Abstract
The association of α-amino-β-methylaminopropionic acid (BMAA) with elevated incidence of amyotrophic lateral sclerosis/Parkinson’s disease complex (ALS/PDC) was first identified on the island of Guam. BMAA has been shown to be produced across the cyanobacterial order and its detection has been reported in a variety of aquatic and terrestrial environments worldwide, suggesting that it is ubiquitous. Various in vivo studies on rats, mice, chicks and monkeys have shown that it can cause neurodegenerative symptoms such as ataxia and convulsions. Zebrafish research has also shown disruption to neural development after BMAA exposure. In vitro studies on mice, rats and leeches have shown that BMAA acts predominantly on motor neurons. Observed increases in the generation of reactive oxygen species (ROS) and Ca2+ influx, coupled with disruption to mitochondrial activity and general neuronal death, indicate that the main mode of activity is via excitotoxic mechanisms. The current review pertaining to the neurotoxicity of BMAA clearly demonstrates its ability to adversely affect neural tissues, and implicates it as a potentially significant compound in the aetiology of neurodegenerative disease. When considering the potential adverse health effects upon exposure to this compound, further research to better understand the modes of toxicity of BMAA and the environmental exposure limits is essential.
Keywords: BMAA, neuron, glia, neural, neurodegeneration, excitotoxicity, ALS, PDC, cycad, toxicology, cyanobacteria, Alzheimer’s, Parkinson’s
1. The Cycad Hypothesis
Medical research attention was drawn towards Guam in 1953 when it was reported that the incidence of an amyotrophic lateral sclerosis/Parkinson’s disease complex (ALS/PDC) within the local Chamorro people was 100 times higher than the rest of the world [1–3]. After failing to identify any clear genetic correlation to this observation, attention was turned to environmental/cultural factors that might be responsible [2,3]. The use of cycad (Cycas circinalis) flour to make tortillas, soups and dumplings by the native Chamorro people [4,5], coupled with various field reports that livestock developed progressive and irreversible ataxia after ingesting cycads [6], led to the suggestion that cycad consumption could be the cause of the human condition [5], and thus the cycad hypothesis was born. In 2007 a group of biostatisticians, led by Borenstein, conducted an in depth population study of the Chamorro people, statistically showing that eating cycads presented the highest associated risk of developing ALS/PDC [7].
2. Proving the BMAA Link
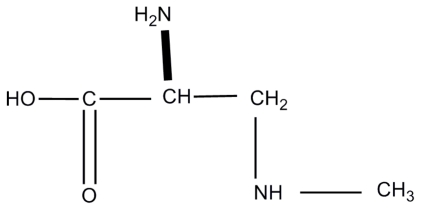
In 1967 Vega and Bell [8] isolated a non-protein amino acid, α-amino-β-methylaminopropionic acid, later renamed BMAA (Figure 1), from cycad seeds. By injecting chemically synthesised BMAA into chicks and rats at 3–7 μmoles/g body weight for chicks, and 6–14 μmoles/g body weight for rats, and subsequently observing weakness, convulsions and general loss of coordination in both animals they determined that BMAA possessed neurotoxic properties [8,9].
Figure 1.
The chemical structure of β-methylaminoalanine (BMAA).
Despite this observation, little attention was paid to BMAA until 1987, when Spencer et al. correlated elevated incidence of ALS in communities in the Kii peninsula of Japan [10] and Irian Jaya (West New Guinea) [11] to the traditional use of cycad pulp and sap in medicinal broths and in concoctions used to treat wounds. They hypothesised that BMAA was the cycad component that caused ALS and possibly Parkinson’s and Alzheimer’s diseases in Guam and elsewhere [12]. The group then conducted a major experiment in which they fed BMAA (100 to 250 mg/kg) to macaques for up to 12 weeks and observed a variety of symptoms indicating that the animals were suffering from neurodegeneration [13]. This was quickly questioned by Duncan et al. [14], who suggested that more than 80% of BMAA was removed from cycad seeds during processing, and by Garruto et al. [15] who calculated (based on the knowledge of the time) that the doses used were equivalent to a 70 kg man consuming 1500 kg of cycad flour. These claims led many to believe that BMAA could not possibly be the causative agent, especially when considering the report that at least 85% of the free BMAA was removed from the flour with a single wash, thereby making it impossible to consume toxic quantities [16].
In 2002, Cox and Sacks [17] rejuvenated the idea by proposing a biomagnification process of BMAA accumulation involving flying foxes that fed on cycads. The flying foxes were part of the traditional diet of the Chamorro people, meaning that the concentrations of BMAA actually consumed by humans were much higher than previously thought. Monson et al. [18] compiled further evidence correlating increases in flying fox consumption with increases in ALS incidence, thereby providing support for this idea. Whilst the native species of flying foxes are now almost extinct in Guam, testing of dried skin samples from museum specimens revealed BMAA concentrations equivalent (per weight) to up to 1014 kg of processed cycad flour [19], supporting the bioaccumulation hypothesis.
At this point in time the source of BMAA in cycads was unknown. In 2003 Cox et al. [20] revealed that BMAA was present in coralloid roots of cycads, but not in roots with normal morphology. It was already known that cyanobacteria lived in the coralloid roots of cycad plants, where they exist as nitrogen fixing symbionts [21]. Cyanobacteria isolated from coralloidal roots were then found to produce BMAA [20]. Subsequent testing of a variety of cyanobacterial species revealed that over 90% of all genera tested, encompassing all five sections of this phylum, produced BMAA [22,23]. The group of Marler et al. [24] have suggested that cycad plants can produce BMAA in normal roots lacking symbiotic interaction. While this raises doubts over cyanobacterial involvement, they do suggest that inoculation with cyanobacteria may induce increased production of BMAA by the cycad roots.
In 2003, studies conducted by Banack and Cox [25] showed that, within cycad plants, BMAA was concentrated in the seeds, which are ground up to make flour, that is then washed and used for cooking. Another study conducted by Murch et al. [26] detected BMAA in the brain tissues of six out of six Chamorro people who had died of ALS/PDC as well as one asymptomatic Chamorro individual (although at lower concentrations). Interestingly the same study also found BMAA at significant concentrations in the brain tissue of two Canadians who had died of Alzheimer’s disease (AD), suggesting that BMAA may play a role in various types of neurodegenerative disease [26]. These brain tissue samples had been fixed in paraformaldehyde prior to storage in a 15% buffered sucrose maintenance solution [26]. In this, and an associated study, BMAA was detected in protein precipitates, revealing that it can exist in an unknown peptide bound form that had not previously been quantified. The concentrations of this bound form were 10–240 fold higher than those of free BMAA, showing that the accumulated levels were much higher than was previously thought [27]. HPLC and mass spectrometry were used to identify and quantify BMAA in tested samples with a detection limit of 100 pmol [26,27]. These findings were brought into question in 2005 when Montine et al. [28] conducted their own study and failed to detect any free BMAA in any brain samples taken from the Chamorro ALS/PDC victims or US AD victims. The same research group again failed to detect free or protein-associated BMAA in similar post mortem brain samples in 2009 [29]. These observations led them to conclude that BMAA did not accumulate in brain tissues and therefore could not cause neurodegeneration. The tissue samples used in these studies had been flash frozen in liquid nitrogen and stored at −80 °C, without the use of any fixative or preservative. Both groups prepared their samples using trichloracetic acid (TCA) protein precipitation techniques, however a different HPLC-fluorescence detection (HPLC-FD) method was used by Montine et al. [28] with a claimed detection limit of 1 pmol (100 times more sensitive than the method of Murch et al. [26]). The different methods used by the group of Montine et al. [28,29] have been suggested by some parties [30,31] to be responsible for a lack of detection, rather than an actual lack of BMAA presence in tested samples. The 6-Aminoquinolyl-N-hydroxysuccinimidyl carbamate method used by Murch et al. [26] is more stable than the other methods [32], and is the preferred method for analysis of amino acids [33]. Instability of the method used by Montine et al. might explain the lack of detection.
In 2006 Banack et al. [4] tested various organs, including the brain and muscle samples of flying foxes from Guam as well as the nearby islands of Yap and Samoa, finding significant levels of BMAA contained in all Guamanian samples, as well as detectable levels in most samples from Yap. They also proposed that consumption of other cycad foraging animals, such as wild deer and boars, could increase BMAA ingestion in the Chamorros. A study by Pablo et al. [34] detected high concentrations of BMAA in 49 out of 50 postmortem brain samples from ALS and AD sufferers in North America, and importantly, no BMAA was detected in healthy controls. This provided further evidence that bioaccumulation of BMAA in neurodegenerative disease sufferers may be a global concern.
In 2007, Banack et al. [35] employed five different detection methods to show that laboratory cultures of free-living marine Nostoc species produce BMAA, proving that it could be produced globally. The ubiquitous nature of cyanobacteria means that given the right conditions, bioaccumulation of BMAA could potentially occur in any of the greatly varying environments in which cyanobacteria are found [26]. This presumption is supported by the growing number of reports of BMAA detection in different environments. Bioaccumulation of BMAA has been shown in aquatic species such as zooplankton, fish, mussels and oysters in the Baltic Sea [36], as well as in food chains in South Florida [37]. BMAA has also been detected in fresh water lakes in China [38], and in desert dust from the Middle East [39]. The potential for bioaccumulation is supported by the in vivo uptake of BMAA by the aquatic macrophyte Ceratophyllum demersum in an experimental system [40], and bioconcentration in the zooplankton Daphnia magna [41].
3. Neurodegeneration is Caused by Excitotoxicity
Excitatory amino acids (EAAs) act as neurotransmitters within the nervous system [42]. Their action is performed by binding to EAA receptors that are present on all nerve cells, particularly concentrated in the synapses. EAA receptors mediate excitatory synaptic transmission via control of the flow of ions, most notably Ca2+, K+, Na+, Mg2+ and Cl− [43]. Malfunctions in this system can lead to neurons being damaged and fatally compromised, a process known as excitotoxicity [44]. Excitotoxic cell death involves prolonged depolarization of neurons, changes in intracellular calcium concentrations, and the activation of enzymatic and nuclear mechanisms of cell death [45]. The main EAA receptors are quisqualate/α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-d-apartate (NMDA) and metabolic glutamate receptors (mGluR), all of which are activated by glutamate and similar substances. A review by Doble [45] explained all these concepts and activities in great detail. The idea that excitotoxicity is a main player in neurodegenerative disease is supported by many studies that have shown that there is an increased level of glutamate found in the cerebrospinal fluid of ALS patients [46–50].
4. Summary of the Multiple Mechanisms of BMAA Activity
With substantial and ever growing evidence that BMAA does play a role in the onset and progression of neurodegenerative diseases, the most important question is; what mode of activity does BMAA exert? Although BMAA had not yet been discovered, Dastur [51] fed cycad flour pancakes to Rhesus monkeys in 1964, observing various effects including muscle atrophy and neurodegeneration. Although given that cycad flour was used rather than pure BMAA, these effects may have potentially been influenced by other compounds present in the mixture. Immediately following the discovery of BMAA in 1967, Bell et al. [52,53] also conducted some very basic toxicity assays by intraperitoneally injecting BMAA into chicks and rats and observing the development of neurological symptoms via impairment of normal physical function in both cases. These findings were repeated in 1972 by Polsky et al. [54], with addition of mice as test subjects. In all cases all the animals suffered the same symptoms, namely weakness, convulsions and general lack of coordination. After this study no productive research using BMAA was conducted until 1987, when the revolutionary investigation of Spencer et al. [13] was reported. In those studies macaques were fed 100–350 mg/kg BMAA daily for up to thirteen weeks, resulting in corticomotoneuronal dysfunction, Parkinsonian features and behavioural abnormalities. The 1991 study of Rakonczay et al. [55] and 1993 study of Matsuoka et al. [56] produced similar findings, with BMAA injected rats displaying acute physical impairment including poor balance, poor coordination and convulsions. Contrary to these observations, the 1989 study of Perry et al. [57] fed high doses of BMAA (15.5 g/kg total, 500 mg/kg or 1000 mg/kg doses) to mice over an 11 week period, and observed no behavioural abnormalities during the course of the experiment. Analysis of brain and liver samples collected post euthanasia failed to find any evidence of neurochemical or neuropathological changes in the any of the sample animals [57]. Similarly, in 2006 the group of Cruz-Aguado et al. [58] fed 28 mg/kg of BMAA, which was an exposure level they deemed to be an accurate environmental representation, to mice daily for 30 days. In this study they found no indication that neurological damage had occurred [58]. In the critical review by Karamyan and Speth [31], the authors raise doubts over the methods used by Perry et al. [57] to observe behavioural differences, and over the doses used by Cruz-Aguado et al. [58], as possible explanations for their negative observations. It was also suggested by Banack et al. [59] that the mouse model may be a poor model to demonstrate the neurotoxicity of BMAA. It is evident that the bulk of early research was focused on either detecting BMAA in known (deceased) neurodegenerative sufferers, or observing behavioural changes in lab animals fed or injected with BMAA. While this information was useful it did nothing to explain the actual mechanisms of BMAA activity.
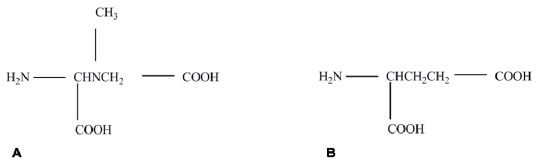
The first mechanistic BMAA research was performed in 1988 when Weiss and Choi [60] discovered that BMAA only displayed activity in vitro when a physiological concentration (10 mM and above) of bicarbonate (HCO3−) ions were present in the media. This discovery was soon followed by Richter and Mena’s [61] observation that BMAA inhibited glutamate binding in the synaptic junctions of rat brains at 1 mM, but only in the presence of 20–25 mM bicarbonate ions. The observation that effective inhibition of glutamate receptors was not achieved by BMAA at the extremely high level of 10 mM, independent of bicarbonate ions, supported the findings of Weiss and Choi [60], that HCO3− was required for BMAA activity to occur. Follow up experiments showed that BMAA could bind to NMDA and non-NMDA receptors on mouse cortical neurons [62]. The dependence of BMAA on HCO3− was a critical discovery as it greatly affected the results of experiments conducted using freshly isolated tissues where experimental reagents are generally simple and defined, and often did not contain HCO3−. Using these leads Myers and Nelson [63] identified a β-carbamate of BMAA (formed in the presence of bicarbonate), that shares structural characteristics with glutamic acid (glutamate, see Figure 2). This led to an explanation of a mechanism of activity, as it suggested that BMAA may have the ability to inhibit glutamate receptors. From this point on all researchers used media and/or buffers supplemented with a minimum of 20 mM bicarbonate in all active in vitro assays.
Figure 2.
Comparison of the structure of (A) β-carbamate (BMAA adduct) and (B) glutamtic acid (glutamate).
In 1990 Lindström et al. [64] gave intracerebral injections (10 or 400 μg) of BMAA to mice and after one week they noticed a decrease in noradrenalin (NA) levels in the hypothalamus, while there was no effect on dopamine or serotonin levels. No physical or behavioural effects were observed in the exposed animals. They suggested that the decrease in NA levels in the tissue may have been the result of BMAA activity on NMDA receptors, causing a release of NA. Copani et al. [65] conducted a thorough investigation of BMAA binding capabilities and specificities by performing in vitro assays. Brain slices and mixed primary cultures taken from 8-day old rats were exposed to BMAA at 1 mM in conjunction with various neural metabolites and antagonists of NMDA. Their results indicated that BMAA acts as a mixed agonist of metabotropic and NMDA receptors, and as seen in other studies, BMAA activity was enhanced by the presence of bicarbonate ions at 25 mM [65]. The groups of Rakonczay et al. [55] and Matsuoka et al. [56] performed a series of binding assays using various receptor antagonists after giving intracerebroventricular injections of BMAA (500 μg/day, for up to 60 days) to rats. Their results indicated that BMAA has a mixed agonistic effect on EAA, NMDA and quisqualate/AMPA receptors in the synapse. In 1991–1992 Duncan’s research group conducted a number of experiments relating to the body’s ability to take up BMAA after oral exposure and transport and accumulate it in the brain. When cynomologous monkeys were orally dosed with BMAA, a maximum of 20% of the administered dose was metabolized, and no greater than 2.1% was excreted indicating that approximately 80% of orally consumed BMAA was absorbed into systemic circulation [66]. The 1998 study by Kisby et al. [67] reported that BMAA was detected in the cerebrospinal fluid of orally dosed monkeys, and in the brain tissue of intraperitoneally dosed rats, suggesting that BMAA is able to cross the blood-brain barrier. In a later study, Duncan et al. [68] demonstrated in rats that, after intravenous injection, acute BMAA levels in the brain peaked at eight h post administration. They also demonstrated that BMAA is taken up into the brain by the large neutral amino acid carrier of the blood-brain barrier, which suggests that uptake may be sensitive to the same factors that affect neutral amino acid transport such as diet, metabolism, disease and age [69]. In essence this means that BMAA uptake into the brain may be increased in times of stress.
Brownson et al. [70] assayed rat brain cells for changes in the concentration of Ca2+ in the presence of BMAA (5 mM) with or without HCO3− ions. This experiment indicated that there was a small increase in intracellular Ca2+ concentration with BMAA only, but a large increase when BMAA and HCO3− were added together. This further supports the belief that BMAA is dependant on HCO3− as a cofactor and that the correspnding β-carbamate is the active compound. It also suggests another potential mechanism of activity as impairment to intracellular calcium homeostasis has been shown to cause disruptions in Ca2+-dependant cascades that lead to neuronal cell death and neurodiseases [71,72].
The study of Nedeljkov et al. [73] measured the membrane input resistance of the nerve cells of the leech Haemopis sanguisuga after treatment with BMAA (100 μM–10 mM) and HCO3− (20 mM). A significant reduction in input membrane resistance was measured, indicating that BMAA depolarizes the cell by increasing membrane permeability and conductance.
In 2007, Buenz and Howe [74] intracranially injected 10 μL of 100 mM BMAA into mice that were then euthanized at 24 h post exposure. This study showed that BMAA caused injury to hippocampal neurons. They also demonstrated that BMAA increasingly caused a degree of cell death in NSC-34 cells (a mouse derived spinal motor neuron-like cell line) as the amount of BMAA administered increased from 100 μM up to 1 mM. A study conducted by Lobner et al. in 2007 [75] showed that BMAA at concentrations as low as 10 μM can potentiate neuronal injury caused by other known neurotoxins such as amyloid-β and MPP+. This observation holds great significance determining that very low concentrations of BMAA (orders of magnitude lower than previously thought) can potentially cause serious neurological damage if other factors are involved. This study also showed that BMAA has three-fold activity by causing excitotoxicity on NMDA and metabotropic glutamate receptor subtype 5 (mGluR5) receptors, and via oxidative stress. This supports the notion that BMAA may play a role in a variety of different neurodegenerative conditions. Rao et al. [76] concluded that low concentrations of BMAA (30 μM) selectively injure motor neurons via excitotoxic activation of AMPA/kainite receptors. They also showed that BMAA induces increases in Ca2+ concentrations and the generation of selective reactive oxygen species (ROS) in motor neurons, with minimal effect on other spinal neurons. Liu et al. [77] validated the three-fold activity of BMAA described by Lobner et al. [75], as well as suggesting that the mechanism BMAA uses to induce oxidative stress is through inhibition of the cystine/glutamate antiporter system Xc−, leading to glutathione depletion and oxidative stress [77]. In 2009, Nunn and Ponnusamy [78] found that 2,3-diaminopropionic acid, the dimethylated product of BMAA, and methylamine were formed in liver and kidney preparations from rats exposed to 10 mM BMAA for 24 h in vitro. It is worth noting that this product was not formed in brain tissues in this study. This provides evidence of yet another method of toxicity by BMAA, although the test dose is potentially too high for the result to be environmentally significant. Production of methylamine is significant as it has been shown to produce a state of oxidative stress in rats [79]. In 2009, Karlsson et al. [80] injected radioactively labelled BMAA into frogs and mice, then euthanized the animals at 30 min, 1 h, 3 h, 24 h and 12 days post injection. The results showed that BMAA interacts/binds with melanin, particularly during its synthesis, and increasingly bioaccumulates in melanin and neuromelanin-containing cells over time. The authors proposed that this may provide a link between BMAA and the PDC symptom of pigmentary retinopathy [80]. Also in 2009, Lopicic et al. [81] showed that 1 mM BMAA (with 20 mM bicarbonate) causes in vitro membrane potential depolarization of leech nerve cells by action on non-NMDA ionotropic glutamate receptors. A concomitant increase in cell membrane input conductance, as well as an increase in Na+ activity and a decrease in K+ activity was noted. This indicated that, in addition to AMPA/kainite receptors, BMAA could initiate excitotoxicity through the activation of other non-NMDA ionotropic glutamate receptors. In 2009, Santucci et al. [82] injected 5–10 nM BMAA into the eyes of mice, that were then euthanized between 4 and 24 h post administration. Increases in retinal neuron death and the production of ROS were observed in this study. Also in 2009, Purdie et al. [83] exposed zebrafish embryos to BMAA at up to 50,000 μg/L (approx. 300 mu;M) for 5 days. This exposure resulted in a range of neuromuscular and developmental abnormalities, which could be directly related to disruptions to the glutamatergic signalling pathways.
In 2010, Cucchiaroni et al. [84] found rat neurons exposed to 1 or 3 mM BMAA displayed increases in the production of ROS, influx of Ca2+ and a massive release of cytochrome-c (cyt-c) into the cytosol. This study also demonstrated that activity was predominantly mediated via mGluR1 receptors. These observations indicate disruption to mitochondrial activity, excitotoxicity, and induction of apoptosis induced by exposure to BMAA. More recently Karlsson et al. [85] injected 50 and 200 mg/kg BMAA into neonatal rats and found that it inhibited neural development leading to long-term cognitive impairment and supporting the zebrafish data implicating BMAA as a developmental neurotoxin [83]. Most recently, Lee and McGeer [86] exposed three different neuronderived human cancer cell lines to BMAA. Interestingly they observed that BMAA did not cause damage to human neurons and concluded that the hypothesis of BMAA causing neurodegeneration in humans was not tenable [86]. It should however be noted, that the cell lines they used were highly proliferative immortalized cells that differ significantly in physiological characteristics from normal neurons in vivo. Summaries of both in vivo and in vitro investigations into the bioactivity of BMAA are presented in Tables 1 and 2 respectively.
Table 1.
A chronological summary of mechanisms of BMAA activity determined by in vivo research.
| Routez of exposure | Species | Dose level, exposure time | Research group and date | Observations |
|---|---|---|---|---|
| Intraperitoneal injections | Rat Chicken |
6–14 μmoles/g body weight 3–7 μmoles/g body weight |
Vega and Bell. 1967 | Weakness, convulsions and uncoordination |
| Intraperitoneal injections | Rat Chicken Mouse |
6–14 μmoles/g body weight 3–7 μmoles/g body weight 6–14 μmoles/g body weight |
Polski et al. 1972 | Weakness, convulsions and uncoordination |
| Perorally Intraperitoneal injections | Monkey Rat |
100–350 mg/kg, 12 months 500 mg/kg daily, 14 days |
Kisby et al. 1988 | BMAA can cross from gut to blood BMAA can cross the blood brain barrier |
| Gavage | Monkey | 100–350 mg/kg daily, up to 10 weeks | Spencer et al. 1987 | Corticomotoneuronal dysfunction, Parkinsonian features and behavioural abnormalities |
| Gavage | Cynomologous monkey | 500 mg/kg daily, 18 days, then 500 mg/kg 2 daily, 28 days, then 100mg/kg 2 daily, 30 days |
Perry et al. 1989 | No behavioral or physiological effects observed |
| Intracerebral injections | Rat | 10 μg or 400 μg/150–200 g rat | Lindström et al. 1990 | Activation of NMDA receptor, release noradrenalin from cells |
| Intracerebroventricular injections | Rat | 500 μg/day 200–250 g body weight, 10–60 days |
Rakonczay et al. 1990 Matsuoka et al. 1993 |
Agonistic effects on NMDA, EAA and AMPA receptors in synapse Physical impairment. Mixed agonistic receptor activity |
| Gavage and intravenous injections | Cynomologous monkey, rat | 2 mg/kg gavage; 1 mg/kg iv 100 mg/kg gavage; 24–400 mg/kg iv |
Duncan et al. 1991–1992 | 80% of ingested BMAA enters systemic circulation. BMAA can cross the blood brain barrier. BMAA is transported by neutral amino acid carriers so uptake can be influenced by diet, metabolism, disease and age |
| Dosed feed pellets | Mouse | 28 mg/kg daily, 30 days | Cruz-Aguado et al. 2006 | No motor, cognitive or neuropathological effect observed |
| Intracranial injections | Mouse | 10 μL of 100 mM, 24 h | Buenz and Howe. 2007 | Injury to hippocampal neurons |
| Intravenous and subcutaneous injections | Mouse and frog | 7.3 μg/kg, 30 min, 1 h, 3 h, 24 h, 12 days | Karlsson et al. 2009 | BMAA interacts/binds melanin, particularly during synthesis, and accumulates in melanin and neuromelanin containing cells increasingly over time |
| Ocular injections | Mouse | 5–10 nmol, 4, 8 and 24 h | Santucci et al. 2009 | Retinal neuron death and production of ROS |
Table 2.
A chronological summary of mechanisms of BMAA activity determined by in vitro research.
| Experimental model | Species | Dose level, exposure time | Research group and date | Conclusion |
|---|---|---|---|---|
| Primary cortical neurons | Mouse | 3 mM, 1 h With and without 10–24 mM HCO3− | Weiss and Choi, 1988 | BMAA activity is dependent on bicarbonate at a min. of 20mM |
| Primary cortical neurons | Mouse | 300 μM–3 mM, 24 h | Weiss et al. 1989 | BMAA has activity on NMDA and non-NMDA receptors |
| Primary cortical neurons | Rat | 1 mM | Richter and Mena, 1989 | Inhibition of glutamate binding in synapse, impaired neuron function |
| Chemical assay | - | Myers and Nelson, 1990 | Formation of bicarbonate adduct with structural similarity to glutamate | |
| Brain slices | Rat | 1 mM, acute | Copani et al. 1991 | BMAA acts as a mixed agonist of metabotropic and NMDA receptors |
| Minced brain | Rat | 5 mM, acute | Brownson et al. 2002 | Impairment of intracellular calcium ion homeostasis. Possible neuronal death. Effects on calcium dependent cascades |
| Primary nerve cells | Leech | 1–10 mM, acute | Nedeljkov et al. 2005 | Depolarisation of cell, impaired nerve function. Membrane permeabilisation. Activity via glutamate receptors |
| Primary embryonic spinal cord culture | Mouse | 30–1000 μM, 20–24 h | Rao et al. 2006 | Increase on calcium ion concentration and ROS. Selective damage to motor neurons |
| Primary mixed cortical cells | Mouse | 0.1–10 mM, 24 h 3 mM, 3 h (DCFDA) | Lobner et al. 2007 | Potentiation of other insults, makes cells more sensitive to other compounds. Increase in ROS |
| NSC-34 cells | Mouse | 50–1000 μM, 18 h | Buenz and Howe 2007 | Dose dependent death of NSC-34 cells |
| Primary mixed cortical cell cultures | Mouse | 3 mM, 3 h | Liu et al. 2009 | Induction of oxidative stress is through inhibition of the cystine/glutamate antiporter system Xc− |
| Brain slices. Brain, liver, kidney homogenates | Rat | 10 mM, 30 min for slices 1 h for homogenates |
Nunn and Ponnusamy, 2009 | The dimethylated product of BMAA, 2,3-diaminopropionic acid was formed in liver and kidney (but not brain) preparations |
| Nerve cells | Leech | 100–3000 μM, acute | Lopicic et al. 2009 | Action on non-NMDA ionotropic glutamate receptors, with a concomitant increase in cell membrane input conductance, as well as an increase in Na+ activity and a decrease in K+ activity. Possible initiation of excitotoxicity through activation of non-NMDA ionotropic glutamate receptors |
| Brain slices | Rat | 100–10000 μM, acute | Cucchiaroni et al. 2010 | BMAA activates mGluR1 receptors to cause neuronal degeneration Massive release of cyt-c into cytosol |
When reviewing BMAA literature it quickly becomes clear that there are large differences in opinion. When Borenstein et al. [7] proposed their correlation supporting the role of cycads (potential involvement of BMAA), Steele and McGeer [87] raised doubt over the statistics. When Duncan et al. [14] indicated that greater than 80% of BMAA is removed from cycad flour during processing (washing), Cheng and Banack [88] claimed that due to sampling methods, the amount of BMAA detected by Duncan et al. [14] has been underestimated by 7- to 30-fold and based on the assumption that BMAA is washed away, another candidate compound such as β-sitosterol β-d-glucoside (BSSG) is suggested to play the same proposed role [89]. Various studies have been conducted to indicate that BSSG does display neurotoxic properties [90,91]. Interestingly despite suggestion that BMAA and BSSG are alternatives for each other, involvement of either or both, would support the same cycad hypothesis.
5. A Summary of the Mode of Action of BMAA based on the Current Literature
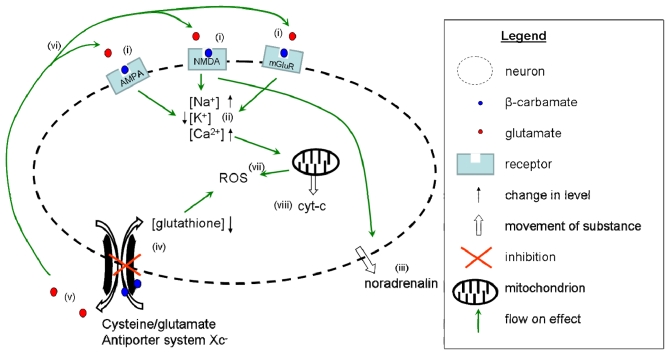
The studies listed in Tables 1 and 2, while executed on vastly different test models with varying measurement parameters, can be combined to generate an image of the mechanisms of action of BMAA on a primary motor neuron as illustrated in Figure 3. After being orally consumed, 80% of ingested BMAA passes from the gut into the blood stream [66]. BMAA then crosses the blood-brain barrier via large neutral amino acid carriers [68]. The physiological concentrations of bicarbonate ions (10 mM and above) reacts with BMAA to form a β-carbamate [60]. In this form, BMAA can compete in binding various glutamate receptors, such as NMDA receptors [55,56,64,65], AMPA receptors [55,56], and metabotropic and ionotropic glutamate receptors [65,73,81,84] (Figure 3i). Activation of the various glutamate receptors leads to shifts in cellular ion concentrations resulting in increases in Na+ [81] and Ca2+ [70,76,84], and a decrease in K+ [81] concentrations (Figure 3ii). Activation also causes the cell to become depolarised [73] leading to permeabilisation of the cell membrane, resulting in the release of noradrenalin [64] (Figure 3iii). BMAA also inhibits the cysteine/glutamate antiporter system Xc− [77] (Figure 3iv), preventing the uptake of cysteine, resulting in glutathione depletion, which contributes to increases in oxidative stress. At the same time the system Xc− increases the release of glutamate from the cell (Figure 3v), which can then bind to glutamate receptors increasing damage by excitotoxicity [77] (Figure 3vi). Increases in intracellular Ca2+ concentrations disrupt normal mitochondrial function leading to the release of ROS into the cytoplasm, thereby contributing to the observed increases in ROS [75,76,82,84] (Figure 3vii). In addition, cytochrome-c is released from the mitochondria [84] (Figure 3viii) resulting in the induction of apoptosis.
Figure 3.
Illustrative summary of the modes of action of BMAA on neurons. In vivo, BMAA is present as a β-carbamate (represented by the blue dots), which binds to NMDA, AMPA and mGlu receptors (i). Activation of glutamate receptors results in an increase in the levels of Na+ and Ca2+ in the cell, accompanied by a reduction in K+ (ii). The cell becomes depolarised and the membrane becomes permeable, as illustrated by the dotted line, and combined with NMDA receptor activity, noradrenalin is released from the cell as a result (iii). The cysteine/glutamate antiporter system Xc− is inhibited, as indicated by the red X (iv), leading to intracellular depletion of glutathione and an increase in ROS. This inhibition also causes an increase in the release of glutamate (v), which then binds to receptors to induce further excitotoxicity (vi). All these mechanisms combine to cause an increase in the generation of ROS (vii). The elevation of Ca2+ leads to overload of the mitochondria resulting in a massive release of cyt-c into the cytosol (viii).
6. Concluding Remarks
Whilst the incidence of ALS-PDC on Guam was 100 times that of the world average, it peaked at 120 cases per 100,000 people, meaning that the majority of individuals thought to be exposed to BMAA still escaped disease. Clearly there is still much to learn about the role(s) that BMAA plays in neurodegeneration. Karamyan and Speth have reviewed the available literature on the evidence for and against the involvement of BMAA in the development of ALS/PDC [31]. They concluded that the majority of studies indicate that BMAA is toxic. It is worth noting that the two studies [57,58] that observed no effect both utilized oral administration methods, perhaps implying reduced toxicity via this delivery method. One must also consider that the severe effects observed by Spencer et al. [13] were also obtained with oral dosing.
When considering all the published data, it appears certain that BMAA can contribute to the onset and progression of neurodegenerative disease in certain susceptible individuals. It would be useful to focus on better understanding the proposed mechanisms of BMAA activity, as well as identifying new as yet undescribed mechanisms that might play an important role in the overall potency of BMAA. Without a sound understanding of how BMAA truly works, it is impossible to predict the level of risk it poses with any significant degree of confidence. One question posed in the review by Karamyan and Speth [31] that is likely to be answered in the affirmative was “are there interactions between BMAA and other exogenous substances with possible synergetic toxicity?”. The potential dangers of BMAA acting as an accessory or combinatorial toxin, rather than being highly toxic as a sole entity, were indicated by Lobner et al. [75] when they demonstrated that BMAA can potentiate the activity of other insults. As BMAA has been shown to be co-present with other cyanotoxins, such as microcystin, anatoxin-a, nodularin and saxitoxin [92], this potentiation capability, may implicate BMAA as an important factor when considering the management strategies of these other toxins. The debate between BSSG and BMAA appears to be very polarized, with acceptance of one causative agent completely ruling out the significance of the other. It may however be more logical to consider the idea that as the two compounds were isolated from the same source, they are likely to be present together environmentally, and could therefore act in a combination, potentially far more potent than either agent alone, to induce neurological damage. There is little doubt that if present in sufficient concentrations, BMAA exerts multiple modes of neurotoxic activity, with perhaps further modes yet to be defined. With growing reports of its presence in varied environments it is important that research to understand the complete nature of BMAA toxicity continue. Equipped with a greater knowledge and understanding of the mechanisms of BMAA toxicity, we will be able to more accurately evaluate and assess the human health risks posed by exposure to this cyanotoxin.
References
- 1.Arnold A, Edgren DC, Palladino VS. Amyotrophic lateral sclerosis; fifty cases observed on Guam. J Nerv Ment Dis. 1953;117:135–139. [PubMed] [Google Scholar]
- 2.Kurland LT, Mulder DW. Epidemiologic investigations of amyotrophic lateral sclerosis. I. Preliminary report on geographic distribution and special reference to the Mariana Islands, including clinical and pathologic observations. Neurology. 1954;4:438–448. doi: 10.1212/wnl.4.6.438. [DOI] [PubMed] [Google Scholar]
- 3.Kurland LT, Mulder DW. Epidemiologic investigations of amyotrophic lateral sclerosis. I. Preliminary report on geographic distribution, with special reference to the Mariana Islands, including clinical and pathologic observations. Neurology. 1954;4:355–378. doi: 10.1212/wnl.4.5.355. [DOI] [PubMed] [Google Scholar]
- 4.Banack SA, Murch SJ, Cox PA. Neurotoxic flying foxes as dietary items for the Chamorro people, Marianas Islands. J Ethnopharmacol. 2006;106:97–104. doi: 10.1016/j.jep.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 5.Whiting MG. Food Practices in Als Foci in Japan, the Marianas, and New Guinea. Fed Proc. 1964;23:1343–1345. [PubMed] [Google Scholar]
- 6.Whiting M, Spatz M, Matsumoto H. Research progress on cycads. Econ Bot. 1966;20:98–102. [Google Scholar]
- 7.Borenstein AR, Mortimer JA, Schofield E, Wu Y, Salmon DP, Gamst A, Olichney J, Thal LJ, Silbert L, Kaye J, et al. Cycad exposure and risk of dementia, MCI, and PDC in the Chamorro population of Guam. Neurology. 2007;68:1764–1771. doi: 10.1212/01.wnl.0000262027.31623.b2. [DOI] [PubMed] [Google Scholar]
- 8.Vega A, Bell EA. α-Amino-β-methylaminopropionic acid, a new amino acid from seeds of Cycas circinalis. Phytochemistry. 1967;6:759–762. [Google Scholar]
- 9.Vega A, Bell EA, Nunn PB. The preparation of l- and d-α-amino-β-methylaminopropionic acids and the identification of the compound isolated from Cycas circinalis as the l-isomer. Phytochemistry. 1968;7:1885–1887. [Google Scholar]
- 10.Spencer PS, Ohta M, Palmer VS. Cycad use and motor neurone disease in Kii peninsula of Japan. Lancet. 1987;2:1462–1463. doi: 10.1016/s0140-6736(87)91159-7. [DOI] [PubMed] [Google Scholar]
- 11.Spencer PS, Palmer VS, Herman A, Asmedi A. Cycad use and motor neurone disease in Irian Jaya. Lancet. 1987;2:1273–1274. doi: 10.1016/s0140-6736(87)91883-6. [DOI] [PubMed] [Google Scholar]
- 12.Spencer PS, Nunn PB, Hugon J, Ludolph A, Roy DN. Motorneurone disease on Guam: Possible role of a food neurotoxin. Lancet. 1986;1:965. doi: 10.1016/s0140-6736(86)91059-7. [DOI] [PubMed] [Google Scholar]
- 13.Spencer PS, Nunn PB, Hugon J, Ludolph AC, Ross SM, Roy DN, Robertson RC. Guam amyotrophic lateral sclerosis-parkinsonism-dementia linked to a plant excitant neurotoxin. Science. 1987;237:517–522. doi: 10.1126/science.3603037. [DOI] [PubMed] [Google Scholar]
- 14.Duncan MW, Kopin IJ, Garruto RM, Lavine L, Markey SP. 2-Amino-3 (methylamino)-propionic acid in cycad-derived foods is an unlikely cause of amyotrophic lateral sclerosis/Parkinsonism. Lancet. 1988;2:631–632. doi: 10.1016/s0140-6736(88)90671-x. [DOI] [PubMed] [Google Scholar]
- 15.Garruto R, Yanagihara R, Gajdusek DC. Cycads and amyotrophic lateral sclerosis/Parkinsonism dementia. Lancet. 1988;332:1079–1079. doi: 10.1016/s0140-6736(88)90099-2. [DOI] [PubMed] [Google Scholar]
- 16.Duncan MW, Steele JC, Kopin IJ, Markey SP. 2-Amino-3-(methylamino)-propanoic acid (BMAA) in cycad flour: An unlikely cause of amyotrophic lateral sclerosis and parkinsonism-dementia of Guam. Neurology. 1990;40:767–772. doi: 10.1212/wnl.40.5.767. [DOI] [PubMed] [Google Scholar]
- 17.Cox PA, Sacks OW. Cycad neurotoxins, consumption of flying foxes, and ALS-PDC disease in Guam. Neurology. 2002;58:956–959. doi: 10.1212/wnl.58.6.956. [DOI] [PubMed] [Google Scholar]
- 18.Monson CS, Banack SA, Cox PA. Conservation implications of chamorro consumption of flying foxes as a possible cause of amyotrophic lateral sclerosis/Parkinsonism dementia complex in guam. Conserv Biol. 2003;17:678–686. [Google Scholar]
- 19.Banack SA, Cox PA. Biomagnification of cycad neurotoxins in flying foxes: Implications for ALS-PDC in Guam. Neurology. 2003;61:387–389. doi: 10.1212/01.wnl.0000078320.18564.9f. [DOI] [PubMed] [Google Scholar]
- 20.Cox PA, Banack SA, Murch SJ. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc Natl Acad Sci USA. 2003;100:13380–13383. doi: 10.1073/pnas.2235808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams D. The Ecology of Cyanobacteria. Kluwer Academic; New York, NY, USA: 2002. pp. 523–561. [Google Scholar]
- 22.Cox PA, Banack SA, Murch SJ, Rasmussen U, Tien G, Bidigare RR, Metcalf JS, Morrison LF, Codd GA, Bergman B. Diverse taxa of cyanobacteria produce β-N-methylamino-l-alanine, a neurotoxic amino acid. Proc Natl Acad Sci USA. 2005;102:5074–5078. doi: 10.1073/pnas.0501526102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esterhuizen M, Downing TG. Beta-N-methylamino-l-alanine (BMAA) in novel South African cyanobacterial isolates. Ecotoxicol Environ Saf. 2008;71:309–313. doi: 10.1016/j.ecoenv.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Marler TE, Snyder LR, Shaw CA. Cycas micronesica (Cycadales) plants devoid of endophytic cyanobacteria increase in [beta]-methylamino-l-alanine. Toxicon. 2010;56:563–568. doi: 10.1016/j.toxicon.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Banack SA, Cox PA. Distribution of the neurotoxic nonprotein amino acid BMAA in Cycas micronesica. Bot J Linn Soc. 2003;143:165–168. [Google Scholar]
- 26.Murch SJ, Cox PA, Banack SA, Steele JC, Sacks OW. Occurrence of β-methylamino-l-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol Scand. 2004;110:267–269. doi: 10.1111/j.1600-0404.2004.00320.x. [DOI] [PubMed] [Google Scholar]
- 27.Murch SJ, Cox PA, Banack SA. A mechanism for slow release of biomagnified cyanobacterial neurotoxins and neurodegenerative disease in Guam. Proc Natl Acad Sci USA. 2004;101:12228–12231. doi: 10.1073/pnas.0404926101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montine TJ, Li K, Perl DP, Galasko D. Lack of beta-methylamino-l-alanine in brain from controls, AD, or Chamorros with PDC. Neurology. 2005;65:768–769. doi: 10.1212/01.wnl.0000174523.62022.52. [DOI] [PubMed] [Google Scholar]
- 29.Snyder LR, Cruz-Aguado R, Sadilek M, Galasko D, Shaw CA, Montine TJ. Lack of cerebral BMAA in human cerebral cortex. Neurology. 2009;72:1360–1361. doi: 10.1212/WNL.0b013e3181a0fed1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradley WG, Mash DC. Beyond Guam: The cyanobacteria/BMAA hypothesis of the cause of ALS and other neurodegenerative diseases. Amyotroph Lateral Scler. 2009;10:7–20. doi: 10.3109/17482960903286009. [DOI] [PubMed] [Google Scholar]
- 31.Karamyan VT, Speth RC. Animal models of BMAA neurotoxicity: A critical review. Life Sci. 2008;82:233–246. doi: 10.1016/j.lfs.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 32.Cohen SA, de Antonis KM. Applications of amino acid derivatization with 6-aminoquinolyl- N-hydroxysuccinimidyl carbamate. Analysis of feed grains, intravenous solutions and glycoproteins. J Chromatogr A. 1994;661:25–34. doi: 10.1016/0021-9673(93)E0821-B. [DOI] [PubMed] [Google Scholar]
- 33.Crimmins DL, Cherian R. Increasing the sensitivity of 6-aminoquinolyl-Nhydroxysuccinimidyl carbamate amino acid analysis: A Simple Solution. Anal Biochem. 1997;244:407–410. doi: 10.1006/abio.1996.9930. [DOI] [PubMed] [Google Scholar]
- 34.Pablo J, Banack SA, Cox PA, Johnson TE, Papapetropoulos S, Bradley WG, Buck A, Mash DC. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer’s disease. Acta Neurol Scand. 2009;120:216–225. doi: 10.1111/j.1600-0404.2008.01150.x. [DOI] [PubMed] [Google Scholar]
- 35.Banack SA, Johnson HE, Cheng R, Cox PA. Production of the Neurotoxin BMAA by a Marine Cyanobacterium. Mar Drugs. 2007;5:180–196. doi: 10.3390/md504180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jonasson S, Eriksson J, Berntzon L, Spáčil Z, Ilag LL, Ronnevi L-O, Rasmussen U, Bergman B. Transfer of a cyanobacterial neurotoxin within a temperate aquatic ecosystem suggests pathways for human exposure. Proc Natl Acad Sci USA. 2010;107:9252–9257. doi: 10.1073/pnas.0914417107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brand LE, Pablo J, Compton A, Hammerschlag N, Mash DC. Cyanobacterial blooms and the occurrence of the neurotoxin, beta-N-methylamino-l-alanine (BMAA), in South Florida aquatic food webs. Harmful Algae. 2010;9:620–635. doi: 10.1016/j.hal.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li A, Tian Z, Li J, Yu R, Banack SA, Wang Z. Detection of the neurotoxin BMAA within cyanobacteria isolated from freshwater in China. Toxicon. 2010;55:947–953. doi: 10.1016/j.toxicon.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 39.Cox PA, Richer R, Metcalf JS, Banack SA, Codd GA, Bradley WG. Cyanobacteria and BMAA exposure from desert dust: A possible link to sporadic ALS among Gulf War veterans. Amyotroph Lateral Scler. 2009;10:109–117. doi: 10.3109/17482960903286066. [DOI] [PubMed] [Google Scholar]
- 40.Esterhuizen M, Pflugmacher S, Downing TG. [beta]-N-Methylamino-l-alanine (BMAA) uptake by the aquatic macrophyte Ceratophyllum demersum. Ecotoxicol Environ Saf. 2011;74:74–77. doi: 10.1016/j.ecoenv.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Lürling M, Faassen EJ, van Eenennaam JS. Effects of the cyanobacterial neurotoxin β-N-methylamino-l-alanine (BMAA) on the survival, mobility and reproduction of Daphnia magna. J Plankton Res. 2011;33:333–342. [Google Scholar]
- 42.Monaghan DT, Bridges RJ, Cotman CW. The excitatory amino acid receptors: Their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- 43.Smith PF, de Waele C, Vidal PP, Darlington CL. Excitatory amino acid receptors in normal and abnormal vestibular function. Mol Neurobiol. 1991;5:369–387. doi: 10.1007/BF02935559. [DOI] [PubMed] [Google Scholar]
- 44.Curtis DR, Watkins JC. The excitation and depression of spinal neurones by structurally related amino acids. J Neurochem. 1960;6:117–141. doi: 10.1111/j.1471-4159.1960.tb13458.x. [DOI] [PubMed] [Google Scholar]
- 45.Doble A. The role of excitotoxicity in neurodegenerative disease: Implications for therapy. Pharmacol Ther. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- 46.Shaw PJ. Molecular and cellular pathways of neurodegeneration in motor neurone disease. J Neurol Neurosurg Psychiatry. 2005;76:1046–1057. doi: 10.1136/jnnp.2004.048652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strong MJ, Kesavapany S, Pant HC. The pathobiology of amyotrophic lateral sclerosis: A proteinopathy? J Neuropathol Exp Neurol. 2005;64:649–664. doi: 10.1097/01.jnen.0000173889.71434.ea. [DOI] [PubMed] [Google Scholar]
- 48.Cozzolino M, Ferri A, Teresa Carri M. Amyotrophic lateral sclerosis: From current developments in the laboratory to clinical implications. Antioxid Redox Signal. 2008;10:405–444. doi: 10.1089/ars.2007.1760. [DOI] [PubMed] [Google Scholar]
- 49.Boillee S, Vande Velde C, Cleveland DW. ALS: A disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 50.Majoor-Krakauer D, Willems PJ, Hofman A. Genetic epidemiology of amyotrophic lateral sclerosis. Clin Genet. 2003;63:83–101. doi: 10.1046/j.0009-9163.2002.00001.x. [DOI] [PubMed] [Google Scholar]
- 51.Dastur DK. Cycad toxicity in monkeys: Clinical, pathological, and biochemical aspects. Fed Proc. 1964;23:1368–1369. [PubMed] [Google Scholar]
- 52.Bell EA, Vega A, Nunn PB. Neurotoxic Effects of α-Amino-β-methyl-aminopropionic Acid. Proceedings of the Fifth Conference on Cycad Toxicity; Miami, FL, USA. 24–25 April 1967. [Google Scholar]
- 53.Nunn PB, Vega A, Bell EA. Neurotoxic effects of alpha-amino-beta-methylaminopropionic acid. Biochem J. 1968;106:15. [Google Scholar]
- 54.Polsky FI, Nunn PB, Bell EA. Distribution and toxicity of alpha-amino-beta-methylaminopropionic acid. Fed Proc. 1972;31:1473–1475. [PubMed] [Google Scholar]
- 55.Rakonczay Z, Matsuoka Y, Giacobini E. Effects of l-beta-N-methylamino-l-alanine (l-BMAA) on the cortical cholinergic and glutamatergic systems of the rat. J Neurosci Res. 1991;29:121–126. doi: 10.1002/jnr.490290114. [DOI] [PubMed] [Google Scholar]
- 56.Matsuoka Y, Rakonczay Z, Giacobini E, Naritoku D. l-beta-methylaminoalanine-induced behavioral changes in rats. Pharmacol Biochem Behav. 1993;44:727–734. doi: 10.1016/0091-3057(93)90191-u. [DOI] [PubMed] [Google Scholar]
- 57.Perry TL, Bergeron C, Biro AJ, Hansen S. Beta-N-methylamino-l-alanine. Chronic oral administration is not neurotoxic to mice. J Neurol Sci. 1989;94:173–180. doi: 10.1016/0022-510x(89)90227-x. [DOI] [PubMed] [Google Scholar]
- 58.Cruz-Aguado R, Winkler D, Shaw CA. Lack of behavioral and neuropathological effects of dietary [beta]-methylamino-l-alanine (BMAA) in mice. Pharmacol Biochem Behav. 2006;84:294–299. doi: 10.1016/j.pbb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 59.Banack SA, Caller TA, Stommel EW. The cyanobacteria derived toxin beta-N-methylamino-l-alanine and amyotrophic lateral sclerosis. Toxins. 2010;2:2837–2850. doi: 10.3390/toxins2122837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiss JH, Choi DW. Beta-N-methylamino-l-alanine neurotoxicity: Requirement for bicarbonate as a cofactor. Science. 1988;241:973–975. doi: 10.1126/science.3136549. [DOI] [PubMed] [Google Scholar]
- 61.Richter KE, Mena EE. l-beta-Methylaminoalanine inhibits [3H]glutamate binding in the presence of bicarbonate ions. Brain Res. 1989;492:385–388. doi: 10.1016/0006-8993(89)90925-6. [DOI] [PubMed] [Google Scholar]
- 62.Weiss JH, Koh JY, Choi DW. Neurotoxicity of beta-N-methylamino-l-alanine (BMAA) and beta-N-oxalylamino-l-alanine (BOAA) on cultured cortical neurons. Brain Res. 1989;497:64–71. doi: 10.1016/0006-8993(89)90970-0. [DOI] [PubMed] [Google Scholar]
- 63.Myers TG, Nelson SD. Neuroactive carbamate adducts of beta-N-methylamino-l-alanine and ethylenediamine. Detection and quantitation under physiological conditions by 13C NMR. J Biol Chem. 1990;265:10193–10195. [PubMed] [Google Scholar]
- 64.Lindstrom H, Luthman J, Mouton P, Spencer P, Olson L. Plant-derived neurotoxic amino acids (beta-N-oxalylamino-l-alanine and beta-N-methylamino-l-alanine): Effects on central monoamine neurons. J Neurochem. 1990;55:941–949. doi: 10.1111/j.1471-4159.1990.tb04582.x. [DOI] [PubMed] [Google Scholar]
- 65.Copani A, Canonico PL, Catania MV, Aronica E, Bruno V, Ratti E, van Amsterdam FT, Gaviraghi G, Nicoletti F. Interaction between beta-N-methylamino-l-alanine and excitatory amino acid receptors in brain slices and neuronal cultures. Brain Res. 1991;558:79–86. doi: 10.1016/0006-8993(91)90716-9. [DOI] [PubMed] [Google Scholar]
- 66.Duncan MW, Markey SP, Weick BG, Pearson PG, Ziffer H, Hu Y, Kopin IJ. 2-Amino-3-(methylamino)propanoic acid (BMAA) bioavailability in the primate. Neurobio Aging. 1992;13:333–337. doi: 10.1016/0197-4580(92)90047-2. [DOI] [PubMed] [Google Scholar]
- 67.Kisby GE, Roy DN, Spencer PS. Determination of beta-N-methylamino-l-alanine (BMAA) in plant (Cycas circinalis L.) and animal tissue by precolumn derivatization with 9-fluorenylmethyl chloroformate (FMOC) and reversed-phase high-performance liquid chromatography. J Neurosci Methods. 1988;26:45–54. doi: 10.1016/0165-0270(88)90128-8. [DOI] [PubMed] [Google Scholar]
- 68.Duncan MW, Villacreses NE, Pearson PG, Wyatt L, Rapoport SI, Kopin IJ, Markey SP, Smith QR. 2-amino-3-(methylamino)-propanoic acid (BMAA) pharmacokinetics and blood-brain barrier permeability in the rat. J Pharmacol Exp Ther. 1991;258:27–35. [PubMed] [Google Scholar]
- 69.Smith QR, Nagura H, Takada Y, Duncan MW. Facilitated transport of the neurotoxin, beta-N-methylamino-l-alanine, across the blood-brain barrier. J Neurochem. 1992;58:1330–1337. doi: 10.1111/j.1471-4159.1992.tb11346.x. [DOI] [PubMed] [Google Scholar]
- 70.Brownson DM, Mabry TJ, Leslie SW. The cycad neurotoxic amino acid, β-N-methylamino-l-alanine (BMAA), elevates intracellular calcium levels in dissociated rat brain cells. J Ethnopharmacol. 2002;82:159–167. doi: 10.1016/s0378-8741(02)00170-8. [DOI] [PubMed] [Google Scholar]
- 71.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 72.Meldrum B, Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990;11:379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- 73.Nedeljkov V, Lopicic S, Pavlovic D, Cemerikic D. Electrophysiological Effect of β-N-Methylamino-l-Alanine on Retzius Nerve Cells of the Leech Haemopis sanguisuga. Ann N Y Acad Sci. 2005;1048:349–351. doi: 10.1196/annals.1342.034. [DOI] [PubMed] [Google Scholar]
- 74.Buenz EJ, Howe CL. Beta-methylamino-alanine (BMAA) injures hippocampal neurons in vivo. Neuro Toxicol. 2007;28:702–704. doi: 10.1016/j.neuro.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 75.Lobner D, Piana PMT, Salous AK, Peoples RW. [beta]-N-methylamino-l-alanine enhances neurotoxicity through multiple mechanisms. Neurobiol Dis. 2007;25:360–366. doi: 10.1016/j.nbd.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rao SD, Banack SA, Cox PA, Weiss JH. BMAA selectively injures motor neurons via AMPA/kainate receptor activation. Exp Neurol. 2006;201:244–252. doi: 10.1016/j.expneurol.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 77.Liu X, Rush T, Zapata J, Lobner D. [beta]-N-methylamino-l-alanine induces oxidative stress and glutamate release through action on system Xc−. Exp Neurol. 2009;217:429–433. doi: 10.1016/j.expneurol.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 78.Nunn PB, Ponnusamy M. [beta]-N-Methylaminoalanine (BMAA): Metabolism and metabolic effects in model systems and in neural and other tissues of the rat in vitro. Toxicon. 2009;54:85–94. doi: 10.1016/j.toxicon.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 79.Deng Y, Boomsma F, Yu PH. Deamination of methylamine and aminoacetone increases aldehydes and oxidative stress in rats. Life Sciences. 1998;63:2049–2058. doi: 10.1016/s0024-3205(99)80001-0. [DOI] [PubMed] [Google Scholar]
- 80.Karlsson O, Berg C, Brittebo EB, Lindquist NG. Retention of the cyanobacterial neurotoxin β-N-methylamino-l-alanine in melanin and neuromelanin-containing cells—A possible link between Parkinson-dementia complex and pigmentary retinopathy. Pigment Cell Melanoma Res. 2009;22:120–130. doi: 10.1111/j.1755-148X.2008.00508.x. [DOI] [PubMed] [Google Scholar]
- 81.Lopicic S, Nedeljkov V, Cemerikic D. Augmentation and ionic mechanism of effect of [beta]-N-methylamino-l-alanine in presence of bicarbonate on membrane potential of Retzius nerve cells of the leech Haemopis sanguisuga. Comp Biochem Physiol Part A. 2009;153:284–292. doi: 10.1016/j.cbpa.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 82.Santucci S, Zsürger N, Chabry J. β-N-methylamino-l-alanine induced in vivo retinal cell death. J Neurochem. 2009;109:819–825. doi: 10.1111/j.1471-4159.2009.06022.x. [DOI] [PubMed] [Google Scholar]
- 83.Purdie EL, Samsudin S, Eddy FB, Codd GA. Effects of the cyanobacterial neurotoxin [beta]-N-methylamino-l-alanine on the early-life stage development of zebrafish (Danio rerio) Aquat Toxicol. 2009;95:279–284. doi: 10.1016/j.aquatox.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 84.Cucchiaroni ML, Viscomi MT, Bernardi G, Molinari M, Guatteo E, Mercuri NB. Metabotropic glutamate receptor 1 mediates the electrophysiological and toxic actions of the cycad derivative {beta}-N-Methylamino-l-alanine on substantia nigra pars compacta DAergic neurons. J Neurosci. 2010;30:5176–5188. doi: 10.1523/JNEUROSCI.5351-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karlsson O, Roman E, Berg A-L, Brittebo EB. Early hippocampal cell death, and late learning and memory deficits in rats exposed to the environmental toxin BMAA ([beta]-N-methylamino-l-alanine) during the neonatal period. Behav Brain Res. 2011;219:310–320. doi: 10.1016/j.bbr.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 86.Lee M, McGeer PL. Weak BMAA toxicity compares with that of the dietary supplement beta-alanine. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2010.11.024. in press. [DOI] [PubMed] [Google Scholar]
- 87.Steele JC, McGeer PL. The ALS/PDC syndrome of Guam and the cycad hypothesis. Neurology. 2008;70:1984–1990. doi: 10.1212/01.wnl.0000312571.81091.26. [DOI] [PubMed] [Google Scholar]
- 88.Cheng R, Banack SA. Previous studies underestimate BMAA concentrations in cycad flour. Amyotroph Lateral Scler. 2009;10:41–43. doi: 10.3109/17482960903273528. [DOI] [PubMed] [Google Scholar]
- 89.Khabazian I, Bains JS, Williams DE, Cheung J, Wilson JM, Pasqualotto BA, Pelech SL, Andersen RJ, Wang YT, Liu L, et al. Isolation of various forms of sterol beta-d-glucoside from the seed of Cycas circinalis: Neurotoxicity and implications for ALS-parkinsonism dementia complex. J Neurochem. 2002;82:516–528. doi: 10.1046/j.1471-4159.2002.00976.x. [DOI] [PubMed] [Google Scholar]
- 90.Ly PTT, Singh S, Shaw CA. Novel environmental toxins: Steryl glycosides as a potential etiological factor for age-related neurodegenerative diseases. J Neurosci Res. 2007;85:231–237. doi: 10.1002/jnr.21147. [DOI] [PubMed] [Google Scholar]
- 91.Tabata RC, Wilson JM, Ly P, Zwiegers P, Kwok D, van Kampen JM, Cashman N, Shaw CA. Chronic exposure to dietary sterol glucosides is neurotoxic to motor neurons and induces an ALS-PDC phenotype. Neuromol Med. 2008;10:24–39. doi: 10.1007/s12017-007-8020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Metcalf JS, Banack SA, Lindsay J, Morrison LF, Cox PA, Codd GA. Co-occurrence of beta-N-methylamino-l-alanine, a neurotoxic amino acid with other cyanobacterial toxins in British waterbodies, 1990–2004. Environ Microbiol. 2008;10:702–708. doi: 10.1111/j.1462-2920.2007.01492.x. [DOI] [PubMed] [Google Scholar]