Abstract
Genetic variation in AKT1 may be associated with sensitivity to the psychotomimetic effects of cannabis as well as with increased risk for psychotic disorder following cannabis use. Investigation of the effect of this interaction on relevant intermediate phenotypes for psychosis, such as cognition, may help to clarify the underlying mechanism. Thus, verbal memory (visually presented Word Learning Task), sustained attention (Continuous Performance Test, CPT), AKT1 rs2494732 genotype, and cannabis use were examined in a large cohort of patients with psychotic disorder. No evidence was found for AKT1 × cannabis interaction on verbal memory. Cannabis use preceding onset of psychotic disorder did interact significantly with AKT1 rs2494732 genotype to affect CPT reaction time (β=8.0, SE 3.9, p=0.037) and CPT accuracy (β=−1.2, SE 0.4, p=0.003). Cannabis-using patients with the a priori vulnerability C/C genotype were slower and less accurate on the CPT, whereas cannabis-using patients with the T/T genotype had similar or better performance than non-using patients with psychotic disorder. The interaction was also apparent in patients with psychotic disorder who had not used cannabis in the 12 months preceding assessment, but was absent in the unaffected siblings of these patients and in healthy controls. In conclusion, cannabis use before onset of psychosis may have long-lasting effects on measures of sustained attention, even in the absence of current use, contingent on AKT1 rs2494732 genotype. The results suggest that long-term changes in cognition may mediate the risk-increasing effect of the AKT1 × cannabis interaction on psychotic disorder.
Keywords: cannabinoids, cognition, schizophrenia/antipsychotics, neurogenetics, AKT1, cannabis
INTRODUCTION
There is growing evidence from epidemiological studies that cannabis use acts as a component cause for psychotic disorder resulting in an approximate twofold increase in risk (Henquet et al, 2005; Moore et al, 2007). An important consideration regarding the possible causality of the cannabis–psychosis relationship is that only a minority of users develops a psychotic disorder, suggesting that underlying vulnerability is of crucial importance. A recent study by the Dutch Genetic Risk and Outcome in Psychosis (GROUP) consortium examined a national sample of 1100 sib-pairs discordant for psychotic disorder and found evidence that genetic risk for schizophrenia is expressed, at least partly, as vulnerability for the psychotomimetic effect of cannabis (Genetic Risk and Outcome of Psychosis (GROUP) Investigators, 2011).
In addition to transient psychotic experiences and negative symptoms, cannabis disrupts cognitive performance when acutely administered (D'Souza et al, 2004; Solowij and Michie, 2007). Compared with healthy controls, patients with schizophrenia were also found to be more vulnerable to the deleterious effects of cannabis on cognition, specifically memory and learning (D'Souza et al, 2005), again suggesting that vulnerability to schizophrenia may be expressed as an increased sensitivity to the effect of cannabis (D'Souza et al, 2005).
Whether chronic cannabis use may have persistent effects on cognitive performance is still a matter of debate. Some studies have suggested that chronic cannabis use may produce long-lasting cognitive deficits (Bolla et al, 2002; Pope and Yurgelun-Todd, 1996), whereas other have reported effects on cognition that were reversed after Δ9-THC-withdrawal (Fried et al, 2005; Pope et al, 2002). As cognitive underachievement is one of the most replicated findings in patients with schizophrenia (Heinrichs and Zakzanis, 1998) and several studies have also suggested that deterioration of cognition may precede the first episode of psychosis (Seidman et al, 2010; Van Oel et al, 2002; van Winkel et al, 2006, 2007), cannabis-induced cognitive changes may have considerable relevance for the risk-increasing effect of cannabis on psychotic disorder (D'Souza et al, 2005). Consistent with this hypothesis, cannabis use was found to be associated with reduced hippocampal and amygdala volume in healthy controls (Yücel et al, 2008) and with excessive cortical thinning in patients with a psychotic disorder compared with cannabis-using healthy controls (Habets et al, 2011). In addition, cannabis-using patients with schizophrenia were found to have larger reductions of gray matter volume than non-using schizophrenia patients (Rais et al, 2008), especially in areas with high expression of cannabinoid type 1 (CB1) receptors (Rais et al, 2010). The relationship between cannabis and cognition in patients with schizophrenia may be more complex, however, as recent meta-analyses found that in patients with schizophrenia, lifetime cannabis use is associated with better rather than worse cognitive performance (Rabin et al, 2011; Yücel et al, 2010).
Our group recently reported that a single-nucleotide polymorphism (SNP) in AKT1 (rs2494732) moderated the short-term psychotomimetic effects of recent cannabis use, determined by urinalysis, in the healthy siblings of patients with a psychotic disorder (van Winkel and Genetic Risk and Outcome of Psychosis (GROUP) Investigators, 2011; Genetic Risk and Outcome of Psychosis (GROUP) Investigators, 2011). In addition, across different samples and epidemiological approaches of gene–environment interaction, this SNP also consistently moderated risk for psychotic disorder following cannabis use. Carriers of the AKT1 rs2494732 C/C genotype had an approximately twofold odds of being diagnosed with a psychotic disorder, but only in the context of cannabis use (van Winkel and Genetic Risk and Outcome of Psychosis (GROUP) Investigators, 2011).
AKT1 is a protein kinase that is involved in multiple cellular functions including metabolism, cell stress, cell-cycle regulation, and apoptosis (Freyberg et al, 2010). It has a basic role in regulating neuronal cell size and survival (Franke, 2008) and is also a key signaling molecule downstream of the dopamine D2 (DRD2) receptor; decreased AKT1 functionality may result in exacerbated responses to DRD2 receptor stimulation (Arguello and Gogos, 2008). Decreased AKT1 levels have been observed in lymphoblasts and postmortem prefrontal cortex of patients with schizophrenia (Emamian et al, 2004; Thiselton et al, 2008), and several studies have shown evidence for genetic association with schizophrenia (Bajestan et al, 2006; Ikeda et al, 2004; Norton et al, 2007; Schwab et al, 2005; Thiselton et al, 2008), although not all studies were able to confirm this (Ide et al, 2006; Liu et al, 2009).
Cannabinoids are able to activate the AKT1/GSK3 pathway by acting on CB1 and CB2 receptors in vitro (Sanchez et al, 2003) and acute administration of THC in mice also activated AKT1 in vivo (through AKT1 phosphorylation) in several brain areas, including the striatum, independent of dopamine D1 and D2 receptor blockade (Ozaita et al, 2007). Interestingly, a haplotype consisting of the AKT rs3730358, rs1130233, and rs2494732 G-A-C alleles was associated with worse performance on the N-back task in a group of healthy volunteers (Tan et al, 2008). Optimal execution of this task critically depends on proper levels of dopamine within the prefrontal cortex (Winterer and Weinberger, 2004).
Given the above, genetic variation in AKT1 may have a role into the degree to which cannabis use induces cognitive alterations in the trajectory toward psychotic disorder. Detectable genetic moderation of cognitive alterations in cannabis-using individuals with psychotic disorder would provide a biological substrate for the hypothesized etiological relevance of the AKT1 rs2494732 × cannabis interaction. This hypothesis would be further strengthened if this particular interaction would also be detectable in patients with past but not current cannabis use, as this would circumvent confounding by the effects of current use. This study examined these hypotheses in a large sample of patients with psychotic disorder.
MATERIALS AND METHODS
Measures
Cannabis and other drug measures
Cannabis and other drug measures were derived from the Composite International Diagnostic Interview (CIDI) and used consistently with previous papers (Genetic Risk and Outcome of Psychosis (GROUP) Investigators, 2011; van Winkel and Genetic Risk and Outcome of Psychosis (GROUP) Investigators, 2011). The main cannabis measure for the current analysis was CIDI cannabis pattern of use during the lifetime period of heaviest use, restricted to those individuals whose age in the period of most heavy use preceded onset of psychosis (hereafter: CIDI lifetime use: none (0), less than weekly (1), weekly (2), and daily (3)). Onset of psychosis was defined as the first mental health contact for psychosis. Given the relatively low rates of CIDI lifetime cannabis use with a frequency of less than weekly and weekly compared with daily use (see van Winkel and Genetic Risk and Outcome of Psychosis (GROUP) Investigators, 2011), the ‘less than weekly' and ‘weekly' categories were joined for the stratified analyses. CIDI lifetime use was the main cannabis measure since long-term changes in cognition were the subject of the present analyses. A second measure was frequency of cannabis use in the 12 months preceding assessment as reported in the CIDI (none (0), less than weekly (1), weekly (2), and daily (3)). A third measure was recent cannabis use, as established by urinalysis (negative (0) and positive (1)), which was used to control, by exclusion, for the cognitive effects of recent cannabis use. Urinalysis was carried out as a screen for the presence of cannabis at the National Alcohol and Drug Use Jellinek Laboratory. The method used was immunoassays with a cut-off level of 50 ng/ml. In addition, as an integrity parameter, the creatinine level of every sample was measured. Cannabis urine screening has a detection window up to 30 days, but the detection time has been documented in literature to be even longer (up to 3 months), depending on level of cannabis use (Musshoff and Madea, 2006). Given the relatively high cut-off level of 50 ng/ml, a conservative detection window of one month can be inferred. Thus, a positive urinalysis result could indicate acute intoxication at the time of the testing. Amphetamine and cocaine use measures were similar to the cannabis use measures: CIDI lifetime use (none (0), less than weekly (1), weekly (2), and daily (3)) and urinalysis (negative (0) and positive (1)). Significant use of alcohol was defined as drinking >12 units per week in the last 12 months (negative (0) and positive (1)).
Cognitive Assessments
Since a previous study in patients with psychosis and healthy controls found genetic moderation of cannabis-induced cognitive impairments of verbal memory and especially sustained attention (Henquet et al, 2006), these were selected as the cognitive outcome measures. The standardized Dutch version of the visually presented Word Learning Task (WLT) was used to assess memory storage and retrieval of 15 monosyllabic nonrelated words from episodic memory (immediate recall, delayed recall and recognition after 20 min (Van Der Elst et al, 2005). Owing to technical problems, <50% of the sample had reliable data on the WLT-recognition (Meijer et al, submitted), therefore these data were not analyzed. Sustained attention was evaluated with the Continuous Performance Test (CPT) with working memory load, known in the literature as CPT-AX (Nuechterlein and Dawson, 1984). Responses were expressed as the percentage of correct detections (‘accuracy'), reaction time of correct detections and false alarms (Nuechterlein and Dawson, 1984). IQ was measured using the abbreviated version of the Wechsler Adult Intelligence Scale, version III (Blyler et al, 2000). Research assistants responsible for the neuropsychological testing received a comprehensive training, in order to standardize the assessments. The assessment was conducted in standardized conditions: in a fixed order on standardized computers in a quiet environment.
AKT1 Genotype
AKT1 rs2494732 genotype was determined by Sequenom (Hamburg, Germany) using the Sequenom MassARRAY iPLEX platform at the facilities of the manufacturer, as described in previous work (van Winkel and Genetic Risk and Outcome of Psychosis (GROUP) Investigators, 2011).
Sample
In selected representative geographical areas in the Netherlands and Belgium, patients were identified through representative clinicians working in regional psychotic disorder services, whose caseload was screened for inclusion criteria. Subsequently, a group of patients presenting consecutively at these services either as outpatients or in-patients were recruited for the study. Controls were selected through a system of random mailings to addresses in the catchment areas of the cases.
The full GROUP sample consists of 1120 patients with non-affective psychotic disorder, 1057 siblings of these 1120 patients, 919 parents of the patients and their siblings, and 590 unrelated controls. Inclusion criteria were: (i) age range 16 to 50 years, (ii) diagnosis of non-affective psychotic disorder, and (iii) good command of Dutch language. Controls had no first- or second-degree relative with a psychotic disorder as established by the Family Interview for Genetic Studies (NIMH Genetics Initiative, 1992) with the control as the informant. Diagnosis was based on the Diagnostic and Statistical Manual of Mental Disorder-IV (DSM-IV) criteria (American Psychiatric Association, 2000), assessed with the Comprehensive Assessment of Symptoms and History interview (Andreasen et al, 1992) or Schedules for Clinical Assessment for Neuropsychiatry (Wing et al, 1990). Of 1120 patients included, genetic data were available in 801 (76.8% male, mean age 27.9 (SD 8.2)). No large or significant differences in age, sex, CIDI lifetime use or recent use of cannabis, cocaine or amphetamines were found for patients who did or did not provide DNA (van Winkel and Genetic Risk and Outcome of Psychosis (GROUP) Investigators, 2011). The WLT was administered in 763 and the CPT in 714 of the patients for whom genetic data were available. Of the 714 patients for whom both genetic and all cognitive data were available, there were 438 lifetime cannabis users, 335 of whom (76.5%) had experienced their lifetime period of most intensive use before the onset of psychosis. As we intended to analyze the etiological relevance of the hypothesized effects of cannabis use on cognitive alterations in psychotic disorder, patients whose period of heaviest use occurred after onset of psychosis (n=103) were excluded, resulting in a final sample of 654 (WLT)/611 (CPT) patients for analysis (Table 1). In all, 790 siblings and 414 controls were available for the case–sib and case–control comparisons of the WLT, respectively, whereas 738 siblings and 379 controls were available for the sensitivity analysis as regards the CPT.
Table 1. Clinical and Demographic Characteristics of the Sample with a Psychotic Disorder for Whom Both Genetic and Cognitive Data were Available.
| Overall sample (n=611) | No cannabis use (n=272) | Cannabis use (n=339) | p (never users vs users of cannabis) | |
|---|---|---|---|---|
| Age (year) (SD) | 28.4 (8.6) | 30.1 (10.1) | 27.0 (6.9) | <0.0001 |
| Sex (% male) | 75.1 | 60.7 | 86.7 | <0.0001 |
| IQ (SD) | 94.6 (16.5) | 93.9 (17.0) | 95.1 (16.1) | 0.38 |
| Age at onset of psychosis (year) (SD) | 23.0 (7.1) | 24.2 (8.1) | 22.1 (6.0) | 0.0005 |
DSM-IV diagnoses of the patients were: schizophrenia and related disorders (DSM-IV 295.x; n=489, 80%), other psychotic disorders (DSM-IV 297/298; n=110, 18%), psychotic illness in the context of substance-abuse or somatic illness (n=5, 1%) or affective psychosis (n=7, 1%) although fulfilling criteria of clinical diagnosis of non-affective psychosis at study entry; these individuals were retained in the sample assuming subtle diagnostic changes between time point of identification for inclusion and actual assessment that could occur in any patient included in the cohort at any time point, and taking into account the fact that for the focus of underlying genetic liability the diagnostic change would not be relevant (Cardno et al, 2002) (for further details, see Genetic Risk and Outcome of Psychosis (GROUP) Investigators, 2011).
Statistical Analysis
In order to examine the hypothesis of AKT1 × cannabis interaction, the relevant cognitive outcome was regressed on cannabis use, AKT1 rs2494732 genotype, and their interaction. Genotypes were coded 0, 1, or 2 and modeled as a linear effect, because this method can deal with different genotype distributions, including distributions with a low minor allele frequency, as it avoids stratification into small subgroups (Cordell and Clayton, 2005). Cannabis use was similarly analyzed as a linear effect, as also applied in a previous paper (van Winkel and Genetic Risk and Outcome of Psychosis (GROUP) Investigators, 2011). Only patients for whom the most intensive period of use preceded the onset of psychosis, defined as the first mental health contact for psychotic symptoms, were included in the analyses. Given the fact that some families contributed more than one patient, hierarchical clustering of data at the level of family was taken into account using the multilevel random regression XTREG routine in STATA, version 11 (StataCorp, 2009). Analyses concerning the effect of AKT1 genotype on measures of cognition were adjusted for the following a priori confounders: age, sex, and IQ. Analyses involving cannabis (both main effects and interactions with AKT1 genotype) were additionally controlled for cigarette use, alcohol use, recent use of cannabis (by urinalysis), use of amphetamines (lifetime and by urinalysis), and use of cocaine (lifetime and by urinalysis).
As a positive urinalysis result could indicate acute intoxication at the time of the testing, a sensitivity analysis examined AKT1 moderation of cannabis-induced cognitive alterations in patients with established absence of use, in order to separate the hypothesized long-term effects from the effects of recent use. Thus, patients who experienced the period of most intensive use before illness onset, reported no use of cannabis in the last year and for whom urinalysis confirmed the absence of current cannabis use were included in this sensitivity analysis. A second analysis examined whether significant interactions (at p<0.05) were associated with (development of) psychotic disorder by fitting the three-way interaction between AKT1 genotype, CIDI lifetime use and group status, using case–sibling and a case–control comparisons. These analyses were also adjusted for age, sex, IQ, and cigarette use.
RESULTS
Cannabis Use
Cannabis use was highly prevalent in the sample of 611 patients for whom all data were available: only 44.5% of the patients reported never having used cannabis. In all, 38.0% had used cannabis daily in the lifetime period of heaviest use, 10.2% weekly, and 7.4% less than weekly. In addition, 15.9% tested positive for recent cannabis use (urinalysis). Cannabis users were significantly younger than non-users, had an earlier age at onset of psychosis, were more likely to be male but did not differ for intelligence quotient (Table 1). Lifetime cocaine use was reported by 19.7% (frequency of use in the heaviest period of use: less than weekly 3.3%, weekly 6.1%, and daily 9.3%); 0.9% tested positive for recent cocaine use by urinalysis. Lifetime amphetamine use was reported by 18% (frequency of use in the heaviest period of use: less than weekly 4.3%, weekly 5.6%, and daily 8.2%); 0.6% tested positive for recent amphetamine use by urinalysis. There were no large or significant differences in age, sex, IQ, or age at onset of psychosis according to AKT1 rs2494732 genotype.
Verbal Memory
In the patients with cannabis use before illness onset, higher frequency of CIDI lifetime use was not associated with immediate recall (β=−0.01, SE 0.16, p=0.97) or delayed recall (β=−0.03, SE 0.09, p=0.68). AKT1 rs2494732 genotype was associated with immediate recall (β=0.65, SE 0.29, p=0.025), but not delayed recall (β=0.18, SE 0.14, p=0.17). No evidence was found for an AKT1 rs2494732 cannabis interaction on any of the verbal memory measures.
Sustained Attention
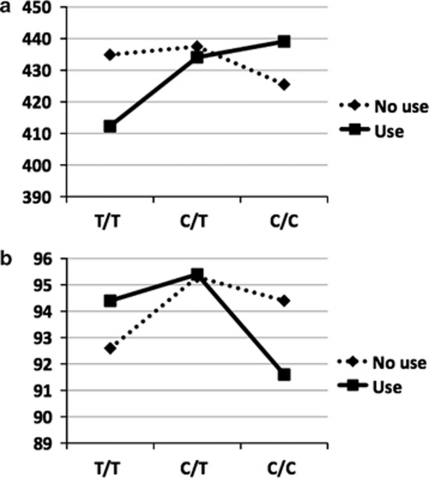
CIDI lifetime use was not associated with CPT reaction time (β=−2.4, SE 2.5, p=0.34), accuracy (β=−0.2, SE 0.3, p=0.43), or false alarms (β=0.1, SE 0.1, p=0.17). The same was true for AKT1 rs2494732 genotype (reaction time: β=6.9, SE 4.3, p=0.12; accuracy: β=−0.6, SE 0.5, p=0.26; false alarms: β=0.2, SE 0.2, p=0.13). CIDI lifetime use interacted significantly with AKT1 rs2494732 genotype in its effect on CPT reaction time (β=8.0, SE 3.9, p=0.037) and accuracy (β=−1.2, SE 0.4, p=0.003), but not false alarms (Table 2). Cannabis users with the C/C genotype performed worst, whereas users with the T/T genotype had similar or better performance than the non-using groups, indicative of qualitative interaction (Table 2, Figure 1). Further analysis revealed that the AKT1 × cannabis interaction was statistically significant only in those with a lifetime history of daily use (accuracy β=−3.76, SE 1.21, p=0.002; reaction time β=24.3, SE 11.8, p=0.039), although the direction of the association was similar in the patients with weekly or less than weekly use (accuracy β=−1.5, SE 1.4, p=0.29; reaction time β=24.6, SE 14.3, p=0.085).
Table 2. Continuous Performance Test Performance as a Function of Cannabis Use and AKT1 rs2494732 Genotype.
|
Cannabis use preceding onset of psychosis (n=601)a | |||||||
|---|---|---|---|---|---|---|---|
|
T/T |
C/T |
C/C |
p interaction | ||||
| Never use (n=103) | Use (n=107) | Never use (n=125) | Use (n=152) | Never use (n=40) | Use (n=74) | ||
| CPT, accuracy (%) (SD) | 92.6 (11.0) | 94.4 (8.7) | 95.3 (8.5) | 95.4 (6.7) | 94.4 (11.4) | 91.6 (14.9) | 0.003 |
| CPT, reaction time (ms) (SD) | 434.9 (76.9) | 412.3 (79.5) | 437.5 (87.5) | 434.1 (82.5) | 425.5 (84.7) | 439.1 (92.2) | 0.037 |
| CPT, false alarms (n) (SD) | 1.16 (4.2) | 0.81 (3.4) | 0.44 (0.8) | 1.01 (4.9) | 0.63 (1.4) | 2.05 (7.0) | 0.455 |
|
Cannabis use preceding onset of psychosis and absence of recent use (N=408)b | |||||||
|
T/T |
C/T |
C/C |
p interaction | ||||
| |
Never use (n=103) |
Former use (n=51) |
Never use (n=125) |
Former use (n=64) |
Never use (n=40) |
Former use (n=25) |
|
| CPT, accuracy (%) (SD) | 92.6 (11.0) | 94.0 (8.3) | 95.3 (8.5) | 96.4 (6.3) | 94.4 (11.4) | 90.6 (16.7) | 0.006 |
| CPT, mean reaction time (ms) (SD) | 434.9 (76.9) | 423.5 (88.9) | 437.5 (87.5) | 429.8 (88.9) | 425.5 (84.7) | 467.1 (85.6) | 0.044 |
Genotyping failed in 10 patients.
Patients without current use: reported no use of cannabis in the last year and urinalysis confirmed the absence of current cannabis use.
Figure 1.
Continuous Performance Test (CPT) performance as a function of cannabis use and AKT1 rs2494732 genotype. (a) CPT–mean reaction time (in ms). (b) CPT–accuracy (% correct).
Sensitivity Analysis in Patients with Established Absence of Use
The sensitivity analysis revealed similar results in patients with a negative urinalysis and reporting absence of cannabis use in the twelve months preceding the diagnostic interview (Table 2). Again, this was only evident in patients with a lifetime history of daily use (accuracy β=−4.33, SE 1.54, p=0.005; reaction time β=34.3, SE 17.5, p=0.050).
Sensitivity Analysis Regarding the Specificity for Psychotic Disorder
In the unaffected siblings, 62.7% reported no cannabis use; of those that did report cannabis use, 13.8% reported less than weekly use, 9.6% reported weekly use, and 13.8% reported daily use in the period of heaviest use. In the healthy controls, 69.9% reported no cannabis use; of those that did report cannabis use, 13.7% reported less than weekly use, 6.6% reported weekly use, and 9.8% reported daily use in the period of heaviest use. The reported AKT1 × cannabis interactions were specific to patients with psychotic disorder, at least for CPT- accuracy (case–sib three-way group × CIDI lifetime use × AKT1 interaction χ2=9.3, p=0.002; case–control three-way group CIDI lifetime use × AKT1 interaction χ2=3.2, p=0.076), indicating that the reported AKT1 × cannabis interaction was not observed in either unaffected siblings (β=0.25, SE 0.37, p=0.51) or controls (β=−0.7, SE 0.46, p=0.88). A similar trend was observed for CPT-reaction time, although this did not reach statistical significance (case–sib three-way interaction χ2=2.7, p=0.10; case–control three-way interaction χ2=0.9, p=0.34).
Explorative Analyses in Patients with the Heaviest Period of Use After Illness Onset
As patients who experienced the heaviest period of use after illness onset were excluded from the analyses reported above, the effects of cannabis use were also explored in this group. In agreement with the results in the patients whose heaviest period of use preceded onset of psychosis, there was no association between CIDI lifetime cannabis use and immediate recall: (β=0.07, SE 0.23, p=0.75) or delayed recall: (β=−0.13, SE 0.10, p=0.22). The same was true for the CPT (reaction time: β=−5.2, SE 3.3, p=0.11; accuracy: β=−0.6, SE 0.4, p=0.12; false alarms: β=0.6, SE 0.4, p=0.13). AKT1 × cannabis interaction analyses were not fitted because of the lack of statistical power in this restricted sample of 103 patients.
DISCUSSION
The relationship between cannabis use, cognition, and psychosis are complex and far from understood. Although most studies agree that acute cannabis use is associated with cognitive impairments in the short-term, the long-term effects of cannabis use remain contentious. In schizophrenia, a recent meta-analysis reported better cognition in cannabis-using patients (Yücel et al, 2010).
This study found no clear associations between lifetime cannabis use and cognitive performance, that is, verbal memory and sustained attention. However, underneath this null finding was significant heterogeneity. Variation in cognitive performance, particularly sustained attention, was associated with a polymorphism in AKT1 (rs2494732) that was also implicated in risk for psychotic disorder following cannabis use in the same sample (van Winkel and Genetic Risk and Outcome of Psychosis (GROUP) Investigators, 2011). Cannabis users with the C/C genotype were slower and less accurate on the CPT, whereas users with the T/T genotype had similar or better performance than non-using groups. The results indicate that the combined risk-increasing effects of AKT1 rs2494732 C/C genotype and cannabis use on psychotic disorder may be accompanied by selective alterations in sustained attention. This is in keeping with the interpretation that in genetically vulnerable individuals, cannabis use may adversely impact on brain function and psychopathology (D'Souza et al, 2005; Genetic Risk and Outcome of Psychosis (GROUP) Investigators, 2011; Habets et al, 2011). The present findings are also in agreement with a previous study that the CPT is sensitive to genetic moderation of cognitive performance by cannabis in patients with schizophrenia (Henquet et al, 2006).
Performance on the CPT critically depends on prefrontal dopamine functioning, which may be in agreement with a reported role of AKT1 in modulating dopamine-related prefrontal cortical structure and functioning (Tan et al, 2008). This is especially interesting, as COMT, another gene important in prefrontal dopamine availability, was also suggested to moderate the effects of cannabis use on expression of psychosis and cognitive performance in previous studies (Caspi et al, 2005; Henquet et al, 2006; O'Tuathaigh et al, 2010). Although the reported cognitive effects of cannabis use were subtle, the present findings are important as they suggest that the psychosis-inducing effects of cannabis may be driven by dopamine-related prefrontal–striatal interactions and that genetic variation in this brain circuit may determine the vulnerability to these effects.
The interpretation that the reported interaction may indicate long-term cognitive effects of cannabis use is supported by findings (i) that it was only present in patients with a lifetime history of daily use, as opposed to weekly or less often use, (ii) that it was specific to patients with a psychotic disorder, and (iii) that it was also apparent in patients who had not used cannabis in the 12 months preceding assessment, as established by the combination of a biological measure (urinalysis) and self-report. The combination of self-report and a biological measure reduces the possibility of identifying cannabis users as non-users (‘false negatives'), thus ensuring the reliability of the reported results.
The current findings may seem contradictory to meta-analyses reporting superior cognitive performance in cannabis-using patients (Rabin et al, 2011; Yücel et al, 2010). In addition, in this study, there was a suggestion that in patients with the AKT1 rs2494732 T/T genotype, cannabis use may be associated with a better performance on measures of sustained attention. Several explanations for superior cognitive abilities in cannabis-using patients have been put forward, including possible neuroprotective effects of cannabis in individuals developing psychotic symptoms (Jockers-Scherubl et al, 2007) and a selective mechanism of causal contribution of cannabis, such that persons with less neurocognitive impairment make a transition to psychotic disorder that they would not have made in the absence of cannabis use (Yücel et al, 2010). In our opinion, the latter explanation seems most plausible and is in agreement with extant literature suggesting that cannabis use is a component cause in the development of psychotic disorder (Murray et al, 2007). Nevertheless, neuroprotective properties of exogenous cannabinoids, mediated by PI3K–AKT1 signaling, have also been documented (Molina-Holgado et al, 2005). Thus, the possibility that cannabis use may have beneficial effects on cognition via AKT1-modulation in certain subgroups at risk for psychosis cannot be discarded.
The results of this study need to be interpreted in the context of the risk for spurious association (Sullivan, 2007) or interaction (van Winkel et al, 2010) in candidate SNP studies. This study analyzed a SNP in AKT1 that was identified by a systematic effort to unravel the genetics of cannabis-induced psychosis (van Winkel and Genetic Risk and Outcome of Psychosis (GROUP) Investigators, 2011). In addition, this study analyzed a relatively large patient sample and used cognitive tests that were found to be sensitive to genetic moderation of cannabis-induced deterioration of cognition in a previous study (Henquet et al, 2006). This should reduce the probability that the reported interaction is spurious; nevertheless, replication in independent samples is required. This study further needs to be interpreted in the context of a number of limitations. The most important limitation is the cross-sectional nature of the study. As we have no assessment of cognition before illness onset, it is not possible to make strong inferences of the causal nature of the reported interaction on measures of cognition. Longitudinal studies are necessary to better understand interactions between the implicated factors. Second, differences in possible confounders related to sampling bias in users versus non-users (eg, cannabis-using patients were younger and more likely to be male) could explain part of the reported interactions. However, in order to confound interactions variables need to be associated with genotype, cannabis use and cognition; conditions that do not apply to a large number of variables. In addition, analyses were covaried for the most important potential confounders. The choice to selectively examine verbal memory and sustained attention can be interpreted as both a limitation and strength of the present work. The decision to analyze these domains was part of a careful strategy to reduce the possibility of spurious genetic association, by examining a SNP that was identified by a systematic effort to unravel the genetics of cannabis-induced psychosis (van Winkel and Genetic Risk and Outcome of Psychosis (GROUP) Investigators, 2011), using a large sample and using cognitive tests that were found to be sensitive to genetic moderation of cannabis-induced cognitive alterations (Henquet et al, 2006). Nevertheless, a larger cognitive battery would have allowed for a more comprehensive examination of the reported AKT1 × cannabis interaction. As the present work suggests that AKT1 moderation of cannabis-induced cognitive alterations may be specific to prefrontal-mediated cognitive tasks, in agreement with a role of AKT1 in regulating dopamine-related prefrontal structure and functioning (Tan et al, 2008), future work could examine this hypothesis more specifically.
In conclusion, cannabis use before onset of psychotic disorder may have long-lasting effects on measures of sustained attention, even in the absence of current use, contingent on AKT1 rs2494732 genotype. The results suggest that long-term changes in cognition may mediate the risk-increasing effect of the AKT1 × cannabis interaction on psychotic disorder. Prospective studies are needed to confirm these findings.
Acknowledgments
The infrastructure for the GROUP study is funded by the Geestkracht program of the Dutch Health Research Council (ZON-MW, Grant number 10-000-1002) and matching funds from participating universities and mental health care organizations (Site Amsterdam: Academic Psychiatric Centre AMC, Ingeest, Arkin, Dijk en Duin, Rivierduinen, Erasmus MC, GGZ Noord Holland Noord; Site Utrecht: University Medical Centre Utrecht, Altrecht, Symfora, Meerkanten, Riagg Amersfoort, Delta; Site Groningen: University Medical Center Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Dimence, Mediant, GGZ De Grote Rivieren and Parnassia Bavo Groep; Site Maastricht: Maastricht University Medical Center, GGZ Eindhoven, GGZ Midden-Brabant, GGZ Oost-Brabant, GGZ Noord- Midden Limburg, Mondriaan Zorggroep, Prins Clauscentrum Sittard, RIAGG Roermond, Universitair Centrum Sint-Jozef Kortenberg, CAPRI University of Antwerp, PC Ziekeren Sint-Truiden, PZ Sancta Maria Sint-Truiden, GGZ Overpelt, OPZ Rekem). We are grateful for the generosity of time and effort by the families who make this GROUP project possible. The research leading to these results has received funding from the European Community′s Seventh Framework Program under grant agreement No. HEALTH-F2-2009-241909 (Project EU-GEI). The analyses were supported by unrestricted grants from Jansen-Cilag, Eli Lilly and Company, Astra-Zeneca, and Lundbeck.
Dr van Winkel has been an unrestricted grant holder with AstraZeneca and Eli Lilly. Dr van Beveren has been an unrestricted grant holder with PsyNova Neurotech Cambridge. Professor De Haan has received research funding from Eli Lilly and honoraria from Eli Lilly, Janssen-Cilag, BMS, and AstraZeneca. Professor van Os is/has been an unrestricted research grant holder with, or has received financial compensation as an independent symposium speaker from Eli Lilly, BMS, Lundbeck, Organon, Janssen-Cilag, GSK, AstraZeneca, Pfizer, and Servier. Dr Cahn is/has been an unrestricted research grant holder with, or has received financial compensation as an independent symposium speaker or as a consultant from Eli Lilly, BMS, Lundbeck, Sanofi-Aventis, Janssen-Cilag, AstraZeneca, and Schering-Plough. Professor Myin-Germeys has received financial compensation as an independent symposium speaker from BMS and Janssen-Cilag. Professor Kahn is/has been an unrestricted research grant holder with, or has received financial compensation as an independent symposium speaker or as a consultant from AstraZeneca, Eli Lilly, Janssen-Cilag, Otsuka, Sinovion, Roche, and Envivo. All other authors declare no conflict of interest.
Contributor Information
Genetic Risk and Outcome of Psychosis (GROUP) Investigators:
René S S Kahn, Don H Linszen, Jim van Os, Durk Wiersma, Richard Bruggeman, Wiepke Cahn, Lieuwe de Haan, Lydia Krabbendam, and Inez Myin-Germeys
References
- American Psychiatric Association (ed) (2000Diagnostic and Statistical Manual of Mental Disorders4th edn. Text revision. American Psychiatric Association: Washington, DC [Google Scholar]
- Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Arguello PA, Gogos JA. A signaling pathway AKTing up in schizophrenia. J Clin Invest. 2008;118:2018–2021. doi: 10.1172/JCI35931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajestan SN, Sabouri AH, Nakamura M, Takashima H, Keikhaee MR, Behdani F, et al. Association of AKT1 haplotype with the risk of schizophrenia in Iranian population. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:383–386. doi: 10.1002/ajmg.b.30291. [DOI] [PubMed] [Google Scholar]
- Blyler CR, Gold JM, Iannone VN, Buchanan RW. Short form of the WAIS-III for use with patients with schizophrenia. Schizophr Res. 2000;46:209–215. doi: 10.1016/s0920-9964(00)00017-7. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Cardno AG, Rijsdijk FV, Sham PC, Murray RM, McGuffin P. A twin study of genetic relationships between psychotic symptoms. Am J Psychiatry. 2002;159:539–545. doi: 10.1176/appi.ajp.159.4.539. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Cordell HJ, Clayton DG. Genetic association studies. Lancet. 2005;366:1121–1131. doi: 10.1016/S0140-6736(05)67424-7. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008;27:6473–6488. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- Freyberg Z, Ferrando SJ, Javitch JA. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry. 2010;167:388–396. doi: 10.1176/appi.ajp.2009.08121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana--a comparison with pre-drug performance. Neurotoxicol Teratol. 2005;27:231–239. doi: 10.1016/j.ntt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Genetic Risk and Outcome of Psychosis (GROUP) Investigators Evidence that familial liability for psychosis is expressed as differential sensitivity to cannabis: an analysis of patient-sibling and sibling-control pairs. Arch Gen Psychiatry. 2011;68:138–147. doi: 10.1001/archgenpsychiatry.2010.132. [DOI] [PubMed] [Google Scholar]
- Habets P, Marcelis M, Gronenschild E, Drukker M, Van Os J. Reduced cortical thickness as an outcome of differential sensitivity to environmental risks in schizophrenia. Biol Psychiatry. 2011;69:487–494. doi: 10.1016/j.biopsych.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31:608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- Henquet C, Rosa A, Krabbendam L, Papiol S, Fananas L, Drukker M, et al. An experimental study of catechol-o-methyltransferase Val158Met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology. 2006;31:2748–2757. doi: 10.1038/sj.npp.1301197. [DOI] [PubMed] [Google Scholar]
- Ide M, Ohnishi T, Murayama M, Matsumoto I, Yamada K, Iwayama Y, et al. Failure to support a genetic contribution of AKT1 polymorphisms and altered AKT signaling in schizophrenia. J Neurochem. 2006;99:277–287. doi: 10.1111/j.1471-4159.2006.04033.x. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Iwata N, Suzuki T, Kitajima T, Yamanouchi Y, Kinoshita Y, et al. Association of AKT1 with schizophrenia confirmed in a Japanese population. Biol Psychiatry. 2004;56:698–700. doi: 10.1016/j.biopsych.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Jockers-Scherubl MC, Wolf T, Radzei N, Schlattmann P, Rentzsch J, Gomez-Carillo de Castro A, et al. Cannabis induces different cognitive changes in schizophrenic patients and in healthy controls. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1054–1063. doi: 10.1016/j.pnpbp.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Liu YC, Huang CL, Wu PL, Chang YC, Huang CH, Lane HY. Lack of association between AKT1 variances versus clinical manifestations and social function in patients with schizophrenia. J Psychopharmacol. 2009;23:937–943. doi: 10.1177/0269881108093840. [DOI] [PubMed] [Google Scholar]
- Meijer J, Simons C, Quee PJ, Verweij K. Genetic Risk and Outcome of Psychosis (GROUP) Investigations (submitted) Cognitive alterations in patients with non-affective psychotic disorder and their unaffected siblings and parents. [DOI] [PubMed]
- Molina-Holgado F, Pinteaux E, Heenan L, Moore JD, Rothwell NJ, Gibson RM. Neuroprotective effects of the synthetic cannabinoid HU-210 in primary cortical neurons are mediated by phosphatidylinositol 3-kinase/AKT signaling. Mol Cell Neurosci. 2005;28:189–194. doi: 10.1016/j.mcn.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Murray RM, Morrison PD, Henquet C, Di Forti M. Cannabis, the mind and society: the hash realities. Nat Rev Neurosci. 2007;8:885–895. doi: 10.1038/nrn2253. [DOI] [PubMed] [Google Scholar]
- Musshoff F, Madea B. Review of biologic matrices (urine, blood, hair) as indicators of recent or ongoing cannabis use. Ther Drug Monit. 2006;28:155–163. doi: 10.1097/01.ftd.0000197091.07807.22. [DOI] [PubMed] [Google Scholar]
- NIMH Genetics Initiative . Family Interview for Genetic Studies (FIGS) National Institute of Mental Health: Rockville, MD; 1992. [Google Scholar]
- Norton N, Williams HJ, Dwyer S, Carroll L, Peirce T, Moskvina V, et al. Association analysis of AKT1 and schizophrenia in a UK case control sample. Schizophr Res. 2007;93:58–65. doi: 10.1016/j.schres.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KHR, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenics disorders. Schizophr Bull. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- O'Tuathaigh CM, Hryniewiecka M, Behan A, Tighe O, Coughlan C, Desbonnet L, et al. Chronic adolescent exposure to delta-9-tetrahydrocannabinol in COMT mutant mice: impact on psychosis-related and other phenotypes. Neuropsychopharmacology. 2010;35:2262–2273. doi: 10.1038/npp.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaita A, Puighermanal E, Maldonado R. Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J Neurochem. 2007;102:1105–1114. doi: 10.1111/j.1471-4159.2007.04642.x. [DOI] [PubMed] [Google Scholar]
- Pope HG., Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Cognitive measures in long-term cannabis users. J Clin Pharmacol. 2002;42:41S–47S. doi: 10.1002/j.1552-4604.2002.tb06002.x. [DOI] [PubMed] [Google Scholar]
- Pope HG., Jr, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA. 1996;275:521–527. [PubMed] [Google Scholar]
- Rabin RA, Zakzanis KK, George TP. The effects of cannabis use on neurocognition in schizophrenia: a meta-analysis. Schizophr Res. 2011;128:111–116. doi: 10.1016/j.schres.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Rais M, Cahn W, van Haren NE, Schnack HG, Caspers E, Hulshoff Pol HE, et al. Excessive brain volume loss over time in cannabis-using first-episode schizophrenia patients. Am J Psychiatry. 2008;165:490–496. doi: 10.1176/appi.ajp.2007.07071110. [DOI] [PubMed] [Google Scholar]
- Rais M, van Haren NE, Cahn W, Schnack HG, Lepage C, Collins L, et al. Cannabis use and progressive cortical thickness loss in areas rich in CB1 receptors during the first five years of schizophrenia. Eur Neuropsychopharmacol. 2010;20:855–865. doi: 10.1016/j.euroneuro.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Sanchez MG, Ruiz-Llorente L, Sanchez AM, Diaz-Laviada I. Activation of phosphoinositide 3-kinase/PKB pathway by CB(1) and CB(2) cannabinoid receptors expressed in prostate PC-3 cells. Involvement in Raf-1 stimulation and NGF induction. Cell Signal. 2003;15:851–859. doi: 10.1016/s0898-6568(03)00036-6. [DOI] [PubMed] [Google Scholar]
- Schwab SG, Hoefgen B, Hanses C, Hassenbach MB, Albus M, Lerer B, et al. Further evidence for association of variants in the AKT1 gene with schizophrenia in a sample of European sib-pair families. Biol Psychiatry. 2005;58:446–450. doi: 10.1016/j.biopsych.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, et al. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry. 2010;67:578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Michie PT. Cannabis and cognitive dysfunction: parallels with endophenotypes of schizophrenia. J Psychiatry Neurosci. 2007;32:30–52. [PMC free article] [PubMed] [Google Scholar]
- StataCorp 2009. Stata/SE statistical software, release 11. StataCorp LP, College Station, TX.
- Sullivan PF. Spurious genetic associations. Biol Psychiatry. 2007;61:1121–1126. doi: 10.1016/j.biopsych.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Tan HY, Nicodemus KK, Chen Q, Li Z, Brooke JK, Honea R, et al. Genetic variation in AKT1 is linked to dopamine-associated prefrontal cortical structure and function in humans. J Clin Invest. 2008;118:2200–2208. doi: 10.1172/JCI34725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiselton DL, Vladimirov VI, Kuo PH, McClay J, Wormley B, Fanous A, et al. AKT1 is associated with schizophrenia across multiple symptom dimensions in the Irish study of high density schizophrenia families. Biol Psychiatry. 2008;63:449–457. doi: 10.1016/j.biopsych.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Elst W, Van Boxtel MPJ, Van Breukelen GJP, Jolles J. Rey′s Verbal Learning Test: normative data for 1855 healthy participants aged 24-81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsycholog Soc. 2005;11:290–302. doi: 10.1017/S1355617705050344. [DOI] [PubMed] [Google Scholar]
- Van Oel CJ, Sitskoorn MM, Cremer MP, Kahn RS. School performance as a premorbid marker for schizophrenia: a twin study. Schizophr Bull. 2002;28:401–414. doi: 10.1093/oxfordjournals.schbul.a006949. [DOI] [PubMed] [Google Scholar]
- van Winkel R, Esquivel G, Kenis G, Wichers M, Collip D, Peerbooms O, et al. Genome-wide findings in schizophrenia and the role of gene-environment interplay. CNS Neurosci Ther. 2010;16:e185–e192. doi: 10.1111/j.1755-5949.2010.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Winkel R, Genetic Risk and Outcome of Psychosis (GROUP) Investigators Family-based analysis of genetic variation underlying psychosis-inducing effects of cannabis: sibling analysis and proband follow-up. Arch Gen Psychiatry. 2011;68:148–157. doi: 10.1001/archgenpsychiatry.2010.152. [DOI] [PubMed] [Google Scholar]
- van Winkel R, Myin-Germeys I, De Hert M, Delespaul P, Peuskens J, van Os J. The association between cognition and functional outcome in first-episode patients with schizophrenia: mystery resolved. Acta Psychiatr Scand. 2007;116:119–124. doi: 10.1111/j.1600-0447.2007.01014.x. [DOI] [PubMed] [Google Scholar]
- van Winkel R, Myin-Germeys I, Delespaul P, Peuskens J, De Hert M, van Os J. Premorbid IQ as a predictor for the course of IQ in first onset patients with schizophrenia: a 10-year follow-up study. Schizophr Res. 2006;88:47–54. doi: 10.1016/j.schres.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, et al. SCAN schedules for clinical assessment in neuropsychiatry. Arch Gen Psychiatry. 1990;47:589–593. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Yücel M, Bora E, Lubman DI, Solowij N, Brewer WJ, Cotton SM, et al. 2010The impact of cannabis use on cognitive functioning in patients with schizophrenia: a meta-analysis of existing findings and new data in a first-episode sample Schizophr Bulldoi: 10.1093/schbul/sbq1079 [DOI] [PMC free article] [PubMed]
- Yücel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, et al. Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]