Abstract
The active metabolite of vitamin D, 1α, 25-dihydroxyvitamin D3 [1,25(OH)2D3], is involved in calcium and phosphate metabolism and exerts a large number of biological effects. Vitamin D3 inhibits parathyroid hormone secretion, adaptive immunity and cell proliferation, and at the same time promotes insulin secretion, innate immunity and stimulates cellular differentiation. The role of vitamin D3 in immunoregulation has led to the concept of a dual function as both as an important secosteroid hormone for the regulation of body calcium homeostasis and as an essential organic compound that has been shown to have a crucial effect on the immune responses. Altered levels of vitamin D3 have been associated, by recent observational studies, with a higher susceptibility of immune-mediated disorders and inflammatory diseases. This review reports the new developments with specific reference to the metabolic and signalling mechanisms associated with the complex immune-regulatory effects of vitamin D3 on immune cells.
Keywords: adaptive immunity, immune cells, immunoregulation, innate immunity, vitamin D3
Introduction
The vitamin D3 [1,25(OH)2D3], is a pleiotropic hormone, which regulates calcium homeostasis of the organism, induces differentiation and inhibits proliferation of various normal and cancer cells.1 Evidence suggests different roles of vitamin D and its active metabolites in a large number of tissues. Nearly every tissue in the body has receptors for the active form of vitamin D, 1,25 dihydroxyvitamin D3 [1,25(OH)2D3] or calcitriol. The immunomodulatory role for 1,25(OH)2D3 was proposed more than 25 years ago. This latest function was essentially based on the finding that monocytes/macrophages from patients affected by the granulomatous disease sarcoidosis constitutively synthesize the active form of vitamin D3 [1,25(OH)2D3] from the precursor 25-hydroxyvitamin D (25OHD), as well as on the data indicating that the receptor for vitamin D (VDR) is detectable in activated, proliferating lymphocytes.2 Nevertheless, only recently has a clearer picture of the function of 1,25(OH)2D3 as a determinant of immune responsiveness been obtained. The crucial role of 1,25(OH)2D3 in the immune system was confirmed by other evidence. First, the intracrine induction of antimicrobial activity by 1,25(OH)2D3 is a pivotal function of the monocyte/macrophage response to infection. Second, sub-optimal vitamin D status is a common peculiarity of many populations throughout the world, with the possible support of monocyte/macrophage metabolism of 25OHD and subsequent synthesis and action of 1,25(OH)2D3.3 These observations suggested a mechanism whereby 1,25(OH)2D3 produced by monocytes could act upon adjacent T cells or B cells, but the consequence of such a system on normal immune regulation is still unclear. Currently, it is know that cutaneous immunity is managed by ultraviolet (UV) irradiation, which affects keratinocytes, antigen-presenting cells, such as epidermal Langerhans cells and T lymphocytes. Peripheral regulatory T cells are responsive to environmental stimuli including UV irradiation. The T-cell effector functions depend on the activation state of Langerhans cells, which can be influenced by UV irradiation. Following their encounter with exogenous antigens the epidermal Langerhans cells migrate to the skin-draining lymph nodes where they present skin-acquired antigens to naive T cells resulting in effector T-cell differentiation. Regulatory T cells induced by UV are expanded by UV-exposed cutaneous Langerhans cells. Recently, it has been shown that epidermal expression of 1,25(OH)2D3 connects the environment to the immune system via expansion of CD4+ CD25+ regulatory T cells.4 After this, T-cell-mediated cutaneous immune responses need to be down-regulated. In this context, CD4+ CD25+ regulatory T cells play an important role in the suppression of cellular immune responses via inhibition of T-cell proliferation. In this scenario 1,25(OH)2D3 is an inhibitor of maturation of dendritic cells (DCs), the most potent antigen-presenting cells, and acts directly on T lymphocytes to inhibit T-cell proliferation.2 The 1,25(OH)2D3 signalling represses the transcription of genes encoding key T helper type 1 (Th1) cytokines, such as interferon-γ (IFN-γ) and interleukin-2 (IL-2).5 The net effect of 1,25(OH)2D3 is to polarize T-helper responses toward a more regulatory Th2 phenotype, which is considered a key component of its capacity to suppress Th1-driven autoimmune responses.2 In the present review, we intend to disclose the basic functions of 1,25(OH)2D3 production. Additionally we will provide an overview on the current knowledge available regarding the molecular mechanisms of action, the role in immunological function and the therapeutic use of vitamin D3 in many diseases characterized by inflammation.
Vitamin D3 synthesis
The two major forms of vitamin D are vitamin D2 (or ergocalciferol) and vitamin D3 [1,25(OH)2D3] also known as cholecalciferol or calcitriol.1 Vitamin D2 is synthesized by plants and fungi, and is not produced in vertebrates, whereas 1,25(OH)2D3 is produced in relatively large quantities in humans and in the majority of vertebrate animals. The main source of 1,25(OH)2D3 occurs in the course of photosynthesis in the skin, in which UV light catalyses the first step in 1,25(OH)2D3 biosynthesis, converting 7-dehydroxycholesterol into pre-vitamin D3, followed by a spontaneous and temperature-dependent isomerization into 1,25(OH)2D3 synthesis. To achieve the biologically active form, vitamin D3 must first be hydroxylated in the liver into 25-hydroxyvitamin D3 [25(OH)D3]6 at the carbon 25-position by 25-hydroxylase. Several cytochrome P450 (CYP) isoforms (including the mitochondrial CYP27A1 and the microsomal CYP2R1, CYP3A4 and CYP2J3) accomplish this hydroxylation step, but CYP2R1 is thought to be the high-affinity 25-hydroxylase.7 The calcidiol is then transported through the bloodstream to the proximal tubule of the kidney, where it is hydrolysed at the 1α-position to form calcitriol (1,25 α-dehydrossicolecalciferol), by the enzyme 25-hydroxyvitamin D-1 α-hydroxylase (CYP27B1), the levels of which are increased by parathyroid hormone secreted by the parathyroid gland, which is the pivotal activator of CYP27B1 in proximal tubule cells.8 Thereafter, the synthesized calcitriol is released into the bloodstream. The preservation of sufficient 25OHD levels in the blood is required for 1,25(OH)2D3 regulation of a large number of physiological functions other than its classic actions in bone mineral metabolism.
Vitamin D3 pathways
The actions of vitamin D on both skeletal function and parathyroid homeostasis may not be exclusively through its endocrine function. The biological action of 1,25(OH)2D3 is performed by binding to the receptors VDR and Retinoid X receptor-α (RXR-α) in the nucleus of various cells of the body.9 The VDR is a nuclear receptor and ligand-activated transcription factor,9 and it is a member of the superfamily of nuclear hormone receptors. It functions as a ligand-activated transcription factor that binds specific DNA sequence elements.10 This receptor is present in many cells of the immune system including monocytes as well as stimulated macrophages, DCs, natural killer cells and activated B and T cells11 and it is involved in many processes such as proliferation and differentiation.12 The VDR is composed of a highly conserved DNA binding domain and an α-helical ligand-binding domain.13 The ligand-bound VDR activates transcription by heterodimerization with RXRs, which is essential for high-affinity DNA binding to cognate vitamin D response elements (VDREs) located in the regulatory regions of 1,25D target genes13 (Fig. 1). Ligand triggered conformational change of VDR–RXR heterodimers results in dissociation of co-repressor proteins such as nuclear receptor co-repressor (NCoR) and facilitates the interaction with members of the CBP/p300 and p160 co-activator families, including steroid receptor co-activators-1, transcriptional intermediary factor 2, and receptor-activated co-activators-3.14 This activated complex then recruits a co-activator complex, known as vitamin D receptor-interacting protein complex and other co-regulatory proteins, which control histone modifications, chromatin remodelling, RNA polymerase II binding, and induce a co-activator exchange in the transcriptional complex of VDR-responsive promoters15 (Fig. 1). Additionally, 1,25(OH)2D activates some intracellular signalling pathways, named ‘rapid’ cellular responses.16 The complex VDR + RXR + 1,25(OH)2D3, targeting the DNA sequence of VDRE requires sufficient level of 1,25(OH)2D to promote VDR signalling. Its ability to bind to a transporter protein, namely, vitamin D binding protein, enables it to reach other districts that will be its target. These effects create an environment suitable for gene transcription.15 Additionally, VDR–RXR interacts with a VDR-interacting repressor at the E-box type element of negative VDREs, comprising a CANNTG-like motif. Such interactions at the E-box induce co-regulator switching, involving dissociation of p300 co-activator and association of the histone deacetylator co-repressor complex, resulting in ligand-induced transrepression.17 Besides expressing abundant 1,25(OH)2D3 receptor (VDR), cells from the parathyroid glands18 and bone-forming osteoblasts19 exhibit significant levels of CYP27B1 activity. As a result, some of the calciotropic actions of vitamin D are the result of localized conversion of 25OHD to 1,25(OH)2D3 and hence result from intracrine, rather than endocrine, signalling by the VDR. Cell-specific or tissue-specific transrepression of CYP27B1 by 1α,25-(OH)2D3 may involve the numerous VDREs found in relatively promoter-proximal locations that enhance chromatin looping and interactions with protein super complexes of differing transcriptional abilities. Other mechanisms of transrepression involve the association between Williams syndrome transcription factor including nucleosome assembly complex, a multifunctional, ATP-dependent chromatin-remodelling complex and chromatin,17 and VDR-induced DNA methylation.20 The 1,25(OH)2D3 intracellular signalling pathways involve the protein kinase C, phosphatidylinositol 3-kinase (PI3-kinase), calcium-dependent pathway, and three groups of mitogen-activated protein kinases.21 So far, it is not clear if 1,25(OH)2D3-induced activation of the signal transduction pathways results in any modifications of VDR protein. It has been proposed that Erk1 is able to phosphorylate VDR.22 Moreover, some data show that in response to 1,25(OH)2D treatment, VDR interacts with PI3-kinase, its downstream element Akt kinase23 and with Src kinase.24 Activation of Src kinase has been documented in different cell line models.25–27 It is not known how activation of Src and PI3-kinase and their physical interaction with VDR can be connected (Fig. 2). Intracellular localization of VDR has been a topic of some controversy because it is not fully established if 1,25(OH)2D3-induced activation of the signal transduction pathways results in any modifications of VDR protein or if VDR is translocated to the cell nucleus in response to 1,25(OH)2D,28 or if it continually shuttles between cytosol and nucleus or if 1,25(OH)2D traps it inside the nucleus29 (Fig. 2). The expression of VDR in the cells increases in response to 1,25(OH)2D3,29 but not in each type of target cell,30 despite all the tissues with VDR being potential targets. The ligand-bound VDR can also repress transcription. For example, in the presence of 1,25(OH)2D3, VDR/RXR heterodimers can displace DNA-bound nuclear factor of activated T cells, and so repress cytokine gene expression.2,11 The classical 1,25-(OH)2D3 genomic response modulates synthesis and accumulation of new proteins and invokes an appropriate cellular response. These events may be suppressed by protein synthesis inhibitors, such as actinomycin D or cycloheximide.31
Figure 1.
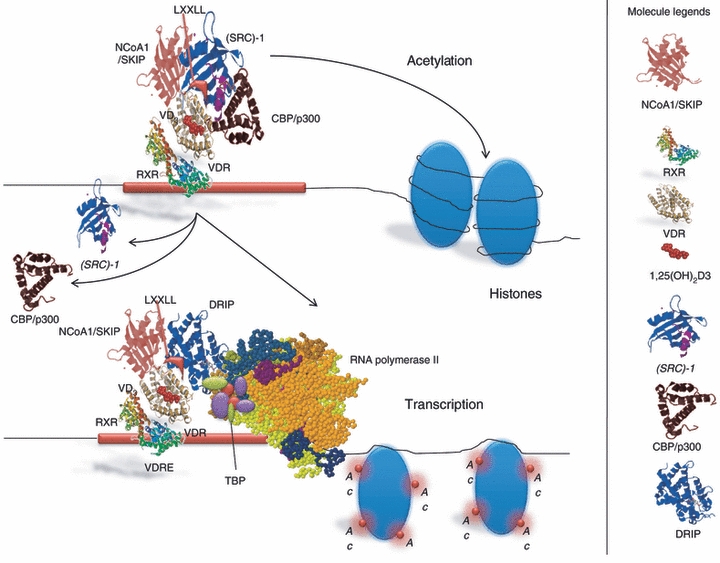
The biological action of 1,25(OH)2D3. The ligand-bound vitamin D receptor (VDR) activates transcription by heterodimerization with RXRs, essential for high-affinity DNA binding to cognate vitamin D response elements (VDREs) located in the regulatory regions of 1,25D target genes. Ligand triggered conformational change of VDR–RXR heterodimers results in dissociation of nuclear receptor co-repressor (NCoR) and facilitates the interaction with members of the CBP/p300 and p160 co-activator families, including steroid receptor co-activators-1 (SRC-1), transcriptional intermediary factor 2 (TIF2), and receptor-activated co-activators-3 (RAC3).This activated complex recruits VDR-interacting protein (DRIP) and other co-regulatory proteins, which control histone modifications, chromatin remodelling, RNA polymerase II binding and transcriptional initiation. The complex VDR+RXR+1,25(OH)2D3, targeting the DNA sequence of VDRE requires sufficient level of 1,25(OH)2D to promote VDR signalling. Acetylated histones relax chromatin structure to make DNA accessible and permit initiation of transcription of the target gene.
Figure 2.
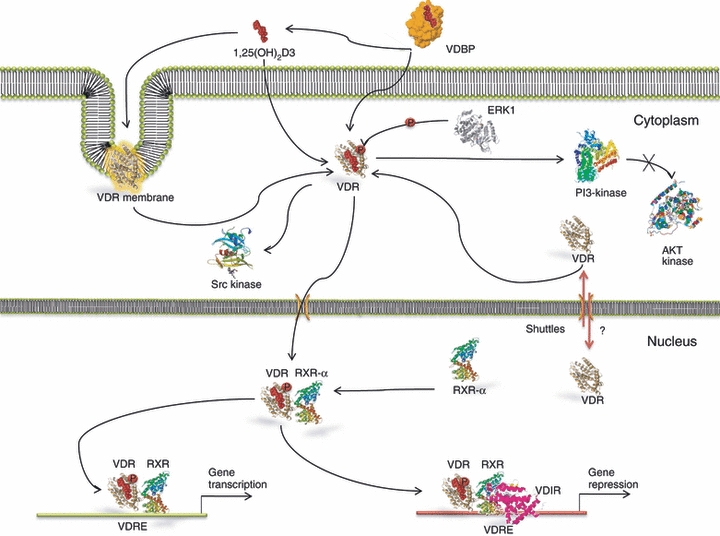
1,25(OH)2D3 intracellular signalling pathway. In response to 1,25(OH)2D treatment viramin D receptor (VDR) interacts with phosphoinositide 3 (PI3) -kinase, its downstream element Akt kinase and with Src kinase. The expression of VDR in the cells increases in response to 1,25(OH)2D3. It is unknown if VDR is translocated to the cell nucleus in response to 1,25(OH)2D, or if it is continuously shuttled between cytosol and nucleus or if 1,25(OH)2D traps it inside the nucleus. In the presence of 1,25(OH)2D3, VDR/RXR heterodimers can displace DNA-bound nuclear factor of activated T cells.
Vitamin D3 and immune cells
The finding that the majority of the immune system cells, including macrophages, B and T lymphocytes, neutrophils and DCs possess VDR,11,32 mainly after activation, produced the idea that vitamin D has pleiotropic effects in immune cells. Some VDR transcription-independent actions play a key role in immune system regulation.
VDR activation in immune cells
In immune cells, activation of VDR leads to production of downstream gene products and elicits potent anti-proliferative, pro-differentiative, and immunomodulatory effects. Several important intracellular pathways have been reported to be inhibited by 1,25(OH)2D3. The suppressive effect of 1,25(OH)2D3 on the nuclear factor-κB (NF-κB) signalling pathway has been observed in T cells, monocytes or macrophages,33 and could influence the expression of various essential secreted molecules on the cell surface. The NF-κB pathway, however, is generally not suppressed by 1,25(OH)2D3 in activated B cells as assessed by the analysis of expression of various genes influenced by this signalling pathway. Evaluating the expression of other transcriptional regulators, including Paired box-5 (PAX-5), B-cell lymphoma 6 (BCL-6), activation-induced cytidine deaminase (AID), B lymphocyte-induced maturation protein 1 (Blimp1), Metastasis-associated protein 3 (MTA3) and IFN-regulatory factor 4 (IRF4), it has been observed that they are unaffected by 1,25(OH)2D3. Whereas the expression of X-box binding protein 1 (XBP1) seems modestly but significantly down-regulated by 1,25(OH)2D3. Furthermore, mRNA expression of endoplasmic reticulum to nucleus signalling 1 (ERN1), which is required for processing XBP1 mRNA to a spliced form that encodes a more stable and active protein,34 is also down-regulated by 1,25(OH)2D3. The inhibitory effect of 1,25(OH)2D3 on the expressions of XBP1 and ERN1 mRNA may, at least in part, explain the greater inhibitory effect of vitamin D on immunoglobulin secretion and detection of plasma cells compared with its more modest inhibition of the differentiation of phenotypically defined plasma cells. Nevertheless, inhibition of the expressions of XBP1 and ERN1 is not sufficient to explain the inhibition of the generation of both plasma cells and post-switched memory cells as well as the ongoing proliferation of activated B cells by 1,25(OH)2D3.35
Vitamin D3 and monocyte/macrophages
The importance of a link between vitamin D3 and innate immunity was underlined by studies showing that monocyte/macrophage responses to bacterial infection were potently stimulated by 1,25(OH)2D3 following induction of both VDR and CYP27B1. Additional studies in patients with the granulomatous disease sarcoidosis provided another link between vitamin D and monocyte/macrophage function. Macrophages isolated from sarcoid granuloma or from lung lavage fluid were capable of synthesizing 1,25(OH)2D3 from precursor 25OHD.36 The presence in sarcoid macrophages of CYP27B1 activity provided an explanation for the elevated circulating levels of 1,25(OH)2D3 frequently found in patients with this disease.37 Macrophage synthesis of 1,25(OH)2D3 seems to be common to granulomatous diseases, as well as several types of tumour involving significant macrophage infiltration.38 Macrophages possess both the enzymes essential to produce locally 1,25(OH)2D3, leading to intracrine and paracrine effects.39 The ability of 1,25(OH)2D3 to stimulate the differentiation of monocytic precursors into more mature, macrophage-like cells was one of the first discoveries suggesting an immunoregulatory role for 1,25(OH)2D3.40 It has been suggested that abundant expression of VDR by monocytes sensitizes these cells to the differentiating effects of 1,25(OH)2D3, providing a ‘fast-forward’ autocrine mechanism for subsequent maturation of cells into macrophages. Of interest, 1,25(OH)2D3 down-regulates the expression of granulocyte–macrophage colony-stimulating factor and stimulates macrophages to produce the immunosuppressant prostaglandin E2. Notably, vitamin D deficiency impairs the ability of macrophages to mature, to produce specific surface antigens by down-regulating the membrane expression of major histocompatibility complex class II (MHC-II) molecules41 to generate the lysosomal enzyme acid phosphatase and to secrete H2O2, essential tools for their antimicrobial function.42 In contrast, the addition of 1,25(OH)2D3 increases expression of macrophage-specific surface antigens and of lysosomal enzyme acid phosphatase. Furthermore, stimulating their ‘oxidative burst’ function enhances chemotaxis and phagocytosis.43 The 1,25(OH)2D3 modulates macrophage responses, preventing them from releasing more inflammatory cytokines and chemokines than required.44 Monocytes isolated from normal human peripheral blood mononuclear cells (PBMCs) are easily able to synthesize 1,25(OH)2D when treated with cytokines such as IFN-γ,45 or bacterial antigens such as lipopolysaccharide.46 The presence of CYP27B1 in macrophages is crucial for the physiological action of 1,25(OH)2D3 in immunoregulation. In activated macrophages CYP27B1 expression is not regulated by Ca2+ homeostatic signals but is regulated by immune inputs, mainly IFN-γ and agonists of the Toll-like receptor (TLR), the pattern recognition receptors.43 Microarray studies demonstrated that signalling through human macrophage TLR1/2 heterodimers stimulated with bacterial lipopeptides induced expression of both CYP27B1 and the VDR.47 Critically, this renders the immune system responsive to circulating levels of 25D.47 Most importantly, in TLR2/1-stimulated human macrophages cultured in the presence of human serum, downstream VDR-driven responses were strongly dependent on serum 1,25(OH)2D3 concentrations47 (Fig. 3). The VDR-driven responses, attenuated or absent in vitamin D-deficient individuals, were restored by 1,25(OH)2D3 supplementation. Consistent with these findings, the 1,25(OH)2D3 levels in serum from African-Americans were markedly lower than those of Caucasian Americans.48 This evidence confirms the demonstration of the dependence of immune responses on circulating 1,25(OH)2D3 levels. The expression of the co-receptor of TLR4, CD14, is strongly regulated by 1,25(OH)2D3 in human cells.49 Several works show a correlation between induction by LPS and expression of CYP27B1 via TLR4/CD14 receptor complexes.43 Moreover, treatment with 1,25(OH)2D3 in human monocytes inhibits the expression of the innate immunity receptors TLR2, TLR4 and TLR9 and alters the TLR9-dependent production of IL-6.50 Promoter-reporter analysis of the events involved in transcriptional regulation of CYP27B1 suggests that TLR4-mediated induction of this enzyme involves JAK-STAT, mitogen-activated protein kinase and NF-κB pathways.43 However, other studies have proposed that TLR2/1 induction by CYP27B1 occurs indirectly as a consequence of TLR2/1-induced IL-15, which is a potent inducer of CYP27B1.51 In a comparable fashion, IL-17 increase 1,25(OH)2D3-mediated induction of cathelicidin, although this response does not seem to involve transcriptional regulation of CYP27B1 or increased VDR sensitivity.52 Treatment of human monocytes with 1,25(OH)2D3 suppressed the expression of both TLR2 and TLR4 mRNA and protein in a time-dependent and dose-dependent manner.53,54 Strikingly, whereas 1,25(OH)2D3 promotes the antimicrobial activities of myeloid cells, this hormone also inhibits TLR2 expression and TLR4 expression on monocytes, so inducing a state of hypo-responsiveness to pathogen-associated molecular patterns. It was thought that this took place as a negative feedback mechanism, preventing excessive TLR activation and inflammation at a later stage of infection.53 Therefore, such down-regulation of pattern recognition receptors by 1,25(OH)2D3 in antigen-presenting cells may contribute to the capacity of 1,25(OH)2D3 to attenuate abnormal Th1-driven inflammatory responses and potential downstream autoimmunity.55 This evidence provides, partially, the molecular basis for the increased production of 1,25(OH)2D3 by macrophages in granulomatous diseases such as sarcoidosis.56 It has been demonstrated that 1,25(OH)2D3 is a direct inducer of expression of the gene encoding NOD2/CARD15/IBD1 in cells of monocytic and epithelial origin.57 This pattern recognition receptor detects muramyl dipeptide (MDP), a lysosomal breakdown product of bacterial peptidoglycan common to Gram-negative and Gram-positive bacteria. The MDP-induced NOD2 activation stimulates NF-κB, which induces expression of the defensin β2 gene.57 One pathway, so far poorly studied, concerns the enzyme 24-hydroxylase (CYP24), which is thought to function by inactivating 1,25(OH)2D3. Remarkably, while expression of CYP24, the mitochondrial enzyme that initiates 1,25(OH)2D3 catabolism, is exquisitely sensitive to the presence of 1,25(OH)2D3, the negative feedback loop appears to be defective in macrophages. The gene for 24-hydroxylase is potently induced by 25OHD following TLR2/1 activation of monocytes.58 A splice variant form (CYP24-SV) that encodes a truncated enzyme lacking the critical N-terminal mitochondrial targeting sequence was proposed as a novel mechanism for induced expression 1,25(OH)2D3 in macrophages.59 However, while expression of CYP24 transcripts is induced by 1,25(OH)2D3 in macrophages as in other cells, the corresponding enzymatic activity is virtually undetectable.59 Although the substrate binding pocket of CYP24-SV is apparently functional, the enzyme, trapped in the cytosol, is catalytically inactive. This suggests that, in macrophages, 1,25(OH)2D3 signalling is maintained over an extended period of time, which would be advantageous for combating intracellular pathogens such as Mycobacterium tuberculosis.51 In fact, 1,25(OH)2D3 significantly attenuates expression of matrix metalloproteinases (MMP) 7 and 10, suppresses secretion of MMP-7 and significantly inhibits secretion and activity of MMP-9 by M. tuberculosis-infected PBMCs. As well 1,25(OH)2D3 induces secretion of IL-10 and prostaglandin E2 from M. tuberculosis-infected PBMCs.60 The 1,25(OH)2D3 dramatically induces genetic expression of antimicrobial peptides (AMPs), such as defensins and cathelicidin (hCAP), in human monocytes, neutrophils and other human cell lines61,62 (Fig. 4a). The AMPs display a broad-spectrum of antimicrobial and antiviral activities including the influenza virus.63 These endogenous antibiotics directly destroy invading microorganisms. Recent reports have underlined the importance of hCAP as a target for vitamin D. The hCAP was identified as a target for transcriptional regulation by 1,25(OH)2D3-liganded VDR, in that its gene promoter contains a functional VDRE61,62 (Fig. 3). This VDRE occurs within a small interchangeable nuclear element sequence, which seems to be present only in the cathelicidin gene promoter of higher primates, suggesting that the 1,25(OH)2D3 regulation of this facet of innate immunity is a recent evolutionary development.64 The induction of hCAP following localized synthesis of 1,25(OH)2D3 has the dual benefit of promoting the generation of autophagosomes while enhancing bacterial killing following fusion with lysosomes to form autolysosomes65 (Fig. 3).
Figure 3.
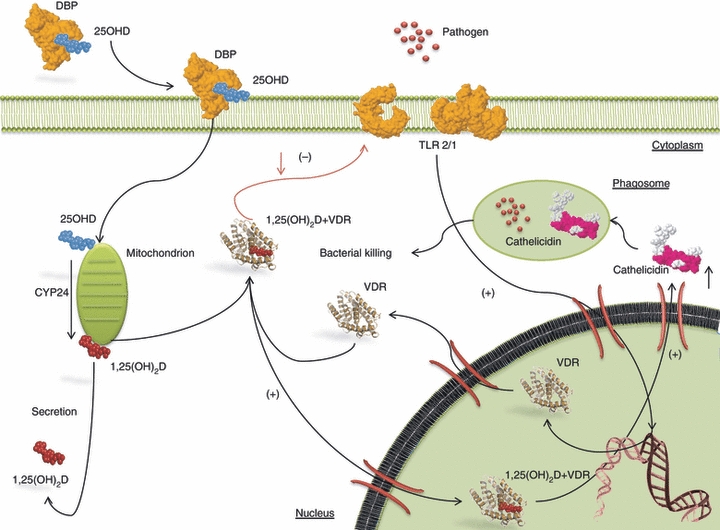
Immune cell responses to pathogens: 25-hydroxyvitamin D (25OHD) binds vitamin D binding protein (VDBP), then is released into the cytosol and converted to 1,25(OH)2D3 in the mitochondria. Vitamin D trigger pathogen-sensing via Toll-like receptor 2/1(TLR2/1) complex. Intracellular 1,25(OH)2D3 generated though action of 25-hydroxyvitamin D-1 α-hydroxylase (CYP27B1) interacts with the 1,25(OH)2D3 receptor (VDR). Activation of the VDR leads to induction of cathelicidin, which is the target for transcriptional regulation by 1,25(OH)2D 3-liganded VDR. The induction of cathelicidin following localized synthesis of 1,25(OH)2D3 promotes the generation of autophagosomes and enhances bacterial killing following generation of autolysosomes.
Figure 4.
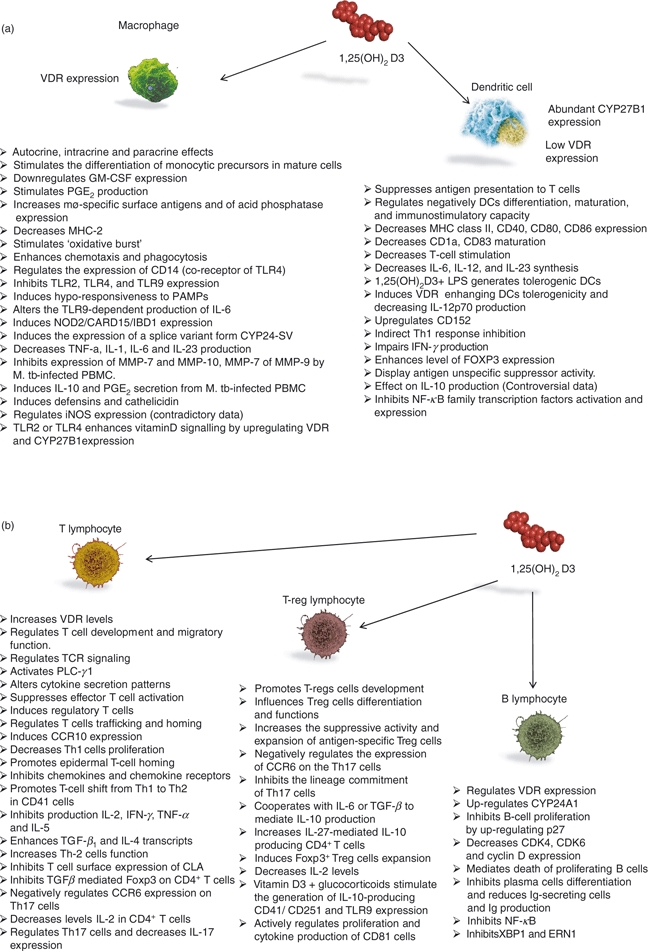
Effects of vitamin D on immune cells: (a) Macrophages and dendritic cells; (b) T lymphocytes, regulatory T (Treg) lymphocytes, B lymphocytes. CCR6, CCR10, chemokine receptor; CLA, conjugated linoleic acid; CDK-4,-6, cyclin-dependent kinase; ERN1, endoplasmic reticulum to nucleus signalling-1; FOXP3, forkhead box P-3; GM-CSF, granulocyte–macrophage colony-stimulating factor; DCs, dendritic cells; CYP24-SV, 24-hydroxylase splice variant form; CYP27B1, 25-hydroxyvitamin D-1 α-hydroxylase; Ig, immunoglobulin; iNOS, inducible nitric oxide synthases; IFN-γ, interferon-γ; IL-1, interleukin-1; LPS, lipopolysaccaride; MHC-2, major histocompatibility complex-2; MMP-7, matrix metalloproteinase-7; M. tb, Mycobacterium tuberculosis; NF-κB, nuclear factor-κB; NOD2/CARD15/IBD1, nucleotide-binding oligomerization domain containing 2/Caspase activation and recruitment domains/inflammatory bowel disease 1; Tregs, regulatory T cells; PAMPs, pathogen-associated molecular patterns; PBMC, peripheral blood mononuclear; PLC-γ1, phospholipase C-γ1; PGE2, prostaglandin E2; TCR, T-cell receptor; TLR-2, toll-like receptor-2; TGF-β1, transforming growth factor-β1; TNF-α, tumour necrosis-α; VDR, 1,25(OH)2D3 receptor; Th-1, T helper type 1; XBP1, X-box binding protein-1.
Conflicting results concern the role of 1,25(OH)2D3 signalling in controlling HIV infection. Notably, hCAP inhibits the replication of a number of HIV isolates63 and the human homologues reduce the infectivity of lentiviral vectors,66 suggesting that vitamin D signalling could induce antiretroviral activity. Also, regulation of inducible nitric oxide synthase (iNOS) potentially contributes to the antimicrobial effects of 1,25(OH)2D3, but different data have been reported so far, ranging from a 1,25(OH)2D3-mediated induction of iNOS expression in a human macrophage-like cell line, to documented inhibitory actions of 1,25(OH)2D3 on this enzyme67 (Fig. 4a). The evidence that the polycyclic aromatic hydrocarbon benzo[a]pyrene, a prominent product of cigarette smoking, attenuates vitamin D-mediated induction of macrophage hCAP in a VDR-dependent fashion by stimulating the expression of 24-hydroxylase and 1,25(OH)2D3 catabolism68 underlines the importance of a connection between vitamin D3 and innate immunity also. The precise mechanism by which this occurs has yet to be determined but these data suggest that some toxic compounds are actively detrimental to 1,25(OH)2D3-mediated immunity.
Vitamin D3 and DCs
The DCs are heterogeneous in terms of their location, phenotype and function. They are generally divided into two groups based on their origin: myeloid (mDCs) and plasmacytoid (pDCs). They express different types of cytokines and chemokines and exert complementary effects on T-cell responses. Typically, mDCs are valuable antigen-presenting cells69 and pDCs are more closely associated with immune tolerance.70 Purified tissue DCs express VDR.71 Data from monocyte-derived DCs (moDCs) prove that the large amounts of VDR expressed in the human monocytes are subsequently down-regulated in the course of moDC differentiation and maturation.72 Whereas CYP27B1 expression and activity increases as the DCs differentiate towards a mature phenotype.73 This mutual organization of CYP27B1 and VDR expression may be helpful in mature antigen-presenting DCs and may be relatively insensitive to 1,25(OH)2D3, thereby permitting induction of an initial T-cell response.73 Successive studies using Langerhans cells, populations of DCs isolated from skin, provided evidence that 1,25(OH)2D3 could operate to attenuate antigen presentation.74 Later, it was found that treatment of human moDCs with 1,25(OH)2D3 or analogues has a profound impact on maturation-induced changes in morphology and function,2,72 thereby suppressing their capacity to present antigen to T cells. The 1,25(OH)2D3 negatively regulates the differentiation, maturation and immunostimulatory capacity of DCs by decreasing the expression of MHC class II, CD40, CD80 and CD8675 and the maturation proteins CD1a and CD83.76 In addition, the 1,25(OH)2D3 decreases the synthesis of IL-6, IL-12 and IL-23.77 The lipopolysaccharide-induced maturation of human moDCs in the presence of 1,25(OH)2D3 generates tolerogenic DCs.76,78 Therefore, induction of VDR on DCs modifies their phenotype and function, enhancing their tolerogenicity in adaptive immune response, and decreasing the production of IL-12p70. When re-stimulated, T cells primed with 1,25(OH)2D3-treated moDCs show up-regulation of CD152 (CTLA-4), impaired proliferation and IFN-γ production, which could not be rescued by high amounts of exogenous IL-2.73,78 The functional analyses showing that treatment with 25OHD3 suppresses DC maturation and inhibits T-cell proliferation confirm the existence of an intracrine pathway for vitamin D similar to that observed for macrophages. Anergic T cells, induced by immature 1,25(OH)2D3-treated moDCs, express enhanced levels of forkhead box protein-3 (FOXP-3) and display antigen unspecific suppressor activity. This T-cell anergy is antigen-unspecific, because re-stimulation with mature DCs derived from a second donor, unrelated to the DCs used for priming, also resulted in T-cell hypo-responsiveness,76 and is in remarkable contrast to the T-cell anergy induced by IL-10 DCs.79 The tolerogenic effects of 1,25(OH)2D3 and its analogues have also been observed on murine DCs in vitro80,81 and in vivo.82,83 Tolerogenic DCs induced by treatment with VDR agonists promote CD4+ CD25+ FoxP3+ T regulatory cells, which are competent to mediate transplantation tolerance and arrest the development of autoimmune diseases.84 The effect on IL-10 production is controversial, several reports describe a decrease, whereas others affirm increased production.78,85 The 1,25(OH)2D3 exerts its modulating function on human mature DCs, moDCs via inhibition of NF-κB activation and expression.86 Of note, all of the in vitro studies demonstrating an effect for 1,25(OH)2D3 on innate immunity have been carried out adding exogenous 1,25(OH)2D3 to cell cultures at levels above the physiological serum range. This information raises the question as to whether there is a role for vitamin D at physiological concentrations in host immune responses. It is possible that local intracrine synthesis of 1,25(OH)2D3 is more effective in achieving these responses. Regarding pDCs no apparent immune response to 1,25(OH)2D3 was observed, this does not preclude a role for vitamin D in the regulation of their reactions. Alternatively, the 1,25(OH)2D3 synthesized by pDCs may regulate tolerance through paracrine effects on VDR-expressing T cells (Fig. 4a).
Vitamin D3 and B lymphocytes
Some investigators reported that resting B cells do not contain detectable amounts of VDR,87 whereas others found that VDR is constitutively expressed on human tonsil B cells and can be up-regulated by activation.88 Interestingly, VDR is expressed on the B-cell lymphoma cell lines SUDHL4 and SUDHL5.89 More recently, it was asserted that VDR mRNA is constitutively expressed in human primary B cells at low levels and is up-regulated following stimulation in the presence of 1,25(OH)2D3.35 This result indicates that B cells may be capable of autocrine/intracrine responses to 1,25(OH)2D3. The finding that VDR expression in B cells is regulated by 1,25(OH)2D3 suggests that vitamin D may exert differential effects on activated versus resting B cells and may also have different effects in individuals with different levels of serum 1,25(OH)2D3. Furthermore, activation of 1,25(OH)2D3 mediated by the up-regulation of the VDR exerts an inhibitory effect on B-cell proliferation, suggesting that a threshold level of VDR engagement might be required for the antiproliferative effect to become apparent. Interestingly, CYP27B1 mRNA was found also expressed by resting B cells and could be further induced by stimulation, but not by 1,25(OH)2D3. However, CYP24A1 was found significantly up-regulated following the incubation of human B cells with 1,25(OH)2D3, suggesting that the activity of vitamin D on B cells might be influenced not only by VDR expression but also by the capacity to degrade the active molecule. In contrast to the VDR, CYP24A1 was not altered by B-cell activation, demonstrating that human B cells can respond to 1,25(OH)2D3 directly. The increased susceptibility of activated B cells to many of the effects of 1,25(OH)2D3 might reflect the up-regulation of VDR but not CYP24A1 by these cells. Then, 25(OH)D3 might be metabolized to 1,25(OH)2D3 by B cells themselves and may represent a source for the extra-renal synthesis of 1,25(OH)2D3.35 This suggests that in conditions such as systemic lupus erythematosus, in which there is a diffuse B-cell activation,90 systemic vitamin D metabolism might be significantly influenced. The precursor, 25(OH)D3, had similar effects on purified B cells compared with the active form, but at higher concentrations. Notably, 1,25(OH)2D3-mediated inhibition of proliferation was associated with apoptosis of the activated and dividing B cells, implying that differentiative events requiring the initial expansion of B cells might be eliminated as a result of the 1,25(OH)2D3-mediated death of proliferating B cells. Using the combination of IL-21 and anti-CD40 stimulation with or without B-cell receptor cross-linking, to induce proliferation and plasma cell differentiation, demonstrated that 1,25(OH)2D3 had inhibitory effects on plasma cell differentiation and immunoglobulin production.35 This effect was not evident when the B cells were treated with 1,25(OH)2D3 after 5 days in culture, indicating that 1,25(OH)2D3 inhibits the generation of plasma cells but not their subsequent persistence. This observation supports the idea that the inhibition of B-cell proliferation by 1,25(OH)2D3 is responsible for the reduction of immunoglobulin-secreting cells and immunoglobulin production. The 1,25(OH)2D3 directly inhibited the proliferation of activated B cells, showing the effect of 1,25(OH)2D3 on cell cycle-related gene expression by B cells.35 Hence the mRNA level of p27, but not of p21 or p18, was up-regulated by 1,25(OH)2D3 in activated human B cells. These data suggested that 1,25(OH)2D3 could inhibit B-cell proliferation by up-regulating p27 and thereby inhibit the cell cycle entry of previously cycling B cells.35 Because 1,25(OH)2D3 also decreased mRNA levels of CDK4 and CDK6 as well as cyclin D, the effect of p27 on ongoing B-cell proliferation might be greater.35 The observation that 1,25(OH)2D3-mediated induction of p27 may limit ongoing B-cell proliferation implies that it modulates B-cell responses. Overall, these results suggested the possibility that the major effects of 1,25(OH)2D3 on plasma cell and memory cell differentiation may result from the suppression of ongoing B-cell proliferation,36 which is required before the differentiation steps can occur91 (Fig. 4b).
Vitamin D3 and T lymphocytes
The 1,25(OH)2D3 regulates T-cell development and migratory function. In quiescent T cells VDR levels are almost undetectable, but the expression increases five times upon activation. Both Th1 and Th2 cells are direct targets of active vitamin D. Direct actions on T cells represent a supplementary or a different route for 1,25(OH)2D3 to shape T-cell responses and to control T-cell antigen receptor signalling,92 which through the alternative p38 pathway induces VDR expression. The VDR binds 1,25(OH)2D3, translocates to the nucleus and activates the gene encoding phospholipase C-γ1(PLC-γ1), which results in the accumulation of PLC-γ1 protein in the cytoplasm of primed T cells approximately 48 hr after the initial activation signals.92 Because PLC-γ1 has a central role in classical T-cell receptor signalling and T-cell activation,93,94 the differences in PLC-γ1 expression in naive and primed T cells might explain the process of functional avidity maturation observed in T cells. Activation of the VDR by 1,25(OH)2D3 alters cytokine secretion patterns, suppresses effector T-cell activation and induces regulatory T cells. Notably, this hormone may also affect other facets of T-cell function. Initial studies suggested that 1,25(OH)2D3 acts to inhibit the migration of T cells to lymph nodes.95 However, more recently, 1,25(OH)2D3 was proposed as an important regulator of lymphocyte trafficking and can exert powerful effects on the homing of T cells to specific tissues. Active 1,25(OH)2D3 influences cell translocation by stimulating T-cell expression of chemokine receptor 10 (CCR10), which recognizes the chemokine CCL27 secreted by epidermal keratinocytes.3 The 1,25(OH)2D3 might also influence the phenotype of T cells, as it preferentially inhibits Th1 cells, which are a subset of CD41 effector T cells closely associated with cellular immune responses.96 In this way the 1,25(OH)2D3 is able to promote the translocation and/or retention of T cells within the skin. In contrast to its positive effect on epidermal T-cell homing, vitamin D seems to exert a negative effect on chemokines and chemokine receptors associated with the gastrointestinal tract.3 Subsequently, it was demonstrated that vitamin D promotes a T-cell shift from Th1 to Th2 and so might help to limit the potential tissue damage associated with Th1 cellular immune responses as demonstrated by the cytokine profile of 1,25(OH)2D3-treated human T cells. The 1,25(OH)2D3 decreases the proliferation of Th1 cells and also inhibits the production of IL-2, IFN-γ, tumour necrosis factor-α and IL-5 of Th1 cells.3,97 Vitamin D administration markedly enhances transforming growth factor-β1 (TGF-β1) and IL-4 transcripts, which results in an immunosuppressive action and increases Th2 cell function.95,98 The Th1 response is blunted by the production of tolerogenic DCs that increase suppressor T-cell action.72 However, it seems likely that this is highly T-cell-selective, as demonstrated in VDR gene knockout mice, which show aberrant gastrointestinal migration of a subset of CD81 cells; this effect seems to be closely linked to the increased risk of colitis in VDR knockout mice.99 The analysis of immune cells from the VDR gene knockout mouse added further complexity because these mice show reduced levels of Th1 cells.100 Hence, although in vitro vitamin D seems broadly to support a shift from Th1 to Th2 in CD41 cells, in vivo its effects on T cells are more complex. By contrast, another study revealed that the 1,25(OH)2D3 inhibits T-cell surface expression of cutaneous lymphocyte-associated antigen, another receptor directing T cells to the skin. Another subset of T-cell lineage distinct from Th1 or Th2 cells, termed Th17 cells because of their capacity to synthesize IL-17,101 plays a crucial role in combating certain pathogens but they have also been linked to tissue damage and inflammation.102 The precise role of vitamin D as a regulator of Th17 cells is not yet clear. It is likely that vitamin D exerts some of its effects on inflammation and autoimmune disease through the regulation of Th17 cells, as demonstrated on animal models in which 1,25(OH)2D3 treatment reduced expression of IL-17,103 and loss of 1,25(OH)2D3 as a result of CYP27B1 gene ablation leads to increased levels of this cytokine104 (Fig. 4b).
Vitamin D3 and regulatory T cells
A fourth group of CD41 T cells exert suppressor rather than effector functions and are known as regulatory T cells or suppressor regulatory T (Treg) cells. Several studies reported that 1,25(OH)2D3 potently modulates the T-cell phenotype, promoting the development of T-reg cells.105 Specifically, topical application of 1,25(OH)2D3 affects the differentiation and functions of Treg cells, increasing the suppressive activity and the in vivo expansion of antigen-specific Treg cells.106,107 This finding is supported by the observation that oral administration of 1,25(OH)2D3 significantly reduced the number of lymphocytes in the central nervous system (CNS) of mice with induced experimental autoimmune encephalomyelitis (EAE).108 The CD4+ T cells are highly infiltrated in EAE-induced mice whereas 1,25(OH)2D3-treated mice present extremely low numbers of CD4+ T cells in their CNS. There are two hypotheses to explain the absence of lymphocytes in the CNS after 1,25(OH)2D3 treatment. First, 1,25(OH)2D3 causes death of activated T cells in the absence of Th17-polarizing conditions. Second, regulation of Th17 cell recruitment occurs via chemokine and chemokine receptors. As expected, 1,25(OH)2D3 negatively regulates the expression of CCR6 on the Th17 cells that had been activated by both TGF-β and IL-6. The CCR6–CCL20 axis seems to play an essential role in controlling the entry of Th17 cells into the CNS, so mediating the initiation of EAE.108 The inhibitory effect of 1,25(OH)2D3 seems to be similar to that of IL-27, which inhibits the lineage commitment of Th17 cells109,110 and induces IL-10 production, which, in turn, suppresses EAE initiation.111 The combination of 1,25(OH)2D3 and dexamethasone (Dex) increase the frequency at which IL-10-producing regulatory T cells are generated.112 Further, 1,25(OH)2D3 fails to inhibit EAE in IL-102/2 or IL-10R2/2 B6 mice.113 However, an in vitro study showed that 1,25(OH)2D3 alone failed to induce IL-10 production in activated T cells.114 Additional factors are required to protect against EAE through the IL-10 effect. The 1,25(OH)2D3 helps TGF-β to mediate IL-10 production and strongly enhances the generation of IL-27-mediated IL-10-producing CD4+ T cells.114 Both the finding that IL-27 is a good inducer of IL-10-producing T cells and that 1,25(OH)2D3 possesses synergistic effects under Th17-polarizing conditions indicate that 1,25(OH)2D3 requires the presence of TGF-β and IL-6 to increase the number of IL-27-mediated IL-10-producing T cells. It is possible that 1,25(OH)2D3 cooperates with IL-27 to protect against EAE through IL-10. The generation of Th17 cells blocked by IL-27 is dependent on the transcription factor STAT1.109 It appears that 1,25(OH)2D3-mediated suppression of Th1 and Th17 cell generation occurs by induction of Foxp3+ Treg-cell expansion. In contrast, It has been shown that the expression of TGF-β-mediated Foxp3 is inhibited by 1,25(OH)2D3 via a VDR signal on CD4+ T cells.114 Moreover, it has been shown that in vitro treatment of 1,25(OH)2D3 results in decreased levels of IL-2 production by activated CD4+ T cells.115 Interleukin-2 might be crucial for inhibiting Treg-cell differentiation by 1,25(OH)2D3.114 Although IL-2 blocks the inhibitory role of 1,25(OH)2D3 on Treg-cell generation, 1,25(OH)2D3 and IL-2 synergistically constrain IL-17 production in CD4+ T cells. However, the direct effect of 1,25(OH)2D3 on the function and differentiation of T cells is basically unknown because VDR is expressed at low levels in naive T cells.116 These inhibitory effects of 1,25(OH)2D3 are most pronounced in the effector/memory T cells, which do express VDR, or are mediated by 1,25(OH)2D3-treated DCs. Overall, the VDR signal on the CD4+ T cells inhibits the expression of IL-17, IL-2, Foxp3 and CCR6 but enhances the expression of IL-10. It has been suggested that VDR activation modulates CCR6 expression and leads to a functional hypo-responsiveness to CCL20. The 1,25(OH)2D3-treated DCs induce Treg cells via independence of an inhibitory receptor immunoglobulin-like transcript 3 (ILT3) molecule, which is required for Treg-cell induction.78 The 1,25(OH)2D3, in conjunction with glucocorticoids, potently stimulated the generation of IL-10-producing CD41/CD251 and TLR9 expression by Treg cells.112 Subsequent reports indicated that the preferential differentiation of Treg cells is a pivotal mechanism connecting 1,25(OH)2D3 to adaptive immunity, with beneficial effects for autoimmune disease and host–graft rejection.113,117 This immunosuppressive mechanism is likely to be mediated by the induction of tolerogenic DCs,72,82,118 but direct effects on T cells may also be important.55 In contrast to CD41 cells, CD81 cells show a poor anti-proliferative response to 1,25(OH)2D3.119 Although the literature on T effector cells is abundant, our understanding of the effects of vitamin D on CD81 suppressor T cells remains limited. However, the notion that CD81 cells express VDR abundantly is proved and suggests that they are potential targets for 1,25(OH)2D3. Further reports have shown that 1,25(OH)2D3 actively regulates proliferation and cytokine production by CD81 cells120 following specific immune stimuli.121 Despite this, the 1,25(OH)2D3 does not seem to have a significant effect on animal disease models such as EAE in which CD81 cells have been implicated 122 (Fig. 4b).
Vitamin D3 deficiency and bacterial infection
The anti-inflammatory role of vitamin D has been documented in various bacterial infections.92 In the 1980s, it was demonstrated that vitamin D enhanced bactericidal activity of human macrophages against M. tuberculosis, the causative agent of tuberculosis.123 This discovery led to a new era of interest regarding the role of 1,25(OH)2D3 in determining pathogenesis and the immune response to bacterial pathogens. Interestingly, Liu et al.47 proposed a key mechanism of how vitamin D may enhance innate immunity. Recent studies provided new insight into the involvement of vitamin D in the TLR triggering of an anti-microbial response to infections. As reported earlier, pathogens such as M. tuberculosis are phagocytosed by monocytes/macrophages triggering pathogen-sensing via TLR2/1 complex, which, in turn, up-regulates the expression of both VDR and CYP27B1.48 In this way, the 25-hydroxyvitamin D (25OHD) in circulation bound to vitamin D binding protein is released to the monocyte/macrophage and converted to 1,25(OH)2D3 in the mitochondria (Fig. 3). Intracellular 1,25(OH)2D3 generated though action of CYP27B1 then interacts with the VDR and transcriptionally leads to induction of cathelicidin, with multifunctional roles in host defence, and killing of intracellular M. tuberculosis,48 via a VDRE in the hCAP gene promoter. The link between vitamin D-triggered antimicrobial activity in monocytes/macrophages and cathelicidin has been confirmed using small interference RNA inhibition of 1,25(OH)2D3 in increased mycobacterial growth.124 Besides AMPs, also the gene encoding the antimicrobial peptide, defensin β2 another antimicrobial peptide with multiple effector functions within the immune system, was identified as a direct target for 1,25(OH)2D3.62 Exposure to 1,25(OH)2D3 results in a strong induction of these peptides, directly leading to enhanced antimicrobial activity in various cell types, including myeloid cells, keratinocytes, neutrophils and bronchial epithelial cells.62,125,126 The antimicrobial protein β-defensin 4 (DEFB4) also exhibits a gene promoter VDRE but requires co-stimulation by activators of NF-κB, such as IL-1 signalling via the IL-1 receptor, to promote transcriptional up-regulation of DEFB4. Other signalling pathways have also been proposed to participate in the anti-mycobacterial activities of 1,25(OH)2D3. For example, PI3-kinase was found to regulate the antimycobacterial activity of 1,25(OH)2D3 by enhancing the generation of reactive oxygen species in monocytes and macrophages,127 thus augmenting another versatile mechanism of bacterial killing. Induction of both DEFB and hCAP promote bacterial killing in the resulting autolysosome generation (Fig. 3). Importantly, Yuk et al.65 have recently demonstrated the ability of vitamin D to induce autophagy and to mediate co-localization of M. tuberculosis and antimicrobial peptides within auto-phagolysosomes, facilitating the destruction of these bacteria. A novel immunomodulatory role for the 1,25(OH)2D3 in M. tuberculosis infection has been reported in its role to modulate expression of MMP, such as MMP-7, MMP-9 and MMP-10, by PBMCs, while inducing secretion of IL-10 and prostaglandin E2.60,128
Subsequent to vitamin D deficiency the infected macrophages are unable to produce sufficient 1,25-(OH)2D3 to up-regulate production of cathelicidin. Recent investigation shows that 1,25-(OH)2D3-stimulated induction of cathelicidin in cystic fibrosis bronchial epithelial cells causes an increased antibacterial activity against common cystic fibrosis airway pathogens such as Pseudomonas aeruginosa and Bordetella bronchiseptica.125 Based on these results, it has been speculated that the targeted use of inhaled 1,25-(OH)2D3 could augment the expression of cathelicidin on the mucosal surface of bronchial epithelia. Endoscopic studies in humans have demonstrated that β-defensin is secreted in the gastric mucosa after infection by Helicobacter pylori129 and may therefore constitute a major aspect of immune defence against this bacterial pathogen at the mucosal surface. Studies of VDR polymorphisms in humans support the hypothesis that variability in vitamin D status and host genes encoding vitamin D-responsive elements affect the immune response to bacterial pathogens other than M. tuberculosis.130 Therefore, much of what is know from the interaction between host vitamin D status and M. tuberculosis infection can enhance our understanding of the immunomodulatory properties of vitamin D in other bacterial diseases, although more research is required to help generalize this information to other clinical settings.
Vitamin D3 and virus infection
The optimal vitamin D status of the host may contribute key immunoregulatory functions in settings of viral respiratory infection by down-regulating excessive, and therefore toxic, cytokine responses, while allowing for improved clearance of various microbial species.131 The incidence of viral infections typically peaks in the winter months when cutaneous vitamin D synthesis is naturally weakened. In contrast to data available from adult subjects, infections observed in children with inadequate vitamin D stores are more frequently reported as being viral in origin. Several studies have pointed to adequate vitamin D concentrations playing a potential role in protecting against upper and lower respiratory tract infections. One of the first clues of the importance of vitamin D status on the incidence of viral infection came from evidence that in children susceptibility to infection occurs before evident manifestations of nutritional rickets become manifest. Moreover, the risk for acquiring an infection necessitating hospitalization is also reflective of a vitamin-D-insufficient state, rather than a secondary manifestation of the more severe vitamin D deficiency typically documented in cases of nutritional rickets. Vitamin D-related pathways have been studied in terms of their involvement in the host immune response to viral respiratory infections, such as influenza.131,132
Another example of an infection where clinical and genetic evidence collectively suggest that vitamin D may play a role in susceptibility to, as well as control of, the infection is HIV. Although increased prevalence of vitamin D deficiency in HIV-infected patients in comparison with uninfected hosts has been reported, these data remain contradictory.133 Laboratory models of HIV infection have demonstrated that pre-treatment of human monocytes and macrophages with 1,25-(OH)2D prevents HIV infection in certain cell lines134 but increases HIV replication in others.135 Another recent study demonstrated that cathelicidin, which is regulated in part by vitamin D, might directly inhibit the replication of HIV.136 Patients with AIDS with abnormally low serum-1,25D also had shorter survival times than other AIDS patients. These results indicate that serum-1,25D is correlated with the degree of immune deficiency in HIV infection. Low 1,25D is associated with increased incidence of AIDS events and reduced survival time.63 In one study, however, HIV-positive patients supplemented with vitamin D demonstrated a positive impact on their CD4+ T-cell counts.63,137 Further research into the connection between vitamin D and HIV is ongoing and includes studies on the role of vitamin D signalling and the VDR in HIV infection. Studies into VDR gene polymorphisms also support the association between vitamin D and other infectious diseases. Recently it has reported a significant association between genetic susceptibility to respiratory syncytial virus bronchiolitis and several single nucleotide polymorphisms of genes related to innate immune function, including the VDR.138 Children with the ff genotype of the VDR had increased risk of acquiring an acute lower respiratory tract infection, predominantly viral bronchiolitis.139 Other single nucleotide polymorphisms in this gene have been associated with susceptibility to tuberculosis,140 and the VDR has been implicated in down-regulating IL-12 and IFN-γ production.141
Further directions and concluding remarks
Based on the findings exposed so far, vitamin D supplementation may hold therapeutic promise in many diseases characterized by inflammation, including chronic infections, malignancies, cardiovascular diseases and autoimmune disorders such as rheumatoid arthritis, type 1 diabetes, inflammatory bowel disease and multiple sclerosis. To obtain immunomodulating effects in vitro requires local concentrations of 1,25(OH)2D3 of about 10−10 m. To achieve such concentrations in vivo requires supra-physiological doses of 1,25(OH)2D3, which are associated with the undesired risk of hypercalcaemia. Consequently, novel therapeutic strategies point toward vitamin D analogues that do not induce hypercalcaemia,142 which may exert considerable immunomodulatory activity at non-hypercalcaemic dosages and may have therapeutic potential for immune disorders or transplant rejection. In patients with Crohn's disease a vitamin D analogue activates VDR in PBMCs, affecting proliferation and exerting an immunosuppressive effect on tumour necrosis factor-α production, which is mediated by NF-κB down-regulation.143 Beneficial therapeutic effects of the supplementation of vitamin D were observed in the patients with systemic lupus erythematosus, in which an efficient tetanus toxoid immunization on vitamin D supplementation induced powerful specific antibody titres.144 Vitamin D may be responsible for reducing the development and severity of autoimmune and allergic diseases. The potential use of vitamin D to augment the innate immune response in atopic dermatitis has been examined extensively.145 Other clinical trials under examination have been the combination of 1,25(OH)2D3 with immunomodulators such as cyclosporine or bisphosphonates.146 Encouraging results have been obtained with both 1,25(OH)2D3 and Dex-DCs for their durable and differential tolerogenic features, acting via different mechanisms. Both could be useful to specifically down-regulate unwanted immune responses and to induce immune tolerance. The modulated DCs appear suitable as adjuvant in antigen-specific clinical vaccination intervention strategies.147 An experimental murine model of Th1-mediated colitis clearly indicated that combining Dex with calcitriol represents an attractive new immune modulatory treatment regimen, substantially because it promotes Treg cell functions with distinct increases of the regulatory set of IL-10, TGF-β, FoxP3, and cytotoxic T-lymphocyte antigen 4.148 Additionally, in prophylactic experiments evaluating the effect on cytokine secretion by DCs it was found that pretreatment with a combination of Dex and 1,25(OH)2D3 inhibits entero-antigen specific T-cell differentiation, prevents weight loss and ameliorates gut pathology upon CD4+ CD25 T-cell transfer in the SCID mouse model of colitis.149 A combination of 1,25(OH)2D3 plus Dex as well as Lactobacillus plantarum induces IL-10 production by human and murine DCs. Both 1,25(OH)2D3/Dex and Lactobacillus plantarum polarize naive T cells towards IL-10-expressing T cells, through distinct mechanisms. As adjuvant, they both enhance sublingual immunotherapy efficacy in a murine asthma model.150 Further studies reported that the combined administration of a corticosteroid drug and allergen extract suppressed the early clinical and immunological effects of specific immunotherapy and that 1,25(OH)2D3 prevented this ‘adverse’ influence of steroids. Topically applied 1,25(OH)2D3 enhances the immunoregulatory ability of CD4+ CD25+ T cells residing in the skin-draining lymph nodes of mice. However, in vitro treatment with 1,25(OH)2D3 did not modify the expression of 84 tested cytokine and cytokine-related mRNAs. It was only in the presence of IL-2 that 1,25(OH)2D3 increased the expression of genes including IL-2 and TLR4. Further, 1,25(OH)2D3 enhanced the ability of IL-2 to stimulate CD4+ CD25+ cells to proliferate in vitro and also regulate contact hypersensitivity responses on adoptive transfer into naive mice. Therefore, 1,25(OH)2D3 enabled by IL-2 can enhance the regulatory potential of CD4+ CD25+ T cells to control immune disease. The CD11c+ cells from the skin-draining lymph nodes of 1,25(OH)2D3-treated mice induced a significantly smaller ear-swelling response in a Th17-mediated model of contact hypersensitivity. The CD4+ CD25+ cells isolated from the ear-draining lymph nodes of mice that received ear injections of CD11c+ cells from donor mice topically treated with 1,25(OH)2D3 suppressed effector cell proliferation more potently. In addition, ear-draining lymph node cells from recipients of CD11c+ cells from 1,25(OH)2D3-treated mice produced increased IL-4. The CD11c+ cells from the skin-draining lymph nodes of mice treated with topical 1,25(OH)2D3 expressed increased levels of indoleamine 2,3-dioxygenase messenger RNA, a molecule by which topical 1,25(OH)2D3 may enhance the ability of DCs to control the suppressive function of CD4+ CD25+ cells.151,152
Several studies have also examined the influence of vitamin D or its derivatives on vaccine responses in humans, mainly conducted in immunocompromised patients, in particular patients on haemodialysis, because vitamin D deficiency is common in this group. The administration of calcitriol at a site adjacent to influenza vaccination did not enhance humoral immunity in these subjects.153 Vitamin D-based therapies at earlier stages of chronic kidney disease may impact the immune status of patients who progress to require dialysis or transplantation.154 Post-transplant osteoporosis in renal transplant patients is still a grave problem. In renal transplant patients who use low-dose methylprednisolone and new immunosuppressive agents together, low doses of 1,25(OH)2D3 and calcium replacement for 1 year provides a reduction in lumbar spine, femoral neck and femoral total bone loss, prevents bone loss and contributes to the normalization of parathyroid hormone levels.154
Interestingly, it has been reported that VDR agonists possess immunoregulatory properties. In particular, for their pronounced tolerogenic properties induced in DCs, leading to enhanced Treg-cell development, are the most used topical agents in the treatment of psoriasis and contribute to the beneficial activity in a variety of autoimmune disease and graft rejection models, highlighting their applicability to the treatment of chronic inflammatory conditions sustained by autoreactive or alloreactive immune responses.84
Current trials focused for non-specific immunomodulation on 1,25(OH)2D3 and thiazolidinediones in patients with slowly progressive insulin-dependent diabetes mellitus and in latent autoimmune diabetes are also promising.155
The expanding recognition of the association between vitamin D deficiency and tuberculosis (TB) risk and of the pleiotropic immunomodulatory functions of 1,25(OH)2D3 has led to evaluation of vitamin D as an adjunctive therapy in the treatment of active TB.48 As active TB infection is associated with deficiencies in l-arginine and vitamin D, a possible clinical application of both l-arginine and vitamin D as candidate adjunctive TB treatments has been proposed. There is a reasonable basis for clinical trials investigating the safety and efficacy of both l-arginine and vitamin D, singly or together as adjunctive immunotherapies in TB. Human macrophages use activated vitamin D and l-arginine-derived NO to kill TB bacilli. Expression of the CD3ζ chain of the T-cell receptor requires l-arginine, T cells need l-arginine to function optimally. Vitamin D and NO might both attenuate excessive organ pathology resulting from an amplified cell-mediated immune response. Therefore, the l-arginine and vitamin D immunological pathways could have differential importance during stages of TB infection or among individuals depending on prevailing genetic and cytokine influences. Whether l-arginine and vitamin D can reduce time to sputum clearance and culture negativity, administration of these agents would have potential advantages such as reduced transmission and improved outcome. Furthermore, with the increasing prevalence of multidrug-resistant TB, agents ameliorating innate mycobacterial killing mechanisms might improve treatment rates in drug-resistant disease.156
In conclusion, although the implications of 1,25(OH)2D3 synthesis in the maintenance of immune homeostasis are still under investigation, the current idea of its function as ‘more than just a key-player in bone-formation’ seems reasonable. In general, immune cells are not only targets for active 1,25(OH)2D3, but are able to activate this hormone in a local fashion, arguing for its autocrine or paracrine role within the immune system. The general hypothesis of linking inadequate 1,25(OH)2D3 levels to immune anomalies, such as increased infection rates, discussed in this review may stimulate the development of new ideas. A deeper understanding, not only on the mechanisms inducing immuno-modulatory actions, but also, on the consequences of VDR agonists, and particularly hypocalcaemic 1,25(OH)2D3 analogues, as plausible candidates for the prevention or treatment of infections could offer an attractive strategy to improve autoimmune disease management and reduce the risk of developing infections.
Disclosures
The authors have no conflicts of interests.
References
- 1.Holick MF. Vitamin D: a millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 2.Van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxy1,25(OH)2D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced 1,25(OH)2D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–93. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 4.Loser K, Beissert S. Regulation of cutaneous immunity by the environment: an important role for UV irradiation and vitamin D. Int Immunopharmacol. 2009;9:587–9. doi: 10.1016/j.intimp.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Alroy I, Towers T, Freedman LP. Transcriptional repression of the interleukin-2 gene by 1,25(OH)2D3: direct inhibition NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol Cell Biol. 1995;15:5789–99. doi: 10.1128/mcb.15.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Luca HF. Vitamin D: the vitamin and the hormone. Fed Proc. 1974;33:2211–9. [PubMed] [Google Scholar]
- 7.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA. 2004;101:7711–5. doi: 10.1073/pnas.0402490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jurutka PW, Bartik L, Whitfield GK, et al. Vitamin D receptor: key roles in bone mineral pathophysiology, molecular mechanism of action, and novel nutritional ligands. J Bone Miner Res. 2007;22(Suppl 2):V2–10. doi: 10.1359/jbmr.07s216. [DOI] [PubMed] [Google Scholar]
- 9.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chawla A, Repa J, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–70. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 11.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxy1,25(OH)2D3 receptors in human leukocytes. Science. 1983;221:1181–3. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 12.Lin R, White JH. The pleiotropic actions of vitamin D. Bioessays. 2004;26:21–8. doi: 10.1002/bies.10368. [DOI] [PubMed] [Google Scholar]
- 13.Carlberg C, Polly P. Gene regulation by 1,25(OH)2D3. Crit Rev Eukaryot Gene Expr. 1998;8:19–42. doi: 10.1615/critreveukargeneexpr.v8.i1.20. [DOI] [PubMed] [Google Scholar]
- 14.Torchia J, Glass C, Rosenfeld MG. Co-activators and co-repressors in the integration of transcriptional responses. Curr Opin Cell Biol. 1998;10:373–83. doi: 10.1016/s0955-0674(98)80014-8. [DOI] [PubMed] [Google Scholar]
- 15.Rachez C, Lemon BD, Suldan Z, et al. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature. 1999;398:824–8. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- 16.Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81:1269–304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- 17.Fujiki R, Kim MS, Sasaki Y, Yoshimura K, Kitagawa H, Kato S. Ligand-induced transrepression by VDR through association of WSTF with acetylated histones. EMBO J. 2005;24:3881–94. doi: 10.1038/sj.emboj.7600853. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Meir T, Levi R, Lieben L, Libutti S, Carmeliet G, Bouillon R, Silver J, Naveh-Many T. Deletion of the vitamin D receptor specifically in the parathyroid demonstrates a limited role for the receptor in parathyroid physiology. Am J Physiol Renal Physiol. 2009;297:F1192–8. doi: 10.1152/ajprenal.00360.2009. [DOI] [PubMed] [Google Scholar]
- 19.van Driel M, Koedam M, Buurman CJ, Hewison M, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP. Evidence for auto/paracrine actions of vitamin D in bone:1 α-hydroxylase expression and activity in human bone cells. FASEB J. 2006;13:2417–9. doi: 10.1096/fj.06-6374fje. [DOI] [PubMed] [Google Scholar]
- 20.Kim MS, Fujiki R, Kitagawa H, Kato S. 1α, 25(OH)2 D3-induced DNA methylation suppresses the human CYP27B1 gene. Mol Cell Endocrinol. 2007;265–266:168–73. doi: 10.1016/j.mce.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Rao J, Studzinski GP. Inhibition of p38 MAP kinase activity up-regulates multiple MAP kinase pathways and potentiates 1,25-dihydroxyvitamin D(3)-induced differentiation of human leukemia HL60 cells. Exp Cell Res. 2000;258:425–37. doi: 10.1006/excr.2000.4939. [DOI] [PubMed] [Google Scholar]
- 22.Cordes T, Diesing D, Becker S, Diedrich K, Reichrath J, Friedrich M. Modulation of MAPK ERK1 and ERK2 in VDR-positive and -negative breast cancer cell lines. Anticancer Res. 2006;26:2749–53. [PubMed] [Google Scholar]
- 23.Zhang X, Zanello LP. Vitamin D receptor-dependent 1 α,25(OH)2 1,25(OH)2D3-induced anti-apoptotic PI3K/AKT signaling in osteoblasts. J Bone Miner Res. 2008;23:1238–48. doi: 10.1359/JBMR.080326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buitrago C, Vazquez G, De Boland AR, Boland RL. Activation of Src kinase in skeletal muscle cells by 1, 1,25-(OH2-vitamin D3 correlates with tyrosine phosphorylation of the vitamin D receptor (VDR) and VDR-Src interaction. J Cell Biochem. 2000;79:274–81. doi: 10.1002/1097-4644(20001101)79:2<274::aid-jcb100>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 25.Carvallo L, Henríquez B, Paredes R, et al. 1α,25-dihydroxy 1,25(OH)2D3-enhanced expression of the osteocalcin gene involves increased promoter occupancy of basal transcription regulators and gradual recruitment of the 1α,25-dihydroxy 1,25(OH)2D3 receptor-SRC-1 coactivator complex. J Cell Physiol. 2008;214:740–9. doi: 10.1002/jcp.21267. [DOI] [PubMed] [Google Scholar]
- 26.Han S, Li T, Ellis E, Strom S, Chiang JY. A novel bile acid-activated vitamin D receptor signaling in human hepatocytes. Mol Endocrinol. 2010;24:1151–64. doi: 10.1210/me.2009-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Kovalenko P, Cui M, Desmet M, Clinton SK, Fleet JC. Constitutive activation of the mitogen-activated protein kinase pathway impairs vitamin D signaling in human prostate epithelial cells. J Cell Physiol. 2010;224:433–42. doi: 10.1002/jcp.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michigami T, Suga A, Yamazaki M, Shimizu C, Cai G, Okada S, Ozono K. Identification of amino acid sequence in the hinge region of human vitamin D receptor that transfers a cytosolic protein to the nucleus. J Biol Chem. 1999;274:33531–8. doi: 10.1074/jbc.274.47.33531. [DOI] [PubMed] [Google Scholar]
- 29.Prufer K, Barsony J. Retinoid X receptor dominates the nuclear import and export of the unliganded vitamin D receptor. Mol Endocrinol. 2002;16:1738–51. doi: 10.1210/me.2001-0345. [DOI] [PubMed] [Google Scholar]
- 30.Studzinski GP, Wang X, Ji Y, Wang Q, Zhang Y, Kutner A, Harrison JS. The rationale for deltanoids in therapy for myeloid leukemia: role of KSR-MAPK-C/EBP pathway. J Steroid Biochem Mol Biol. 2005;7:47–55. doi: 10.1016/j.jsbmb.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3:27–41. doi: 10.1038/nrd1283. [DOI] [PubMed] [Google Scholar]
- 32.Baeke F, Korf H, Overbergh L, van Etten E, Verstuyf A, Gysemans C, Mathieu C. Human T lymphocytes are direct targets of 1,25-dihydroxy1,25(OH)2D3 in the immune system. J Steroid Biochem Mol Biol. 2010;121:221–7. doi: 10.1016/j.jsbmb.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 33.Bao BY, Ting HJ, Hsu JW, Yasmin-Karim S, Messing E, Lee YF. Down-regulation of NF-κB signals is involved in loss of 1α, 25-dihydroxyvitamin responsiveness. J Steroid Biochem Mol Biol. 2010;120:11–21. doi: 10.1016/j.jsbmb.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 34.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–6. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 35.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxy1,25(OH)2D3 on human B cell differentiation. J Immunol. 2007;179:1634–47. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 36.Adams JS, Gacad MA. Characterization of 1 α-hydroxylation of 1,25(OH)2D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J Exp Med. 1985;161:755–65. doi: 10.1084/jem.161.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papapoulos SE, Clemens TL, Fraher LJ, Lewin IG, Sandler LM, O'Riordan JL. 1, 25 dihydroxycholecalciferol in the pathogenesis of the hypercalcaemia of sarcoidosis. Lancet. 1979;1:627–30. doi: 10.1016/s0140-6736(79)91076-6. [DOI] [PubMed] [Google Scholar]
- 38.Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxy1,25(OH)2D3-1α-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316–21. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 39.Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin North Am. 2010;39:365–9. doi: 10.1016/j.ecl.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abe E, Miyaura C, Tanaka H, et al. 1 α,25-dihydroxy1,25(OH)2D3 promotes fusion of mouse alveolar macrophages both by a direct mechanism and by a spleen cell-mediated indirect mechanism. Proc Natl Acad Sci U S A. 1983;80:5583–7. doi: 10.1073/pnas.80.18.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu H, Soruri A, Gieseler RK, Peters JH. 1,25-Dihydroxy1,25(OH)2D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand J Immunol. 1993;38:535–40. doi: 10.1111/j.1365-3083.1993.tb03237.x. [DOI] [PubMed] [Google Scholar]
- 42.Abu-Amer Y, Bar-Shavit Z. Impaired bone marrow-derived macrophage differentiation in vitamin D deficiency. Cell Immunol. 1993;151:356–68. doi: 10.1006/cimm.1993.1245. [DOI] [PubMed] [Google Scholar]
- 43.Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin-D-3-1 α-hydroxylase in human monocytes. J Bone Miner Res. 2006;21:37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- 44.Alappat L, Valerio M, Awad AB. Effect of vitamin D and β-sitosterol on immune function of macrophages. Int Immunopharmacol. 2010;10:1390–6. doi: 10.1016/j.intimp.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Koeffler HP, Reichel H, Bishop JE, Norman AW. γ-Interferon stimulates production of 1,25-dihydroxy1,25(OH)2D3 by normal human macrophages. Biochem Biophys Res Commun. 1985;127:596–603. doi: 10.1016/s0006-291x(85)80202-3. [DOI] [PubMed] [Google Scholar]
- 46.Reichel H, Koeffler HP, Bishop JE, Norman AW. 25-Hydroxy1,25(OH)2D3 metabolism by lipopolysaccharide-stimulated normal human macrophages. J Clin Endocrinol Metab. 1987;64:1–9. doi: 10.1210/jcem-64-1-1. [DOI] [PubMed] [Google Scholar]
- 47.Liu PT, Stenger S, Li H, et al. Toll-like receptor riggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 48.O'Dell SN, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: Third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–92. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 49.Oberg F, Botling J, Nilsson K. Functional antagonism between 1,25(OH)2D3 and retinoic acid in the regulation of CD14 and CD23 expression during monocytic differentiation of U-937 cells. J Immunol. 1993;150:3487–95. [PubMed] [Google Scholar]
- 50.Dickie LJ, Church LD, Coulthard LR, Mathews RJ, Emery P, McDermott MF. 1,25(OH)2D3 down-regulates intra-cellular Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology (Oxford) 2010;49:1466–71. doi: 10.1093/rheumatology/keq124. [DOI] [PubMed] [Google Scholar]
- 51.Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, Modlin RL. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115–20. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peric M, Koglin S, Kim S-M, et al. IL-17A enhances 1,25(OH)2D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol. 2008;181:8504–12. doi: 10.4049/jimmunol.181.12.8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sadeghi K, Wessner B, Laggner U, et al. 1,25(OH)2D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36:361–70. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 54.Scherberich JE, Kellermeyer M, Ried C, Hartinger A. 1-α-calcidol modulates major human monocyte antigens and toll-like receptors TLR 2 and TLR4 in vitro. Eur J Med Res. 2005;10:179–82. [PubMed] [Google Scholar]
- 55.Urry Z, Xystrakis E, Richards DF, et al. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1α,25-dihydroxy1,25(OH)2D3 abrogates regulatory function. J Clin Invest. 2009;119:387–98. doi: 10.1172/JCI32354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iannuzzi MC, Rybicki BA, Teirstein AS. Medical progress: sarcoidosis. N Engl J Med. 2007;357:2153–65. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 57.Wang TT, Dabbas B, Laperriere D, et al. Direct and indirect induction by 1,25-dihydroxy1,25(OH)2D3 of the NOD2/CARD15-defensin β2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285:2227–31. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams JS, Ren S, Liu PT, et al. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182:4289–95. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ren S, Nguyen L, Wu S, Encinas C, Adams JS, Hewison M. Alternative splicing of vitamin D-24-hydroxylase: a novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. J Biol Chem. 2005;280:20604–11. doi: 10.1074/jbc.M414522200. [DOI] [PubMed] [Google Scholar]
- 60.Coussens A, Timms PM, Boucher BJ, et al. 1α,25-dihydroxy1,25(OH)2D3 inhibits matrix metalloproteinases induced by Mycobacterium tuberculosis infection. Immunology. 2009;127:539–48. doi: 10.1111/j.1365-2567.2008.03024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxy1,25(OH)2D3. FASEB J. 2005;19:1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 62.Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxy1,25(OH)2D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 63.Bergman P, Walter-Jallow L, Broliden K, Agerberth B, Soderlund J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr HIV Res. 2007;5:410–5. doi: 10.2174/157016207781023947. [DOI] [PubMed] [Google Scholar]
- 64.Gombart AF, Saito T, Koeffler HP. Exaptation of an ancient Alu short interspersed element provides a highly conserved vitamin D-mediated innate immune response in humans and primates. BMC Genomics. 2009;10:321. doi: 10.1186/1471-2164-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yuk JM, Shin DM, Lee HM, et al. 1,25(OH)2D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–43. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Steinstraesser L, Tippler B, Mertens J, et al. Inhibition of early steps in the lentiviral replication cycle by cathelicidin host defense peptides. Retrovirology. 2005;2:2. doi: 10.1186/1742-4690-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rockett KA, Brookes R, Udalova I, Vidal V, Hill AV, Kwiatkowski D. 1,25-Dihydroxy1,25(OH)2D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect Immun. 1998;66:5314–21. doi: 10.1128/iai.66.11.5314-5321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsunawa M, Amano Y, Endo K, Uno S, Sakaki T, Yamada S, Makishima M. The aryl hydrocarbon receptor activator benzo[a]pyrene enhances 1,25(OH)2D3 catabolism in macrophages. Toxicol Sci. 2009;109:50–8. doi: 10.1093/toxsci/kfp044. [DOI] [PubMed] [Google Scholar]
- 69.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 70.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 71.Brennan A, Katz DR, Nunn JD, Barker S, Hewison M, Fraher LJ, O'Riordan JL. Dendritic cells from human tissues express receptors for the immunoregulatory 1,25(OH)2D3 metabolite, dihydroxycholecalciferol. Immunology. 1987;61:457–61. [PMC free article] [PubMed] [Google Scholar]
- 72.Adorini L, Penna G, Giarratana N, Roncari A, Amuchastegui S, Daniel KC, Uskokovic M. Dendritic cells as key targets for immunomodulation by Vitamin D receptor ligands. J Steroid Biochem Mol Biol. 2004;90:437–41. doi: 10.1016/j.jsbmb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 73.Hewison M, Freeman L, Hughes SV, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170:5382–90. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 74.Dam TN, Møller B, Hindkjaer J, Kragballe K. The Vitamin D3 analog calcipotriol suppresses the number and antigen-presenting function of Langerhans cells in normal human skin. J Investig Dermatol Symp Proc. 1996;1:72–7. [PubMed] [Google Scholar]
- 75.Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, Bergamini A. 1Alpha,25-dihydroxy1,25(OH)2D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. 2009;45:190–7. doi: 10.1016/j.cyto.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 76.Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, Allavena P, Di Carlo V. 1,25(OH)2D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–51. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 77.Pedersen AW, Holmstrøm K, Jensen SS, Fuchs D, Rasmussen S, Kvistborg P, Claesson MH, Zocca MB. Phenotypic and functional markers for 1 α,25-dihydroxyvitamin D(3)-modified regulatory dendritic cells. Clin Exp Immunol. 2009;157:48–59. doi: 10.1111/j.1365-2249.2009.03961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, Adorini L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxy1,25(OH)2D3. Blood. 2005;106:3490–7. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- 79.Steinbrink K, Graulich E, Kubsch S, Knop J, Enk AH. CD4+ and CD8+ anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–76. doi: 10.1182/blood.v99.7.2468. [DOI] [PubMed] [Google Scholar]
- 80.Griffin MD, Xing N, Kumar R. Gene expression profiles in dendritic cells conditioned by 1α,25-dihydroxy1,25(OH)2D3 analog. J Steroid Biochem Mol Biol. 2004;89–90:443–8. doi: 10.1016/j.jsbmb.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 81.van EE, Decallonne B, Bouillon R, Mathieu C. NOD bone marrow-derived dendritic cells are modulated by analogs of 1,25-dihydroxy1,25(OH)2D3. J Steroid Biochem Mol Biol. 2004;89–90:457–9. doi: 10.1016/j.jsbmb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 82.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 α,25-dihydroxy1,25(OH)2D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:1945–53. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 83.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1α,25 dihydroxy1,25(OH)2D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:6800–5. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol. 2009;70:345–52. doi: 10.1016/j.humimm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 85.Penna G, Amuchastegui S, Giarratana N, Daniel KC, Vulcano M, Sozzani S, Adorini L. 1,25-dihydroxy1,25(OH)2D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 2007;178:145–53. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 86.Dong X, Lutz W, Schroeder TM, Bachman LA, Westendorf JJ, Kumar R, Griffin MD. Regulation of relB in dendritic cells by means of modulated association of vitamin D receptor and histone deacetylase 3 with the promoter. Proc Natl Acad Sci USA. 2005;102:16007–12. doi: 10.1073/pnas.0506516102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heine G, Anton K, Henz BM, Worm M. 1,25-dihydroxy1,25(OH)2D3 inhibits anti-CD40 plus IL-4-mediated IgE production in vitro. Eur J Immunol. 2002;32:3395–404. doi: 10.1002/1521-4141(200212)32:12<3395::AID-IMMU3395>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 88.Morgan JW, Kouttab N, Ford D, Maizel AL. Vitamin D-mediated gene regulation in phenotypically defined human B cell subpopulations. Endocrinology. 2000;141:3225–34. doi: 10.1210/endo.141.9.7666. [DOI] [PubMed] [Google Scholar]
- 89.Hickish T, Cunningham D, Colston K, Millar BC, Sandle J, Mackay AG, Soukop M, Sloane J. The effect of 1,25-dihydroxyvitamin-D(3) on lymphoma cell-lines and expression of vitamin-D receptor in lymphoma. Br J Cancer. 1993;68:668–72. doi: 10.1038/bjc.1993.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grammer AC, Lipsky PE. B cell abnormalities in systemic lupus erythematosus. Arthritis Res Ther. 2003;5:S22–7. doi: 10.1186/ar1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vernino L, McAnally LM, Ramberg J, Lipsky PE. Generation of nondividing high-rate Ig-secreting plasma-cells in cultures of human B-cells stimulated with anti-Cd3-activated T-cells. J Immunol. 1992;148:404–10. [PubMed] [Google Scholar]
- 92.von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010;11:344–9. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- 93.Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat Rev Immunol. 2004;4:301–8. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- 94.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Topilski I, Flaishon L, Naveh Y, Harmelin A, Levo Y, Shachar I. The anti-inflammatory effects of 1,25-dihydroxy1,25(OH)2D3 on Th2 cells in vivo are due in part to the control of integrin mediated T lymphocyte homing. Eur J Immunol. 2004;34:1068–76. doi: 10.1002/eji.200324532. [DOI] [PubMed] [Google Scholar]
- 96.Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxy1,25(OH)2D3: preferential inhibition of Th1 functions. J Nutr. 1995;125:1704S–8S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- 97.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4-positive T cells. J Cell Biochem. 2003;89:922–32. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 98.Vidyarani M, Selvaraj P, Jawahar MS, Narayanan PR. 1,25-Dihydroxy1,25(OH)2D3 modulated cytokine response in pulmonary tuberculosis. Cytokine. 2007;40:128–34. doi: 10.1016/j.cyto.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 99.Yu S, Bruce D, Froicu M, Weaver V, Cantorna MT. Failure of T cell homing, reduced CD4/CD8αα intraepithelial lymphocytes, and inflammation in the gut of vitamin D receptor KO mice. Proc Natl Acad Sci U S A. 2008;105:20834–9. doi: 10.1073/pnas.0808700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O'Kelly J, Hisatake J, Hisatake Y, Bishop J, Norman A, Koeffler HP. Normal myelopoiesis but abnormal T lymphocyte responses in vitamin D receptor knockout mice. J Clin Invest. 2002;109:1091–9. doi: 10.1172/JCI12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weaver CT, Hatton RD, Mangan PR. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 102.Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Semin Immunol. 2007;19:362–71. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of TNBS colitis with calcitriol is associated with a change of a Th1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2007;323:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 104.Liu N, Nguyen L, Chun RF, et al. Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology. 2008;149:4799–808. doi: 10.1210/en.2008-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jeffery LE, Burke F, Mura M, et al. 1,25-Dihydroxy1,25(OH)2D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–67. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gorman S, Kuritzky LA, Judge MA, Dixon KM, McGlade JP, Mason RS, Finlay-Jones JJ, Hart PH. Topically applied 1,25-dihydroxy1,25(OH)2D3 enhances the suppressive activity of CD4+-CD25+ cells in the draining lymph nodes. J Immunol. 2007;179:6273–83. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 107.Ghoreishi M, Bach P, Obst J, Komba M, Fleet JC, Dutz JP. Expansion of antigen-specific regulatory T cells with the topical vitamin D analog calcipotriol. J Immunol. 2009;182:6071–8. doi: 10.4049/jimmunol.0804064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reboldi A, Coisne C, Baumjohann D, et al. CC chemokine receptor 6-regulated entry of Th-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–23. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 109.Amadi-Obi A, Yu CR, Liu X, et al. Th17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 110.Diveu C, McGeachy MJ, Boniface K, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–56. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 111.Fitzgerald DC, Zhang GX, El-Behi M, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–9. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 112.Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Spach KM, Nashold FE, Dittel BN, Hayes CE. IL-10 signaling is essential for 1,25-dihydroxy1,25(OH)2D3-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:6030–7. doi: 10.4049/jimmunol.177.9.6030. [DOI] [PubMed] [Google Scholar]
- 114.Chang JH, Cha HR, Lee DS, Seo KY, Kweon MN. 1,25-Dihydroxy1,25(OH)2D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. PLoS ONE. 2010;5:12925. doi: 10.1371/journal.pone.0012925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bhalla AK, Amento EP, Krane SM. Differential effects of 1,25-dihydroxy1,25(OH)2D3 on human lymphocytes and monocyte/macrophages: inhibition of interleukin-2 and augmentation of interleukin-1 production. Cell Immunol. 1986;98:311–22. doi: 10.1016/0008-8749(86)90291-1. [DOI] [PubMed] [Google Scholar]
- 116.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–98. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gregori S, Giarratana N, Smiroldo S. 1α,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 118.Dong X, Bachman LA, Kumar R, Griffin MD. Generation of antigen-specific, interleukin-10-producing T-cells using dendritic cell stimulation and steroid hormone conditioning. Transpl Immunol. 2003;11:323–33. doi: 10.1016/S0966-3274(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 119.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374:334–8. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 120.Willheim M, Thien R, Schrattbauer K, et al. Regulatory effects of 1α,25-dihydroxy1,25(OH)2D3 on the cytokine production of human peripheral blood lymphocytes. J Clin Endocrinol Metab. 1999;84:3739–44. doi: 10.1210/jcem.84.10.6054. [DOI] [PubMed] [Google Scholar]
- 121.Iho S, Iwamoto K, Kura F, Okuno Y, Takahashi T, Hoshino T. Mechanism in 1,25(OH)2D3-induced suppression of helper/suppressor function of CD4/CD8 cells to immunoglobulin production in B cells. Cell Immunol. 1990;127:12–5. doi: 10.1016/0008-8749(90)90110-d. [DOI] [PubMed] [Google Scholar]
- 122.Meehan TF, De Luca HF. CD8 (1) T cells are not necessary for 1 α,25-dihydroxyvitamin D(3) to suppress experimental autoimmune encephalomyelitis in mice. Proc Natl Acad Sci USA. 2002;99:5557–60. doi: 10.1073/pnas.082100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rook GA, Steele J, Fraher L, Barker S, Karmali R, O'Riordan J, Stanford J. 1,25(OH)2D3, γ interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57:159–63. [PMC free article] [PubMed] [Google Scholar]
- 124.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–3. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 125.Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3) J Cyst Fibros. 2007;6:403–10. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gombart AF, O'Kelly J, Saito T, Koeffler HP. Regulation of the CAMP gene by 1,25(OH)2D3 in various tissues. J Steroid Biochem Mol Biol. 2007;103:552–5. doi: 10.1016/j.jsbmb.2006.12.095. [DOI] [PubMed] [Google Scholar]
- 127.Sly LM, Lopez M, Nauseef WM, Reiner NE. 1α,25-Dihydroxy1,25(OH)2D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Biol Chem. 2001;276:35482–93. doi: 10.1074/jbc.M102876200. [DOI] [PubMed] [Google Scholar]
- 128.Anand SP, Selvaraj P. Effect of 1, 25 dihydroxyvitamin D(3) on matrix metalloproteinases MMP-7, MMP-9 and the inhibitor TIMP-1 in pulmonary tuberculosis. Clin Immunol. 2009;133:126–31. doi: 10.1016/j.clim.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 129.Wehkamp J, Schauber J, Stange EF. Defensins and cathelicidins in gastrointestinal infections. Curr Opin Gastroenterol. 2007;23:32–8. doi: 10.1097/MOG.0b013e32801182c2. [DOI] [PubMed] [Google Scholar]
- 130.Malik MH, Jury F, Bayat A, Ollier WE, Kay PR. Genetic susceptibility to total hip arthroplasty failure: a preliminary study on the influence of matrix metalloproteinase 1, IL-6 polymorphisms and vitamin D receptor. Ann Rheum Dis. 2007;66:1116–20. doi: 10.1136/ard.2006.062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–40. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grant WB. Variations in vitamin D production could possibly explain the seasonality of childhood respiratory infections in Hawaii. Pediatr Infect Dis J. 2008;27:853. doi: 10.1097/INF.0b013e3181817bc1. [DOI] [PubMed] [Google Scholar]
- 133.Rodríguez M, Daniels B, Gunawardene S, Robbins GK. High frequency of vitamin D deficiency in ambulatory HIV-positive patients. AIDS Res Hum Retroviruses. 2009;25:9–14. doi: 10.1089/aid.2008.0183. [DOI] [PubMed] [Google Scholar]
- 134.Van Den Bout-Van Den Beukel CJ, Fievez L, Michels M, et al. Vitamin D deficiency among HIV type 1-infected individuals in the Netherlands: effects of antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24:1375–82. doi: 10.1089/aid.2008.0058. [DOI] [PubMed] [Google Scholar]
- 135.Connor RI, Rigby WF. 1 α,25-Dihydroxy1,25(OH)2D3 inhibits productive infection of human monocytes by HIV-1. Biochem Biophys Res Commun. 1991;176:852–9. doi: 10.1016/s0006-291x(05)80264-5. [DOI] [PubMed] [Google Scholar]
- 136.Kizaki M, Ikeda Y, Simon KJ, Nanjo M, Koeffler HP. Effect of 1,25-dihydroxy1,25(OH)2D3 and its analogs on human immunodeficiency virus infection in monocytes/macrophages. Leukemia. 1993;7:1525–30. [PubMed] [Google Scholar]
- 137.Haug CJ, Müller F, Rollag H, Aukrust P, Degré M, Frøland SS. The effect of 1,25-1,25(OH)2D3 on maturation of monocytes from HIV-infected patients varies with degree of immunodeficiency. APMIS. 1996;104:539–48. doi: 10.1111/j.1699-0463.1996.tb04909.x. [DOI] [PubMed] [Google Scholar]
- 138.Villamor E. A potential role for vitamin D on HIV infection? Nutr Rev. 2006;64:226–33. doi: 10.1301/nr.2006.may.226-233. [DOI] [PubMed] [Google Scholar]
- 139.Janssen R, Bont L, Siezen CL, et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J Infect Dis. 2007;196:826–34. doi: 10.1086/520886. [DOI] [PubMed] [Google Scholar]
- 140.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case–control study. Lancet. 2000;355:618–21. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 141.Lemire JM. Immunomodulatory actions of 1,25-dihydroxy1,25(OH)2D3. J Steroid Biochem Mol Biol. 1995;53:599–602. doi: 10.1016/0960-0760(95)00106-a. [DOI] [PubMed] [Google Scholar]
- 142.Zügel U, Steinmeyer A, May E, Lehmann M, Asadullah K. Immunomodulation by a novel, dissociated Vitamin D analogue. Exp Dermatol. 2009;18:619–27. doi: 10.1111/j.1600-0625.2009.00845.x. [DOI] [PubMed] [Google Scholar]
- 143.Stio M, Martinesi M, Bruni S, et al. The Vitamin D analogue TX 527 blocks NF-κB activation in peripheral blood mononuclear cells of patients with Crohn's disease. J Steroid Biochem Mol Biol. 2007;103:51–60. doi: 10.1016/j.jsbmb.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 144.Heine G, Drozdenko G, Lahl A, et al. Efficient tetanus toxoid immunization on vitamin D supplementation. Eur J Clin Nutr. 2011;65:329–34. doi: 10.1038/ejcn.2010.276. [DOI] [PubMed] [Google Scholar]
- 145.Searing DA, Leung DY. Vitamin D in atopic dermatitis, asthma and allergic diseases. Immunol Allergy Clin North Am. 2010;30:397–9. doi: 10.1016/j.iac.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Geller JL, Adams JS. Vitamin D therapy. Curr Osteoporos Rep. 2008;6:5–11. doi: 10.1007/s11914-008-0002-z. [DOI] [PubMed] [Google Scholar]
- 147.Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by 1,25(OH)2D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009;39:3147–59. doi: 10.1002/eji.200839103. [DOI] [PubMed] [Google Scholar]
- 148.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune Modulatory Treatment of Trinitrobenzene Sulfonic Acid Colitis with Calcitriol Is Associated with a Change of a T Helper (Th) 1/Th17 to a Th2 and Regulatory T cell Profile. J Pharmacol Exp Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 149.Pedersen AE, Schmidt EG, Gad M, Poulsen SS, Claesson MH. Dexamethasone/1α-25-dihydroxyvitamin D3-treated dendritic cells suppress colitis in the SCID T-cell transfer model. Immunology. 2009;127:354–64. doi: 10.1111/j.1365-2567.2008.02996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Van Overtvelt L, Lombardi V, Razafindratsita A, Saint-Lu N, Horiot S, Moussu H, Mascarell L, Moingeon P. IL-10-inducing adjuvants enhance sublingual immunotherapy efficacy in a murine asthma model. Int Arch Allergy Immunol. 2008;145:152–62. doi: 10.1159/000108140. [DOI] [PubMed] [Google Scholar]
- 151.Majak P, Rychlik B, Stelmach I. The effect of oral steroids with and without 1,25(OH)2D3 on early efficacy of immunotherapy in asthmatic children. Clin Exp Allergy. 2009;39:1830–41. doi: 10.1111/j.1365-2222.2009.03357.x. [DOI] [PubMed] [Google Scholar]
- 152.Gorman S, Judge MA, Hart PH. Topical 1,25-dihydroxy1,25(OH)2D3 subverts the priming ability of draining lymph node dendritic cells. Immunology. 2010;131:415–25. doi: 10.1111/j.1365-2567.2010.03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sahin G, Yasar NS, Sirmagul B, Bal C, Yalcin AU. The effect of low-dose cholecalciferol and calcium treatment on posttransplant bone loss in renal transplant patients: a prospective study. Ren Fail. 2008;30:992–9. doi: 10.1080/08860220802406369. [DOI] [PubMed] [Google Scholar]
- 154.Mathieu C, Jafari M. Immunomodulation by 1,25-dihydroxy1,25(OH)2D3: therapeutic implications in hemodialysis and renal transplantation. Clin Nephrol. 2006;66:275–83. doi: 10.5414/cnp66275. [DOI] [PubMed] [Google Scholar]
- 155.Pozzilli P, Guglielmi C. Immunomodulation for the prevention of SPIDDM and LADA. Ann N Y Acad Sci. 2006;1079:90–8. doi: 10.1196/annals.1375.012. [DOI] [PubMed] [Google Scholar]
- 156.Ralph AP, Kelly Paul M, Anstey NM. l-Arginine and vitamin D: novel adjunctive immunotherapies in tuberculosis. Trends Microbiol. 2008;16:336–44. doi: 10.1016/j.tim.2008.04.003. [DOI] [PubMed] [Google Scholar]


