Abstract
Background
Sepsis impairs hypoxic pulmonary vasoconstriction (HPV) in patients and animal models contributing to systemic hypoxemia. Levels of cysteinyl leukotrienes are elevated in the bronchoalveolar lavage fluid of patients with sepsis, but the contribution of cysteinyl leukotrienes to the impairment of HPV is uncertain.
Methods
Wild-type mice, mice deficient in leukotriene C4 synthase, the enzyme responsible for cysteinyl leukotriene synthesis, and mice deficient in cysteinyl leukotriene receptor 1 were studied at 18 h after challenge with either saline or endotoxin. HPV was measured by the increase in left pulmonary vascular resistance induced by left mainstem bronchus occlusion. Levels of cysteinyl leukotrienes were determined in the bronchoalveolar lavage fluid.
Results
In the bronchoalveolar lavage fluid of all three strains, cysteinyl leukotrienes were not detectable after saline challenge; whereas endotoxin challenge increased cysteinyl leukotriene levels in wild-type mice and mice deficient of cysteinyl leukotriene receptor 1, but not in mice deficient of leukotriene C4 synthase. HPV did not differ between the three mouse strains after saline challenge (120±26, 114±16, and 115±24%, respectively; mean±SD). Endotoxin challenge markedly impaired HPV in wild-type mice (41±20%) but only marginally in mice deficient in leukotriene C4 synthase (96±16%, P<0.05 vs. wild-type mice), thereby preserving systemic oxygenation. While endotoxin modestly decreased HPV in mice deficient in cysteinyl leukotriene receptor 1 (80±29%, P<0.05 vs. saline challenge), the magnitude of impairment was markedly less than in endotoxin-challenged wild-type mice.
Conclusion
Cysteinyl leukotrienes importantly contribute to endotoxin-induced impairment of HPV in part via a cysteinyl leukotriene receptor 1-dependent mechanisms.
INTRODUCTION
Hypoxic pulmonary vasoconstriction (HPV) is an essential vasomotor response to alveolar hypoxia, diverting blood flow from poorly ventilated lung regions to better ventilated areas thereby improving ventilation-perfusion matching and raising the partial pressure of oxygen in the systemic circulation 1,2. HPV is impaired in patients with acute lung injury and the adult respiratory distress syndrome (ARDS) 3. Among patients with acute lung injury/ARDS, sepsis-induced ARDS has the highest mortality rate 4. Experimental endotoxemia has also been shown to impair HPV 5-12. However, the precise mechanisms by which endotoxin impairs HPV are incompletely understood 13-16.
Among the inflammatory mediators implicated in the impairment of HPV are the leukotrienes5-7,9-12,17. Leukotrienes are lipid mediators that are rapidly generated from arachidonic acid. Arachidonic acid is converted to the unstable intermediate leukotriene A4 (LTA4) by 5-lipoxygenase 18,19. LTA4 can be converted by either LTA4 hydrolase to leukotriene B4 (LTB4) 20, or it can be conjugated with glutathione by LTC4 synthase to form leukotriene C4 (LTC4) 21-23. LTC4 is converted by sequential hydrolysis to leukotriene D4 (LTD4) and leukotriene E4 (LTE4) 24. LTC4, LTD4, and LTE4 are collectively called the cysteinyl leukotrienes (cysLTs). The cysLT bind to the cysteinyl leukotriene receptors 1, 2, 3 or the cysteinyl leukotriene receptor E4 25-28, whereas LTB4 mediates its effects by binding to LTB4 receptors 1 or 2 29,30.
LTB4 and the cysLTs display different functions during inflammation. LTB4 is a potent chemokinetic and chemoattractant agent for polymorphonuclear neutrophils, while the cysLTs increase vascular permeability and stimulate bronchoconstriction and mucus secretion 31,32. Noncardiogenic pulmonary edema and the intrapulmonary accumulation of polymorphonuclear neutrophils are key features of acute lung injury/ARDS 33,34. The generation of leukotrienes by leukocytes is enhanced during sepsis and leukotriene levels are elevated in the bronchoalveolar lavage fluid obtained from patients with ARDS 35,36. These observations suggest the possibility that leukotrienes participate in the pathogenesis of acute lung injury and the impairment of HPV.
In a previous study, we showed that mice congenitally deficient in 5-lipoxygenase are protected from the impairment of HPV that follows endotoxin challenge 10. Furthermore, congenital deficiency of either LTA4 hydrolase or LTB4 receptor 1 did not preserve HPV in endotoxemic mice, suggesting that LTB4 does not contribute to the impairment of HPV in endotoxin-challenged mice. In contrast, the pharmacological inhibition of the cysteinyl leukotriene receptor 1 (CysLT1), using MK571, completely protected wild-type (WT) mice from endotoxin-induced impairment of HPV 10. However, MK571 has multiple targets such as the multidrug resistance protein-1 and the purinergic receptors 1,2,4 and 6 37,38, therefore, we sought to clarify the role of cysLTs and CysLT1 in the impairment of HPV by endotoxin using mice congenitally deficient in either LTC4 synthase (LTC4S-/-) or the CysLT1 receptor (CysLT1-/-). We hypothesized that cysLTs contribute to the impairment of HPV after endotoxin challenge and that they exert their effect via the CysLT1 receptor-dependent mechanisms.
MATERIALS AND METHODS
All animal experiments were approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital, Boston, Massachusetts. LTC4S-/- and CysLT1-/- mice were generated as described previously 39,40. LTC4S-/- and CysLT1-/- mice were backcrossed onto a C57BL/6J background for nine generations. WT mice (C57BL6/J) were purchased from Jackson Laboratory (Bar Harbor, ME). The studies were conducted in male WT, LTC4S-/-, and CysLT1-/- mice. Mice weighing between 21 and 27 g were matched for body weight and then intravenously challenged with either saline or lipopolysaccharide (Escherichia coli O111:B4, Sigma, St Louis, MO; 10 mg/kg, dissolved in saline 0.1 ml/10 g body weight).
Measurement of Hypoxic Pulmonary Vasoconstriction
To assess HPV, the change of slope of the left lung pulmonary blood flow-pressure relationship in response to acute left lung alveolar hypoxia was measured in nine animals per group, as described previously 10,41. Briefly, 18 h after challenge with either saline or lipopolysaccharide, mice were anesthetized and mechanically ventilated with a respiratory rate of 100 breaths/min and a tidal volume of 10 ml/kg body weight at an inspired oxygen fraction of 1.0. The peak inspiratory pressure was approximately 10 cm H2O and the positive end-expiratory pressure 2 cm H2O. An arterial line was placed in the left carotid artery, and a left-sided thoracotomy was performed. A custom-made polyethylene catheter was positioned in the main pulmonary artery, and a flow probe was placed around the left pulmonary artery. Heart rate, systemic arterial pressure, pulmonary arterial pressure, and left pulmonary arterial blood flow were continuously measured and recorded (DI 720; Dataq Instruments, Akron, OH). Left lung alveolar hypoxia and collapse was induced by occluding the left mainstem bronchus. To estimate left pulmonary vascular resistance, the inferior vena cava was transiently occluded to decrease left pulmonary arterial blood flow by approximately 50%. Left pulmonary vascular resistance was calculated from the slope of the left pulmonary arterial blood flow/pulmonary arterial pressure relationship. The increase in left pulmonary vascular resistance induced by occlusion of the left mainstem bronchus was expressed as the percentage increase from baseline left pulmonary vascular resistance to left pulmonary vascular resistance after 5 min of occlusion of the left mainstem bronchus. After all hemodynamic measurements were obtained, blood was sampled from the left carotid artery, anticoagulated with heparin, and arterial blood gas analyses were performed using a Rapid Lab 840 (Chiron Diagnostics, Medfield, MA).
The following exclusion criteria were employed: a preparation time over 60 min, at baseline a mean blood pressure below 60 mmHg and a heart rate below 400 beats/min, and inadvertent displacement of the arterial line or the flow probe.
Circulating Leukocyte Count
In additional mice (in each group 6 or 7 animals), blood was obtained via an arterial line 18 h after challenge with saline or lipopolysaccharide. Erythrocytes were hemolyzed using a Unopette ® (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ), and leukocytes were counted with a hemocytometer (Hausser Scientific, Horsham, PA).
Myeloperoxidase Assay
Infiltration of polymorphonuclear neutrophils into the lungs was estimated by measuring myeloperoxidase levels at 18 hours after saline (n = 6 in each group) or lipopolysaccharide challenge (WT (n = 5), LTC4S-/- (n = 7), and CysLT1-/- (n = 7)), as described previously 42.
Lung Wet / Dry Weight Ratio
In additional experiments, mice were euthanized with pentobarbital (0.1 mg/kg intraperitoneal) at 18 h after challenge with saline (WT (n = 5), LTC4S-/- (n = 5), CysLT1-/- (n = 4)) or lipopolysaccharide (WT (n = 9), LTC4S-/- (n = 10), CysLT1-/- (n = 10)). Both lungs were removed, blotted, and immediately weighed. The tissue was dried in a microwave oven for 60 min and reweighed. The lung wet/dry weight ratio was expressed as a percentage of dry to wet weight.
Bronchoalveolar Lavage Fluid
The lungs of mice challenged with either saline or lipopolysaccharide 18 h earlier were lavaged with 3 × 1 ml of ice-cold phosphate buffered saline. The recovered bronchoalveolar lavage fluid was pooled and centrifuged at 1,500 rpm for 10 min at 4°C. The supernatant was snap frozen and stored at –80°C until the measurement of leukotriene concentration. Samples were taken after challenge with either saline (WT (n = 4), LTC4S-/- (n = 5), CysLT1-/- (n = 5) or lipopolysaccharide (n = 6).
Measurement of Leukotriene Concentration
The samples of bronchoalveolar lavage fluid were thawed and acidified to a pH of 3.5. LTB4 and cysLTs were extracted with methyl formate and methanol, respectively. Leukotriene levels were quantified in duplicate using enzyme immunoassay kits following the manufacture's instructions (Neogen Corporation, Lexington, KY).
Statistical Analysis
Data are expressed as mean ± SD. P values less than 0.05 were considered statistically significant. Statistical analyses were performed using Sigma Stat 3.0 (Systat Software Inc, Richmond, CA). For the comparison between saline and lipopolysaccharide or the genotypes of WT, LTC4S-/-, and CysLT1-/-, data were analyzed using a two-way ANOVA with post-hoc Bonferroni tests (two-tailed) for normally distributed data or using a Kruskal-Wallis Test (two-tailed) with a post-hoc Bonferroni test for nonnormally distributed data. Hemodynamic changes between before and during occlusion of the left mainstem bronchus were compared with a paired t-test (two-tailed).
RESULTS
Hemodynamic Measurements Before and During Unilateral Hypoxia
At 18 h all saline challenged mice survived whereas approximately 50% of the lipopolysaccharide-challenged mice had died. Before inducing left lung hypoxia by occlusion of the left mainstem bronchus, the values of heart rate, systemic arterial pressure, pulmonary arterial pressure, and left pulmonary arterial blood flow did not differ between the mouse genotypes at 18 h after challenge with either saline or LPS (table 1). During occlusion of the left mainstem bronchus, the heart rate, systemic arterial pressure, and pulmonary arterial pressure were not different between saline-and lipopolysaccharide-challenged mice. A comparison between before and during occlusion of the left mainstem bronchus showed that the pulmonary arterial pressure increased and left pulmonary arterial blood flow decreased in all mice, whereas heart rate and systemic arterial pressure did not change, suggesting that the changes in pulmonary arterial pressure and left pulmonary arterial blood flow were not attributable to hemodynamic instability.
TABLE 1.
Hemodynamic Measurements
| WT | LTC4S-/- | CysLT1-/- | |||||
|---|---|---|---|---|---|---|---|
| Sal | LPS | Sal | LPS | Sal | LPS | ||
| HR (bpm) | Baseline | 515±37 | 497±38 | 487±41 | 485±26 | 489±44 | 504±50 |
| LMBO | 508±39 | 500±27 | 473±38 | 484±26 | 485±47 | 493±55 | |
| SAP (mmHg) | Baseline | 79±11 | 85±9 | 81±7 | 85±12 | 79±6 | 89±10‡ |
| LMBO | 81±12 | 82±11 | 80±8 | 84±9 | 78±9 | 86±9 | |
| PAP (mmHg) | Baseline | 15±2 | 15±2 | 15±1 | 16±1‡ | 16±1 | 15±1 |
| LMBO | 18±1† | 17±2† | 17±1† | 18±2† | 18±2† | 18±2† | |
| QLPA ml/min | Baseline | 2.4±0.3 | 2.3±0.2 | 2.4±0.2 | 2.3±0.3 | 2.4±0.2 | 2.4±0.1 |
| LMBO | 1.5±0.3† | 2.0±0.2‡† | 1.7±0.2¥† | 1.7±0.2† | 1.6±0.2† | 1.8±0.2† | |
Hemodynamic measurements before (baseline) and during occlusion of the left mainstem bronchus in WT, LTC4S-/-, and CysLT1-/- mice at 18 h after challenge with either saline or lipopolysaccharide (n = 9 per group). All values at baseline or during occlusion of the left mainstem bronchus were compared for challenge and for genotype by two way ANOVA. A paired t-test was used for the comparison before and during occlusion of the left mainstem bronchus.
P < 0.05 differs vs. saline-challenged mice of respective genotype
P < 0.05 vs. lipopolysaccharide-challenged WT mice
P < 0.05 vs. BL
bpm = beats/min; CysLT1-/- = mice congenitally deficient in the cysteinyl leukotriene receptor 1; HR = heart rate; LMBO = occlusion of the left mainstem bronchus; LPS = lipopolysaccharide; LTC4S-/- = mice congenitally deficient in leukotriene C4 synthase ml/min = milliliter per minute; mmHg = millimeters of mercury; PAP = mean pulmonary arterial pressure; QPLA = flow rate in left pulmonary artery; Sal = saline; SAP = mean systemic arterial pressure; WT = wild type mice. All data mean ± SD.
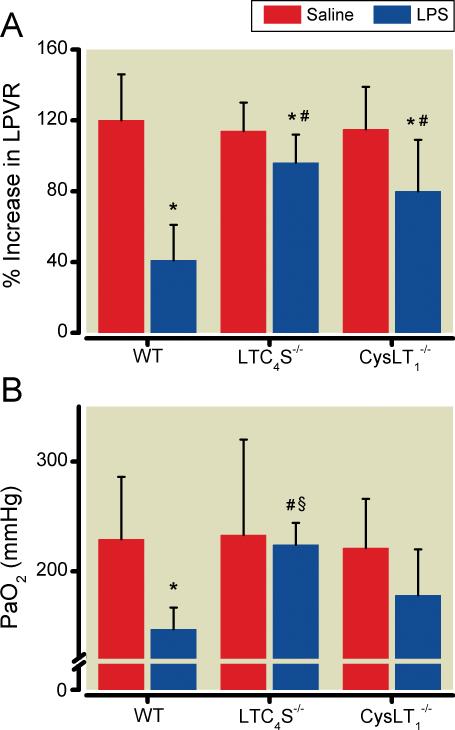
HPV was assessed as the percentage change of left pulmonary vascular resistance in response to occlusion of the left mainstem bronchus (fig. 1A). Saline-challenged mice of all three genotypes (WT, LTC4S-/-, and CysLT1-/-) demonstrated a marked increase of left pulmonary vascular resistance in response to occlusion of the left mainstem bronchus. As expected, challenge with lipopolysaccharide markedly impaired the increase of left pulmonary vascular resistance during occlusion of the left mainstem bronchus in WT mice as compared to saline-treated WT mice (P < 0.05). In contrast, in LTC4S-/- mice, the increase in left pulmonary vascular resistance induced by left mainstem bronchus occlusion was largely preserved after challenge with lipopolysaccharide. In CysLT1-/- mice, challenge with lipopolysaccharide modestly impaired the increase in left pulmonary vascular resistance in response to the occlusion of the left mainstem bronchus occlusion (P < 0.05 vs. saline-challenged CysLT1-/- mice). However, the increase in left pulmonary vascular resistance was significantly greater in lipopolysaccharide-challenged CysLT1-/- mice than in lipopolysaccharide-challenged WT mice (P < 0.05, fig. 1A).
Figure 1.
(A) Occlusion of the left mainstem bronchus-induced increase of left pulmonary vascular resistance in WT, LTC4S-/-, and CysLT1-/- mice at 18 hours after challenge with either saline or lipopolysaccharide (n=9 in each group). (B) Values of oxygen in the arterial blood during occlusion of the left mainstem bronchus at the end of the HPV measurements. *P<0.05 differs vs. saline-challenged mice of the respective genotype, # P<0.05 differs vs. lipopolysaccharide-challenged WT mice, § P<0.05 differs vs. lipopolysaccharide-challenged CysLT1-/- mice. CysLT1-/-, mice congenitally deficient in the cysteinyl leukotriene receptor 1; LPS, lipopolysaccharide; LPVR, left pulmonary vascular resistance; LTC4S-/-, mice congenitally deficient in leukotriene C4 synthase; mmHg, millimeters of mercury; PaO2, level of oxygen in the arterial blood; WT, wild type mice. All data mean ± SD.
Preserved HPV is Associated with a Higher Systemic Arterial Oxygen Tension during occlusion of the left mainstem bronchus
To estimate the impact of HPV on systemic arterial oxygenation, arterial blood gas tensions were measured during occlusion of the left mainstem bronchus at the end of each HPV experiment (fig. 1B and table 2). The systemic arterial partial pressure of oxygen (PaO2) during occlusion of the left mainstem bronchus did not differ between the genotypes after saline challenge. However, after occlusion of the left mainstem bronchus, PaO2 was markedly less in endotoxin-challenged WT mice than in saline-challenged WT mice (P < 0.05). In contrast, the PaO2 after occlusion of the left mainstem bronchus in lipopolysaccharide-challenged LTC4S-/- mice was similar to the level in saline-challenged LTC4S-/- mice and greater than that measured in both lipopolysaccharide-challenged WT mice and lipopolysaccharide-challenged CysLT1-/- mice (P < 0.05 for both). In lipopolysaccharide-challenged CysLT1-/- mice, the PaO2 during occlusion of the left mainstem bronchus tended to be higher than in lipopolysaccharide-challenged WT mice (P > 0.05).
TABLE 2.
Arterial Blood Gas Analyses
| WT | LTC4S-/- | CysLT1-/- | ||||
|---|---|---|---|---|---|---|
| Sal | LPS | Sal | LPS | Sal | LPS | |
| PaO2 (mmHg) | 229±57 | 147±20‡ | 233±87 | 224±20¥‡‡ | 221±45 | I78±42 |
| PaCO2 (mmHg) | 30.5±5.2 | 31.0±5.6 | 29.6±7.3 | 31.0±6.5 | 32.9±8.7 | 29.3±5.3 |
| pHa | 7.35±0.08 | 7.11±0.08‡ | 7.33±0.06 | 7.07±0.05‡ | 7.34±0.07 | 7.11±0.07‡ |
| BE (mmol/l) | -7.5±3.3 | -21.1 ±5.5‡ | -9.8±2.9 | -20.5±2.8‡ | -7.7±3.2 | -19.4±3.6‡ |
| Hb (g/dl) | 13.4±1.1 | 13.1±0.8 | 13.7±1.0 | 13.9±0.7 | 13.5±0.8 | 14.1±0.6 |
Arterial blood gas analyses at the end of the hemodynamic studies during occlusion of the left mainstem bronchus in WT, LTC4S-/-, and CysLT1-/- mice after challenge with either saline or lipopolysaccharide.
P < 0.05 vs. saline-challenged mice of respective genotype
P < 0.05 vs. lipopolysaccharide-challenged WT mice, n = 9 in each group
P < 0.05 vs. LPS-challenged CysLT1-/-mice.
BE = base excess; CysLT1-/- = mice congenitally deficient in the cysteinyl leukotriene receptor 1; g/dl = gram per deciliter; Hb = hemoglobin; LPS = lipopolysaccharide; LTC4S-/- = mice congenitally deficient in leukotriene C4 synthase; mmHg = millimeters of mercury; mmol/l = millimoles per liter; PaCO2 = level of carbon dioxide in the arterial blood; PaO2 = level of oxygen in the arterial blood; pHa = arterial pH; Sal = saline; WT = wild type mice. All data mean ± SD.
There were no differences in the values of the arterial partial pressure of carbon dioxide between the genotypes after challenge with saline or lipopolysaccharide. The changes in pHa and the base excess were smaller in each of the three genotypes after saline challenge than after lipopolysaccharide challenge. Hemoglobin concentrations were similar in all mice.
Endotoxin promotes pulmonary infiltration of polymorphonuclear neutrophils and increases cysLT levels in the bronchoalveolar lavage fluid
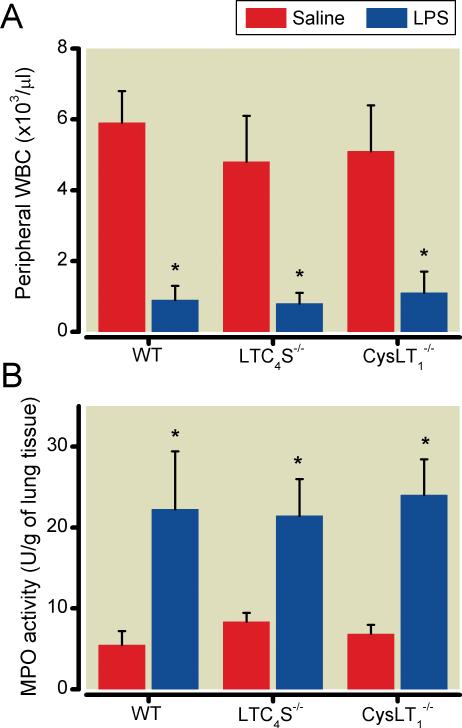
Challenge with lipopolysaccharide markedly decreased the concentration of circulating leukocytes in all three mouse strains (fig. 2A). There was no difference in the circulating leukocyte concentration between WT, LTC4S-/-, and CysLT1-/- mice after intravenous challenge with lipopolysaccharide.
Figure 2.
(A) In WT (n=6), LTC4S-/-(n=6), and CysLT1-/- mice (n=7) the circulating leukocyte concentrations were markedly reduced after lipopolysaccharide challenge compared with WT (n=6), LTC4S-/- (n=7), and CysLT1-/- (n=6) mice after saline challenge. (B) Lung tissue myeloperoxidase activity was greater in lipopolysaccharide-treated WT (n=5), LTC4S-/- (n=7), and CysLT1-/- (n=7) mice than in saline-treated WT (n=6), LTC4S-/- (n=6), and CysLT1-/- (n=6) mice. Blood and tissue samples were taken 18 hours after lipopolysaccharide-challenge. *P<0.05 differs vs. saline-challenged mice of the respective genotype. CysLT1-/-, mice congenitally deficient in the cysteinyl leukotriene receptor 1; LPS, lipopolysaccharide; LTC4S-/-, mice congenitally deficient in leukotriene C4 synthase; MPO, myeloperoxidase; WBC, white blood cell count; WT, wild type mice. All data mean ± SD.
In all three genotypes, the myeloperoxidase activity of the right lung was more than 3-fold higher at 18 h after lipopolysaccharide challenge than after saline challenge (fig. 2B). Lung myeloperoxidase activity levels in lipopolysaccharide-challenged CysLT1-/- mice were greater than the levels measured in lipopolysaccharide-challenged WT mice (p < 0.05).
The lung wet-to-dry weight ratio did not differ between WT, LTC4S-/-, and CysLT1-/- mice after challenge with saline (4.4±0.2, (n = 5); 4.4 ± 0.2, (n = 5); 4.5 ± 0.3, (n = 4), respectively). Challenge with lipopolysaccharide did not alter the wet-to-dry weight ratio when compared to saline challenge in WT, LTC4S-/-, and CysLT1-/- mice (4.5 ± 0.3, (n = 9); 4.4 ± 0.3, (n = 10); 4.4 ± 0.2, (n = 10).
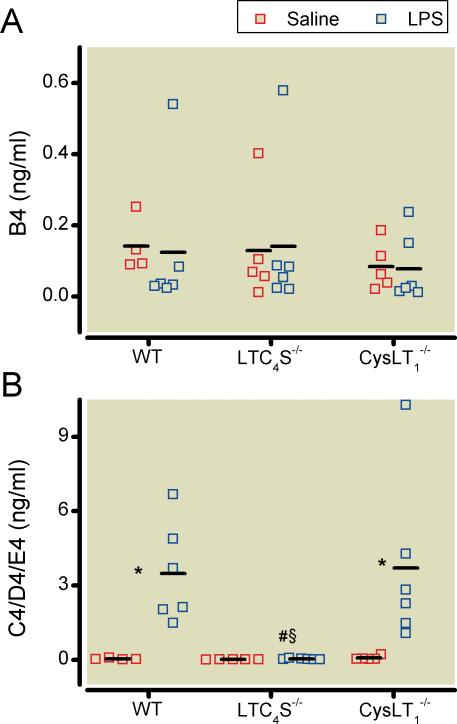
In all three genotypes, there were no differences in the bronchoalveolar lavage fluid LTB4 levels after challenge with saline or lipopolysaccharide (fig. 3A). In contrast, cysLT concentrations in the bronchoalveolar lavage fluid of the same mice were much higher in WT and CysLT1-/- mice after endotoxin challenge than after saline challenge. No cysLTs were detectable in bronchoalveolar lavage fluid obtained from LTC4S-/- mice (fig. 3B).
Figure 3.
The concentration of LTB4 in bronchoalveolar lavage fluid did not differ between saline-challenged WT (n=4), LTC4S-/-(n=5), and CysLT1-/- (n=5) mice and lipopolysaccharide-challenged WT (n=6), LTC4S-/-(n=6), and CysLT1-/- (n=6) mice 18 hours after challenge. (B) In the same mice, levels of cysLTs (LTC4/D4/E4) in the bronchoalveolar lavage fluid were higher in WT and CysLT1-/- mice after lipopolysaccharide challenge than in saline-challenged WT and CysLT1-/- mice. As expected, no cysLTs were detectable in bronchoalveolar lavage fluid from the LTC4S-/- mice after challenge with either saline or lipopolysaccharide. * P<0.05 differs vs. saline-challenged mice of the respective genotype, # P<0.05 differs vs. lipopolysaccharide-challenged WT mice, § P<0.05 differs vs. lipopolysaccharide-challenged CysLT1-/- mice. B4, cysteinyl leukotriene B4; C4/D4/E4, cysteinyl leukotriene C4/D4/E4; CysLT1-/-, mice congenitally deficient in the cysteinyl leukotriene receptor 1; LPS, lipopolysaccharide; LTC4S-/-, mice congenitally deficient in leukotriene C4 synthase; WT, wild type mice. The levels of LTB4 and cysLT are depicted as individual values with arithmetic means.
DISCUSSION
Our data show that cysLTs play an important role in endotoxin-induced impairment of HPV. A congenital deficiency of cysteinyl leukotriene synthesis largely protects septic mice from lipopolysaccharide-induced impairment of HPV and preserves systemic arterial oxygenation. Activation of the CysLT1 receptor contributes significantly to the impairment of HPV after endotoxin challenge, since mice lacking the CysLT1 receptor are to a great extent protected from the lipopolysaccharide-induced attenuation of HPV.
After a saline challenge, both LTC4S-/- and CysLT1-/- mice demonstrated the same marked increase of left pulmonary vascular resistance that was observed in WT mice. In a previous study, we reported that HPV was similarly preserved in mice deficient in either 5-lipoxygenase or LTA4 hydrolase under normal (nonseptic) conditions 10. Taken together, these results confirm that neither LTB4 nor cysLTs are required for the pulmonary vasoconstrictor response to hypoxia in healthy lung.
As reported previously, we observed that endotoxin challenge markedly impaired HPV in WT mice 10. In the current study, we found that a congenital deficiency of cysLT synthesis largely preserves HPV after endotoxin challenge. Since cysLTs can bind to cysteinyl leukotriene receptors 1, 2, 3, or E4 25-28, we sought to clarify the role of the CysLT1 receptor in endotoxin-induced impairment of HPV by using CysLT1 receptor-deficient mice. We found that CysLT1 deficiency significantly attenuates the endotoxin-induced impairment of HPV compared to lipopolysaccharide-challenged WT mice albeit to a lesser extent than did a complete deficiency of cysLT synthesis. It is possible that activation of cysteinyl leukotriene receptors 2 and/or cysteinyl leukotriene receptors 3 by cysteinyl leukotrienes may have contributed to the impairment of HPV in CysLT1-/- mice 43,44. Taken together our results show that cysLTs impair HPV after endotoxin challenge and that they exert their effects in major part via CysLT1.
We previously reported that 5-lipoxygenase deficiency prevented the impairment of HPV by endotoxin challenge associated with a reduction in the endotoxin-induced increase in pulmonary myeloperoxidase levels 10. To learn if cysLTs impair HPV by inducing pulmonary polymorphonuclear leukocytes accumulation, the peripheral leukocytes concentration and pulmonary myeloperoxidase levels were measured in WT, LTC4S-/-, and CysLT1-/- mice at 18 h after endotoxin challenge. In all three genotypes, the leukocytes concentrations were markedly decreased, and pulmonary myeloperoxidase levels were increased. On the other hand, HPV was impaired in WT mice but not in LTC4S-/- mice. Taken together these results suggest that the recruitment of leukocytes to the lung after endotoxin challenge is mediated by LTB4 but not by cysLTs and that the accumulation of leukocytes in the lung per se does not contribute to the impairment of HPV.
Lärfars and colleagues showed that leukotrienes can cause nitric oxide release from polymorphonuclear leukocytes 45. Nitric oxide is a potent vasodilator that acts primarily via stimulation of soluble guanylate cyclase. In a previous study, we reported that mice deficient of inducible nitric synthase had preserved HPV after endotoxin challenge 9. Furthermore, pharmacological inhibition of soluble guanylate cyclase attenuated the endotoxin-induced impairment of HPV in an isolated, perfused, and ventilated mouse lung 12. It is possible that cysLTs contribute to the endotoxin-induced impairment of HPV by causing vasodilation via the nitric oxide pathway.
Levels of cysLTs are elevated in the bronchoalveolar lavage fluid of patients with ARDS 35, and cysLTs are known to increase vascular permeability 31,32. We sought to determine whether the impairment of HPV by endotoxin was associated with elevated levels of cysLTs in bronchoalveolar lavage fluid and with increased pulmonary microvascular permeability. Eighteen hours after challenge with lipopolysaccharide, cysLT levels were markedly increased in bronchoalveolar lavage fluid obtained from WT and CysLT1-/- mice, whereas in the bronchoalveolar lavage fluid of LTC4-/- mice, as expected, no cysLTs were detectable. In all three genotypes, endotoxin challenge did not increase lung wet-to-dry weight ratios. These observations suggest that cysLTs did not impair HPV by increasing permeability in the current study.
The molecular mechanisms underlying HPV remain elusive 13-16. However, the current theories of how oxygen tension is sensed by the pulmonary arteries center around the biosynthesis of radical oxygen species and the cellular redox state. In a previous study from our laboratory we showed that oxygen radical scavengers attenuated the impairment of HPV after lipopolysaccharide challenge 11. In animal models of either Indomethacin-induced gastric ulcers or skin flap ischemia reperfusion injury, the cysLT receptor antagonist Montelukast exerted antioxidant effects 46,47. Taken together, it is possible that the deficiency of cysLT synthesis prevented endotoxin-induced impairment of HPV by reducing oxidative stress.
The current study demonstrates that cysLTs contribute to the endotoxin-induced impairment of HPV in a rodent model. However, our study has limitations. The administration of lipopolysaccharide is widely used as an animal model of sepsis but the lipopolysaccharide component of the bacterial cell wall does not cause all of the complex inflammatory processes seen in clinical sepsis 5-12. Our results are also limited due to the small number of animals used and relatively large standard deviations in some experiments.
In summary, we have identified a key role for cysLTs in endotoxin-induced impairment of HPV using two strains of genetically-modified mice. We found that a congenital deficiency of LTC4S almost completely protected mice from endotoxin-induced impairment of HPV, whereas deficiency of the CysLT1 receptor significantly attenuated the endotoxin-induced impairment of HPV. Endotoxin-induced activation of cysLT pathway compromized HPV thereby reducing systemic arterial oxygenation. The protective effects of cysLT deficiency were independent of changes in both pulmonary polymorphonuclear leukocytes accumulation and the presence of pulmonary edema. The current results suggest that, cysLTs may be additional therapeutic targets in the treatment or prevention of the sepsis-induced impairment of HPV.
Summary statement: In sepsis, hypoxic pulmonary vasoconstriction is impaired and levels of cysteinyl leukotrienes in the bronchial alveolar fluid are elevated. However, the precise role of cysteinyl leukotrienes in sepsis-induced impairment of hypoxic pulmonary vasoconstriction is uncertain.
Acknowledgments
Funding: This study was supported by the grants P01HL36110 (K. Frank Austen), R01HL74352 (Kenneth D. Bloch), R01GM79360 (Fumito Ichinose), R01HL090630 (Yoshihide Kanaoka), and R01HL42397 (Warren M. Zapol) from the National Institutes of Health, Bethesda, Maryland.
Footnotes
Department to which the work should be attributed
Department of Anesthesia and Critical Care, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts
Department of Medicine and Division of Rheumatology, Immunology and Allergy, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts
Disclosure:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Thomas HM, 3rd, Garrett RC. Strength of hypoxic vasoconstriction determines shunt fraction in dogs with atelectasis. J Appl Physiol. 1982;53:44–51. doi: 10.1152/jappl.1982.53.1.44. [DOI] [PubMed] [Google Scholar]
- 2.Brimioulle S, LeJeune P, Naeije R. Effects of hypoxic pulmonary vasoconstriction on pulmonary gas exchange. J Appl Physiol. 1996;81:1535–43. doi: 10.1152/jappl.1996.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 3.Dantzker DR, Brook CJ, Dehart P, Lynch JP, Weg JG. Ventilation-perfusion distributions in the adult respiratory distress syndrome. Am Rev Respir Dis. 1979;120:1039–52. doi: 10.1164/arrd.1979.120.5.1039. [DOI] [PubMed] [Google Scholar]
- 4.Sharma S, Kumar A. Septic shock, multiple organ failure, and acute respiratory distress syndrome. Curr Opin Pulm Med. 2003;9:199–209. doi: 10.1097/00063198-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Weir EK, Mlczoch J, Reeves JT, Grover RF. Endotoxin and prevention of hypoxic pulmonary vasoconstriction. J Lab Clin Med. 1976;88:975–83. [PubMed] [Google Scholar]
- 6.Hales CA, Sonne L, Peterson M, Kong D, Miller M, Watkins WD. Role of thromboxane and prostacyclin in pulmonary vasomotor changes after endotoxin in dogs. J Clin Invest. 1981;68:497–505. doi: 10.1172/JCI110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang SW, Feddersen CO, Henson PM, Voelkel NF. Platelet-activating factor mediates hemodynamic changes and lung injury in endotoxin-treated rats. J Clin Invest. 1987;79:1498–509. doi: 10.1172/JCI112980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theissen JL, Loick HM, Curry BB, Traber LD, Herndon DN, Traber DL. Time course of hypoxic pulmonary vasoconstriction after endotoxin infusion in unanesthetized sheep. J Appl Physiol. 1991;70:2120–5. doi: 10.1152/jappl.1991.70.5.2120. [DOI] [PubMed] [Google Scholar]
- 9.Ullrich R, Bloch KD, Ichinose F, Steudel W, Zapol WM. Hypoxic pulmonary blood flow redistribution and arterial oxygenation in endotoxin-challenged NOS2-deficient mice. J Clin Invest. 1999;104:1421–9. doi: 10.1172/JCI6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ichinose F, Zapol WM, Sapirstein A, Ullrich R, Tager AM, Coggins K, Jones R, Bloch KD. Attenuation of hypoxic pulmonary vasoconstriction by endotoxemia requires 5-lipoxygenase in mice. Circ Res. 2001;88:832–8. doi: 10.1161/hh0801.089177. [DOI] [PubMed] [Google Scholar]
- 11.Baboolal HA, Ichinose F, Ullrich R, Bloch KD, Zapol WM. Reactive oxygen species scavengers attenuate endotoxin-induced impairment of hypoxic pulmonary vasoconstriction in mice. Anesthesiology. 2002;97:1227–33. doi: 10.1097/00000542-200211000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Spohr F, Busch CJ, Teschendorf P, Weimann J. Selective inhibition of guanylate cyclase prevents impairment of hypoxic pulmonary vasoconstriction in endotoxemic mice. J Physiol Pharmacol. 2009;60:107–12. [PubMed] [Google Scholar]
- 13.Paky A, Michael JR, Burke-Wolin TM, Wolin MS, Gurtner GH. Endogenous production of superoxide by rabbit lungs: Effects of hypoxia or metabolic inhibitors. J Appl Physiol. 1993;74:2868–74. doi: 10.1152/jappl.1993.74.6.2868. [DOI] [PubMed] [Google Scholar]
- 14.Marshall C, Mamary AJ, Verhoeven AJ, Marshall BE. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 1996;15:633–44. doi: 10.1165/ajrcmb.15.5.8918370. [DOI] [PubMed] [Google Scholar]
- 15.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–66. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 16.Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res. 2002;90:1307–15. doi: 10.1161/01.res.0000024689.07590.c2. [DOI] [PubMed] [Google Scholar]
- 17.Stevens T, Morris K, McMurtry IF, Zamora M, Tucker A. Pulmonary and systemic vascular responsiveness to TNF-alpha in conscious rats. J Appl Physiol. 1993;74:1905–10. doi: 10.1152/jappl.1993.74.4.1905. [DOI] [PubMed] [Google Scholar]
- 18.Rouzer CA, Matsumoto T, Samuelsson B. Single protein from human leukocytes possesses 5-lipoxygenase and leukotriene A4 synthase activities. PNAS. 1986;83:857–61. doi: 10.1073/pnas.83.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon RA, Diehl RE, Opas E, Rands E, Vickers PJ, Evans JF, Gillard JW, Miller DK. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990;343:282–4. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- 20.Evans JF, Dupuis P, Ford-Hutchinson AW. Purification and characterisation of leukotriene A4 hydrolase from rat neutrophils. Biochim Biophys Acta. 1985;840:43–50. doi: 10.1016/0304-4165(85)90160-6. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson DW, Ali A, Vaillancourt JP, Calaycay JR, Mumford RA, Zamboni RJ, Ford-Hutchinson AW. Purification to homogeneity and the N-terminal sequence of human leukotriene C4 synthase: A homodimeric glutathione S-transferase composed of 18-kDa subunits. PNAS. 1993;90:2015–9. doi: 10.1073/pnas.90.5.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam BK, Penrose JF, Freeman GJ, Austen KF. Expression cloning of a cDNA for human leukotriene C4 synthase, an integral membrane protein conjugating reduced glutathione to leukotriene A4. PNAS. 1994;91:7663–7. doi: 10.1073/pnas.91.16.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welsch DJ, Creely DP, Hauser SD, Mathis KJ, Krivi GG, Isakson PC. Molecular cloning and expression of human leukotriene-C4 synthase. PNAS. 1994;91:9745–9. doi: 10.1073/pnas.91.21.9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter BZ, Shi ZZ, Barrios R, Lieberman MW. γ-glutamyl leukotrienase, a γ-glutamyl transpeptidase gene family member, is expressed primarily in spleen. J Biol Chem. 1998;273:28277–85. doi: 10.1074/jbc.273.43.28277. [DOI] [PubMed] [Google Scholar]
- 25.Lynch KR, O'Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, Coulombe N, Abramovitz M, Figueroa DJ, Zeng Z, Connolly BM, Bai C, Austin CP, Chateauneuf A, Stocco R, Greig GM, Kargman S, Hooks SB, Hosfield E, Williams DL, Jr, Ford-Hutchinson AW, Caskey CT, Evans JF. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–93. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 26.Heise CE, O'Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, Stocco R, Bellefeuille JN, Abramovitz M, Cheng R, Williams DL, Jr, Zeng Z, Liu Q, Ma L, Clements MK, Coulombe N, Liu Y, Austin CP, George SR, O'Neill GP, Metters KM, Lynch KR, Evans JF. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem. 2000;275:30531–6. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- 27.Maekawa A, Kanaoka Y, Xing W, Austen KF. Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. PNAS. 2008;105:16695–700. doi: 10.1073/pnas.0808993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paruchuri S, Jiang, Feng C, Francis SA, Plutzky J, Boyce J. Leukotriene E4 activates peroxisome proliferator-activated receptor γ and induces prostaglandin D2 generation by human mast cells. J Biol Chem. 2008;283:16477–87. doi: 10.1074/jbc.M705822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–4. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 30.Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T. A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J Exp Med. 2000;192:421–32. doi: 10.1084/jem.192.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis RA, Austen KF. The biologically active leukotrienes. Biosynthesis, metabolism, receptors, functions, and pharmacology. J Clin Invest. 1984;73:889–97. doi: 10.1172/JCI111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323:645–55. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- 33.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319–23. [Google Scholar]
- 34.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 35.Stephenson AH, Lonigro AJ, Hyers TM, Webster RO, Fowler AA. Increased concentrations of leukotrienes in bronchoalveolar lavage fluid of patients with ARDS or at risk for ARDS. Am Rev Respir Dis. 1988;138:714–9. doi: 10.1164/ajrccm/138.3.714. [DOI] [PubMed] [Google Scholar]
- 36.Baenkler M, Leykauf M, John S. Functional analysis of eicosanoids from white blood cells in sepsis and SIRS. J Physiol Pharmacol. 2006;57(Suppl 12):25–33. [PubMed] [Google Scholar]
- 37.Mamedova L, Capra V, Accomazzo MR, Gao ZG, Ferrario S, Fumagalli M. CysLT1 leukotriene receptor antagonists inhibit the effects of nucleotides acting at P2Y receptors. Biochem Pharmacol. 2005;71:115–25. doi: 10.1016/j.bcp.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. PNAS. 2006;103:16394–9. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanaoka Y, Maekawa A, Penrose JF, Austen KF, Lam BK. Attenuated zymosan-induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J Biol Chem. 2001;276:22608–13. doi: 10.1074/jbc.M103562200. [DOI] [PubMed] [Google Scholar]
- 40.Maekawa A, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J Biol Chem. 2002;277:20820–4. doi: 10.1074/jbc.M203163200. [DOI] [PubMed] [Google Scholar]
- 41.Ichinose F, Ullrich R, Sapirstein A, Jones RC, Bonventre JV, Serhan CN, Bloch KD, Zapol WM. Cytosolic phospholipase A(2) in hypoxic pulmonary vasoconstriction. J Clin Invest. 2002;109:1493–500. doi: 10.1172/JCI14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hellman J, Roberts JD, Jr, Tehan MM, Allaire JE, Warren HS. Bacterial peptidoglycan-associated lipoprotein is released into the bloodstream in gram-negative sepsis and causes inflammation and death in mice. J Biol Chem. 2002;277:14274–80. doi: 10.1074/jbc.M109696200. [DOI] [PubMed] [Google Scholar]
- 43.Ortiz JL, Gorenne I, Cortijo J, Seller A, Labat C, Sarria B, Abram TS, Gardiner PJ, Morcillo E, Brink C. Leukotriene receptors on pulmonary vascular endothelium. Br J Pharmacol. 1995;115:1382–6. doi: 10.1111/j.1476-5381.1995.tb16627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paruchuri S, Tashimo H, Feng C, Maekawa A, Xing W, Jiang Y, Kanaoka Y, Conley P, Boyce JA. Leukotriene E4-induced pulmonary inflammation is mediated by P2Y12 receptor. JEM. 2009;206:2543–55. doi: 10.1084/jem.20091240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lärfars G, Lantoine F, Devynck MA, Palmblad J, Gyllenhammer H. Activation of nitric oxide release and oxidative metabolism by leukotrienes B4, C4, and D4 in human polymorphonuclear leukocytes. Blood. 1999;93:1399–405. [PubMed] [Google Scholar]
- 46.Dengiz GO, Odabasoglu F, Halici Z, Cadirci E, Suleyman H. Gastroprotective and antioxidant effects of montelukast on indomethacin-induced ulcer in rats. J Pharmacol Sci. 2007;105:94–102. doi: 10.1254/jphs.fp0070122. [DOI] [PubMed] [Google Scholar]
- 47.Gideroglu K, Yilamz F, Aksoy F, Bugdayci G, Saglam I, Yimaz F. Montelukast protects axial pattern rat skin flaps against ischemia/reperfusion injury. J Surg Res. 2009;157:181–6. doi: 10.1016/j.jss.2008.07.031. [DOI] [PubMed] [Google Scholar]