Abstract
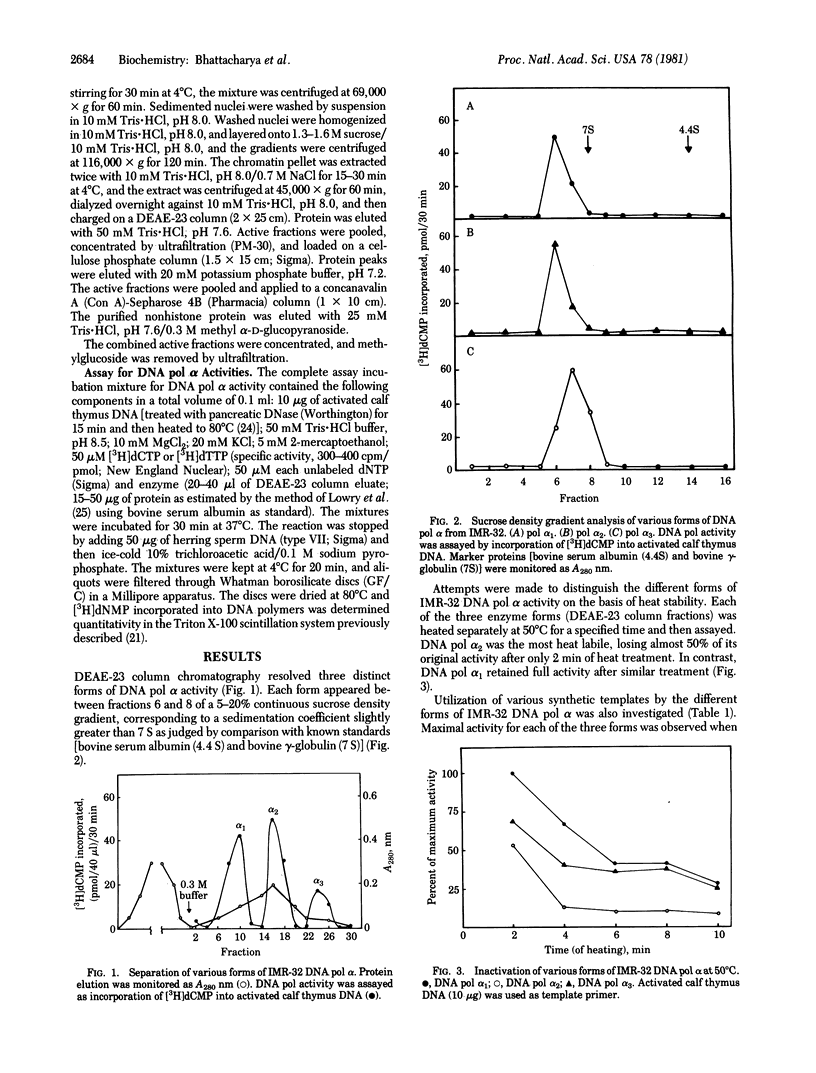
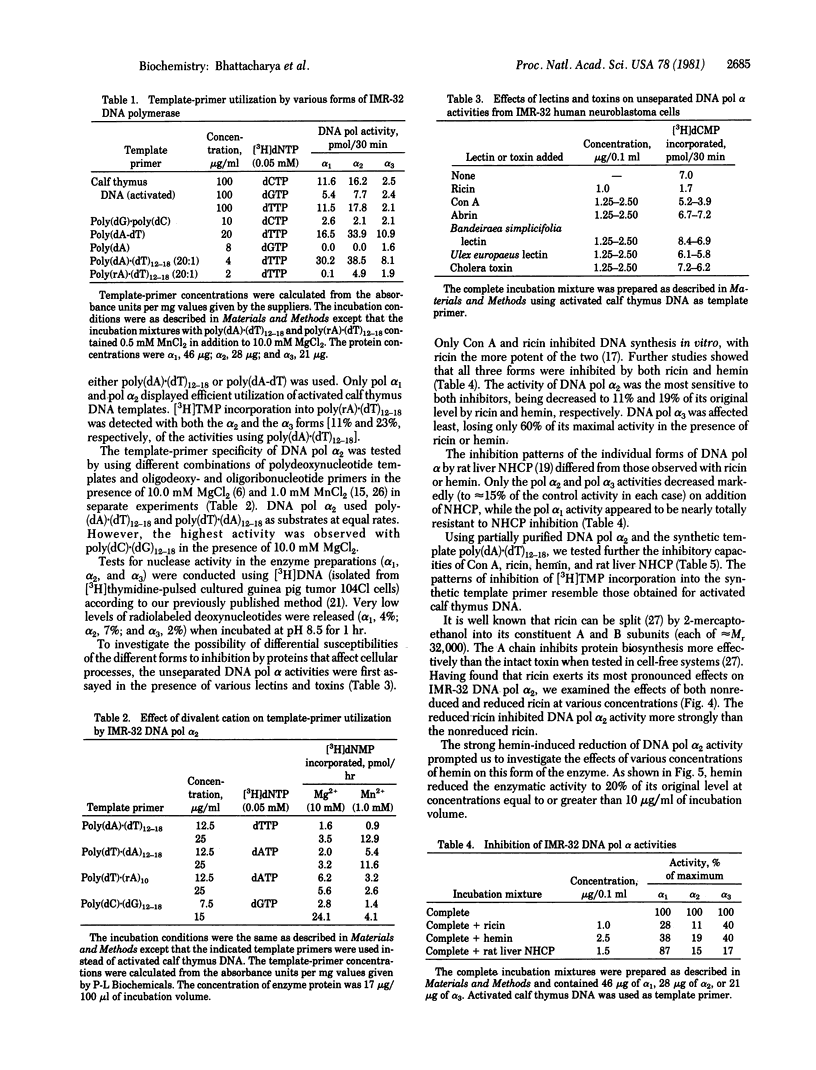
Three forms of DNA polymerase (pol) alpha from human neuroblastoma IMR-32 were separated by DEAE column chromatography. All sedimented at approximately 7 S in 5-20% continuous sucrose density gradients. All were heat labile, with pol alpha 2 the most (90% inactivated) and pol alpha 3 the least (50% inactivated) sensitive to heating for 5 min at 50 degrees C. pol alpha 1 and alpha 2 efficiently utilized activated calf thymus DNA as template. The most active form, pol alpha 2, used both poly(dA).(dT)12-18 and poly(dT).(dA)12-18 as template at equal rates. Differential inhibition of DNA polymerase alpha activities was examined in the presence of ricin, hemin, and a nonhistone chromatin protein. All three polymerases were inhibited by both ricin (nonreduced) and hemin, with pol alpha 2 the most (80-90%) and pol alpha 3 the least (60%) sensitive in each case. In contrast, only pol alpha 2 and alpha 3 activities were inhibited (80-85%) by rat liver nonhistone chromatin protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APOSHIAN H. V., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. IX. The polymerase formed after T2 bacteriophage infection of Escherichia coli: a new enzyme. J Biol Chem. 1962 Feb;237:519–525. [PubMed] [Google Scholar]
- Abell C. W., Kamp C. W., Johnson L. D. Effects of phytohemagglutinin and isoproterenol on DNA synthesis in lymphocytes from normal donors and patients with chronic lymphocytic leukemia. Cancer Res. 1970 Mar;30(3):717–723. [PubMed] [Google Scholar]
- Bhattacharya P., Basu S. DNA polymerase activities in differentiating mouse neuroblastoma N-18 cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1289–1293. doi: 10.1073/pnas.75.3.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P., Moskal J. R., Basu S. Embryonic chicken brain and mouse neuroblastoma cells N1E-115 and N-18 contain an inhibitor of acid deoxyribonuclease. Proc Natl Acad Sci U S A. 1977 Mar;74(3):842–845. doi: 10.1073/pnas.74.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P., Simet I., Basu S. Inhibition of human neuroblastoma DNA polymerase activities by plant lectins and toxins. Proc Natl Acad Sci U S A. 1979 May;76(5):2218–2221. doi: 10.1073/pnas.76.5.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieri-Bonniot F., Schuerch A. R. Molecular heterogeneity of DNA polymerase alpha from P815 mouse mastocytoma cells. FEBS Lett. 1978 Dec 1;96(1):192–196. doi: 10.1016/0014-5793(78)81092-8. [DOI] [PubMed] [Google Scholar]
- Bolden A., Noy G. P., Weissbach A. DNA polymerase of mitochondria is a gamma-polymerase. J Biol Chem. 1977 May 25;252(10):3351–3356. [PubMed] [Google Scholar]
- Bollum F. J. Mammalian DNA polymerases. Prog Nucleic Acid Res Mol Biol. 1975;15(0):109–144. doi: 10.1016/s0079-6603(08)60118-x. [DOI] [PubMed] [Google Scholar]
- Byrnes J. J., Downey K. M., Esserman L., So A. G. Mechanism of hemin inhibition of erythroid cytoplasmic DNA polymerase. Biochemistry. 1975 Feb 25;14(4):796–799. doi: 10.1021/bi00675a023. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. A chemical model for transcriptional initiation of DNA replication. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1354–1360. doi: 10.1016/s0006-291x(72)80124-4. [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Bohn E. W., Planck S. R., Wilson S. H. Mouse DNA polymerase alpha. Subunit structure and identification of a species with associated exonuclease. J Biol Chem. 1979 Nov 25;254(22):11678–11687. [PubMed] [Google Scholar]
- Dube D. K., Kunkel T. A., Seal G., Loeb L. A. Distinctive properties of mammalian DNA polymerases. Biochim Biophys Acta. 1979 Feb 27;561(2):369–382. doi: 10.1016/0005-2787(79)90145-x. [DOI] [PubMed] [Google Scholar]
- Duguet M., Méchali M., Rossignol J. M. A rapid technique to separate DNA polymerase-alpha and -beta activity from a cytosol extract. Anal Biochem. 1978 Aug 1;88(2):399–405. doi: 10.1016/0003-2697(78)90437-2. [DOI] [PubMed] [Google Scholar]
- Ebert P. S., Ikawa Y. Induction of delta-aminolevulinic acid synthetase during erythroid differentiation of cultured leukemia cells. Proc Soc Exp Biol Med. 1974 Jun;146(2):601–604. doi: 10.3181/00379727-146-38155. [DOI] [PubMed] [Google Scholar]
- Hachmann H. J., Lezius A. G. High-molecular-weight DNA polymerases from mouse myeloma. Purification and properties of three enzymes. Eur J Biochem. 1975 Jan 2;50(2):357–366. doi: 10.1111/j.1432-1033.1975.tb09811.x. [DOI] [PubMed] [Google Scholar]
- Hedblom M. L., Cawley D. B., Boguslawski S., Houston L. L. Binding of ricin A chain to rat liver ribosomes: relationship to ribosome inactivation. J Supramol Struct. 1978;9(2):253–268. doi: 10.1002/jss.400090210. [DOI] [PubMed] [Google Scholar]
- Hesslewood I. P., Holmes A. M., Wakeling W. F., Johnston I. R. Studies on the purification and properties of a 6.8-S DNA polymerase activity found in calf-thymus DNA polymerase-alpha fraction. Eur J Biochem. 1978 Mar;84(1):123–131. doi: 10.1111/j.1432-1033.1978.tb12148.x. [DOI] [PubMed] [Google Scholar]
- Holmer A. M., Hesslewood I. P., Johnston I. R. The occurrence of multiple activities in the high-molecular-weight DNA polymerase fraction of mammalian tissues. A preliminary study of some of their properties. Eur J Biochem. 1974 Apr 16;43(3):487–499. doi: 10.1111/j.1432-1033.1974.tb03436.x. [DOI] [PubMed] [Google Scholar]
- Holmes A. M., Hesslewood I. P., Johnston I. R. Evidence that DNA polymerase-alpha of calf thymus contains a subunit of molecular weight 155000. Eur J Biochem. 1976 Feb 16;62(2):229–235. doi: 10.1111/j.1432-1033.1976.tb10152.x. [DOI] [PubMed] [Google Scholar]
- Ishii D. N., Maniatis G. M. Haemin promotes rapid neurite outgrowth in cultured mouse neuroblastoma cells. Nature. 1978 Jul 27;274(5669):372–374. doi: 10.1038/274372a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin J. Y., Pao C. C., Ju S. T., Tung T. C. Polyribosome disaggregation in rat liver following administration of the phytotoxic proteins, abrin and ricin. Cancer Res. 1972 May;32(5):943–947. [PubMed] [Google Scholar]
- Masaki S., Yoshida S. 10-S DNA polymerase from calf thymus which copies both poly(rA) . oligo(dT) and activated DNA. Biochim Biophys Acta. 1978 Nov 21;521(1):74–88. doi: 10.1016/0005-2787(78)90250-2. [DOI] [PubMed] [Google Scholar]
- Matsukage A., Bohn E. W., Wilson S. H. Multiple forms of DNA polymerase in mouse myeloma. Proc Natl Acad Sci U S A. 1974 Feb;71(2):578–582. doi: 10.1073/pnas.71.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momparler R. L., Rossi M., Labitan A. Partial purification and properties of two forms of deoxyribonucleic acid polymerase from calf thymus. J Biol Chem. 1973 Jan 10;248(1):285–293. [PubMed] [Google Scholar]
- NOWELL P. C. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960 May;20:462–466. [PubMed] [Google Scholar]
- Ochoa S., de Haro C. Regulation of protein synthesis in eukaryotes. Annu Rev Biochem. 1979;48:549–580. doi: 10.1146/annurev.bi.48.070179.003001. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Pihl A. Different biological properties of the two constituent peptide chains of ricin, a toxic protein inhibiting protein synthesis. Biochemistry. 1973 Jul 31;12(16):3121–3126. doi: 10.1021/bi00740a028. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Refsnes K., Pihl A. Mechanism of action of the toxic lectins abrin and ricin. Nature. 1974 Jun 14;249(458):627–631. doi: 10.1038/249627a0. [DOI] [PubMed] [Google Scholar]
- Ono Y., Enomoto T., Yamada M. Variation of two forms of DNA polymerase-alpha during a HeLa cell cycle. Gan. 1979 Aug;70(4):527–532. [PubMed] [Google Scholar]
- Ono Y., Hanaoka F. E., Yamada M. Separation of two forms of HeLa DNA polymerase alpha with different binding affinity to DNA. Gan. 1978 Apr;69(2):207–212. [PubMed] [Google Scholar]
- Onozaki K., Hayatsu H., Ukita T. Inhibition of protein synthesis in mouse myeloma cells by Ricinus communis toxin. Biochim Biophys Acta. 1975 Sep 12;407(1):99–107. doi: 10.1016/0005-2787(75)90027-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez L. V., Becker F. F. Rat liver chromatin. Fractionation into eu- and heterochromatin with localization of ribosomal genes. Arch Biochem Biophys. 1976 Apr;173(2):428–437. doi: 10.1016/0003-9861(76)90280-0. [DOI] [PubMed] [Google Scholar]
- Tanabe K., Takahashi T. Studies on the molecular species of DNA polymerase extracted from rat ascites hepatoma cells. J Biochem. 1976 Jan;79(1):85–90. doi: 10.1093/oxfordjournals.jbchem.a131061. [DOI] [PubMed] [Google Scholar]
- Trachsel H., Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: purification and characterization of heme-reversible translational inhibitor. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3654–3658. doi: 10.1073/pnas.75.8.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach A. The functional roles of mammalian DNA polymerase. Arch Biochem Biophys. 1979 Dec;198(2):386–396. doi: 10.1016/0003-9861(79)90511-3. [DOI] [PubMed] [Google Scholar]
- Wickremasinghe R. G., Hesslewood I. P., Holmes A. M., Johnston I. R. Differential N-ethylmaleimide inhibition of two enzymes of the DNA alpha-polymerase-fraction from calf thymus. FEBS Lett. 1977;78(1):139–142. doi: 10.1016/0014-5793(77)80291-3. [DOI] [PubMed] [Google Scholar]
- Wilson S. H., Matsukage A., Bohn E. W., Chen Y. C., Sivarajan M. Polynucleotide recognition by DNA alpha-polymerase. Nucleic Acids Res. 1977 Nov;4(11):3981–3996. doi: 10.1093/nar/4.11.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Kondo T., Ando T. Multiple molecular species of cytoplasmic DNA polymerase from calf thymus. Biochim Biophys Acta. 1974 Jul 24;353(4):463–474. doi: 10.1016/0005-2787(74)90052-5. [DOI] [PubMed] [Google Scholar]


