Abstract
Preterm premature rupture of membranes is responsible for one third of preterm births. Ehlers-Danlos syndrome (EDS) is associated with preterm premature rupture of membranes in humans. Notably, an EDS variant is caused by a genetic mutation resulting in abnormal secretion of biglycan and decorin, two small leucine-rich proteoglycans highly expressed in reproductive tissues. Because biglycan/decorin null mutant (Bgn−/−Dcn−/−) mice demonstrate phenotypic changes similar to EDS, we utilized this model to test whether either or both biglycan and decorin play a role in the attainment of successful term gestation. Wild-type, biglycan null mutant, decorin null mutant and biglycan/decorin null mutant pregnancies were assessed for length of gestation, pup and placenta weight and litter size. Quantitative real-time polymerase chain reaction was performed to measure biglycan and decorin gene expression and immunohistochemistry was performed to assess protein expression in placenta and fetal membranes at embryonic day E12, E15 and E18. Bgn−/−Dcn−/− dams displayed preterm birth, whereas the possession of at least two biglycan or decorin wild-type alleles was protective of preterm birth. Bgn−/−Dcn−/− pups were decreased at postnatal day P1 but not at E18. Biglycan and decorin were upregulated in the placenta in each other’s absence and were developmentally regulated in fetal membranes, suggesting that these two proteoglycans demonstrate genetic complementation and contribute to gestational success in a dose dependent manner. Thus, the biglycan/decorin null mutant mouse is a model of genetically induced preterm birth and perinatal loss. This model presents novel targets for preventive or therapeutic manipulation of preterm birth.
Introduction
Despite significant advances in the care of pregnant mothers and of low birth weight infants, preterm birth is the leading cause of newborn morbidity and mortality and the main cause of hospitalization in the first year of life in the United States. Moreover, despite numerous interventions, the incidence of prematurity has shown no significant improvement over the last two decades (Ananth & Vintzileos 2006). Risk factors for prematurity include adverse socio-demographic status, ethnicity, infection, stress, trauma and prior history of a premature birth. However, the majority of preterm births are unexplained (Romero et al. 1994).
Preterm premature rupture of fetal membranes (PPROM) is estimated to account for 40% of preterm births (Steer 2005). While recent research has focused on inflammation leading to activation of matrix metalloproteinases (Parry & Strauss 1998, Menon & Fortunato 2004) as a mechanism, it appears implausible that infection is the only etiologic factor, since the majority of patients have no clinical evidence for an ongoing inflammatory process. Several mechanisms have been proposed to play a role in the process of preterm premature rupture of fetal membranes, including physical stress, biochemical alterations and apoptosis (Parry & Strauss 1998, Menon & Fortunato 2004, Arikat et al. 2006, El Khwad et al. 2006, Moore et al. 2006). Risk factors for PPROM mirror those for preterm birth and include genetic susceptibility and infection (Parry & Strauss 1998).
Infants with Ehlers-Danlos syndrome have a significantly increased incidence of preterm birth from PPROM in comparison to their unaffected siblings (Barabas 1966, Yen et al. 2006). Ehlers-Danlos syndrome is a heterogeneous group of rare inherited connective tissue disorders associated with a decrease in tensile strength and integrity of skin, joints, and other connective tissues.
In patients afflicted with the progeroid variant of Ehlers-Danlos syndrome, the molecular basis of the connective tissue anomaly is a mutation of xylosylprotein-4β-galactosyltransferase I, an enzyme that is necessary for the posttranslational glycosylation of biglycan and decorin, two small leucine-rich proteoglycans (SLRPs) (Schaefer & Iozzo 2008) that are involved in regulating collagen fibrillogenesis, cell growth and inflammatory responses (Reed & Iozzo 2002). This mutation leads to the abnormal secretion of biglycan and decorin core protein lacking glycosaminoglycan side chains (Kresse et al. 1987, Quentin et al. 1990).
Mice deficient in biglycan, decorin, or both, model the phenotype of Ehlers-Danlos syndrome, displaying connective tissue anomalies of skin, bone and tendon (Danielson et al. 1997, Xu et al. 1998).
Biglycan is a small leucine-rich proteoglycan that is a component of the extracellular matrix in a variety of tissues including skin, bone, tendon and connective tissue (Young et al. 2002, Wadhwa et al. 2004). Its core protein contains two chondroitin or dermatan sulfate side chains (Bowe et al. 2000). Biglycan binds to collagen VI, TGF-α, TGF-β, chordin and BMP-4 (Hildebrand et al. 1994, Wiberg et al. 2001, Wiberg et al. 2003, Chen et al. 2004, Hayashi et al. 2005, Moreno et al. 2005). Decorin is a SLRP protein with one chondroitin or dermatan sulfate side chain that demonstrates ~55% homology with biglycan (Iozzo & Murdoch 1996) and also interacts with a number of extracellular matrix constituents and growth factors (Iozzo & Schaefer 2010).
Biglycan and decorin are expressed in a variety of gestational tissues in humans and mice. They are expressed in the human placenta, where they are increased in association with gestational diabetes mellitus (Chen et al. 2007). Biglycan is expressed in the pregnant mouse uterus (San Martin et al. 2003, San Martin & Zorn 2003), while both biglycan and decorin are decreased in the human myometrium during labor (Hjelm et al. 2002). In decorin homozygous null mutant mice, the decidualized stroma of the uterus shows abnormal collagen architecture with large diameters and irregular contours (Sanches et al. 2010). Furthermore, these SLRPs are the most abundant proteoglycans expressed in human fetal membranes (Gogiel & Jaworski 2000, Meinert et al. 2001, Gogiel et al. 2003, Valiyaveettil et al. 2004). After labor, biglycan increases, while decorin decreases in fetal membranes (Meinert et al. 2007). However, little is known about the role that these proteoglycans play in the maintenance of gestation.
Thus, we hypothesize that biglycan and decorin are necessary for the attainment of successful full term gestation and that the absence of both biglycan and decorin will lead to preterm birth.
Results
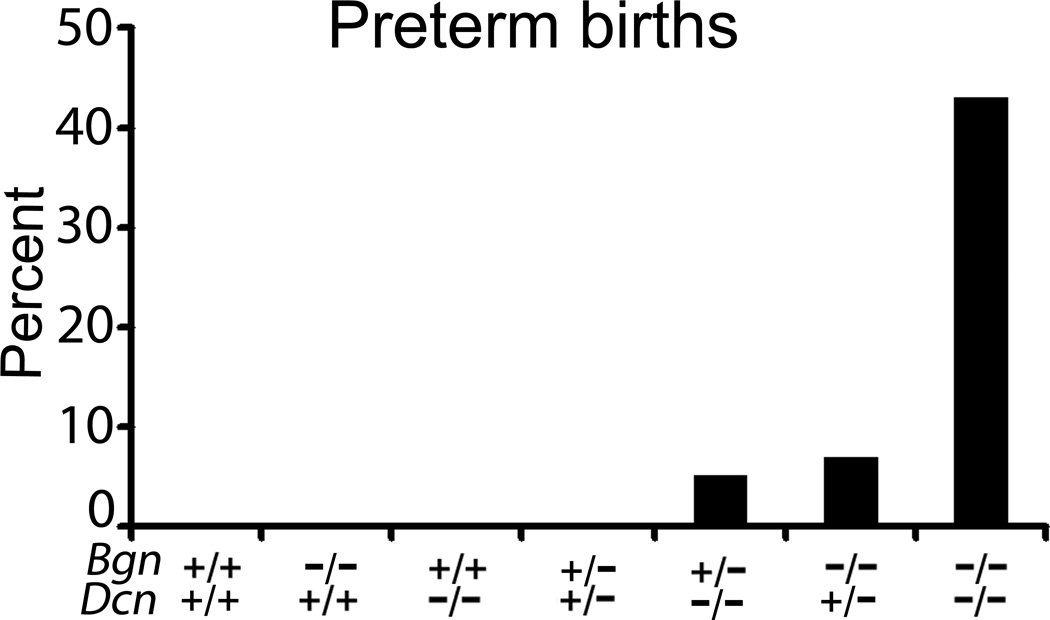
First, we evaluated the reproductive capacity of the following females compared to the wild-type (Bgn+/+Dcn+/+): biglycan homozygous null mutant and decorin homozygous null mutant mice (Bgn−/−Dcn+/+ and Bgn+/+Dcn−/−), the biglycan heterozygous / decorin heterozygous null mutant (Bgn+/−Dcn+/−), the biglycan heterozygous / decorin homozygous null mutant (Bgn+/−Dcn−/−), the biglycan homozygous / decorin heterozygous null mutant (Bgn−/−Dcn+/−) and the biglycan homozygous / decorin homozygous null mutant (Bgn−/−Dcn−/−). Wild-type females, biglycan homozygous null mutant females and decorin homozygous null mutant females were bred with males of their own genotype. Bgn+/−Dcn+/−, Bgn+/−Dcn−/−, Bgn−/−Dcn+/− and Bgn−/−Dcn−/− females were bred with Bgn−/0Dcn+/− males as described in the Materials and Methods section. We observed that the Bgn−/−Dcn+/+, Bgn+/+Dcn−/−, Bgn+/−Dcn+/− as well as the wild-type female did not display preterm birth (defined as birth before E18). The Bgn+/−Dcn−/− and Bgn−/−Dcn+/− females had a significantly increased risk of preterm birth compared to the wild-type, while the Bgn−/−Dcn−/− had further significantly increased risk of preterm birth (Fig. 1) (P=0.0002). This increase in rate of preterm birth displayed an inversely linear relationship to the number of SLRP (biglycan or decorin) alleles per genotype (Table 1) (P<0.0001).
Figure 1.
Percentage of preterm births (before embryonic day 18) per mouse genotype. Biglycan and decorin are necessary for the maintenance of gestation to full term in a dose dependent and compensatory manner. Biglycan homozygous null mutants, decorin homozygous null mutants and biglycan heterozygous / decorin heterozygous null mutant are not at increased risk of preterm birth. Biglycan heterozygous / decorin homozygous null mutants and biglycan homozygous / decorin heterozygous null mutants are at increased risk of preterm birth, while biglycan homozygous / decorin homozygous null mutants are at further increased risk of preterm birth. P=0.0002. Chi-square test. Bgn+/+Dcn+/+ n=13; Bgn−/−Dcn+/+ n=33; Bgn+/+Dcn−/− n=11; Bgn+/−Dcn+/− n=14; Bgn+/−Dcn−/− n=20; Bgn−/−Dcn+/− n=30; Bgn−/−Dcn−/− n=7. Bgn=biglycan; Dcn=decorin.
Table 1.
The percentage of preterm mouse births (<embryonic day 18) increases with decreasing number of maternal biglycan and decorin alleles
| SLRP alleles | Preterm births*** | Total births |
|---|---|---|
| 4 | 0 (0%) | 13 |
| 2 | 0 (0%) | 55 |
| 1 | 3 (6%) | 50 |
| 0 | 3 (43%) | 7 |
Chi-square for linear trend test: p<0.0001
SLRP: small leucine rich proteoglycan
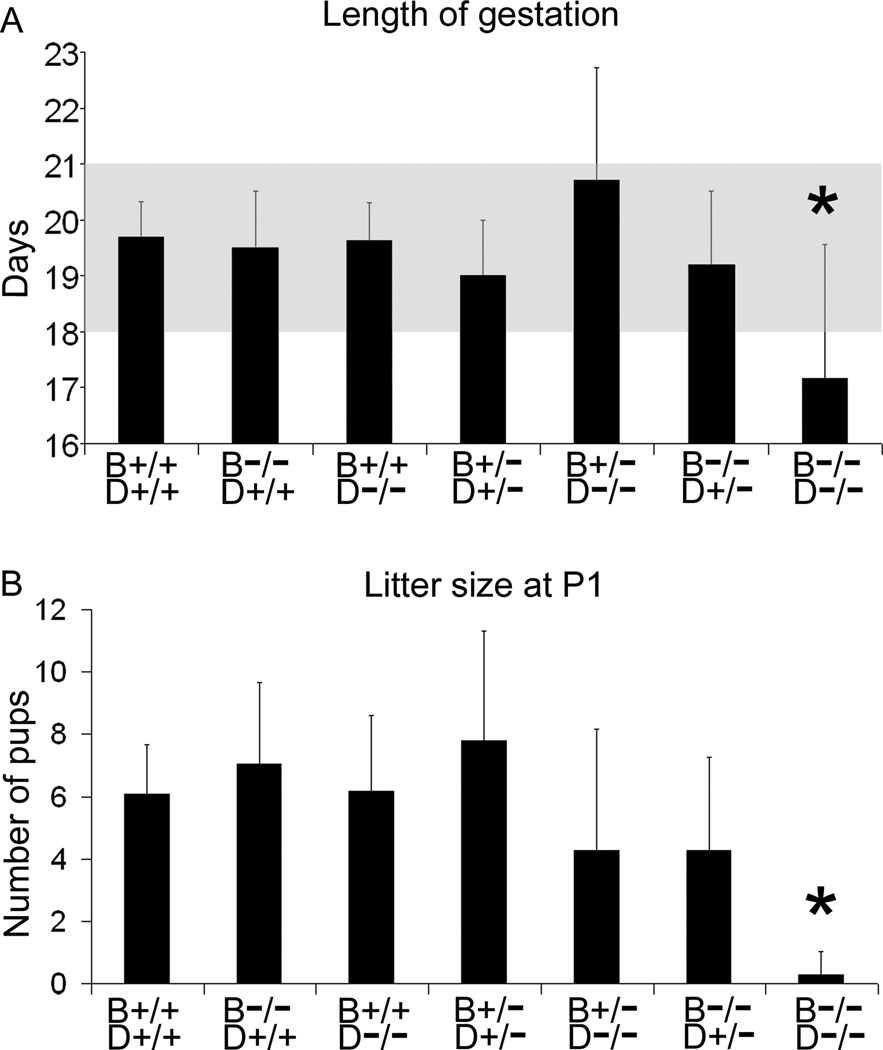
Furthermore, we observed that the Bgn−/−Dcn−/− dams displayed a significantly decreased gestational length (Fig. 2A) (P<0.001) as well as a significantly reduced litter size (defined as pups surviving the first 24 h after birth) compared to the control wild-type (Fig. 2B) (P<0.001).
Figure 2.
(A) Bgn−/−Dcn−/− female mice display a statistically significant decrease in length of gestation compared to all other genotypes. P<0.001. Bgn+/+Dcn+/+ n=13; Bgn−/−Dcn+/+ n=33; Bgn+/+Dcn−/− n=11; Bgn+/−Dcn+/− n=13; Bgn+/−Dcn−/− n=14; Bgn−/−Dcn+/− n=20; Bgn−/−Dcn−/− n=7. Shading: range of normal length of pregnancy. (B) Number of mouse pups per litter surviving beyond 24 h of age. Bgn−/−Dcn−/− dams display a significantly decreased litter size at 24 h. P<0.001. Bgn+/+Dcn+/+ n=11; Bgn−/−Dcn+/+ n=17; Bgn+/+Dcn−/− n=32; Bgn+/−Dcn+/− n=54; Bgn+/−Dcn−/− n=34; Bgn−/−Dcn+/− n=19; Bgn−/−Dcn−/− n=7. Error bars = SEM. P = postnatal day. Bgn=biglycan; Dcn=decorin. One-way ANOVA with Holm-Sidak contrast was performed for both sets of data.
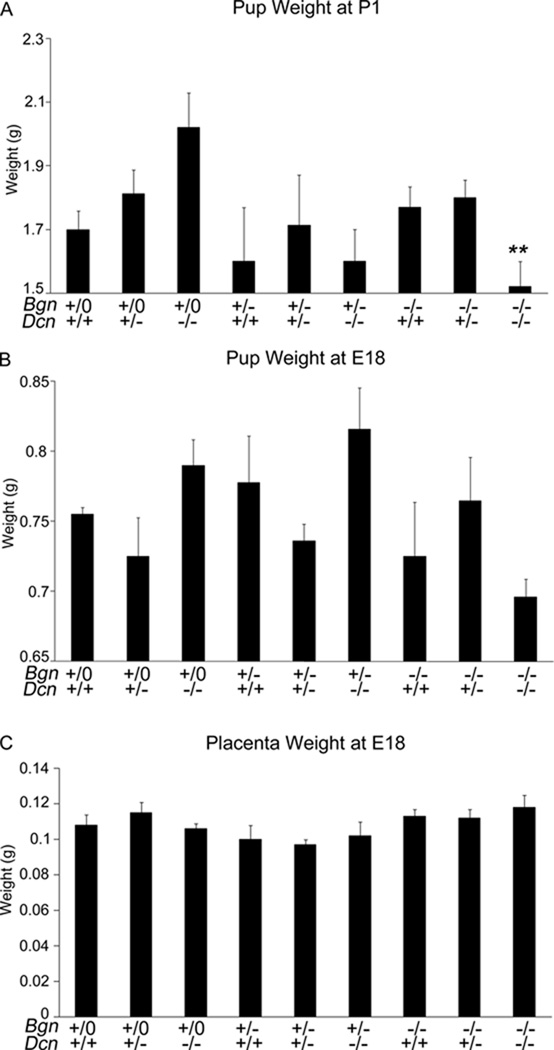
In mixed litters of Bgn+/−Dcn+/− females mated with Bgn−/0Dcn+/− males, the mean litter size decreased significantly from E18 to P1 (Table 2A). Concurrently, the number of biglycan homozygous / decorin homozygous null mutant (Bgn−/−Dcn−/−) pups was as expected by Mendelian calculations at E18, but was significantly lower than expected at P1, suggesting a perinatal loss of Bgn−/−Dcn−/− pups as a cause of the decrease in litter size (Table 2B) (P<0.001). Furthermore, Bgn−/−Dcn−/− pups displayed lower weights at P1 than their littermate controls and displayed a trend toward lower weights at E18 that was not statistically significant, while their placental weights at E18 were not different from littermate controls (Fig. 3A, B and C) (P=0.0014, P=0.2068 and P=0.858 respectively).
Table 2.
| A. The mean litter size for Bgn+/−Dcn+/− dams is decreased at postnatal day 1 compared to embryonic day 18. | ||
|---|---|---|
| Genotype | Embryonic day 18 (n) | Postnatal day 1 (n) |
| Bgn+/−Dcn +/− | 9.3 (10) | 6.6 (14)* |
| B. The number of Bgn−/−Dcn−/− mouse pups is unchanged prenatally but decreased postnatally compared to Mendelian distribution. | |||
|---|---|---|---|
| Genotype | Embryonic day 18 (n) | Postnatal day 1 (n) | Mendelian expected |
| Bgn+/0Dcn+/+ | 5% (5) | 7% (10) | 6.25% |
| Bgn+/0Dcn+/− | 17% (16) | 14% (19) | 12.50% |
| Bgn+/0Dcn−/− | 5% (5) | 4% (5) | 6.25% |
| Bgn+/−Dcn+/+ | 9% (8) | 12% (16) | 6.25% |
| Bgn+/−Dcn+/− | 12% (11) | 23% (31) | 12.50% |
| Bgn+/−Dcn−/− | 6% (6) | 6% (8) | 6.25% |
| Bgn−/−Dcn+/+ or Bgn−/0Dcn+/+ | 8% (7) | 10% (13) | 12.50% |
| Bgn−/−Dcn+/− or Bgn−/0Dcn+/− | 22% (20) | 21% (28) | 25% |
| Bgn−/−Dcn−/− or Bgn−/0Dcn−/− | 16% (15) | 3% (4)*** | 12.50% |
p<0.05
p<0.001
Figure 3.
(A) Biglycan heterozygous / decorin heterozygous null mutant mouse dams’ litters were weighed on postnatal day 1. Bgn−/−Dcn−/− pups display significantly lower weight compared to littermates of other genotypes. P=0.0014. Pregnant Bgn+/−Dcn+/− dams: n=12; Litters: 14; Total pups in litters: 93; Bgn+/+Dcn+/+ n=7; Bgn+/+Dcn+/− n=16; Bgn+/+Dcn−/− n=14; Bgn+/−Dcn+/+ n=4; Bgn+/−Dcn+/− n=7; Bgn+/−Dcn−/− n=2; Bgn−/−Dcn+/+ n=13; Bgn−/−Dcn+/− n=25; Bgn−/−Dcn−/− n=5. (B) Biglycan heterozygous / decorin heterozygous null mutant mouse dams’ litters were weighed on embryonic day 18. As opposed to postnatal day 1 pup weights, there is no significant difference in pup weight at E18 between littermates of various genotypes. Pregnant Bgn+/−Dcn+/− dams: n=7; Total pups in litters 63: Bgn+/+Dcn+/+ n=2; Bgn+/+Dcn+/− n=8; Bgn+/+Dcn−/− n=5; Bgn+/−Dcn+/+ n=9; Bgn+/−Dcn+/− n=8; Bgn+/−Dcn−/− n=5; Bgn−/−Dcn+/+ n=6; Bgn−/−Dcn+/− n=15; Bgn−/−Dcn−/− n=5. (C) Biglycan heterozygous / decorin heterozygous null mutant mouse dams’ placenta weight was measured on embryonic day 18. As opposed to postnatal day 1 pup weights, there is no significant difference in placenta weight between littermates of various genotypes. Pregnant Bgn+/−Dcn+/− dams: n=10; Total pups in litters 93: Bgn+/+Dcn+/+ n=8; Bgn+/+Dcn+/− n=11; Bgn+/+Dcn−/− n=6; Bgn+/−Dcn+/+ n=5; Bgn+/−Dcn+/− n=16; Bgn+/−Dcn−/− n=5; Bgn−/−Dcn+/+ n=7; Bgn−/−Dcn+/− n=20; Bgn−/−Dcn−/− n=15. Bgn−/− refers to Biglycan−/− or Biglycan−/0 depending on gender. Error bars = SEM. P = postnatal day. Bgn=biglycan; Dcn=decorin. Mixed statistical model: genotype as a fixed effect, litter as a random effect, specific contrasts of Bgn−/−Dcn−/− versus all other genotypes.
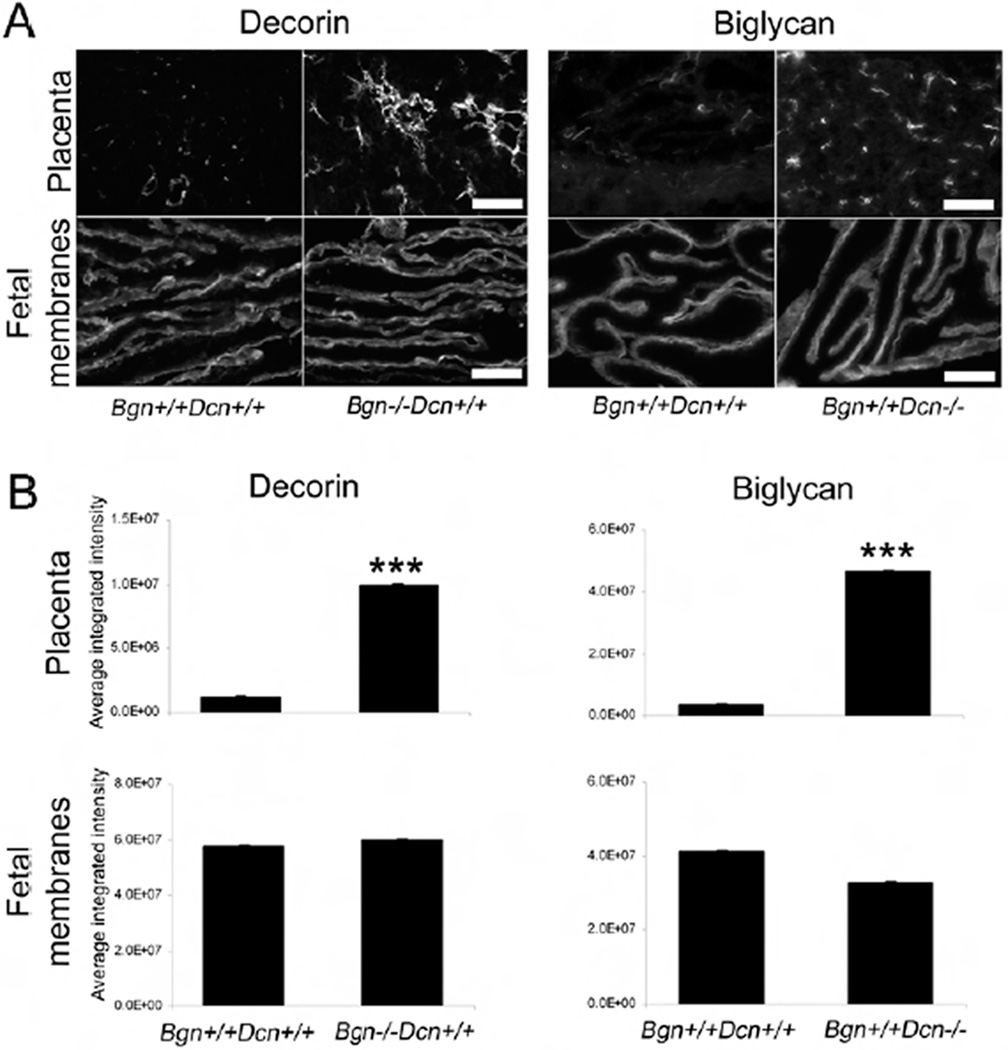
As a first step in pinpointing the compensatory mechanisms that allow for normal gestational phenotypes in null mutants that possess at least two of four wild-type SLRP alleles, we performed immunohistochemical analysis of gestational tissues at E18. We assessed decorin expression and localization in the placenta and fetal membranes in the presence and absence of biglycan, as well as the expression and localization of biglycan in the same tissues in the presence and absence of decorin. Each experiment was repeated three times. On comparison of placental sections of wild-type and biglycan homozygous null mutant mice, we noted a robust upregulation of decorin in the absence of biglycan. Conversely, biglycan was upregulated in the decorin homozygous null mutant placenta (Fig. 4A). We did not note a change in biglycan expression in decorin homozygous null mutant fetal membranes or in decorin expression in biglycan homozygous null mutant fetal membranes. Our results were similar for all tissue types at E12 and E15, but the biglycan and decorin upregulation in the placenta was less striking than at E18 (data not shown). Quantitative analysis revealed a statistically significant increase in decorin immunofluorescence in the biglycan homozygous null mutant placenta compared to the wild-type (P<0.001), as well as a statistically significant increase in biglycan immunofluorescence in the decorin homozygous null mutant (P<0.001), but no difference in fetal membranes (Fig. 4B).
Figure 4.
(A) Immunohistochemical comparison of biglycan expression in wild-type and decorin homozygous null mutant mouse placenta and fetal membranes as well as decorin expression in wild-type and biglycan homozygous null mutant mouse placenta and fetal membranes. There is increased biglycan signal in the absence of decorin and increased decorin signal in the absence of biglycan in the placenta. No compensatory upregulation is noted in the fetal membranes. Placenta decorin staining 10×, scale bar 500 µm. All others 20×, scale bar 100 µm. The photomicrographs are representative of one placenta each from three pregnant females per experimental group. (B) Quantitative analysis of immunofluorescence showing a significant increase in decorin signal in the absence of biglycan (P<0.001) and a significant increase in biglycan signal in the absence of decorin (P<0.001) in the placenta but no difference in fetal membranes. Student’s t-test. The graphs are representative of 5–15 images from one placenta each from three pregnant females per experimental group. Error bars = SEM. Bgn=biglycan; Dcn=decorin.
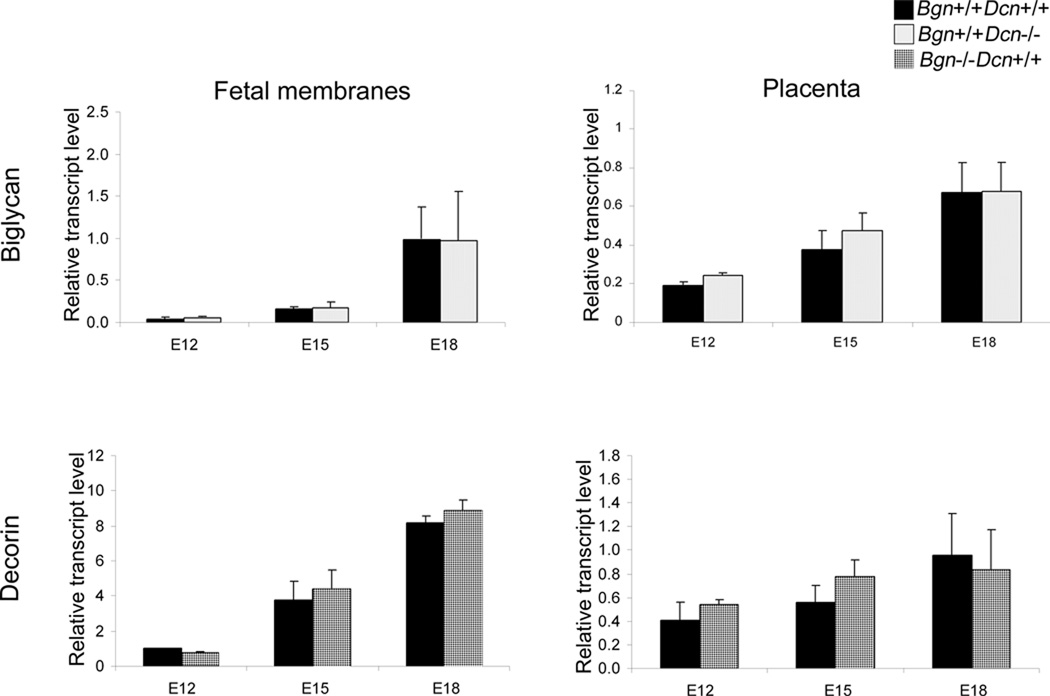
Next, in order to assess whether the protein compensation between biglycan and decorin in the placenta was also present at the transcript level, we delineated gene expression of these SLRPs in mouse gestational tissues during the course of gestation. We measured biglycan mRNA levels in the wild-type and decorin homozygous null mutant and decorin mRNA levels in the wild-type and biglycan homozygous null mutant in placenta and fetal membranes at E12, E15 and E18 via quantitative real time PCR. Each experiment was repeated three times. Our data indicate that biglycan is developmentally regulated in fetal membranes (P=0.017) and placenta (P=0.005), and decorin is developmentally regulated in fetal membranes (P<0.001) but not in the placenta (P=0.188). However, there is no significant difference in biglycan transcript levels in the presence or absence of decorin in either of the gestational tissue types. Likewise, there is no difference in decorin transcript levels in the presence or absence of biglycan in either of the gestational tissue types (Fig. 5).
Figure 5.
Levels of biglycan mRNA in wild-type and decorin homozygous null mutant mouse placenta and fetal membranes, as well as decorin mRNA in wild-type and biglycan homozygous null mutant mouse placenta and fetal membranes were determined using qRT-PCR at progressive gestational ages. Biglycan and decorin are developmentally regulated in fetal membranes (P=0.017 and P<0.001 respectively) and biglycan is developmentally regulated in placenta (P=0.005). Biglycan and decorin do not compensate for each other at the transcript level in the biglycan homozygous null mutant or the decorin homozygous null mutant. Two-way ANOVA using genotype or gestational age as main effects, genotype with gestational age as interaction effect. n=3 samples from 3 pregnant dams per condition. Error bars = SEM. E=embryonic day. Bgn=biglycan; Dcn=decorin.
Finally, when comparing mating frequency (defined as the number of days of mating necessary for the formation of a plug that leads to clinically apparent pregnancy), there were no differences between Bgn−/−Dcn+/+, Bgn+/+Dcn−/− and Bgn−/−Dcn−/− dams compared to Bgn+/+Dcn+/+ dams (P=0.195) (Suppl. Fig. 1A), while the rate of progression from plugging to viable pregnancy demonstrated a significant decrease in the Bgn+/+Dcn−/− and Bgn−/−Dcn−/− dams but not in Bgn−/−Dcn+/+ dams compared to Bgn+/+Dcn+/+ dams (P=0.045, P=0.022 and P=0.153 respectively) (Suppl. Fig. 1B). On comparison of prenatal litter size as well as number of viable versus resorbing embryo implantation sites at E12, E15 and E18, we observed no significant difference in Bgn−/−Dcn+/+ and Bgn+/+Dcn−/− dams compared to Bgn+/+Dcn+/+ dams (P=0.574 and P=0.385 respectively) (Suppl. Fig. 2A and B).
Discussion
To our knowledge, this is the first established mouse model of a genetic mechanism of spontaneous preterm birth. In other models of preterm birth, for example the interleukin-18, interleukin-10 and recombination activating gene 1 knockout models, preterm birth was linked to an environmental insult and was not spontaneous (Robertson et al. 2006, Wang et al. 2006, Bizargity et al. 2009, Thaxton et al. 2009). Furthermore, to our knowledge, this is the first mouse model of genetic preterm birth that displays a correlate to a human disease associated with preterm birth.
The underlying cause of the progeroid variant of Ehlers-Danlos syndrome, a disorder associated with decreased connective tissue tensile strength, is a mutation in the enzyme xylosylprotein-4β-galactosyl-transferase I (Kresse et al. 1987, Quentin et al. 1990). This mutation prevents posttranslational glycosylation of biglycan and decorin, resulting in the secretion of non-glycosylated abnormal biglycan and decorin protein cores (Kresse et al. 1987, Quentin et al. 1990). Infants born with Ehlers-Danlos syndrome have a significantly elevated incidence of preterm birth from preterm premature rupture of fetal membranes in comparison to their unaffected siblings (Barabas 1966). Thus, the biglycan homozygous / decorin homozygous null mutant mouse (Bgn−/−Dcn−/−) is a novel model of human preterm birth caused by genetic predisposition.
The significance of this model extends beyond its utility as a model for preterm birth secondary to the rare Ehlers-Danlos syndrome. Otherwise healthy women with a history of recurrent preterm birth secondary to PPROM of unknown etiology have skin morphology similar to patients with Ehlers-Danlos syndrome (Hermanns-Le et al. 2005). This finding suggests the existence of a spectrum of clinical presentations in which Ehlers-Danlos syndrome with its full scope of connective tissue abnormalities represents the extreme form and phenotypically healthy women with recurrent PPROM represent the mild end of the spectrum. Mutations in biglycan and decorin may be part of undiagnosed predisposing genetic factors for preterm birth. Hence, our model has the potential to provide insight into novel mechanisms that predispose phenotypically healthy women to “idiopathic” PPROM.
Our studies indicate that biglycan and decorin contribute to the attainment of full term gestation in a gene dose dependent manner. The loss of two of the four possible wild-type SLRP alleles in the dam does not change the attainment of full term gestation when compared to the wild-type dam. However, the loss of three wild-type alleles, and even more dramatically, the loss of all four wild-type alleles, results in a significantly increased risk of preterm birth. These observations suggest that the complete absence of both biglycan and decorin wild-type alleles results in dysregulation of processes that are protective of preterm birth.
Decorin plays a role in trophoblast migration (Xu et al. 2002, Iacob et al. 2008). In the placenta, we observed an increase in biglycan expression in the absence of decorin and an increase in decorin expression in the absence of biglycan. Thus, we suggest that the appropriate birth weight in the biglycan homozygous null mutant and decorin homozygous null mutant compared to the biglycan homozygous / decorin homozygous null mutant female, as well as successful gestational outcomes are a function of compensatory SLRP upregulation in the placenta. This is an indication of some functional overlap between the two proteoglycans. Furthermore, the attainment of full term gestation seen in the biglycan homozygous null mutant, decorin homozygous null mutant and the biglycan heterozygous / decorin heterozygous null mutant (Bgn−/−Dcn+/+, Bgn+/+Dcn−/− and Bgn+/−Dcn+/−) compared to the impaired preterm birth reproductive phenotype of the biglycan heterozygous / decorin homozygous null mutant, the biglycan homozygous / decorin heterozygous null mutant and the biglycan homozygous / decorin homozygous null mutant (Bgn−/−Dcn+/−, Bgn+/−Dcn−/− and Bgn−/−Dcn−/−) may also be a function of compensatory SLRP upregulation. This ability to compensate for each other in reproductive tissues is similar to the genetic compensation that has been reported in skin, bone, muscle, kidney and cornea (Ameye & Young 2002, Corsi et al. 2002, Zhang et al. 2009) between biglycan and decorin, as well as in tendon between another set of SLRPs, lumican and fibromodulin (Svensson et al. 1999). Interestingly, we did not observe similar compensatory upregulation of protein expression in the fetal membranes. This could be a result of compensatory mechanisms not being present in the fetal membranes. Alternately, it may be a reflection of the fact that given the abundance of both biglycan and decorin in fetal membranes (Parry & Strauss 1998), a possible compensatory increase may not be appreciable with the techniques utilized.
The compensatory biglycan and decorin protein upregulation we observed in the placenta of decorin homozygous null mutant and biglycan homozygous null mutant dams was not present at the gene transcript level, suggesting that the compensation is secondary to post-transcriptional processes such as increased mRNA stabilization or decreased protein degradation and not increased gene expression. Also, we observed that both biglycan and decorin are developmentally regulated in fetal membranes and biglycan, but not decorin, is developmentally regulated in the placenta. This again suggests an important role in the maintenance of successful gestation, as well as underlining that biglycan and decorin act via discrete mechanisms.
Biglycan homozygous / decorin homozygous null mutant (Bgn−/−Dcn−/−) pups have lower birth weights and a trend toward lower late gestational (E18) weights than their littermate controls, but not lower placental weights. This suggests that the absence of both biglycan and decorin in the pup leads to in utero growth restriction, or conversely, that having at least partial biglycan or decorin competence is protective for appropriate fetal and early postnatal growth. However, biglycan and decorin are not necessary for appropriate placental growth. This observation is consistent with infants born with Ehlers-Danlos syndrome, who display low birth weight (Roop & Brost 1999), as well as with infants with in utero growth restriction, who display decreased placental biglycan and decorin expression (Murthi et al. 2010, Swan et al. 2010). Given that mean litter size decreases between E18 and P1 and subsequently less than the Mendelian expected numbers of biglycan homozygous / decorin homozygous null mutant (Bgn−/−Dcn−/−) pups are present in litters of Bgn+/−Dcn+/− females mated with Bgn−/0Dcn+/− males at P1, we suspect that the same mechanisms that lead to low birth weight also lead to late fetal or early postnatal loss. The genotype of the placenta and fetal membranes reflects that of the fetus and not of the dam, emphasizing the fact that it is the complete absence of both biglycan and decorin in the fetus that is associated with significant growth restriction and not the maternal genotype (Bgn+/−Dcn+/−), which is identical for all littermates.
Beyond their structural roles, both biglycan and decorin have been implicated in a host of signaling pathways that may provide insight into their mechanisms of action in the placenta leading to in utero growth restriction as well as in fetal membranes leading to preterm birth.
Among these pathways are the transforming growth factor β (TGF-β) - bone morphogenetic protein (BMP) - Smad pathway and the Wnt - WISP pathway. TGF-β signals via the Smad family of intracellular transcription factors, demonstrates cross-talk with the Wnt-WISP pathway at a number of levels, and plays a crucial role in embryonic development as well as a multitude of other processes (Guo & Wang 2009). Biglycan and decorin interact with these pathways in a number of ways. Their differential expression is regulated by these growth factors (reviewed in (Kinsella et al. 2004)). They both bind TGF-β and decorin modulates its activity via negative feedback mechanisms (Bassols & Massague 1988, Okuda et al. 1990, Yamaguchi et al. 1990, Border et al. 1992), while both biglycan and decorin bind WISP-1 and modulate its activity (Desnoyers et al. 2001). Also, decorin plays a role in placental trophoblast antimigratory activity (Iacob et al. 2008). Thus, the absence of both biglycan and decorin may lead to dysregulation of placental growth via complex and system-specific interactions within the TGF-β – BMP - Smad and the Wnt - WISP pathways.
Another possible mechanism of biglycan and decorin’s role in in utero growth restriction is their function in anticoagulation. The placentae of infants with in utero growth restriction display decreased biglycan and decorin as well as thrombosis (Murthi et al. 2010, Swan et al. 2010, Fuke et al. 1994), while the glycosaminoglycan chains on biglycan and decorin display anticoagulant activity (He et al. 2008). Thus, the absence of biglycan and decorin in the placenta may lead to thrombosis and fibrosis of the placenta with subsequent in utero growth restriction.
Biglycan is also a ligand for the transmembrane receptors toll-like receptor 2 and 4, thus playing a role in inflammatory cascade activation (Schaefer et al. 2005). Via this pathway, biglycan may play a role in modulating an inflammatory response whose dysregulation may lead to PPROM or in utero growth restriction.
The underlying etiology of the preterm birth in our model is particularly challenging to ascertain. It is unclear whether it is the fetal genotype, the maternal genotype, or a combination of both that is causative of the phenotype. In patients with connective tissue anomalies secondary to Ehlers-Danlos syndrome, preterm birth is caused by PPROM. Changes in collagen content and/or structure have been proposed to play a role in the pathogenesis of PPROM (Parry & Strauss 1998). Biglycan and decorin are highly expressed in fetal membranes (Meinert et al. 2007) and bind collagens in the extracellular matrix (Brown & Vogel 1989, Hildebrand et al. 1994, Wiberg et al. 2001, Wiberg et al. 2003, Chen et al. 2004, Hayashi et al. 2005, Moreno et al. 2005). Thus, we hypothesize that the complete absence of both biglycan and decorin leads to decreased fetal membrane tensile strength and ultimately PPROM as the mechanism for the preterm birth observed in the biglycan homozygous / decorin homozygous null mutant (Bgn−/−Dcn−/−) dams. Additionally, biglycan and decorin are differentially expressed in fetal membranes (Meinert et al. 2007). Decorin decreases prior to parturition as membrane tensile strength decreases, while biglycan increases (Meinert et al. 2007). Thus, given biglycan’s role in signaling in other tissues (Chen et al. 2004, Moreno et al. 2005, Schaefer et al. 2005), it is possible that biglycan has a functional role beyond its biophysical, structural role in fetal membranes.
Another possible mechanism of biglycan and decorin’s role in PPROM involves their interactions with matrix metalloproteinases (MMPs). MMPs are proteases that are involved in extracellular matrix remodeling associated with various physiological and pathological processes (Malemud 2006). A compelling body of evidence suggests that matrix metalloproteinases play a significant role in the pathogenesis of PPROM (Menon & Fortunato 2004, Vadillo-Ortega & Estrada-Gutierrez 2005). The mechanism by which MMPs are thought to contribute to the pathogenesis of PPROM is through the degradation of the extracellular matrix of fetal membranes. Proteoglycans and collagens are present in the fetal membranes (Parry & Strauss 1998). The substrate affinities of MMP-2, -3, -8, -9 and -13, all of which are expressed in fetal membranes, suggest that they target the various collagens as well as the proteoglycans within the fetal membrane extracellular matrix (Vadillo-Ortega & Estrada-Gutierrez 2005). Also, decorin induces MMP-1, -2 and -14 synthesis (Schonherr et al. 2001). Furthermore, biglycan as well as decorin are associated with increased expression of MMP-1 (Huttenlocher et al. 1996). These observations suggest that biglycan and decorin may play a role in the process of tissue remodeling via interactions with MMPs, leading to PPROM.
Another possible mechanism of biglycan and decorin’s role in PPROM involves their interactions with the TGF-β – BMP – Smad signaling pathway described above. The TGF-β – Smad – biglycan/decorin signaling pathway is involved in the development of aortic aneurysms in humans, with Smad2 levels correlating with extracellular matrix elastic fiber destruction (Gomez et al. 2009). Humans with Ehlers-Danlos Syndrome (Barabas 1972), biglycan homozygous null mutant (Bgn−/−Dcn+/+) mice (Heegaard et al. 2007) and biglycan homozygous / decorin heterozygous null mutant mice (Bgn−/−Dcn+/−) (unpublished observations) are at increased risk of developing aortic aneurysm and subsequent rupture. Aneurysms and subsequent aortic rupture are caused by the weakening of the vessel’s connective tissue extracellular matrix; similarly, PPROM is caused by breakdown of fetal membrane connective tissue extracellular matrix. Biglycan is decreased in human aortic aneurysms and decorin is dyslocalized (Tamarina et al. 1998, Theocharis & Karamanos 2002), while biglycan is increased and decorin is decreased in ruptured fetal membranes (Meinert et al. 2007). TGF-β, via its interactions with biglycan and decorin (reviewed in (Kinsella et al. 2004)), is involved in the regulation of collagens and MMPs (Ignotz & Massague 1986, Ma et al. 1999). Aortic rupture and rupture of fetal membranes display pathophysiologic similarities that involve biglycan and decorin. Thus, the TGF-β – Smad – biglycan/decorin signaling pathway may lead to PPROM via interactions with the MMPs and collagens (reviewed in (Kinsella et al. 2004)), mirroring its role in aortic rupture pathophysiology.
Thus, we hypothesize that biglycan and decorin may play a role in reproduction by playing a significant role in maintaining the structural integrity of the fetal membrane extracellular matrix. Dysregulation by physiologic and/or pathologic processes via any or all of the aforementioned structural/signaling pathways may lead to tissue remodeling and subsequently to preterm premature rupture of membranes.
Nonetheless, based on the present data, we cannot rule out mechanisms other than PPROM leading to preterm birth. The maternal biglycan homozygous / decorin homozygous null mutant (Bgn−/−Dcn−/−) genotype may cause uterine or cervical pathology leading to the preterm birth. Alternately, the genotype of the individual pups and their placenta and fetal membranes, be that biglycan homozygous / decorin homozygous null mutant (Bgn−/−Dcn−/−) or others, may be leading to placental pathologies such as preeclampsia or to fetal membrane pathologies such as PPROM or chorioamnionitis, which then lead to preterm birth. Possibly, it is the interaction of both maternal and fetal gestational tissue pathology, each with its own individual genotype, which is leading to the observed preterm birth. Given the genetically complex nature of the maternal and fetal genotypes and thus the variety of possible genotype combinations that contribute to the gestational outcome, future experiments need to be thoughtfully geared towards controlling for the contribution of maternal and individual pup genetics on preterm birth outcomes. This would provide valuable insight into the mechanism by which biglycan and decorin contribute to the attainment of full term gestation.
In summary, we present a novel gene dose dependent mouse model of genetic preterm birth and in utero growth restriction and show that biglycan and decorin exhibit compensatory mechanisms. In addition to preterm birth, the loss of biglycan and decorin results in perinatal fetal loss and low birth weight. Thus, this model presents novel targets for preventive or therapeutic manipulation of preterm birth. We hope that future experiments with this model will allow for mechanistic insight into the initiators of preterm birth, as well as the discrete roles of both biglycan and decorin in gestation.
Materials and Methods
Mouse nomenclature
Biglycan homozygous null mutant: Bgn−/−Dcn+/+ female; Bgn−/0Dcn+/+ male.
Decorin homozygous null mutant: Bgn+/+Dcn−/− female; Bgn+/0Dcn−/− male.
Biglycan heterozygous / decorin heterozygous null mutant: Bgn+/−Dcn+/− female.
Biglycan heterozygous / decorin homozygous null mutant: Bgn+/−Dcn−/− female.
Biglycan homozygous / decorin heterozygous null mutant: Bgn−/−Dcn+/− female; Bgn−/0Dcn+/− male.
Biglycan homozygous / decorin homozygous null mutant: Bgn−/−Dcn−/− female; Bgn−/0Dcn−/− male.
Mouse husbandry
C3H wild-type mice were purchased from Jackson Laboratories (Bar Harbor, ME). A biglycan homozygous null mutant breeding pair (generated by Marian Young(Xu et al. 1998)) was a gift from Justin Fallon. A decorin heterozygous null mutant (Bgn+/+Dcn+/−) breeding pair was mated to the birth of homozygous pups, which were then bred to establish the decorin homozygous null mutant colony. Mice were housed under standard conditions. A biglycan homozygous null mutant female was crossed with a decorin homozygous null mutant male to establish breeding pairs in which the females were heterozygous for both biglycan and decorin (biglycan heterozygous / decorin heterozygous null mutant: Bgn+/−Dcn+/−) and the males were heterozygous for decorin but homozygous knockouts for biglycan (Bgn−/0Dcn+/−), given that biglycan is an X-chromosomal gene. These pairs were mated to breed mixed genotype litters. The Bgn−/0Dcn+/− males were also used for mating with biglycan heterozygous / decorin homozygous null mutant and biglycan homozygous / decorin heterozygous null mutant females (Bgn+/−Dcn−/−, Bgn−/−Dcn+/−) as well as biglycan homozygous / decorin homozygous null mutant females (Bgn−/−Dcn−/−). We chose this breeding strategy given that our observations demonstrate that while males with at least one SLRP allele have mating success that is not significantly different from the wild-type, the biglycan homozygous / decorin homozygous null mutant male (Bgn−/0Dcn−/−) is not capable of producing a pregnancy (data not shown). Breeding pairs were set up in the evening. Plugs were checked the following morning and every morning thereafter. The day of the plug was defined as embryonic day 0. Cages were observed each morning for litters. The morning that a litter was observed was defined as the day of birth. Pairs were set up for mating at 5–7 weeks of age. A subgroup of wild-type, biglycan homozygous null mutant and decorin homozygous null mutant pregnant dams were sacrificed at E12, E15 and E18. Litter size, as well as viable and nonviable embryo numbers were recorded.
Data was collected on the number of days of mating to copulatory plug, on length of pregnancy from plug to birth and on litter size. Pups were weighed at P1. Mixed genotype litters were tattooed for identification after weighing, then tail snipped at P4-7 for genotyping. A subgroup of the biglycan heterozygous / decorin heterozygous null mutant pregnant dams (Bgn+/−Dcn+/−) was sacrificed at E18. Pup and placental wet weight as well as litter size were recorded. Pups were tail snipped for genotyping. IACUC approval was obtained.
Sample size
Figure 1Preterm birth per genotype Pregnant dams: Bgn+/+Dcn+/+ n=13; Bgn−/−Dcn+/+ n=33; Bgn+/+Dcn−/− n=11; Bgn+/−Dcn+/− n=14; Bgn+/−Dcn−/− n=20; Bgn−/−Dcn+/− n=30; Bgn−/−Dcn−/− n=7
Table 1Preterm birth per allele Pregnant dams: 4 SLRP wild-type allele dams n=13; 2 SLRP wild-type allele dams n=55; 1 SLRP wild-type allele dams n=50; 0 SLRP wild-type allele dams n=7
Figure 2ALength of gestation Pregnant dams: Bgn+/+Dcn+/+ n=13; Bgn−/−Dcn+/+ n=33; Bgn+/+Dcn−/− n=11; Bgn+/−Dcn+/− n=13; Bgn+/−Dcn−/− n=14; Bgn−/−Dcn+/− n=20; Bgn−/−Dcn−/− n=7
Figure 2BLitter size Pregnant dams: Bgn+/+Dcn+/+ n=11; Bgn−/−Dcn+/+ n=17; Bgn+/+Dcn−/− n=32; Bgn+/−Dcn+/− n=54; Bgn+/−Dcn−/− n=34; Bgn−/−Dcn+/− n=19; Bgn−/−Dcn−/− n=7
Figure 3APup weight at P1 Pregnant Bgn+/−Dcn+/− dams: n=12; Litters: 14; Total pups in litters: 93; Bgn+/+Dcn+/+ n=7; Bgn+/+Dcn+/− n=16; Bgn+/+Dcn−/− n=14; Bgn+/−Dcn+/+ n=4; Bgn+/−Dcn+/− n=7; Bgn+/−Dcn−/− n=2; Bgn−/−Dcn+/+ n=13; Bgn−/−Dcn+/− n=25; Bgn−/−Dcn−/− n=5
Figure 3BPup weight at E18 Pregnant Bgn+/−Dcn+/− dams: n=7; Total pups in litters 63: Bgn+/+Dcn+/+ n=2; Bgn+/+Dcn+/− n=8; Bgn+/+Dcn−/− n=5; Bgn+/−Dcn+/+ n=9; Bgn+/−Dcn+/− n=8; Bgn+/−Dcn−/− n=5; Bgn−/−Dcn+/+ n=6; Bgn−/−Dcn+/− n=15; Bgn−/−Dcn−/− n=5
Figure 3CPlacenta weight at E18 Pregnant Bgn+/−Dcn+/− dams: n=10; Total pups in litters 93: Bgn+/+Dcn+/+ n=8; Bgn+/+Dcn+/− n=11; Bgn+/+Dcn−/− n=6; Bgn+/−Dcn+/+ n=5; Bgn+/−Dcn+/− n=16; Bgn+/−Dcn−/− n=5; Bgn−/−Dcn+/+ n=7; Bgn−/−Dcn+/− n=20; Bgn−/−Dcn−/− n=15
Table 2AE18 litter size Pregnant Bgn+/−Dcn+/− dams: n=10; Total pups in litters n=93: Bgn+/+Dcn+/+ n=8; Bgn+/+Dcn+/− n=11; Bgn+/+Dcn−/− n=6; Bgn+/−Dcn+/+ n=5; Bgn+/−Dcn+/− n=16; Bgn+/−Dcn−/− n=5; Bgn−/−Dcn+/+ n=7; Bgn−/−Dcn+/− n=20; Bgn−/−Dcn−/− n=15
Table 2AP1 litter size Pregnant Bgn+/−Dcn+/− dams: n=12; Total litters: 14; Total pups in litters n=93: Bgn+/+Dcn+/+ n=7; Bgn+/+Dcn+/− n=16; Bgn+/+Dcn−/− n=14; Bgn+/−Dcn+/+ n=4; Bgn+/−Dcn+/− n=7; Bgn+/−Dcn−/− n=2; Bgn−/−Dcn+/+ n=13; Bgn−/−Dcn+/− n=25; Bgn−/−Dcn−/− n=5
Table 2BMendelian distribution of genotype at E18 Pregnant Bgn+/−Dcn+/− dams: n=10; Total pups in litters n=93: Bgn+/+Dcn+/+ n=8; Bgn+/+Dcn+/− n=11; Bgn+/+Dcn−/− n=6; Bgn+/−Dcn+/+ n=5; Bgn+/−Dcn+/− n=16; Bgn+/−Dcn−/− n=5; Bgn−/−Dcn+/+ n=7; Bgn−/−Dcn+/− n=20; Bgn−/−Dcn−/− n=15
Table 2BMendelian distribution of genotype at P1 Pregnant Bgn+/−Dcn+/− dams n=20; Total pups in litters n=134: Bgn+/0Dcn+/+ n=10; Bgn+/0Dcn+/− n=19; Bgn+/0Dcn−/− n=5; Bgn+/−Dcn+/+ n=16; Bgn+/−Dcn+/− n=31; Bgn+/−Dcn−/− n=8; Bgn−/−Dcn+/+ n=13; Bgn−/−Dcn+/− n=28; Bgn−/−Dcn−/− n=4
Figure 4Protein localization Pregnant dams: Bgn+/+Dcn+/+ n=3; Bgn−/−Dcn+/+ n=3; Bgn+/+Dcn−/− n=3; One placenta and one fetal membrane sample was used from each pregnant dam.
Figure 5Gene expression Pregnant dams: Bgn+/+Dcn+/+ n=3; Bgn−/−Dcn+/+ n=3; Bgn+/+Dcn−/− n=3; One placenta and one fetal membrane sample was used from each pregnant dam.
Supplementary Figure 1ADays to plug formation Pregnant dams: Bgn+/+Dcn+/+ n=26; Bgn−/−Dcn+/+ n=25; Bgn+/+Dcn−/− n=27; Bgn−/−Dcn−/− n=7
Supplementary Figure 1BProgression of plug to pregnancy Pregnant dams: Bgn+/+Dcn+/+ n=27; Bgn−/−Dcn+/+ n=31; Bgn+/+Dcn−/− n=37; Bgn−/−Dcn−/− n=12
Supplementary Figure 2APrenatal litter size Pregnant dams at E12: Bgn+/+Dcn+/+ n=4; Bgn−/−Dcn+/+ n=5; Bgn+/+Dcn−/− n=7; Pregnant dams at E15: Bgn+/+Dcn+/+ n=4; Bgn−/−Dcn+/+ n=5; Bgn+/+Dcn−/− n=6; Pregnant dams at E18: Bgn+/+Dcn+/+ n=3; Bgn−/−Dcn+/+ n=4; Bgn+/+Dcn−/− n=6
Supplementary Figure 2BResorbed implantation sites Pregnant dams at E12: Bgn+/+Dcn+/+ n=4; Bgn−/−Dcn+/+ n=5; Bgn+/+Dcn−/− n=7; Pregnant dams at E15: Bgn+/+Dcn+/+ n=4; Bgn−/−Dcn+/+ n=5; Bgn+/+Dcn−/− n=6; Pregnant dams at E18: Bgn+/+Dcn+/+ n=3; Bgn−/−Dcn+/+ n=4; Bgn+/+Dcn−/− n=6
Genotyping
A 3-mm tail biopsy specimen was obtained for each pup within a mixed genotype litter at P4-7 or at weaning. Genomic DNA was extracted from each tail biopsy sample using the High Pure PCR Template Preparation Kit (Roche, Mannheim, Germany). Polymerase chain reaction (PCR) was performed to identify the decorin and biglycan alleles using the Taq DNA Polymerase kit (New England Biolabs, Ipswich, MA) and the PTC-200 thermal cycler. The PCR product was run on a 1.8% w/v agarose gel to visualize the following diagnostic bands. The decorin PCR produced bands of 161 bp for the wild-type allele and 238 bp for the knockout allele. The biglycan PCR produced bands of 212 bp and 310 bp for the wild-type and knockout alleles, respectively.
RNA and cDNA preparation
Wild-type, biglycan homozygous null mutant and decorin homozygous null mutant mouse placenta and fetal membranes were dissected at three prenatal timepoints (E12, E15 and E18) in 0.1mol l−1 phosphate-buffered saline, pH 7.4, snap-frozen in liquid nitrogen and stored at −80°C. RNA extraction was performed using the Trizol method (Invitrogen, Carlsbad, CA). Genomic DNA was removed by incubating the RNA sample with DNase I (Invitrogen, Carlsbad, CA) for 30 min at 37°C with subsequent RNA re-extraction with Trizol. The purified RNA was converted to cDNA using the Superscript III First-Strand Synthesis System Kit (Invitrogen, Carlsbad, CA).
Quantitative PCR analysis
qPCR reactions were performed on the ABI PRISM 7000 real-time thermocycler (Applied Biosystems, Foster City, CA) and on the Eppendorf Mastercycler epgradient S (Eppendorf, Hamburg, Germany) using the SYBR-Green method (Invitrogen, Carlsbad, CA). Primers were designed using Primer-Blast primer design software (National Library of Medicine, Bethesda, MD). GAPDH was used as a normalizer. Melting point analysis of the product was performed to ensure the absence of alternative products or primer dimers. Data analysis was performed using the comparative Ct method with a validation experiment. qPCR analysis was performed in triplicate. n=3 samples from 3 dams per genotype.
Primer sequences
qPCR biglycan forward: ATTGCCCTACCCAGAACTTGAC; qPCR biglycan reverse: GCAGAGTATGAACCCTTTCCTG; qPCR decorin forward: TTCCTACTCGGCTGTGAGTC; qPCR decorin reverse: AAGTTGAATGGCAGAACGC; qPCR GAPDH forward: CTCACAATTTCCATCCCAGAC; qPCR GAPDH reverse: TTTTTGGGTGCAGCGAAC; biglycan genotyping PCR wild-type allele forward TGATGAGGAGGCTTCAGGTT; biglycan genotyping PCR wild-type allele reverse GCAGTGTGGTGTCAGGTGAG; biglycan genotyping PCR knockout allele forward TGTGGCTACTCACCTTGCTG; biglycan genotyping PCR knockout allele reverse GCCAGAGGCCACTTGTGTAG; decorin genotyping PCR allele forward CCTTCTGGCACAAGTCTCTTGG; decorin genotyping PCR wild-type allele reverse TCGAAGATGACACTGGCATCGG; decorin genotyping PCR knockout allele reverse TGGATGTGGAATGTGTGCGAG. All primers were provided by Invitrogen (Carlsbad, CA).
Immunohistochemistry
Wild-type, biglycan homozygous null mutant and decorin homozygous null mutant mouse placenta and fetal membranes were dissected at three prenatal timepoints (E12, E15 and E18) in 0.1 mol l−1 phosphate-buffered saline, pH 7.4. The specimens were then flash frozen in isopentane and stored at −80°C. The frozen tissue was cryostat sectioned to 10 µm thickness, mounted on slides and stored at −20°C. Sections were fixed in 1% v/v paraformaldehyde and stained with primary antibodies using the Mouse on Mouse immunostaining kit for monoclonal antibodies (Vector Laboratories, Burlingame, CA), then incubated with primary antibody overnight at 4°C and with secondary antibody for 30 min at room temperature. Sections were mounted with Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Fluorescent microscopy to evaluate the samples was performed using an inverted stage Nikon Eclipse TE2000-E microscope equipped with epifluorescent filters and a Nikon Plan Apo 20× and 40× and a Plan Fluor 10× objective lens (Yokohama, Japan). Images were acquired using a Coolsnap HQ cooled CCD camera (Roper Scientific, Ottobrunn, Germany) and MetaVue software (Molecular Devices, Downingtown, PA). Experiments were repeated three times with tissue samples from three dams per genotype.
Antibodies
The following antibodies were used: polyclonal rabbit anti-mouse biglycan antibody LF-159 (a gift from Larry Fisher), polyclonal goat anti-mouse decorin antibody (R&D Systems, Minneapolis, MN). Secondary antibody labeling was performed with goat anti-rabbit IgG conjugated to Alexa 488 (Invitrogen, Carlsbad, CA) for biglycan and rabbit anti-goat IgG conjugated to CY3 (Sigma-Aldrich, St. Louis, MO) for decorin. Mouse IgG and rabbit IgG (Vector Laboratories, Burlingame, CA), respectively, were used as controls.
Quantitative immunofluorescence analysis
Images of tissue sections were analyzed as follows: Between 5 and 15 images per sample were collected using constant exposure and microscope settings. Images were analyzed using MetaVue software (Molecular Devices, Downingtown, PA). Image sets were thresholded to remove background and the integrated intensity was calculated for each image and the average was calculated for each sample. Error bars represent Standard Error of the Mean. For both placenta and fetal membranes, decorin immunofluorescence of wild-type was compared to the biglycan homozygous null mutant and biglycan immunofluorescence of wild-type was compared to the decorin homozygous null mutant. Image analysis was repeated on placenta and fetal membrane samples from three pregnant females per genotype and antibody.
Statistical analysis
The occurrence of preterm births across genotypes was analyzed using the chi-square test, and the relationship of preterm birth to number of alleles was analyzed using the chi-square for linear trend test. Analysis of gestational length and prenatal litter size was conducted using one-way ANOVA with a planned contrast of all genotypes versus wild-type using the Holm-Sidak method. Comparison of the number of biglycan homozygous / decorin homozygous null mutant pups with the Mendelian expected number was done using the one way chi-square test. Analysis of pre- and postnatal pup weights and placental weights was conducted with mixed models, with genotype as a fixed effect and litter as a random effect, in order to control for the correlation of measures within litters. Specific planned contrasts were also done in these models, comparing biglycan homozygous / decorin homozygous null mutant pups to all other genotypes. Analysis of E18 vs. P1 litter size as well as average integrated fluorescence intensity was performed using the Student’s t-test. Analysis of relative ratios was done with two-way ANOVA using genotype and gestational age as main effects, as well as a genotype × gestational age interaction effect. Analysis of days to plug formation was done with Cox regression with robust sandwich covariance matrix estimate to adjust for clustered data per mother. Analysis of progression of plug to pregnancy was done with Poisson regression with generalized estimating equations to adjust for correlated observations. Analysis of resorbed implantation sites was done with negative binomial regression. The software used for these analyses was Sigmaplot version 11.0 and SAS version 9.1.
Supplementary Material
Supplementary Figure 1 (A) Mean number of days from pairing to plug formation according to genotype. There was no difference in the number of days for wild-type females, biglycan homozygous null mutants, decorin homozygous null mutants, or biglycan homozygous/decorin homozygous null mutants. Cox regression. Bgn+/+Dcn+/+ n=26; Bgn−/−Dcn+/+ n=25; Bgn+/+Dcn−/− n=27; Bgn−/−Dcn−/− n=7. Error bars = SEM. (B) Rate of progression of copulatory plug to viable pregnancy. There is no difference in the progression from plug to viable pregnancy in biglycan homozygous null mutant females compared to wild-type females. There is a significant decrease in progression for both decorin homozygous null mutants (P=0.045) and biglycan homozygous/decorin homozygous null mutants (P=0.022) compared to wild-type females. Poisson regression. Bgn+/+Dcn+/+ n=27; Bgn−/−Dcn+/+ n=31; Bgn+/+Dcn−/− n=37; Bgn−/−Dcn−/− n=12. Bgn=biglycan; Dcn=decorin.
Supplementary Figure 2 (A) Prenatal litter size per genotype at E12, E15, and E18. There is no difference in litter size for wild-type dams, biglycan homozygous null mutant dams, or decorin homozygous null mutant dams at these gestational ages. Two-way ANOVA using genotype or gestational age as main effects, genotype with gestational age as interaction effect. (B) Percent of resorbed implantation sites per genotype at E12, E15, and E18. There is no difference in the number of resorbed versus viable implantation sites for wild-type dams, biglycan homozygous null mutant dams, or decorin homozygous null mutant dams at these gestational ages. Negative binomial regression. Pregnant dams at E12: Bgn+/+Dcn+/+ n=4; Bgn−/−Dcn+/+ n=5; Bgn+/+Dcn−/− n=7; Pregnant dams at E15: Bgn+/+Dcn+/+ n=4; Bgn−/−Dcn+/+ n=5; Bgn+/+Dcn−/− n=6; Pregnant dams at E18: Bgn+/+Dcn+/+ n=3; Bgn−/−Dcn+/+ n=4; Bgn+/+Dcn−/− n=6. E = embryonic day. Error bars = SEM. Bgn=biglycan; Dcn=decorin.
Acknowledgments
We thank Larry Fisher for the generous gift of the anti-biglycan antibodies; Justin Fallon for generously providing a biglycan knockout mouse breeding pair; Sunil Shaw, Joseph Bliss and Edward Chien for valuable discussions.
Funding
This work was supported by NICHD 1K08HD054676, NCRR 5P20RR018728-08 and NCI R01CA39481.
Footnotes
Declaration of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Disclaimer. This is not the definitive version of record of this article. This manuscript has been accepted for publication in Reproduction, but the version presented here has not yet been copy edited, formatted or proofed. Consequently, the journal accepts no responsibility for any errors or omissions it may contain. The definitive version is available at [insert DOI link][insert year of publication] Society for Reproduction and Fertility.
References
- Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107R–116R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med. 2006;19:773–782. doi: 10.1080/14767050600965882. [DOI] [PubMed] [Google Scholar]
- Arikat S, Novince RW, Mercer BM, Kumar D, Fox JM, Mansour JM, Moore JJ. Separation of amnion from choriodecidua is an integral event to the rupture of normal term fetal membranes and constitutes a significant component of the work required. Am J Obstet Gynecol. 2006;194:211–217. doi: 10.1016/j.ajog.2005.06.083. [DOI] [PubMed] [Google Scholar]
- Barabas AP. Ehlers-Danlos syndrome: associated with prematurity and premature rupture of foetal membranes; possible increase in incidence. Br Med J. 1966;5515:682–684. doi: 10.1136/bmj.2.5515.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabas AP. Vascular complications in the Ehlers-Danlos syndrome, with special reference to the "arterial type" or Sack's syndrome. J Cardiovasc Surg (Torino) 1972;13:160–167. [PubMed] [Google Scholar]
- Bassols A, Massague J. Transforming growth factor beta regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J Biol Chem. 1988;263:3039–3045. [PubMed] [Google Scholar]
- Bizargity P, Del Rio R, Phillippe M, Teuscher C, Bonney EA. Resistance to lipopolysaccharide-induced preterm delivery mediated by regulatory T cell function in mice. Biol Reprod. 2009;80:874–881. doi: 10.1095/biolreprod.108.074294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992;360:361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- Bowe MA, Mendis DB, Fallon JR. The small leucine-rich repeat proteoglycan biglycan binds to alpha-dystroglycan and is upregulated in dystrophic muscle. J Cell Biol. 2000;148:801–810. doi: 10.1083/jcb.148.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DC, Vogel KG. Characteristics of the in vitro interaction of a small proteoglycan (PG II) of bovine tendon with type I collagen. Matrix. 1989;9:468–478. doi: 10.1016/s0934-8832(11)80016-8. [DOI] [PubMed] [Google Scholar]
- Chen CP, Chang SC, Vivian Yang WC. High glucose alters proteoglycan expression and the glycosaminoglycan composition in placentas of women with gestational diabetes mellitus and in cultured trophoblasts. Placenta. 2007;28:97–106. doi: 10.1016/j.placenta.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Chen XD, Fisher LW, Robey PG, Young MF. The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. Faseb J. 2004;18:948–958. doi: 10.1096/fj.03-0899com. [DOI] [PubMed] [Google Scholar]
- Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17:1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnoyers L, Arnott D, Pennica D. WISP-1 binds to decorin and biglycan. J Biol Chem. 2001;276:47599–47607. doi: 10.1074/jbc.M108339200. [DOI] [PubMed] [Google Scholar]
- El Khwad M, Pandey V, Stetzer B, Mercer BM, Kumar D, Moore RM, Fox J, Redline RW, Mansour JM, Moore JJ. Fetal membranes from term vaginal deliveries have a zone of weakness exhibiting characteristics of apoptosis and remodeling. J Soc Gynecol Investig. 2006;13:191–195. doi: 10.1016/j.jsgi.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Fuke Y, Aono T, Imai S, Suehara N, Fujita T, Nakayama M. Clinical significance and treatment of massive intervillous fibrin deposition associated with recurrent fetal growth retardation. Gynecol Obstet Invest. 1994;38:5–9. doi: 10.1159/000292434. [DOI] [PubMed] [Google Scholar]
- Gogiel T, Bankowski E, Jaworski S. Proteoglycans of Wharton's jelly. Int J Biochem Cell Biol. 2003;35:1461–1469. doi: 10.1016/s1357-2725(03)00128-6. [DOI] [PubMed] [Google Scholar]
- Gogiel T, Jaworski S. Proteoglycans of human umbilical cord arteries. Acta Biochim Pol. 2000;47:1081–1091. [PubMed] [Google Scholar]
- Gomez D, Al Haj Zen A, Borges LF, Philippe M, Gutierrez PS, Jondeau G, Michel JB, Vranckx R. Syndromic and non-syndromic aneurysms of the human ascending aorta share activation of the Smad2 pathway. J Pathol. 2009;218:131–142. doi: 10.1002/path.2516. [DOI] [PubMed] [Google Scholar]
- Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Liu CY, Jester JJ, Hayashi M, Wang IJ, Funderburgh JL, Saika S, Roughley PJ, Kao CW, Kao WW. Excess biglycan causes eyelid malformation by perturbing muscle development and TGF-alpha signaling. Dev Biol. 2005;277:222–234. doi: 10.1016/j.ydbio.2004.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Giri TK, Vicente CP, Tollefsen DM. Vascular dermatan sulfate regulates the antithrombotic activity of heparin cofactor II. Blood. 2008;111:4118–4125. doi: 10.1182/blood-2007-12-127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heegaard AM, Corsi A, Danielsen CC, Nielsen KL, Jorgensen HL, Riminucci M, Young MF, Bianco P. Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation. 2007;115:2731–2738. doi: 10.1161/CIRCULATIONAHA.106.653980. [DOI] [PubMed] [Google Scholar]
- Hermanns-Le T, Pierard G, Quatresooz P. Ehlers-Danlos-like dermal abnormalities in women with recurrent preterm premature rupture of fetal membranes. Am J Dermatopathol. 2005;27:407–410. doi: 10.1097/01.dad.0000175529.42615.42. [DOI] [PubMed] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302(Pt 2):527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelm AM, Barchan K, Malmstrom A, Ekman-Ordeberg GE. Changes of the uterine proteoglycan distribution at term pregnancy and during labour. Eur J Obstet Gynecol Reprod Biol. 2002;100:146–151. doi: 10.1016/s0301-2115(01)00476-6. [DOI] [PubMed] [Google Scholar]
- Iacob D, Cai J, Tsonis M, Babwah A, Chakraborty C, Bhattacharjee RN, Lala PK. Decorin-mediated inhibition of proliferation and migration of the human trophoblast via different tyrosine kinase receptors. Endocrinology. 2008;149:6187–6197. doi: 10.1210/en.2008-0780. [DOI] [PubMed] [Google Scholar]
- Ignotz RA, Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- Iozzo RV, Murdoch AD. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. Faseb J. 1996;10:598–614. [PubMed] [Google Scholar]
- Kinsella MG, Bressler SL, Wight TN. The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Crit Rev Eukaryot Gene Expr. 2004;14:203–234. doi: 10.1615/critreveukaryotgeneexpr.v14.i3.40. [DOI] [PubMed] [Google Scholar]
- Kresse H, Rosthoj S, Quentin E, Hollmann J, Glossl J, Okada S, Tonnesen T. Glycosaminoglycan-free small proteoglycan core protein is secreted by fibroblasts from a patient with a syndrome resembling progeroid. Am J Hum Genet. 1987;41:436–453. [PMC free article] [PubMed] [Google Scholar]
- Ma C, Tarnuzzer RW, Chegini N. Expression of matrix metalloproteinases and tissue inhibitor of matrix metalloproteinases in mesothelial cells and their regulation by transforming growth factor-beta1. Wound Repair Regen. 1999;7:477–485. doi: 10.1046/j.1524-475x.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–1701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- Meinert M, Eriksen GV, Petersen AC, Helmig RB, Laurent C, Uldbjerg N, Malmstrom A. Proteoglycans and hyaluronan in human fetal membranes. Am J Obstet Gynecol. 2001;184:679–685. doi: 10.1067/mob.2001.110294. [DOI] [PubMed] [Google Scholar]
- Meinert M, Malmstrom A, Tufvesson E, Westergren-Thorsson G, Petersen AC, Laurent C, Uldbjerg N, Eriksen GV. Labour induces increased concentrations of biglycan and hyaluronan in human fetal membranes. Placenta. 2007;28:482–486. doi: 10.1016/j.placenta.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Menon R, Fortunato SJ. The role of matrix degrading enzymes and apoptosis in rupture of membranes. J Soc Gynecol Investig. 2004;11:427–437. doi: 10.1016/j.jsgi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Moore RM, Mansour JM, Redline RW, Mercer BM, Moore JJ. The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta. 2006;27:1037–1051. doi: 10.1016/j.placenta.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Moreno M, Munoz R, Aroca F, Labarca M, Brandan E, Larrain J. Biglycan is a new extracellular component of the Chordin-BMP4 signaling pathway. Embo J. 2005;24:1397–1405. doi: 10.1038/sj.emboj.7600615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthi P, Faisal FA, Rajaraman G, Stevenson J, Ignjatovic V, Monagle PT, Brennecke SP, Said JM. Placental biglycan expression is decreased in human idiopathic fetal growth restriction. Placenta. 2010;31:712–717. doi: 10.1016/j.placenta.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Okuda S, Languino LR, Ruoslahti E, Border WA. Elevated expression of transforming growth factor-beta and proteoglycan production in experimental glomerulonephritis. Possible role in expansion of the mesangial extracellular matrix. J Clin Invest. 1990;86:453–462. doi: 10.1172/JCI114731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry S, Strauss JF., 3rd Premature rupture of the fetal membranes. N Engl J Med. 1998;338:663–670. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- Quentin E, Gladen A, Roden L, Kresse H. A genetic defect in the biosynthesis of dermatan sulfate proteoglycan: galactosyltransferase I deficiency in fibroblasts from a patient with a progeroid syndrome. Proc Natl Acad Sci U S A. 1990;87:1342–1346. doi: 10.1073/pnas.87.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19:249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Skinner RJ, Care AS. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J Immunol. 2006;177:4888–4896. doi: 10.4049/jimmunol.177.7.4888. [DOI] [PubMed] [Google Scholar]
- Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- Roop KA, Brost BC. Abnormal presentation in labor and fetal growth of affected infants with type III Ehlers-Danlos syndrome. Am J Obstet Gynecol. 1999;181:752–753. doi: 10.1016/s0002-9378(99)70524-7. [DOI] [PubMed] [Google Scholar]
- San Martin S, Soto-Suazo M, De Oliveira SF, Aplin JD, Abrahamsohn P, Zorn TM. Small leucine-rich proteoglycans (SLRPs) in uterine tissues during pregnancy in mice. Reproduction. 2003;125:585–595. doi: 10.1530/rep.0.1250585. [DOI] [PubMed] [Google Scholar]
- San Martin S, Zorn TM. The small proteoglycan biglycan is associated with thick collagen fibrils in the mouse decidua. Cell Mol Biol (Noisy-le-grand) 2003;49:673–678. [PubMed] [Google Scholar]
- Sanches JC, Jones CJ, Aplin JD, Iozzo RV, Zorn TM, Oliveira SF. Collagen fibril organization in the pregnant endometrium of decorin-deficient mice. J Anat. 2010;216:144–155. doi: 10.1111/j.1469-7580.2009.01170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Gotte M, Malle E, Schaefer RM, Grone HJ. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonherr E, Schaefer L, O'Connell BC, Kresse H. Matrix metalloproteinase expression by endothelial cells in collagen lattices changes during co-culture with fibroblasts and upon induction of decorin expression. J Cell Physiol. 2001;187:37–47. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1048>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Steer P. The epidemiology of preterm labour. Bjog. 2005;112 Suppl 1:1–3. doi: 10.1111/j.1471-0528.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- Svensson L, Aszodi A, Reinholt FP, Fassler R, Heinegard D, Oldberg A. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem. 1999;274:9636–9647. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- Swan BC, Murthi P, Rajaraman G, Pathirage NA, Said JM, Ignjatovic V, Monagle PT, Brennecke SP. Decorin expression is decreased in human idiopathic fetal growth restriction. Reprod Fertil Dev. 2010;22:949–955. doi: 10.1071/RD09240. [DOI] [PubMed] [Google Scholar]
- Tamarina NA, Grassi MA, Johnson DA, Pearce WH. Proteoglycan gene expression is decreased in abdominal aortic aneurysms. J Surg Res. 1998;74:76–80. doi: 10.1006/jsre.1997.5201. [DOI] [PubMed] [Google Scholar]
- Thaxton JE, Romero R, Sharma S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J Immunol. 2009;183:1144–1154. doi: 10.4049/jimmunol.0900788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theocharis AD, Karamanos NK. Decreased biglycan expression and differential decorin localization in human abdominal aortic aneurysms. Atherosclerosis. 2002;165:221–230. doi: 10.1016/s0021-9150(02)00231-9. [DOI] [PubMed] [Google Scholar]
- Vadillo-Ortega F, Estrada-Gutierrez G. Role of matrix metalloproteinases in preterm labour. Bjog. 2005;112 Suppl 1:19–22. doi: 10.1111/j.1471-0528.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- Valiyaveettil M, Achur RN, Muthusamy A, Gowda DC. Characterization of chondroitin sulfate and dermatan sulfate proteoglycans of extracellular matrices of human umbilical cord blood vessels and Wharton's jelly. Glycoconj J. 2004;21:361–375. doi: 10.1023/B:GLYC.0000046276.77147.b2. [DOI] [PubMed] [Google Scholar]
- Wadhwa S, Embree MC, Bi Y, Young MF. Regulation, regulatory activities, and function of biglycan. Crit Rev Eukaryot Gene Expr. 2004;14:301–315. doi: 10.1615/critreveukaryotgeneexpr.v14.i4.50. [DOI] [PubMed] [Google Scholar]
- Wang X, Hagberg H, Mallard C, Zhu C, Hedtjarn M, Tiger CF, Eriksson K, Rosen A, Jacobsson B. Disruption of interleukin-18, but not interleukin-1, increases vulnerability to preterm delivery and fetal mortality after intrauterine inflammation. Am J Pathol. 2006;169:967–976. doi: 10.2353/ajpath.2006.050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg C, Hedbom E, Khairullina A, Lamande SR, Oldberg A, Timpl R, Morgelin M, Heinegard D. Biglycan and decorin bind close to the n-terminal region of the collagen VI triple helix. J Biol Chem. 2001;276:18947–18952. doi: 10.1074/jbc.M100625200. [DOI] [PubMed] [Google Scholar]
- Wiberg C, Klatt AR, Wagener R, Paulsson M, Bateman JF, Heinegard D, Morgelin M. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J Biol Chem. 2003;278:37698–37704. doi: 10.1074/jbc.M304638200. [DOI] [PubMed] [Google Scholar]
- Xu G, Guimond MJ, Chakraborty C, Lala PK. Control of proliferation, migration, and invasiveness of human extravillous trophoblast by decorin, a decidual product. Biol Reprod. 2002;67:681–689. doi: 10.1095/biolreprod67.2.681. [DOI] [PubMed] [Google Scholar]
- Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S, Bonadio J, Boskey A, Heegaard AM, Sommer B, Satomura K, Dominguez P, Zhao C, Kulkarni AB, Robey PG, Young MF. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet. 1998;20:78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- Yen JL, Lin SP, Chen MR, Niu DM. Clinical features of Ehlers-Danlos syndrome. J Formos Med Assoc. 2006;105:475–480. doi: 10.1016/S0929-6646(09)60187-X. [DOI] [PubMed] [Google Scholar]
- Young MF, Bi Y, Ameye L, Chen XD. Biglycan knockout mice: new models for musculoskeletal diseases. Glycoconj J. 2002;19:257–262. doi: 10.1023/A:1025336114352. [DOI] [PubMed] [Google Scholar]
- Zhang G, Chen S, Goldoni S, Calder BW, Simpson HC, Owens RT, McQuillan DJ, Young MF, Iozzo RV, Birk DE. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem. 2009;284:8888–8897. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 (A) Mean number of days from pairing to plug formation according to genotype. There was no difference in the number of days for wild-type females, biglycan homozygous null mutants, decorin homozygous null mutants, or biglycan homozygous/decorin homozygous null mutants. Cox regression. Bgn+/+Dcn+/+ n=26; Bgn−/−Dcn+/+ n=25; Bgn+/+Dcn−/− n=27; Bgn−/−Dcn−/− n=7. Error bars = SEM. (B) Rate of progression of copulatory plug to viable pregnancy. There is no difference in the progression from plug to viable pregnancy in biglycan homozygous null mutant females compared to wild-type females. There is a significant decrease in progression for both decorin homozygous null mutants (P=0.045) and biglycan homozygous/decorin homozygous null mutants (P=0.022) compared to wild-type females. Poisson regression. Bgn+/+Dcn+/+ n=27; Bgn−/−Dcn+/+ n=31; Bgn+/+Dcn−/− n=37; Bgn−/−Dcn−/− n=12. Bgn=biglycan; Dcn=decorin.
Supplementary Figure 2 (A) Prenatal litter size per genotype at E12, E15, and E18. There is no difference in litter size for wild-type dams, biglycan homozygous null mutant dams, or decorin homozygous null mutant dams at these gestational ages. Two-way ANOVA using genotype or gestational age as main effects, genotype with gestational age as interaction effect. (B) Percent of resorbed implantation sites per genotype at E12, E15, and E18. There is no difference in the number of resorbed versus viable implantation sites for wild-type dams, biglycan homozygous null mutant dams, or decorin homozygous null mutant dams at these gestational ages. Negative binomial regression. Pregnant dams at E12: Bgn+/+Dcn+/+ n=4; Bgn−/−Dcn+/+ n=5; Bgn+/+Dcn−/− n=7; Pregnant dams at E15: Bgn+/+Dcn+/+ n=4; Bgn−/−Dcn+/+ n=5; Bgn+/+Dcn−/− n=6; Pregnant dams at E18: Bgn+/+Dcn+/+ n=3; Bgn−/−Dcn+/+ n=4; Bgn+/+Dcn−/− n=6. E = embryonic day. Error bars = SEM. Bgn=biglycan; Dcn=decorin.