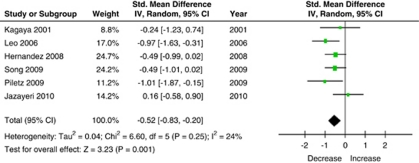
Interleukin-1 Beta
A total of six studies (n=115 subjects) measured levels of IL-1β and were included in this meta-analysis (Figure 3). Using the random-effects model, there was a significant effect of antidepressant treatment on IL-1β levels (SMD=−0.52 (95% CI: −0.83, −0.20), Z=3.23, p<0.001), and there was minimal heterogeneity in the studies that measured changes in IL-1β levels (τ2=0.04; χ2=6.6, df=5, p=0.25, I2=24%). Of the six studies that measured IL-1β levels, five used an SSRI for treatment; therefore no stratification by antidepressant class was performed.
Figure 3.
Effect of antidepressant treatment on IL-1 serum levels.
DISCUSSION
Meta-analysis of the studies included here failed to show a significant overall effect of pharmacological antidepressant treatment for MDD on serum levels of TNFα and IL-6. On the other hand, it appears that antidepressant treatment reduces IL-1β levels. Using the less stringent fixed-effects model, we found a small effect of antidepressant treatment on IL-6 levels. Similarly, excluding two outliers, the effect of antidepressant treatment on IL-6 levels was statistically significant. Stratified subgroup analysis suggested that SSRI treatment specifically may be associated with a reduction in IL-6 levels, and perhaps with a reduction in TNFα levels (when using the less stringent fixed-effects model rather than the random-effects model); however, the results of subgroup analyses are based on a small subsample of studies and must therefore be considered with that caveat in mind.
As a result of the homogeneity in the degree of improvement, no meta-regression was performed. In a large (n=100) study, there was no change in TNFα serum levels in responders or non-responders to SSRI treatment, despite a large difference in the change in depression severity (74% reduction in responders vs 33% reduction in non-responders) (Eller et al, 2008). In a follow-up study, non-responders were treated with bupropion, and among those who responded there was a 63% reduction in depression severity, but no change in TNFα levels (Eller et al, 2009). Overall, neither this meta-analysis nor Eller's data support the hypothesis that serum levels of TNFα decrease as a result of improvement in depressive symptoms, or that a decrease in TNFα levels is required for the antidepressant effect. This may suggest that increased TNFα levels in depression are due to an inherent dysfunction in the immune system and not related to the effects of MDD on the brain.
This meta-analysis suggests that there may be an effect of antidepressants on IL-6 levels, specifically of SSRI treatment. One study found a negative correlation between the reduction in depression severity and the change in serum IL-6 (Yoshimura et al, 2009). In cardiac patients with depression, both SSRI treatment (Pizzi et al, 2009) and cognitive-behavioral therapy (Doering et al, 2007) was associated with a reduction in IL-6 levels. The degree of elevation in IL-6 levels in MDD (d=0.25 (95% CI: 0.18, 0.31) (Howren et al, 2009)) and weighted mean difference=1.8 pg/ml (95% CI: 1.23 to 2.33) (Dowlati et al, 2010) is consistent with the degree of reduction in levels with SSRI treatment found here (SMD=−1.9).
Antidepressant treatment appears to have an effect in lowering levels of IL-1β, a cytokine for which evidence of an elevation in depression is controversial (Dowlati et al, 2010). The degree of elevation in IL-1β levels in one study (d=0.35 (95% CI: 0.03, 0.67)) (Howren et al, 2009) is consistent with the reduction seen here with antidepressant treatment (SMD=−0.62). Studies of depression co-morbid with other conditions have found that SSRIs lower IL-1β levels, including post-traumatic stress disorder (Tucker et al, 2004) and renal failure (Lee et al, 2004). Recently, Himmerich et al (2010) found that blood levels of IL-1β became undetectable in patients with depression after treatment with antidepressants. At the same time, the proportion of regulatory T lymphocytes (Treg) increased. As Tregs suppress innate immunity, this may be a mechanism through which antidepressant treatment reduces IL-1β levels, although the converse is also possible, that is, a reduced level if IL-1β permits differentiation of Tregs (Himmerich et al, 2010). Interestingly, a recent study found that tryptophan metabolites can enhance Treg differentiation (Yan et al, 2010); how this may relate to serotonin reuptake inhibition remains to be determined.
In depression, levels of TNFα, IL-6, and IL-1β are elevated (Howren et al, 2009; Dowlati et al, 2010), however, the reasons for this are not understood. An elevated level of circulating inflammatory cytokines may occur because of a dysfunction in the brain's anti-inflammatory mechanisms, which include the hypothalamic–pituitary–adrenal axis, the efferent vagus (Bierhaus et al, 2003; Pavlov and Tracey, 2005; Shaked et al, 2009), and noradrenergic innervation (Selmeczy et al, 2003). It has long been known that the human brain can modulate IL-1β levels in the periphery (Keppel et al, 1993). Psychological stress can increase peripheral inflammation in humans (Bierhaus et al, 2003; Dickerson et al, 2004; Gundersen et al, 2006; Pace et al, 2006) by interfering with the brain's ability to modulate systemic inflammation. People with low parasympathetic tone show impaired inhibition of TNFα levels after exercise (Weber et al, 2010), indicating poor control of inflammation by the autonomic nervous system. However, if a lack of autonomic control of inflammation were the cause of elevated inflammatory cytokine levels in depression, remission of depressive symptoms would be expected to reduce levels, which the current meta-analysis does not unequivocally show. That is, in the studies included here there was a mean 50% reduction in depressive symptoms (regardless of the class of antidepressant used); however, only SSRIs appeared to have a potential effect on cytokine levels. This suggests that, if elevated levels of these inflammatory cytokines are due to an immune system abnormality (genetic or acquired) in depression or to excess stimulation by pathogen-associated molecules (eg, endotoxin leakage from the gut), treatment with antidepressants may not have an effect on TNFα and IL-6 levels, which is more consistent with our results.
In the studies included here, there was a significant improvement in depression severity (50% reduction); however, this occurred despite unchanged levels of TNFα and IL-6. This suggests that, even if elevated levels of TNFα and IL-6 contribute to some depressive symptoms (eg, fatigue), treatment with antidepressants may improve such symptoms (presumably through an effect on the brain) without lowering levels of circulating cytokine levels. This is consistent with a recent study in which we showed that pretreatment with an SSRI reduced endotoxin-induced depressive symptoms without an effect on levels of TNFα and IL-6 (Hannestad et al, 2011). It is also possible that in depression certain symptoms do not improve completely because of continued elevations in these cytokines. For instance, IL-6 levels were associated with refractoriness to antidepressant treatment in one study (Yoshimura et al, 2009).
The studies included here indicate that SSRI treatment specifically may decrease levels of IL-1β, IL-6, and possibly TNFα, although these results must be interpreted with caution because of the low number of studies that used SSRIs only. On the other hand, it appears that the SNRIs venlafaxine and duloxetine are associated with an increase in levels of, respectively, TNFα (Piletz et al, 2009) and IL-6 (Fornaro et al, 2011). This difference between SSRIs and SNRIs is consistent with the known pro-inflammatory effects of norepinephrine on innate immune cells (Thayer and Sternberg, 2010).
It is important to point out several limitations of this meta-analysis. There was large heterogeneity between studies that measured levels of TNFα and IL-6, and there was significant evidence for publication bias for TNFα studies. It is possible that different pharmacological treatments for depression affect cytokine levels differently as suggested by the stratified subgroup analysis; however, the large degree of heterogeneity remains and the sources of this heterogeneity are not clear. We do not believe assay differences were responsible for the heterogeneity because most studies used ELISA to measure cytokine levels. Similarly, the baseline depression severity of the subjects included in the studies was very similar and therefore an unlikely source of heterogeneity. This meta-analysis was performed with study-level data. Future meta-analyses of patient-level data may clarify some of these questions, which in turn can answer some critical questions about the association between MDD, antidepressant treatment, and cytokine levels.
In summary, this meta-analysis showed that, overall, there was no effect of pharmacological antidepressant treatment on serum levels of TNFα, while there was an effect on IL-1β levels and possibly on IL-6 levels. Stratified analysis suggests a possible effect of SSRIs on levels of IL-6 and TNFα. Finally, the data analyzed here are not consistent with the notion that the state of depression ‘causes' elevated levels of inflammatory cytokines, because levels do not decrease even when patients show improvement in symptoms.
Acknowledgments
We thank Dr Reiji Yoshimura for providing additional data for inclusion in this review. We acknowledge the National Institute of Drug Addiction (K12DA00167; JH), the National Institute of Mental Health (Yale Child Study Center Research Training Program and 1K23MH091240-01; MB), the APIRE/Eli Lilly Psychiatric Research Fellowship (MB), the AACAP/Eli Lilly Pilot Research Award (MB), NARSAD (MB and JH), and UL1 RR024139 from the National Center for Research Resources, a component of the National Institutes of Health, and NIH roadmap for Medical Research (MB and JH).
The authors declare no conflict of interest.
References
- Basterzi AD, Aydemir C, Kisa C, Aksaray S, Tuzer V, Yazici K, et al. IL-6 levels decrease with SSRI treatment in patients with major depression. Hum Psychopharmacol. 2005;20:473–476. doi: 10.1002/hup.717. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals. J Affect Disord. 2009;119:181–185. doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Lin WW, Chen YJ, Mao WC, Hung YJ. Antidepressant effects on insulin sensitivity and proinflammatory cytokines in the depressed males. Mediators Inflamm. 2010;2010:573594. doi: 10.1155/2010/573594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaGioia N, Hannestad J. A critical review of human endotoxin administration as an experimental paradigm of depression. Neurosci Biobehav Rev. 2010;34:130–143. doi: 10.1016/j.neubiorev.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, Aziz N, Kim KH, Fahey JL. Immunological effects of induced shame and guilt. Psychosom Med. 2004;66:124–131. doi: 10.1097/01.psy.0000097338.75454.29. [DOI] [PubMed] [Google Scholar]
- Doering LV, Cross R, Vredevoe D, Martinez-Maza O, Cowan MJ. Infection, depression, and immunity in women after coronary artery bypass: a pilot study of cognitive behavioral therapy. Altern Ther Health Med. 2007;13:18–21. [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller T, Vasar V, Shlik J, Maron E. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:445–450. doi: 10.1016/j.pnpbp.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Eller T, Vasar V, Shlik J, Maron E. Effects of bupropion augmentation on pro-inflammatory cytokines in escitalopram-resistant patients with major depressive disorder. J Psychopharmacol. 2009;23:854–858. doi: 10.1177/0269881108091077. [DOI] [PubMed] [Google Scholar]
- Fornaro M, Martino M, Battaglia F, Colicchio S, Perugi G. Increase in IL-6 levels among major depressive disorder patients after a 6-week treatment with duloxetine 60 mg/day: a preliminary observation. Neuropsychiatr Dis Treat. 2011;7:51–56. doi: 10.2147/NDT.S16382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommberger UH, Bauer J, Haselbauer P, Fraulin A, Riemann D, Berger M. Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci. 1997;247:228–233. doi: 10.1007/BF02900219. [DOI] [PubMed] [Google Scholar]
- Gundersen Y, Opstad PK, Reistad T, Thrane I, Vaagenes P. Seven days' around the clock exhaustive physical exertion combined with energy depletion and sleep deprivation primes circulating leukocytes. Eur J Appl Physiol. 2006;97:151–157. doi: 10.1007/s00421-006-0150-8. [DOI] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Ortiz N, Pittman B, Bhagwagar Z. Citalopram reduces endotoxin-induced fatigue. Brain Behav Immun. 2011;25:256–259. doi: 10.1016/j.bbi.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez ME, Mendieta D, Martinez-Fong D, Loria F, Moreno J, Estrada I, et al. Variations in circulating cytokine levels during 52 week course of treatment with SSRI for major depressive disorder. Eur Neuropsychopharmacol. 2008;18:917–924. doi: 10.1016/j.euroneuro.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Himmerich H, Binder EB, Kunzel HE, Schuld A, Lucae S, Uhr M, et al. Successful antidepressant therapy restores the disturbed interplay between TNF-alpha system and HPA axis. Biol Psychiatry. 2006;60:882–888. doi: 10.1016/j.biopsych.2006.03.075. [DOI] [PubMed] [Google Scholar]
- Himmerich H, Milenovic S, Fulda S, Plumakers B, Sheldrick AJ, Michel TM, et al. Regulatory T cells increased while IL-1beta decreased during antidepressant therapy. J Psychiatr Res. 2010;44:1052–1057. doi: 10.1016/j.jpsychires.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Hinze-Selch D, Schuld A, Kraus T, Kuhn M, Uhr M, Haack M, et al. Effects of antidepressants on weight and on the plasma levels of leptin, TNF-alpha and soluble TNF receptors: a longitudinal study in patients treated with amitriptyline or paroxetine. Neuropsychopharmacology. 2000;23:13–19. doi: 10.1016/S0893-133X(00)00089-0. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Jazayeri S, Keshavarz SA, Tehrani-Doost M, Djalali M, Hosseini M, Amini H, et al. Effects of eicosapentaenoic acid and fluoxetine on plasma cortisol, serum interleukin-1beta and interleukin-6 concentrations in patients with major depressive disorder. Psychiatry Res. 2010;178:112–115. doi: 10.1016/j.psychres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Kagaya A, Kugaya A, Takebayashi M, Fukue-Saeki M, Saeki T, Yamawaki S, et al. Plasma concentrations of interleukin-1beta, interleukin-6, soluble interleukin-2 receptor and tumor necrosis factor alpha of depressed patients in Japan. Neuropsychobiology. 2001;43:59–62. doi: 10.1159/000054867. [DOI] [PubMed] [Google Scholar]
- Keppel WH, Regan DH, Heffeneider SH, McCoy S, Ramsey F. Effects of behavioral stimuli on plasma interleukin-1 activity in humans at rest. J Clin Psychol. 1993;49:777–789. doi: 10.1002/1097-4679(199311)49:6<777::aid-jclp2270490605>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Kraus T, Haack M, Schuld A, Hinze-Selch D, Koethe D, Pollmacher T. Body weight, the tumor necrosis factor system, and leptin production during treatment with mirtazapine or venlafaxine. Pharmacopsychiatry. 2002;35:220–225. doi: 10.1055/s-2002-36390. [DOI] [PubMed] [Google Scholar]
- Kubera M, Kenis G, Bosmans E, Zieba A, Dudek D, Nowak G, et al. Plasma levels of interleukin-6, interleukin-10, and interleukin-1 receptor antagonist in depression: comparison between the acute state and after remission. Pol J Pharmacol. 2000;52:237–241. [PubMed] [Google Scholar]
- Lee SK, Lee HS, Lee TB, Kim DH, Koo JR, Kim YK, et al. The effects of antidepressant treatment on serum cytokines and nutritional status in hemodialysis patients. J Korean Med Sci. 2004;19:384–389. doi: 10.3346/jkms.2004.19.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo R, Di Lorenzo G, Tesauro M, Razzini C, Forleo GB, Chiricolo G, et al. Association between enhanced soluble CD40 ligand and proinflammatory and prothrombotic states in major depressive disorder: pilot observations on the effects of selective serotonin reuptake inhibitor therapy. J Clin Psychiatry. 2006;67:1760–1766. doi: 10.4088/jcp.v67n1114. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, et al. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. 1995;34:301–309. doi: 10.1016/0165-0327(95)00028-l. [DOI] [PubMed] [Google Scholar]
- Mikova O, Yakimova R, Bosmans E, Kenis G, Maes M. Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis. Eur Neuropsychopharmacol. 2001;11:203–208. doi: 10.1016/s0924-977x(01)00081-5. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Piletz JE, Halaris A, Iqbal O, Hoppensteadt D, Fareed J, Zhu H, et al. Pro-inflammatory biomakers in depression: treatment with venlafaxine. World J Biol Psychiatry. 2009;10:313–323. doi: 10.3109/15622970802573246. [DOI] [PubMed] [Google Scholar]
- Pizzi C, Mancini S, Angeloni L, Fontana F, Manzoli L, Costa GM. Effects of selective serotonin reuptake inhibitor therapy on endothelial function and inflammatory markers in patients with coronary heart disease. Clin Pharmacol Ther. 2009;86:527–532. doi: 10.1038/clpt.2009.121. [DOI] [PubMed] [Google Scholar]
- Raison CL, Lowry CA, Rook GA. Inflammation, sanitation, and consternation: loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch Gen Psychiatry. 2010;67:1211–1224. doi: 10.1001/archgenpsychiatry.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Roumestan C, Michel A, Bichon F, Portet K, Detoc M, Henriquet C, et al. Anti-inflammatory properties of desipramine and fluoxetine. Respir Res. 2007;8:35. doi: 10.1186/1465-9921-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmeczy Z, Szelenyi J, Vizi ES. Intact noradrenaline transporter is needed for the sympathetic fine-tuning of cytokine balance. Eur J Pharmacol. 2003;469:175–181. doi: 10.1016/s0014-2999(03)01721-7. [DOI] [PubMed] [Google Scholar]
- Shaked I, Meerson A, Wolf Y, Avni R, Greenberg D, Gilboa-Geffen A, et al. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity. 2009;31:965–973. doi: 10.1016/j.immuni.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Sluzewska A, Rybakowski JK, Laciak M, Mackiewicz A, Sobieska M, Wiktorowicz K. Interleukin-6 serum levels in depressed patients before and after treatment with fluoxetine. Ann NY Acad Sci. 1995;762:474–476. doi: 10.1111/j.1749-6632.1995.tb32372.x. [DOI] [PubMed] [Google Scholar]
- Song C, Halbreich U, Han C, Leonard BE, Luo H. Imbalance between pro- and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: the effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry. 2009;42:182–188. doi: 10.1055/s-0029-1202263. [DOI] [PubMed] [Google Scholar]
- Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O, et al. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin Dev Immunol. 2007;2007:76396. doi: 10.1155/2007/76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Sternberg EM. Neural aspects of immunomodulation: focus on the vagus nerve. Brain Behav Immun. 2010;24:1223–1228. doi: 10.1016/j.bbi.2010.07.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P, Ruwe WD, Masters B, Parker DE, Hossain A, Trautman RP, et al. Neuroimmune and cortisol changes in selective serotonin reuptake inhibitor and placebo treatment of chronic posttraumatic stress disorder. Biol Psychiatry. 2004;56:121–128. doi: 10.1016/j.biopsych.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Tuglu C, Kara SH, Caliyurt O, Vardar E, Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology (Berl) 2003;170:429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Weber CS, Thayer JF, Rudat M, Wirtz PH, Zimmermann-Viehoff F, Thomas A, et al. Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. Eur J Appl Physiol. 2010;109:201–211. doi: 10.1007/s00421-009-1341-x. [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Yan Y, Zhang GX, Gran B, Fallarino F, Yu S, Li H, et al. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol. 2010;185:5953–5961. doi: 10.4049/jimmunol.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Pollak Y, Barak O, Avitsur R, Ovadia H, Bette M, et al. Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacology. 2001;24:531–544. doi: 10.1016/S0893-133X(00)00226-8. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Hori H, Ikenouchi-Sugita A, Umene-Nakano W, Ueda N, Nakamura J. Higher plasma interleukin-6 (IL-6) level is associated with SSRI- or SNRI-refractory depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:722–726. doi: 10.1016/j.pnpbp.2009.03.020. [DOI] [PubMed] [Google Scholar]