Abstract
Despite the impact of schizophrenia and mood disorders, which in extreme cases can lead to death, recent decades have brought little progress in the development of new treatments. Recent studies have shown that Reelin, an extracellular protein that is critical for neuronal development, is reduced in schizophrenia and bipolar disorder patients. However, data on a causal or protective role of Reelin in psychiatric diseases is scarce. In order to study the direct influence of Reelin's levels on behavior, we subjected two mouse lines, in which Reelin levels are either reduced (Reelin heterozygous mice) or increased (Reelin overexpressing mice), to a battery of behavioral tests: open-field, black–white box, novelty-suppressed-feeding, forced-swim-test, chronic corticosterone treatment followed by forced-swim-test, cocaine sensitization and pre-pulse inhibition (PPI) deficits induced by N-methyl--aspartate (NMDA) antagonists. These tests were designed to model some aspects of psychiatric disorders such as schizophrenia, mood, and anxiety disorders. We found no differences between Reeler heterozygous mice and their wild-type littermates. However, Reelin overexpression in the mouse forebrain reduced the time spent floating in the forced-swim-test in mice subjected to chronic corticosterone treatment, reduced behavioral sensitization to cocaine, and reduced PPI deficits induced by a NMDA antagonist. In addition, we demonstrate that while stress increased NMDA NR2B-mediated synaptic transmission, known to be implicated in depression, Reelin overexpression significantly reduced it. Together, these results point to the Reelin signaling pathway as a relevant drug target for the treatment of a range of psychiatric disorders.
Keywords: depression, behavior, cocaine sensitization, pre-pulse inhibition, corticosterone, NR2B
INTRODUCTION
Schizophrenia, mood, and anxiety disorders are devastating diseases with high prevalence and comorbidity rates (Altamura et al, 2011; Kessler et al, 2005). Despite the immense impact of these diseases, knowledge about their pathophysiology is limited. According to the stress–diathesis model, these diseases result from interaction between genetic predisposition and environmental risk factors. Research into how genetic makeup interacts with the environmental factors implicated in the triggering of these diseases is crucial. This implies not only understanding the genetic risk factors but also identifying genetic variations that confer resilience to disease, which may lead to the development of new treatments.
Reelin, an extracellular protein essential for neuronal migration and positioning during development (D'Arcangelo et al, 1995; Tissir and Goffinet, 2003), has received recent attention because of its connection to bipolar disorder and schizophrenia. In fact, comprehensive databases of schizophrenia genetic association studies, such as the SZGR (Jia et al, 2010) and the Stanley Foundation Neuropathology Consortium (Kim and Webster, 2009), place Reelin as a top candidate gene associated with schizophrenia. This link is also supported by several studies showing that Reelin levels are reduced in patients with schizophrenia and bipolar disorder (Fatemi et al, 2000; Torrey et al, 2005), altered by antipsychotic, antidepressant, and mood-stabilizing medication (Fatemi et al, 2009), and also reduced following chronic stress (Lussier et al, 2009). Furthermore, Reelin has also been shown to positively modulate adult-neurogenesis (Kim et al, 2002; Pujadas et al, 2010), a process that when disrupted, has been implicated in cognitive and mood-related behavior dysfunctions (Zhao et al, 2008). Noteworthy, several studies found an association between Reelin variants and schizophrenia or bipolar disorder in women only (Goes et al, 2010; Liu et al, 2010; Shifman et al, 2008).
Although these lines of evidence point to a link between Reelin and psychiatric disorders, other studies do not report an effect of Reelin's allelic variants on brain function (Tost et al, 2010). Moreover, there is scarce direct causal evidence of the involvement of this protein in these diseases. Reelin-deficient mice (known as reeler) are not suitable for behavioral testing because they show severe motor deficits, and reeler heterozygous mice have been challenged as a suitable model for psychiatric diseases. While some studies have shown that heterozygous reeler have cognitive (Brigman et al, 2006; Qiu et al, 2006a) and sensory-motor deficits as measured in the pre-pulse inhibition test (PPI) (Barr et al, 2008; Tueting et al, 1999), others have failed to find differences (Krueger et al, 2006; Podhorna and Didriksen, 2004; Salinger et al, 2003).
Reelin participates in several functions that are altered in psychiatric diseases, such as neuronal development and synapse formation (D'Arcangelo et al, 1995; Tissir and Goffinet, 2003), glutamatergic neurotransmission (Herz and Chen, 2006), Gsk-3β inhibition (Beffert et al, 2002), and adult neurogenesis (Kim et al, 2002; Pujadas et al, 2010). Given these observations, here we tested whether varying Reelin levels alters behavioral phenotypes related to psychiatric disorders (specifically schizophrenia, mood, and anxiety disorders). For this purpose, we used two mouse lines, the relatively well-characterized heterozygous reeler mice (rl/+), and a Reelin overexpressing line (Reelin-OE), generated in our laboratory (Pujadas et al, 2010) in which we focused our attention.
Although the design of animal models that relate to all aspects of a specific psychiatric disease is challenging, various models (with different levels of etiological, face, and predictive validity) are available. In this study, we focused on models that, in addition to presenting predictive validity, are based on environmental risk factors and thus present etiological validity, such as chronic stress linked with the development of ‘helplessness' and psychostimulant use (Nestler and Hyman, 2010). In all the tests performed, we found no differences between rl/+ mice and their wild-type (WT) littermates, either under basal conditions or after corticosterone, cocaine or N-methyl--aspartate (NMDA)-antagonist treatments. In addition, we show that increased Reelin expression does not alter mood-related behaviors under basal conditions. However, Reelin-OE mice showed reduced time floating in the forced-swim-test after chronic stress, reduced sensitization to cocaine, and reduced disruption of PPI after a NMDA antagonist challenge. In addition, we demonstrate that while chronic stress increased NMDA NR2B-mediated currents in the hippocampus, which were shown to mediate some of the behavioral dysfunctions seen after stress (Wong et al, 2007), Reelin overexpression reduced them. This finding is especially relevant since it has recently been reported that NR2B antagonists have antidepressant properties (Li et al, 2010).
SUBJECTS AND METHODS
Animals
Transgenic mice were bred at the Institute for Research in Biomedicine (Barcelona, Spain). Mice were housed in groups (2–6 mice per cage) and maintained on a 12-h light–dark cycle with access to food and water ad libitum. The Reelin-OE mice used in this study have been described previously (Pujadas et al, 2010). This line was obtained by crossing two mouse lines in a tet-off regulated system. The first line (CamKII/tTA) expresses the tTA transactivator under the control of the CamKII-α promoter (Mayford et al, 1996). The second line generated in our laboratory contains a myc-tagged Reelin cDNA construct controlled by a tetO promoter (tetO-rl). Reeler heterozygous mice were obtained from Jackson Laboratories (B6C3Fe a/a-Relnrl/J). Mice were maintained in a C57BL/6J background. Reeler mice were maintained by crossing rl/+ with C57BL/6J mice. These mice were backcrossed with C57BL/6J for >10 generations. CamKII/tTA mice were previously backcrossed into C57BL/6J for >10 generations in the laboratory of Jesus Avila, who generously provided us with these mice. They were then backcrossed with C57BL/6J mice in our laboratory for five more generations. TetO-rl mice were backcrossed with C57BL/6J for five generations. All mice were at least 8 weeks of age at the start of experiments, and behavioral procedures were conducted during the light phase of the cycle, except for the self-administration procedure, which was done in the dark phase. Females were used in the behavioral experiments, except for the self-administration experiments in which males where used. A detailed table with the groups of mice used in each experiment can be found in the Supplementary information (Supplementary Table S1). Experiments were conducted blind to the treatment condition of the mouse, and in compliance with local animal care protocols.
Immunohistochemistry
For Reelin immunohistochemistry, mice were perfused transcardially with ice cold 0.1 M phosphate-buffered saline (PBS) and then 4% paraformaldehyde (PFA). Brains were removed, fixed overnight in PFA, and then transferred to a 30% sucrose solution and stored at 4 °C. Fifty-micrometer coronal sections were cut and free-floating sections were prepared for immunohistochemistry. For immunodetection of Reelin, sections were blocked for 2 h at room temperature (RT) with PBS-T containing 10% of normal goat serum (NGS), 0.2% of gelatin and anti-mouse unconjugated F(ab')2 fragments (1 : 300, Jackson ImmunoResearch). Anti-Reelin primary antibody (ms, 1 : 200, Millipore) was incubated overnight at RT with PBS-T with 0.2% gelatin and 5% NGS. Sections were washed with PBS-T and then incubated for 2 h at RT with biotinylated secondary antibody (1 : 200, Vector Laboratories). After subsequent washes with PBS-T, the sections were incubated for 2 h at RT with streptavidin-HRP (1 : 400, GE Healthcare UK). After washing, the staining was revealed using diaminobenzidine (DAB) and H2O2, with nickel ammonium sulfate added to the solution. Finally, sections were dehydrated and mounted (Eukitt mounting medium).
Actimetry Box Test
Locomotor circadian activity was measured in actimetry boxes (45 × 45 cm; Harvard Apparatus, Panlab, Spain) placed in a sound-proof cupboard (WT, n=6; Reelin-OE, n=6). Movements were monitored via a grid of infrared beams and used as an index of locomotor activity (counts). Counts were integrated every hour and added to obtain total locomotor activity for a 24-h period, maintaining the 12 : 12-h light–dark schedule. All data were collected with Acti-Track software (Harvard Apparatus).
Corticosterone
Mice were given corticosterone (Sigma, St Louis, MO) in drinking water (25 mg/l) for 3 weeks (Gourley et al, 2008). Corticosterone was first diluted in ethanol (25 mg of corticosterone in 2 ml of ethanol) before addition to the water. Mice were tested 1 day after corticosterone treatment (WTReelin−OE, n=6; Reelin-OE, n=5; WTrl/+, n=8; rl/+, n=12).
Open-Field, Novelty-Suppressed-Feeding, Black and White Box, and Forced-Swim-Test
General anxiety and depression-like behaviors were tested using the open-field (OF), novelty-suppressed-feeding (NSF), black–white box (BW), and forced-swim-test (FST) tests (Li et al, 2008; Maeng et al, 2008; Santarelli et al, 2003). The OF test consisted of allowing the mice to explore an open white arena (50 × 50 cm). The time the mice spent in the center vs the time in the periphery of the arena as well as the total distance traveled were recorded over a 10-min trial (WTReelin−OE, n=26; Reelin-OE, n=13; WTrl/+, n=12; rl/+, n=12). In the NSF test, mice were deprived of food for 16 h and placed in a well-lit arena (50 × 50 cm, covered with sawdust) with a food pellet in the center. Latency in feeding was recorded (WTReelin−OE, n=26; Reelin-OE, n=13; WTrl/+, n=7; rl/+, n=10). In the BW box test, mice were placed in the dark compartment of a box (20 × 20 cm) connected to a well-lit, white compartment (25 × 25 cm). The time the mice spent in the white compartment was recorded during the 5 min of the test (WTReelin−OE, n=12; Reelin-OE, n=13; WTrl/+, n=6; rl/+, n=6). The FST is a measure of depression-like behavior and is used to test antidepressant activity. In the FST, mice were placed for 5 min in a 5 l beaker (diameter: 20 cm; water depth: 12 cm) filled with warm water (27 °C) until the 3 l mark. The amount of time the mice spent floating during the last 4 min of the trial was recorded (WTReelin−OE, n=26; Reelin-OE, n=13; WTrl/+, n=6; rl/+, n=6).
Cocaine Sensitization
Mice used in the OF test were subsequently subdivided and used in the cocaine sensitization experiment (WTsaline, n=9; Reelin-OEsaline, n=5; WTcocaine, n=17; Reelin-OEcocaine, n=8; WTsaline−rl/+, n=6; rl/+saline, n=6 WTcocaine−rl/+, n=6; rl/+cocaine, n=6). See Supplementary Table S1 for a detailed description of the groups of animals used. Thus, the first day of habituation corresponds to the OF trial. One week after being subjected to the previous tests, mice were habituated to the OF box for an additional 20 min (habituation day 2). Starting the following day, separate groups of mice were injected intraperitoneally with either cocaine (10 mg/kg in 0.9% NaCl) or saline (0.9% NaCl) for 5 consecutive days and immediately placed in the OF box. Locomotion was recorded for 20 min using the Smart Junior software (Panlab, Spain). Two weeks after the last cocaine injection, mice received a challenge injection and were placed back in the OF box as previously described (Maeng et al, 2008).
Self-Administration
The procedure for cocaine self-administration used in this work was previously described in detail (Boeuf et al, 2009). Briefly, mice were anaesthetized and implanted with an intravenous catheter in the jugular vein. Catheter patency was evaluated at the end of the experiment by infusing 0.1 ml of thiopental (5 mg/ml) through the catheter. Mice that did not show signs of anesthesia 3 s after infusion were removed from the analysis. Three days after surgery, mice were trained to nose-poke (operant chambers ENV-307A-CT, Med-Associates) to acquire intravenous self-administration of cocaine (0.5 μg/ml) on a fixed ratio 1 schedule of reinforcement, during 12 days of daily 1-h sessions (Reelin-OE n=5; WT n=5). The motivational value of cocaine was then assessed using a progressive ratio schedule of reinforcement (escalated series: 1-2-3-5-12-18-27-40) in a single 3-h session.
Rotor-Rod
Mice were first habituated for 1 min to walk on a rotor-rod at 4 r.p.m. (WT, n=5; Reelin-OE, n=5). They were then given three trials with the rotor-rod accelerating from 4 to 40 r.p.m. over 5 min. The average latency to fall was calculated for each mouse.
PPI of Acoustic Startle Response
The PPI of acoustic startle response was measured with a startle response apparatus (Harvard Apparatus). Each mouse was habituated to the equipment by being placed in the restraint device for 5 in a day during 3 days. On the test day, mice were allowed a 15-min habituation to the apparatus with background noise of 62 dB and then exposed to six blocks of seven trial types in pseudo-random order with 30-s inter-trial intervals. The eight trial types were as follows: trial 1 (startle-only trial), 40 ms of a 120 dB, 8000 Hz sound; trials 2–7 (pre-pulse (PP) trials), 40 ms of a 120 dB, 8000 Hz sound preceded for 140 ms by a 20 ms PP sound of 70, 74, 78, 82, 86, or 90 dB; and trial 8, 62 dB background noise. The startle response was recorded for 65 ms, with measurements every 1 ms from the onset of the startle stimulus. The maximum startle amplitude recorded during the 65 ms sampling window was used as the dependent variable. The PPI for each PP trial was defined as 100 ((startle amplitude on PP trials/startle amplitude on pulse alone trials) × 100). One week after baseline PPI testing, the mice were injected with MK-801 (0.15 mg/kg in 0.9% NaCl) and then retested for PPI 10 min later. One week later, the same group was tested after a 0.3 mg/kg MK-801 injection (WTReelin−OE, n=7; Reelin-OE, n=10; WTrl/+, n=9; rl/+, n=9).
Electrophysiology
Transverse brain slices (400 μm thickness) were prepared as described previously (Martin and Buno, 2003) and incubated for >1 h at RT (21–24 °C) in ACSF. The ACSF contained the following (in mM): NaCl 124, KCl 2.69, KH2PO4 1.25, MgSO4 2, NaHCO3 26, CaCl2 2 and glucose 10, and was gassed with 95% O2 and 5% CO2. Slices were transferred to an immersion recording chamber and superfused (2.5 ml/min) with equilibrated ACSF. Extracellular field excitatory postsynaptic potentials (fEPSPs) were recorded with a glass microelectrode (impedances: 2–3 MΩ filled with 1 M NaCl) positioned in stratum radiatum area CA1. Evoked fEPSPs were elicited by stimulation of the Schaeffer collateral fibers with an extracellular bipolar nichrome electrode via a 2100 isolated pulse stimulator (A-M Systems, Carlsborg, WA). The responses to paired-pulse stimulation at a range of interpulse intervals (25–400 ms) were used to measure paired-pulse facilitation. Data were transferred to the hard disk of a Pentium-based computer using a DigiData 1440A interface and the pCLAMP 10.0 software (Molecular Devices, Union City, CA). To measure the contribution of NMDA receptors to fEPSPs, we performed the experiment in the presence of 50 μM bicuculline to isolate EPSPs from gamma-aminobutyric acid A-mediated inhibitory synaptic transmission. Application of AP5 (50 μM), an antagonist of NMDARs, abolished fEPSPs, thereby confirming that the fEPSPs recorded were mediated through NMDARs. In this condition, 6- cyano-7-nitroquinoxaline-2, 3-dione (CNQX; 20 μM) in Mg2+-free external solution was added to pharmacologically isolated NMDA fEPSC components. Train stimuli at 100 Hz were used to assess the synaptic activation of the NMDA receptors by high-frequency afferent stimulation. To measure the contribution of NR2B receptors, we performed recordings in the presence of 3 μM of Ifenprodil. All drugs were purchased from Sigma, except CNQX, DL-2-amino-5-phosphopentanoic acid (AP5), Ifenprodil and Bicuculline, which were all from Tocris Cookson (Bristol, UK).
Statistical Analysis
Statistical differences were established using a two-tailed Student's t-test or ANOVA followed by an LSD post hoc test. Data are expressed as mean±SEM.
RESULTS
Reeler Heterozygous Mice Do Not Exhibit Behavioral Phenotypes Related to Psychiatric Disorders
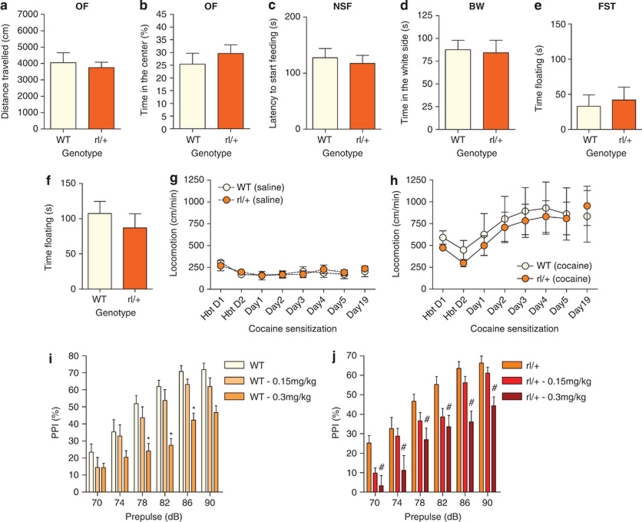
Contradictory findings have been reported regarding behavioral alterations of Reeler heterozygous mice (Barr et al, 2008; Brigman et al, 2006; Krueger et al, 2006; Podhorna and Didriksen, 2004; Qiu et al, 2006a; Salinger et al, 2003; Tueting et al, 1999). In order to assess whether these mice present behavioral alterations related to psychiatric disorders, we first subjected them to a battery of tests currently used as anxiety (OF, NSF, and BW box) and depression (FST) models. Heterozygous reeler mice (rl/+) showed no significant differences from their WT littermates in any of the tests performed (Figures 1a–e).
Figure 1.
Behavioral characterization of reeler heterozygous (rl/+) mice (a–e) Characterization of anxiety- and depression-like behaviors in rl/+ mice. There were no significant differences between rl/+ and WT mice in the distance travelled (a) and in the time spent in the center (b) in the open-field test (OF). Nor there were differences in the latency to feed in the novelty-suppressed feeding test (c: NSF), in the time spent on the white compartment of the black and white box (d: BW) or in the time spent floating in the forced-swim-Test (e: FST). (f) After 3 weeks of corticosterone administration, there were no significant differences in the time spent floating in the FST between rl/+ and WT mice. (g, h) Cocaine sensitization in rl/+ mice. (g) Locomotion values for rl/+ and WT mice treated with saline. (h) Sensitization to continuous cocaine administration in rl/+ and WT mice. (i) Reelin heterozygous WT littermate controls showed significantly reduced PPI after treatment with 0.3 mg/kg MK-801 at PP levels of 78–86 dB. (j) There was a significant effect of the dose on the disruption of PPI in rl/+ mice. PPI was significantly disrupted with the 0.3 mg/kg dose (#). In figure (j), there was a significant effect of the dose, as shown by ANOVA. A posterior LSD test showed that the 0.3 mg/kg dose was significantly different from the other treatments. Significant differences were established at *p<0.05. Data are presented as mean±SEM.
Although diseases like schizophrenia and bipolar disorder have a significant heritable component, interactions between genes and environmental risk factors make a significant contribution to the development of these diseases. One of the major environmental factors that precipitates depression as well as schizophrenic and bipolar disorder episodes is stress (Krishnan and Nestler, 2008). In order to study stress-associated behavioral dysfunctions in mice, we subjected them to a chronic treatment of the stress-associated hormone corticosterone. Next, we used the FST as a measure of developed helplessness-like behavior (Gourley and Taylor, 2009). After 3 weeks of corticosterone administration, there were no differences between stressed WT and heterozygous reeler mice (Figure 1f).
Dopamine agonists and NMDA antagonists induce behavioral dysfunctions, such as PPI and working-memory deficits, similar to those observed in schizophrenia (Geyer and Markou, 1995; Krystal et al, 1994). In addition, the hypothesis that schizophrenia might be related to dopaminergic or glutamatergic dysfunction (Belforte et al, 2009) led to the development of mouse models based on the modulation of these neurotransmitters (Geyer and Markou, 1995; Nestler and Hyman, 2010). Locomotion sensitization to psychostimulants provides a good predictive value of the efficacy of antipsychotic and mood-stabilizing drugs and this approach has been used in schizophrenia and mania research (Geyer and Markou, 1995; Nestler and Hyman, 2010). In order to study manic-like behavior and positive symptoms of schizophrenia in mice, we used the cocaine sensitization model (Figures 1g–h). Heterozygous reeler mice receiving saline treatment had locomotion values similar to those of their WT littermate controls throughout the experiment (Figure 1g). As expected, cocaine-treated rl/+ and WT mice increased their locomotion over the course of the treatment, and we found no significant differences between genotypes (Figure 1h; day: F(7, 70)=8.54, p<0.001). A comparison of locomotion on day 19 between all the groups indicated that animals receiving cocaine showed higher locomotion values, independently of the genotype (drug: F(1, 19)=11.49, p<0.01; LSD test p<0.01).
Deficits in PPI may indicate an incapacity to filter irrelevant information and they are observed in schizophrenic patients (Braff et al, 2001). Thus, PPI has been used as a tool to study schizophrenic-related behavior in mice as it shows good face and predictive value (Geyer and Markou, 1995; Miyakawa et al, 2003). In addition, previous studies have shown that heterozygous reeler mice present PPI deficits (Barr et al, 2008). We thus examined whether PPI was modified in heterozygous reeler mice. No significant disruption of PPI in untreated rl/+ was detected when compared with their WT littermate controls (Figures 1i–j and Supplementary Figure S3a). Startle amplitudes are shown in Supplementary Figure S2.
NMDA antagonist-induced deficits in PPI are used as a model of the positive symptoms of schizophrenia and have good predictive value of the efficacy of anti-psychotic medication (van den Buuse, 2010). Here, we proceeded to treat rl/+ mice and their respective WT littermates with the NMDA antagonist, MK-801, 10 min before testing them for PPI. MK-801 disrupted PPI in a dose-dependent manner in WT (Figure 1i) and rl/+ animals (Figure 1j). In rl/+ WT littermates, there was a significant dose by PP level interaction, showing that PPI was significantly disrupted when using 0.3 mg/kg of MK-801 on the PP levels of 78–86 dB (PP × dose: F(10, 120)=2.96, p<0.01; LSD test, p<0.05). At 0.3 mg/kg, MK-801 also significantly disrupted PPI in rl/+ mice (dose: F(2, 24)=9.72, p<0.0001; LSD test p<0.05). Together, these results suggest that reeler heterozygous mice do not show behavioral alterations related to psychiatric disorders.
Reelin-OE Mice Display Normal Anxiety- and Depression-Related Behaviors Under Basal Conditions
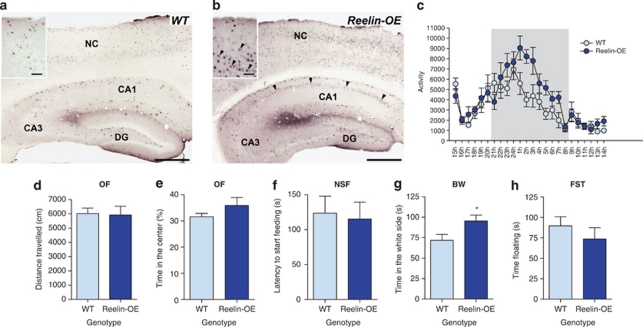
Given that rl/+ mice fail to show behavioral alterations, we tested whether Reelin overexpression is involved in the modulation of schizophrenia-, mood-, and anxiety-related behavioral phenotypes. For this purpose, we used a line generated in our laboratory (Pujadas et al, 2010) that overexpresses Reelin under the control of the CAMKII-α promoter (Reelin-OE). This promoter drives expression exclusively in the postnatal brain in excitatory forebrain neurons. These animals showed widespread expression of Reelin in pyramidal neurons of the neocortex and hippocampus, as well in most striatal neurons (Figures 2a and b). We have previously shown that this 4–6-fold increase in Reelin expression leads to over-activation of the Reelin pathway, as seen by an increase in Dab1 phosphorylation (Pujadas et al, 2010). In contrast to the dramatic cytoarchitectonic alterations and severe motor deficits found in reeler mice (Reelin knock-out), no gross anatomic or behavioral abnormalities were observed in Reelin-OE mice. These mice showed increased activity during the dark phase of their circadian rhythm (Figure 2c; 22–8 h: t(10)=−2.40, p<0.05) and normal locomotion in the OF test (Figure 2d), thereby indicating the absence of motor deficits.
Figure 2.
General characterization of Reelin-OE mice. (a, b) Reelin expression in Reelin-OE mice (b) was higher than in WT mice (a) throughout the postnatal forebrain, including the hippocampus (CA1, CA3, DG) and the cortex (NC). Arrowheads—Reelin overexpressing neurons. Scale bar: 500 μm. Scale bar of insets: 100 μm. (c) Reelin-OE mice showed increased activity during the dark phase of the light cycle. (d–h) Characterization of anxiety- and depression-like behaviors in Reelin-OE mice. There were no significant differences between WT and Reelin-OE mice in most tests (d–e: OF; f: NSF; h: FST). Reelin-OE mice spent significantly more time in the white compartment in the Black–White box test (g: BW). Significant differences were established at *p<0.05. Data are presented as mean±SEM.
Next, we tested whether anxiety-related behaviors were altered in the Reelin-OE mice. We found no differences between Reelin-OE mice and their littermate controls in the OF (Figures 2d and e), in the NSF (Figure 2f) or in the FST (Figure 2h). However, Reelin-OE animals showed reduced anxiety-like behavior in the BW box (Figure 2g; t(23)=−2.27, p<0.05).
Reelin-OE Mice Show Reduced Time Floating in the FST After Chronic Corticosterone Treatment and also Hyperlocomotion Induced by Psychostimulants
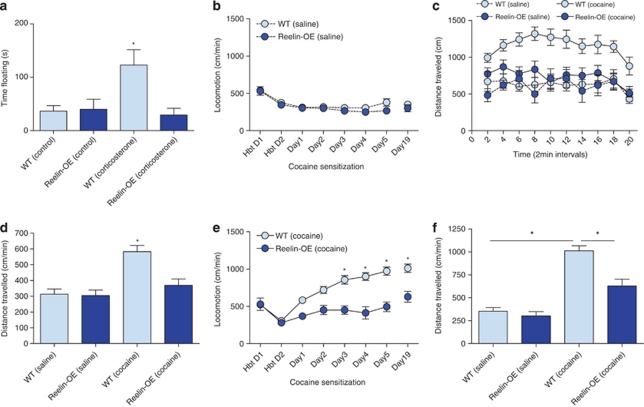
To test whether environmental risk factors like stress and psychostimulant abuse affect Reelin-OE mice differently, we next tested these mice in the FST after chronic corticosterone treatment and in the cocaine sensitization model. Corticosterone-treated WT mice spent more time floating in the FST than controls. This effect was abolished by Reelin overexpression, thereby indicating that Reelin-OE mice were less susceptible to corticosterone-induced helplessness-like behavior (Figure 3a; genotype × treatment: F(1, 27)=7.04, p<0.05; LSD test p<0.05). These data suggest that Reelin overexpression protects against the development of behavioral dysfunctions induced by chronic stress.
Figure 3.
Reduced helplessness-like behavior after corticosterone treatment and cocaine sensitization in Reelin-OE mice. (a) Reelin-OE mice spent less time floating in the FST than WT animals after corticosterone treatment. (b–f) Cocaine sensitization in Reelin-OE mice. (b) There were no differences in locomotion between Reelin-OE mice and their WT controls injected with saline. (c, d) After a single cocaine injection, locomotion of the Reelin-OE mice did not differ from that shown by saline controls during the 20 min of test. (e) Reelin-OE mice presented less cocaine sensitization. (f) Comparison of the locomotion values at day 19. Cocaine-treated WT mice showed locomotion values significantly higher than saline controls and cocaine-treated Reelin-OE mice. Significant differences were established at *p<0.05. Data are presented as mean±SEM.
In the cocaine sensitization model, no differences in locomotion were observed between the Reelin-OE and WT mice receiving saline injections. This observation indicates that there were no differences in novelty-induced hyperlocomotion or in habituation to the novel environment (Figure 3b). On the first day of sensitization, cocaine-injected Reelin-OE mice exhibited locomotion values identical to those of WT and Reelin-OE animals injected with saline (Figures 3c and d). In contrast, WT mice injected with cocaine showed significantly higher locomotion values than all the other groups (genotype × drug: F(1, 35)=4.92, p<0.05; LSD test p<0.05). Over the course of the experiment, WT mice injected with cocaine showed a dramatic increase in locomotion activity. In contrast, Reelin-OE mice showed less sensitivity to continued cocaine administration than their WT littermates (Figure 3e; genotype × day: F(7, 161)=11.80, p<0.0001; LSD test p<0.05). Comparison of the locomotion values at day 19 between cocaine- and saline-treated animals shows that cocaine-treated WT mice presented significantly higher locomotion values than saline controls and cocaine-treated Reelin-OE mice (Figure 3f; genotype × drug: F(1, 35)=5.99, p<0.05; LSD test p<0.05). These findings indicate that the behavioral dysfunctions induced by cocaine were dramatically blocked by Reelin overexpression. The differences observed in cocaine sensitization were not due to differences in the rewarding effects of cocaine or in motor performance, measured in the cocaine self-administration paradigm and in the rotor-rod, respectively (Supplementary Figures S1a–c in Supplementary information).
Taken together, these data show that Reelin-OE mice are resistant to the psychomotor effects of chronic psychostimulant administration.
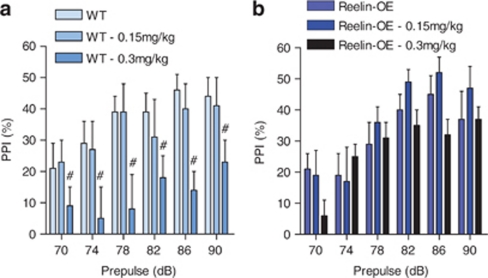
Reelin-OE Prevents PPI Deficits Induced by NMDA Antagonists
PPI deficits are an especially relevant schizophrenia endophenotype given that they can be easily tested in mice. We found no differences in PPI between untreated WT and Reelin-OE mice (Figures 4a and b and Supplementary Figure S3d). Startle amplitudes are shown in Supplementary Figure S2. However, significantly lower PPI levels were observed in WT mice (Reelin-OE littermates) treated with 0.3 mg/kg of MK-801 compared with the control and the 0.15 mg/kg groups (Figure 4a; dose: F(2, 18)=4.01, p<0.05; LDS test p<0.05), while no differences between treated and control Reelin-OE were detected (Figure 4b). This finding indicates that Reelin-OE mice are more resilient to a NMDA antagonist challenge. This observation is highly relevant because this test has good predictive value for the efficacy of antipsychotic medication (van den Buuse, 2010).
Figure 4.
Increased resilience after a MK-801 challenge in Reelin-OE mice. (a) PPI in WT mice (Reelin-OE littermates) was significantly disrupted at a dose of 0.3 mg/kg (#). In figure (a), there was a significant effect of the dose, as shown by ANOVA. A posterior LSD test showed that the 0.3 mg/kg dose was significantly different from the other treatments. (b) MK-801 treatment did not affect PPI in Reelin-OE mice. Data are presented as mean±SEM.
Reelin Increases Glutamatergic Transmission In Vivo
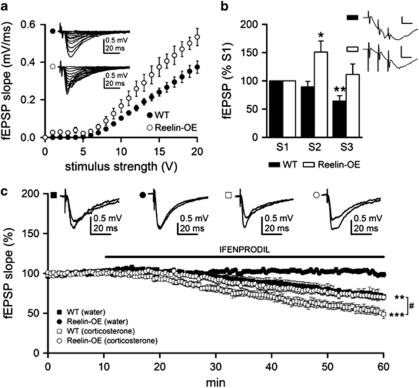
Reelin controls glutamatergic neurotransmission during development (Chen et al, 2005; Groc et al, 2007; Herz and Chen, 2006; Qiu et al, 2006b) and LTP is enhanced in Reelin-OE mice (Pujadas et al, 2010). Given that NMDA receptor hypofunction is often observed in schizophrenic patients (Coyle, 1996), we proceeded to analyze NMDA-mediated transmission in Reelin-OE mice. We first analyzed basal synaptic transmission in acute hippocampal slices from these mice and WT littermates. After stimulating Schaeffer collaterals with isolated stimuli of increasing intensity, neurons were recorded in the CA1 region. Extracellular field recordings showed that Reelin-OE CA1 neurons exhibited increased fEPSP responses, which were statistically significant at high stimulus strengths (Figure 5a; V20: t(20)=−2.18, p<0.05; See also Supplementary Figure S4 in Supplementary information). Using fEPSP recordings evoked by a three-stimulus train (100 Hz), we next examined whether synaptic activation of NMDA receptors was normal in Reelin-OE mice. After the second and third stimuli in the train, the NMDA receptor-mediated component of fEPSP (pharmacologically isolated) was significantly reduced in the WT slices when compared with the first stimuli (Figure 5b; t(6)=3.83, p<0.01). In contrast, there was a significant increase after the second stimulus in the Reelin-OE mice (Figure 5b; t(5)=−2.61, p<0.05). These observations indicate that Reelin overexpression potentiates NMDA-dependent glutamatergic neurotransmission, a process that is altered in several psychiatric disorders (Coyle, 1996).
Figure 5.
Reelin overexpression modifies synaptic activation of NMDA receptors and the function of NR2B subunit. (a) fEPSP slopes for WT (filled circle, n=12 slices from three mice) and Reelin-OE (open circle, n=10 slices from three mice) slices for a given range of stimulus intensities. Note that Reelin-OE CA1 neurons tended to exhibit increased fEPSP responses. Inset shows representative fEPSPs recorded in the stratum radiatum, and evoked by stimulation of the Schaffer collateral-commissural pathway with different intensities in WT (top) and Reelin-OE (bottom) mice. (b) Summary data showing mean NMDA-isolated fEPSP slopes in WT (filled bar; n=7 slices from three mice) and Reelin-OE (open bar; n=6 slices from three mice) slices. The fEPSP slope was significantly smaller in the WT mice for the third stimulus and significantly larger in the Reelin-OE mice for the second stimulus (compared with its respective first stimulus). Significant differences were established at *p<0.05 and **p<0.01. Inset shows representative field responses of NMDA component evoked by a 100 Hz train (three stimuli) in WT (top trace) and Reelin-OE (bottom trace), recorded in the presence of 20 μM CNQX to block the AMPA component. Calibration: 0.5 mV, 10 ms. (c) Time course of 3 μM Ifenprodil effects on normalized mean NMDAR-mediated fEPSP slopes evoked by Schaeffer collateral stimulation in WT control (filled square, n=6 slices from three mice), WT treated with corticosterone (open square, n=6 slices from three mice), Reelin-OE control (filled circles, n=6 slices from three mice), and Reelin-OE treated with corticosterone (open circles, n=6 slices from three mice) slices. Inset shows representative fEPSPs recorded in the absence or presence of Ifenprodil. Significant differences were established at **p<0.01 and ***p<0.001 (with respect to pre-Ifenprodil perfusion baseline slope values) and #p<0.05 (between WT and Reelin-OE treated with corticosterone). Data are presented as mean±SEM.
Reelin Decreases NMDA NR2B-Mediated Currents
Reelin is involved in the NR2B to NR2A developmental switch (Groc et al, 2007). Given that NR2B antagonists act as antidepressant drugs (Li et al, 2010), we next studied NR2B activity in WT and Reelin-OE mice. Perfusion of Ifenoprodil, a specific NR2B antagonist, reduced NMDA-mediated fEPSPs slopes by 30% in control WT slices (t(5)=3.35, p<0.01) while no changes were detected in Reelin-OE slices (Figure 5c). These observations indicate that Reelin overexpression in vivo leads to a dramatic hypofunction of NR2B-containing NMDARs.
Next, we analyzed NMDA-mediated neurotransmission in WT and Reelin-OE mice treated with corticosterone (Figure 5c; see also Supplementary Figure S5 in Supplementary information). In corticosterone-treated WT mice, electrophysiological recordings in the presence of Ifenprodil showed a marked reduction in NMDARs-mediated fEPSPs (nearly 50% of baseline; Figure 5c; t(5)=6.96, p<0.001). Interestingly, Reelin-OE mice treated with corticosterone exhibited fEPSPs that were indistinguishable from those of WT controls (Figure 5c; t(10)=−2.39, p<0.05). Given that NR2B is upregulated after stress and NR2B inhibition is sufficient to prevent behavioral deficits induced by stress (Li et al, 2010; Wong et al, 2007), our results suggest that Reelin overexpression increases resilience to stress through NR2B downregulation.
DISCUSSION
In order to study the role of Reelin in the modulation of behavioral phenotypes related to schizophrenia, mood, and anxiety disorders, we used two mouse lines that express different levels of Reelin: Reelin overexpressing mice (Reelin-OE) and heterozygous Reelin knock-out mice (rl/+). Under basal conditions, Reelin-OE and rl/+ mice presented mood-related behaviors similar to those of WT mice. However, when challenged with environmental stressors implicated in the genesis of psychiatric diseases—chronic stress and psychostimulant abuse—Reelin-OE mice were more resilient. These mice spent less time floating in the FST after chronic corticosterone treatment and less hyperlocomotion induced by cocaine. Furthermore, they were protected against the PPI deficits induced by NMDA antagonists. In addition, we show that Reelin overexpression enhanced glutamatergic transmission while simultaneously reducing NR2B-subunit-dependent NMDA currents.
Intense research effort is underway to identify resilience and risk factors involved in the pathophysiology of psychiatric diseases. Although epidemiological studies provide us with a list of candidate genes, further studies are necessary to identify whether these genes have a causative or protective role or even no role at all. Previous studies have shown that Reelin, an extracellular protein extensively studied during development because of its roles in cortical lamination, is downregulated in patients with several psychiatric diseases and is associated with schizophrenia (Fatemi et al, 2000; Jia et al, 2010; Kim and Webster, 2009). However, behavioral studies on the function of this protein have been hampered by the fact that reeler mice have severe motor deficits. In fact, the absence of Reelin in humans causes a neurological disorder—lissencephaly—characterized by the lack of development of the brain gyri and sulci, which leads to severe neurological impairments (Hong et al, 2000). In contrast, reeler heterozygous mice, which have no motor deficits (Podhorna and Didriksen, 2004), have been studied in some detail. These mice have mild cognitive deficits and higher anxiety levels, with PPI deficits being the most consistently documented behavioral phenotype related to psychiatric diseases (Barr et al, 2008; Tueting et al, 1999). However, these findings have not been replicated by other studies (Podhorna and Didriksen, 2004; Salinger et al, 2003). Furthermore, to our knowledge, there is no strong evidence of depression-like or manic-like behavioral phenotypes in these mice. As most studies in rl/+ mice have been conducted under basal conditions, we tested whether environmental stressors could uncover a behavioral phenotype. However, we found no differences between reeler heterozygous and WT mice in any of the paradigms tested. The absence of difference could be attributable to compensatory effects that commonly prevent the development of a phenotype in heterozygous mice. To overcome this, it would be interesting to repeat these behavioral tests in Reelin conditional knock-out mice. Our results are consistent with those of several other studies reporting no differences between rl/+ and WT mice in the OF and BW tests (Podhorna and Didriksen, 2004) and in the FST with or without corticosterone treatment (Lussier et al, 2010). It is worth noting that discrepancies in the detection of PPI deficits in the rl/+ might result from differences in the experimental setup as referred to in (Barr et al, 2008). In that study, the investigators show deficits in cross-modal PPI in rl/+ but no deficits in acoustic PPI, the procedure we used. In our study, in addition to following a downregulation strategy, we also studied the function of Reelin using the reverse approach, that is, Reelin overexpression. Using this novel approach, not only did we overcome the motor deficits found in the reeler mice but we also show a potential therapeutic use of Reelin pathway activation. Interestingly, Reelin overexpression had only a mild effect on anxiety-related behaviors under basal conditions (a reduction of anxiety-like behavior in the BW box). Psychiatric disorders are believed to result from a combination of genetic and environmental factors. Therefore, we proceeded to test whether Reelin protects against environmental insults. After exposure to chronic corticosterone treatment (a stress-associated hormone), Reelin-OE mice spent less time than WT mice floating in the FST, a paradigm that intents to model the development of helplessness behavior—characteristic of depression in humans—after chronic stress, and that has predictive value for the efficacy of antidepressants. In addition, in the presence of Ifenprodil (NR2B antagonist), slice recordings from corticosterone-treated WT animals showed a significantly higher reduction in fEPSPs than those of non-stressed WT animals. In contrast, fEPSPs of corticosterone-treated Reelin-OE mice slices were similar to those of WT non-stressed controls. Given that NR2B antagonists act as antidepressants (Li et al, 2010), we propose that Reelin may increase resilience to stress-induced behavioral dysfunctions through the downregulation of NR2B-mediated transmission.
Diseases like schizophrenia, mood, and anxiety disorders have high comorbidity levels. For instance, it has been reported that depression has a prevalence of 50% over the lifetime of schizophrenic patients (Altamura et al, 2011). Interestingly, we found that Reelin overexpression not only prevents behavioral dysfunctions after chronic corticosterone treatment, but also decreases hyperlocomotion induced by cocaine and PPI deficits induced by MK-801. Both of these tests—cocaine-induced hyperlocomotion and PPI deficits induced by NMDA antagonists—are used as models of positive symptoms of schizophrenia (Geyer and Markou, 1995; van den Buuse, 2010) and show predictive validity of the effects of antipsychotic and mood-stabilizing medication, as well as face validity in the case of PPI deficits (Geyer and Markou, 1995; Nestler and Hyman, 2010). It is fairly well established that the Reelin signaling pathway leads to Gsk-3β phosphorylation at Ser9, thereby leading to its enzymatic inhibition (Beffert et al, 2002). Several studies have reported that other candidate genes for mental illness, such as DISC1 (Mao et al, 2009) and Neuregulin 1 (Fazzari et al, 2010), regulate behavior through Gsk-3β inhibition. It is therefore possible that some of the behavioral effects of Reelin overexpression are due to Gsk-3β inhibition. In fact, lithium, a Gsk-3β inhibitor commonly used to treat bipolar disorder, also decreases psychostimulant-induced hyperlocomotion (Beaulieu et al, 2009).
In summary, here we show that the overexpression of Reelin in the mouse forebrain protects against the effects of chronic corticosterone and psychostimulant treatments. These observations indicate that this pathway may be an important therapeutic drug target.
Acknowledgments
We thank Lucas Brunso, Roberto Cabrera and the Cajal Physiolomic Unit for technical assistance, Anne Wheeler, Michel van den Oever, Scellig Stone and Paul Frankland for helpful comments, and Tanya Yates for editorial corrections. This work was supported by grants from CIBERNED, Plan Nacional de Drogas, Caixa Catalunya Foundation (Obra Social), MICINN and INCRECyT (European Social Fund and JCCM).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Altamura AC, Serati M, Albano A, Paoli RA, Glick ID, Dell'osso B.2011An epidemiologic and clinical overview of medical and psychopathological comorbidities in major psychoses Eur Arch Psychiatry Clin Neuroscie-pub ahead of print 18 February 2011. [DOI] [PubMed]
- Barr AM, Fish KN, Markou A, Honer WG. Heterozygous reeler mice exhibit alterations in sensorimotor gating but not presynaptic proteins. Eur J Neurosci. 2008;27:2568–2574. doi: 10.1111/j.1460-9568.2008.06233.x. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J Biol Chem. 2002;277:49958–49964. doi: 10.1074/jbc.M209205200. [DOI] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2009;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeuf J, Trigo JM, Moreau PH, Lecourtier L, Vogel E, Cassel JC, et al. Attenuated behavioural responses to acute and chronic cocaine in GASP-1-deficient mice. Eur J Neurosci. 2009;30:860–868. doi: 10.1111/j.1460-9568.2009.06865.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Padukiewicz KE, Sutherland ML, Rothblat LA. Executive functions in the heterozygous reeler mouse model of schizophrenia. Behav Neurosci. 2006;120:984–988. doi: 10.1037/0735-7044.120.4.984. [DOI] [PubMed] [Google Scholar]
- Chen Y, Beffert U, Ertunc M, Tang TS, Kavalali ET, Bezprozvanny I, et al. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci. 2005;25:8209–8216. doi: 10.1523/JNEUROSCI.1951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle JA, McMenomy T.2000Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression Mol Psychiatry 5654–663.571. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD. Chronic psychotropic drug treatment causes differential expression of Reelin signaling system in frontal cortex of rats. Schizophr Res. 2009;111:138–152. doi: 10.1016/j.schres.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Markou A.1995Animal models of psychiatric disordersIn: Bloom FE, Kupfer DJ (eds).Psychopharmacology: The Fourth Generation of Progress Raven Press: New York; 787–798. [Google Scholar]
- Goes FS, Willour VL, Zandi PP, Belmonte PL, MacKinnon DF, Mondimore FM, et al. Sex-specific association of the Reelin gene with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:549–553. doi: 10.1002/ajmg.b.31018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Kiraly DD, Howell JL, Olausson P, Taylor JR. Acute hippocampal brain-derived neurotrophic factor restores motivational and forced swim performance after corticosterone. Biol Psychiatry. 2008;64:884–890. doi: 10.1016/j.biopsych.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Taylor JR.2009Recapitulation and reversal of a persistent depression-like syndrome in rodents Curr Protoc Neurosci 9Unit 9 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Choquet D, Stephenson FA, Verrier D, Manzoni OJ, Chavis P. NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin. J Neurosci. 2007;27:10165–10175. doi: 10.1523/JNEUROSCI.1772-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7:850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- Hong SE, Shugart YY, Huang DT, Shahwan SA, Grant PE, Hourihane JO, et al. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet. 2000;26:93–96. doi: 10.1038/79246. [DOI] [PubMed] [Google Scholar]
- Jia P, Sun J, Guo AY, Zhao Z. SZGR: a comprehensive schizophrenia gene resource. Mol Psychiatry. 2010;15:453–462. doi: 10.1038/mp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Qu T, Kriho V, Lacor P, Smalheiser N, Pappas GD, et al. Reelin function in neural stem cell biology. Proc Natl Acad Sci USA. 2002;99:4020–4025. doi: 10.1073/pnas.062698299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Webster MJ. The Stanley neuropathology consortium integrative database: a novel, web-based tool for exploring neuropathological markers in psychiatric disorders and the biological processes associated with abnormalities of those markers. Neuropsychopharmacology. 2009;35:473–482. doi: 10.1038/npp.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger DD, Howell JL, Hebert BF, Olausson P, Taylor JR, Nairn AC. Assessment of cognitive function in the heterozygous reeler mouse. Psychopharmacology (Berl) 2006;189:95–104. doi: 10.1007/s00213-006-0530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen PL, McGrath J, Wolyniec P, Fallin D, Nestadt G, et al. Replication of an association of a common variant in the Reelin gene (RELN) with schizophrenia in Ashkenazi Jewish women. Psychiatr Genet. 2010;20:184–186. doi: 10.1097/YPG.0b013e32833a220b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier AL, Caruncho HJ, Kalynchuk LE. Repeated exposure to corticosterone, but not restraint, decreases the number of reelin-positive cells in the adult rat hippocampus. Neurosci Lett. 2009;460:170–174. doi: 10.1016/j.neulet.2009.05.050. [DOI] [PubMed] [Google Scholar]
- Lussier AL, Romay-Tallon R, Kalynchuk LE, Caruncho HJ. Reelin as a putative vulnerability factor for depression: examining the depressogenic effects of repeated corticosterone in heterozygous reeler mice. Neuropharmacology. 2010;60:1064–1074. doi: 10.1016/j.neuropharm.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Maeng S, Hunsberger JG, Pearson B, Yuan P, Wang Y, Wei Y, et al. BAG1 plays a critical role in regulating recovery from both manic-like and depression-like behavioral impairments. Proc Natl Acad Sci USA. 2008;105:8766–8771. doi: 10.1073/pnas.0803736105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ED, Buno W. Caffeine-mediated presynaptic long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2003;89:3029–3038. doi: 10.1152/jn.00601.2002. [DOI] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Leiter LM, Gerber DJ, Gainetdinov RR, Sotnikova TD, Zeng H, et al. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci USA. 2003;100:8987–8992. doi: 10.1073/pnas.1432926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podhorna J, Didriksen M. The heterozygous reeler mouse: behavioural phenotype. Behav Brain Res. 2004;153:43–54. doi: 10.1016/j.bbr.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Pujadas L, Gruart A, Bosch C, Delgado L, Teixeira CM, Rossi D, et al. Reelin egulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J Neurosci. 2010;30:4636–4649. doi: 10.1523/JNEUROSCI.5284-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Korwek KM, Pratt-Davis AR, Peters M, Bergman MY, Weeber EJ. Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiol Learn Mem. 2006a;85:228–242. doi: 10.1016/j.nlm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Qiu S, Zhao LF, Korwek KM, Weeber EJ. Differential reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus. J Neurosci. 2006b;26:12943–12955. doi: 10.1523/JNEUROSCI.2561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinger WL, Ladrow P, Wheeler C. Behavioral phenotype of the reeler mutant mouse: effects of RELN gene dosage and social isolation. Behav Neurosci. 2003;117:1257–1275. doi: 10.1037/0735-7044.117.6.1257. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Shifman S, Johannesson M, Bronstein M, Chen SX, Collier DA, Craddock NJ, et al. Genome-wide association identifies a common variant in the reelin gene that increases the risk of schizophrenia only in women. PLoS Genet. 2008;4:e28. doi: 10.1371/journal.pgen.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Tost H, Lipska BK, Vakkalanka R, Lemaitre H, Callicott JH, Mattay VS, et al. No effect of a common allelic variant in the reelin gene on intermediate phenotype measures of brain structure, brain function, and gene expression. Biol Psychiatry. 2010;68:105–107. doi: 10.1016/j.biopsych.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tueting P, Costa E, Dwivedi Y, Guidotti A, Impagnatiello F, Manev R, et al. The phenotypic characteristics of heterozygous reeler mouse. Neuroreport. 1999;10:1329–1334. doi: 10.1097/00001756-199904260-00032. [DOI] [PubMed] [Google Scholar]
- van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr Bull. 2010;36:246–270. doi: 10.1093/schbul/sbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TP, Howland JG, Robillard JM, Ge Y, Yu W, Titterness AK, et al. Hippocampal long-term depression mediates acute stress-induced spatial memory retrieval impairment. Proc Natl Acad Sci USA. 2007;104:11471–11476. doi: 10.1073/pnas.0702308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.